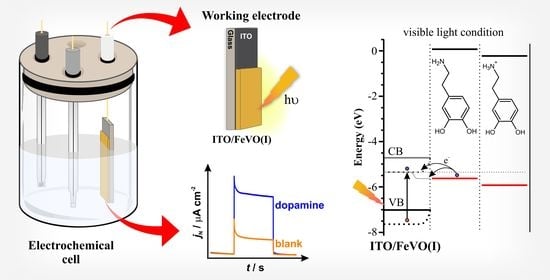
Visible Light Photoelectrochemical Sensor for Dopamine: Determination Using Iron Vanadate Modified Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterization
2.2. Electrochemical and Photoelectrochemical Characterization
2.3. Dopamine Detection
3. Materials and Methods
3.1. Materials
3.2. Construction of ITO/Iron Vanadate Electrode by the SILAR Process
3.3. Physical Characterization
3.4. Photoelectrochemical Characterization
3.5. Electrochemical and Photoelectrochemical Dopamine Detection
3.6. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bromberg-Martin, E.S.; Matsumoto, M.; Hikosaka, O. Dopamine in Motivational Control: Rewarding, Aversive, and Alerting. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.; Fouillioux, C.; Bolívar, A.; Simonovis, N.; Hernández-Hernández, R.; Armas-Hernandez, M.J.; Velasco, M. Dopamine, Hypertension and Obesity. J. Hum. Hypertens. 2002, 16, S13–S17. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Wager, T.D.; Kober, H.; Bliss-Moreau, E.; Barrett, L.F. The Brain Basis of Emotion: A Meta-Analytic Review. Behav. Brain Sci. 2012, 35, 121–143. [Google Scholar] [CrossRef]
- Murray, T.F. Biogenic Amine Transmitters. In Veterinary Psychopharmacology; Wiley: Omaha, NE, USA, 2019; pp. 29–42. [Google Scholar]
- Post, M.R.; Sulzer, D. The Chemical Tools for Imaging Dopamine Release. Cell Chem. Biol. 2021, 28, 748–764. [Google Scholar] [CrossRef]
- Arumugasamy, S.K.; Govindaraju, S.; Yun, K. Electrochemical Sensor for Detecting Dopamine Using Graphene Quantum Dots Incorporated with Multiwall Carbon Nanotubes. Appl. Surf. Sci. 2020, 508, 145294. [Google Scholar] [CrossRef]
- Barbeau, A.; Joffroy, L.G.; Mars, H. Treatment of Parkinson’s Disease with Levodopa and Ro 4-4602. Clin. Pharmacol. Ther. 1971, 12, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.; Engel, J.; Fuxe, K. L-Dopa Induced Changes in Central Monoamine Neurons after Peripheral Decarboxylase Inhibition. J. Pharm. Pharmacol. 2011, 22, 313–316. [Google Scholar] [CrossRef]
- Barbeau, A.; Roy, M. Six-Year Results of Treatment with Levodopa plus Benzerazide in Parkinson’s Disease. Neurology 1976, 26, 399. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, G.; Juge, O.; Birchler, A. Parkinsonian Syndromes. Treatment by Association of L-Dopa plus Decarboxylase Inhibito. Eur. Neurol. 1974, 11, 133–157. [Google Scholar] [CrossRef]
- Vázquez-Guardado, A.; Barkam, S.; Peppler, M.; Biswas, A.; Dennis, W.; Das, S.; Seal, S.; Chanda, D. Enzyme-Free Plasmonic Biosensor for Direct Detection of Neurotransmitter Dopamine from Whole Blood. Nano Lett. 2019, 19, 449–454. [Google Scholar] [CrossRef]
- Hubbard, K.E.; Wells, A.; Owens, T.S.; Tagen, M.; Fraga, C.H.; Stewart, C.F. Determination of Dopamine, Serotonin, and Their Metabolites in Pediatric Cerebrospinal Fluid by Isocratic High Performance Liquid Chromatography Coupled with Electrochemical Detection. Biomed. Chromatogr. 2009, 24, 626–631. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zha, Q.; Zhai, C.; Li, W.; Zeng, L.; Zhu, M. Photo-Electrochemical Detection of Dopamine in Human Urine and Calf Serum Based on MIL-101 (Cr)/Carbon Black. Microchim. Acta 2020, 187, 526. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J. Biosensors and Sensors for Dopamine Detection. View 2021, 2, 20200102. [Google Scholar] [CrossRef]
- da Silva Pelissari, M.R.; Camargo, L.P.; da Silva, P.R.C.; Dall’Antonia, L.H. Fe2V4O13 Photoanode Material: An Interesting Approach to Non-Enzymatic Glucose Oxidation. J. Mater. Sci. 2022, 57, 7173–7190. [Google Scholar] [CrossRef]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for Electrochemical Non-Enzymatic Glucose Biosensors. RSC Adv. 2013, 3, 3487. [Google Scholar] [CrossRef]
- Anshori, I.; Kepakisan, K.A.A.; Nuraviana Rizalputri, L.; Rona Althof, R.; Nugroho, A.E.; Siburian, R.; Handayani, M. Facile Synthesis of Graphene Oxide/Fe3O4 Nanocomposite for Electrochemical Sensing on Determination of Dopamine. Nanocomposites 2022, 8, 155–166. [Google Scholar] [CrossRef]
- Trindade, C.M.B.; Silva, M.K.L.; Cesarino, I. Copper Nanostructures Anchored on Renewable Carbon as Electrochemical Platform for the Detection of Dopamine, Fluoxetine and Escitalopram. Sens. Actuators Rep. 2022, 4, 100107. [Google Scholar] [CrossRef]
- Chu, H.; Sun, X.; Zha, X.; Zhang, Y.; Wang, Y. Synthesis of Core-Shell Structured Metal Oxide@covalent Organic Framework Composites as a Novel Electrochemical Platform for Dopamine Sensing. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129238. [Google Scholar] [CrossRef]
- Ranku, M.N.; Uwaya, G.E.; Fayemi, O.E. Electrochemical Detection of Dopamine at Fe3O4/SPEEK Modified Electrode. Molecules 2021, 26, 5357. [Google Scholar] [CrossRef]
- Hao, W.; Zhang, Y.; Fan, J.; Liu, H.; Shi, Q.; Liu, W.; Peng, Q.; Zang, G. Copper Nanowires Modified with Graphene Oxide Nanosheets for Simultaneous Voltammetric Determination of Ascorbic Acid, Dopamine and Acetaminophen. Molecules 2019, 24, 2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbumannan, V.; Kumar, R.T.R.; Suresh, K. Enhanced Electrochemical Detection of Dopamine by Graphene Oxide/Tungsten Trioxide Nanocomposite. Mater. Sci. Semicond. Process. 2021, 127, 105696. [Google Scholar] [CrossRef]
- Wu, H.; Tan, H.L.; Toe, C.Y.; Scott, J.; Wang, L.; Amal, R.; Ng, Y.H. Photocatalytic and Photoelectrochemical Systems: Similarities and Differences. Adv. Mater. 2019, 32, 1904717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.; Dong, S.; Qiao, J.; Zeng, Z.; Shao, S. A Visible-Light-Driven Photoelectrochemical Sensing Platform Based on the BiVO4/FeOOH Photoanode for Dopamine Detection. Electrochim. Acta 2022, 414, 140207. [Google Scholar] [CrossRef]
- Ibrahim, I.; Lim, H.N.; Mohd Zawawi, R.; Ahmad Tajudin, A.; Ng, Y.H.; Guo, H.; Huang, N.M. A Review on Visible-Light Induced Photoelectrochemical Sensors Based on CdS Nanoparticles. J. Mater. Chem. B 2018, 6, 4551–4568. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.M.R.; Azevedo Neto, N.F.; Camargo, L.P.; Dall’Antonia, L.H. Characterization and Photo-Induced Electrocatalytic Evaluation for BiVO4 Films Obtained by the SILAR Process. Electrocatalysis 2021, 12, 211–224. [Google Scholar] [CrossRef]
- Lucilha, A.C.; Camargo, L.P.; Liberatti, V.R.; Barbosa, E.C.M.; Dall’Antonia, L.H. Zn1-xCoxO vs. Ag-ZnO Photoanodes Design via Combustion: Characterization and Application in Photoelectrocatalysis. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128261. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied Photoelectrocatalysis on the Degradation of Organic Pollutants in Wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Zarei, E.; Ojani, R. Fundamentals and Some Applications of Photoelectrocatalysis and Effective Factors on Its Efficiency: A Review. J. Solid State Electrochem. 2017, 21, 305–336. [Google Scholar] [CrossRef]
- Camargo, L.P.; Lucilha, A.C.; Gomes, G.A.B.; Liberatti, V.R.; Andrello, A.C.; da Silva, P.R.C.; Dall’Antonia, L.H. Copper Pyrovanadate Electrodes Prepared by Combustion Synthesis: Evaluation of Photoelectroactivity. J. Solid State Electrochem. 2020, 24, 1935–1950. [Google Scholar] [CrossRef]
- Prado, T.M.; Carrico, A.; Cincotto, F.H.; Fatibello-Filho, O.; Moraes, F.C. Bismuth Vanadate/Graphene Quantum Dot: A New Nanocomposite for Photoelectrochemical Determination of Dopamine. Sens. Actuators B Chem. 2019, 285, 248–253. [Google Scholar] [CrossRef]
- Yu, L.-M.; Zhu, Y.-C.; Liu, Y.-L.; Qu, P.; Xu, M.-T.; Shen, Q.; Zhao, W.-W. Ferroelectric Perovskite Oxide@TiO2 Nanorod Heterostructures: Preparation, Characterization, and Application as a Platform for Photoelectrochemical Bioanalysis. Anal. Chem. 2018, 90, 10803–10811. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-L.; Jiao, H.-J.; Liu, K.-L.; Wu, X.-M.; Dong, Y.-M.; Li, Z.-J.; Zhang, C. A Novel Strategy for the Construction of Photoelectrochemical Sensors Based on Quantum Dots and Electron Acceptor: The Case of Dopamine Detection. Electrochem. Commun. 2014, 41, 47–50. [Google Scholar] [CrossRef]
- Silva Araújo, M.; Barretto, T.R.; Galvão, J.C.R.; Tarley, C.R.T.; Dall’Antônia, L.H.; Matos, R.; Medeiros, R.A. Visible Light Photoelectrochemical Sensor for Acetaminophen Determination Using a Glassy Carbon Electrode Modified with BiVO4 Nanoparticles. Electroanalysis 2021, 33, 663–671. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, L.; Bei, J.; Xie, M.; Zhu, X.; Chen, T.; Wang, X.; Du, Y.; Yao, Y. An Specific Photoelectrochemical Sensor Based on Pillar [5] Arenes Functionalized Gold Nanoparticles and Bismuth Oxybromide Nanoflowers for Bovine Hemoglobin Recognition. J. Colloid Interface Sci. 2022, 620, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Bai, X.; Zhang, Y.; Gao, K. Photoelectrochemical CdSe/TiO2 Nanotube Array Microsensor for High-Resolution in-Situ Detection of Dopamine. Microchim. Acta 2018, 185, 278. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Q.; Du, X.; Qian, J.; Mao, H.; Wang, K. Visible Light Photoelectrochemical Sensor for Ultrasensitive Determination of Dopamine Based on Synergistic Effect of Graphene Quantum Dots and TiO2 Nanoparticles. Anal. Chim. Acta 2015, 853, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Hun, X.; Wang, S.; Wang, S.; Zhao, J.; Luo, X. A Photoelectrochemical Sensor for Ultrasensitive Dopamine Detection Based on Single-Layer NanoMoS2 Modified Gold Electrode. Sens. Actuators B Chem. 2017, 249, 83–89. [Google Scholar] [CrossRef]
- Ye, C.; Wang, M.Q.; Gao, Z.F.; Zhang, Y.; Lei, J.L.; Luo, H.Q.; Li, N.B. Ligating Dopamine as Signal Lrigger onto the Substrate via Metal-Catalyst-Free Click Chemistry for “Signal-on” Photoelectrochemical Sensing of Ultralow MicroRNA Levels. Anal. Chem. 2016, 88, 11444–11449. [Google Scholar] [CrossRef]
- Jayaprakash, G.K.; Swamy, B.E.K.; Chandrashekar, B.N.; Flores-Moreno, R. Theoretical and Cyclic Voltammetric Studies on Electrocatalysis of Benzethonium Chloride at Carbon Paste Electrode for Detection of Dopamine in Presence of Ascorbic Acid. J. Mol. Liq. 2017, 240, 395–401. [Google Scholar] [CrossRef]
- Tang, D.; Rettie, A.J.E.; Mabayoje, O.; Wygant, B.R.; Lai, Y.; Liu, Y.; Mullins, C.B. Facile Growth of Porous Fe2V4O13 Films for Photoelectrochemical Water Oxidation. J. Mater. Chem. A 2016, 4, 3034–3042. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Y.; Li, H.; Xu, Q.; Meng, X.; Wang, X.; Xiao, M.; Zou, Z. All-Solid-State Z-Scheme System Arrays of Fe2V4O13/RGO/CdS for Visible Light-Driving Photocatalytic CO2 Reduction into Renewable Hydrocarbon Fuel. Chem. Commun. 2015, 51, 800–803. [Google Scholar] [CrossRef]
- Crespo, C.T. Electronic, Magnetic, Optical and Solar Energy Absorption Properties of the Fe2V4O13 Vanadate. Sol. Energy 2019, 183, 345–349. [Google Scholar] [CrossRef]
- Vuk, A.Š.; Orel, B.; Dražič, G.; Decker, F.; Colomban, P. UV-Visible and IR Spectroelectrochemical Studies of FeVO4 Sol-Gel Films for Electrochromic Applications. J. Sol. Gel Sci. Technol. Vol. 2002, 23, 165–181. [Google Scholar] [CrossRef]
- Thaba, K.P.; Mphahlele-Makgwane, M.M.; Kyesmen, P.I.; Diale, M.; Baker, P.G.L.; Makgwane, P.R. Composition-Dependent Structure Evolution of FeVO4 Nano-Oxide and Its Visible-Light Photocatalytic Activity for Degradation of Methylene Blue. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127856. [Google Scholar] [CrossRef]
- Sajid, M.M.; Zhai, H.; Shad, N.A.; Shafique, M.; Afzal, A.M.; Javed, Y.; Khan, S.B.; Ikram, M.; Amin, N.; Zhang, Z. Photocatalytic Performance of Ferric Vanadate (FeVO4) Nanoparticles Synthesized by Hydrothermal Method. Mater. Sci. Semicond. Process. 2021, 129, 105785. [Google Scholar] [CrossRef]
- Melghit, K.; Al-Mungi, A.S. New Form of Iron Orthovanadate FeVO4·1.5H2O Prepared at Normal Pressure and Low Temperature. Mater. Sci. Eng. B 2007, 136, 177–181. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Masjedi-Arani, M.; Mazloom, F. An Easy Sonochemical Route for Synthesis, Characterization and Photocatalytic Performance of Nanosized FeVO4 in the Presence of Aminoacids as Green Capping Agents. J. Mater. Sci. Mater. Electron. 2018, 29, 474–485. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik Der Farbanstriche. Z Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Mandal, H.; Shyamal, S.; Hajra, P.; Bera, A.; Sariket, D.; Kundu, S.; Bhattacharya, C. Development of Ternary Iron Vanadium Oxide Semiconductors for Applications in Photoelectrochemical Water Oxidation. RSC Adv. 2016, 6, 4992–4999. [Google Scholar] [CrossRef]
- Monfort, O. Structure and Properties of Vanadium-Based Oxides. In Introduction to Photochemical Processes for Environmental Purposes; Brigante, M., Ed.; Comenius University Bratislava: Bratislava, Slovakia, 2022; p. 77. ISBN 978-80-223-5380-9. [Google Scholar]
- Allen, J.B.; Larry, R.F.; Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Carrera-Crespo, J.E.; Fuentes-Camargo, I.; Palma-Goyes, R.E.; García-Pérez, U.M.; Vazquez-Arenas, J.; Chairez, I.; Poznyak, T. Unrevealing the Effect of Transparent Fluorine-Doped Tin Oxide (FTO) Substrate and Irradiance Configuration to Unmask the Activity of FTO-BiVO4 Heterojunction. Mater. Sci. Semicond. Process. 2021, 128, 105717. [Google Scholar] [CrossRef]
- Kuroda, M.A.; Tersoff, J.; Nistor, R.A.; Martyna, G.J. Optimal Thickness for Charge Transfer in Multilayer Graphene Electrodes. Phys. Rev. Appl. 2014, 1, 014005. [Google Scholar] [CrossRef]
- Yotsumoto Neto, S.; de Cássia Silva Luz, R.; Santos Damos, F. Photoelectroanalytical Sensor Based on TiO2 Nanoparticles/Copper Tetrasulfonated Phthalocyanine for Detection of Dopamine Exploiting Light Emitting Diode Irradiation. Electroanalysis 2016, 28, 2087–2092. [Google Scholar] [CrossRef]
- Fishman, R.A. Physiology of the Cerebrospinal Fluid. Arch. Neurol. 1968, 18, 459–460. [Google Scholar] [CrossRef]
- Davson, H. Physiology of the Cerebrospinal Fluid; Harcourt Brace/Churchill Livingstone: London, UK, 1967; ISBN 978-0700012909. [Google Scholar]
- Martínez-Huitle, C.A.; Cerro-Lopez, M.; Quiroz, M.A. Electrochemical Behaviour of Dopamine at Covalent Modified Glassy Carbon Electrode with L-Cysteine: Preliminary Results. Mater. Res. 2009, 12, 375–384. [Google Scholar] [CrossRef]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine Oxidation and Autophagy. Parkinsons. Dis. 2012, 2012, 920953. [Google Scholar] [CrossRef]
- Batagin-Neto, A.; Oliveira, E.F.; Graeff, C.F.O.; Lavarda, F.C. Modelling Polymers with Side Chains: MEH-PPV and P3HT. Mol. Simul. 2013, 39, 309–321. [Google Scholar] [CrossRef]
- Allouche, A.-R. Gabedit-A Graphical User Interface for Computational Chemistry Softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Yang, W.; Mortier, W.J. The Use of Global and Local Molecular Parameters for the Analysis of the Gas-Phase Basicity of Amines. J. Am. Chem. Soc. 1986, 108, 5708–5711. [Google Scholar] [CrossRef] [PubMed]
- Maia, R.A.; Ventorim, G.; Batagin-Neto, A. Reactivity of Lignin Subunits: The Influence of Dehydrogenation and Formation of Dimeric Structures. J. Mol. Model. 2019, 25, 228. [Google Scholar] [CrossRef] [PubMed]
- Ayers, P.W.; Morell, C.; De Proft, F.; Geerlings, P. Understanding the Woodward–Hoffmann Rules by Using Changes in Electron Density. Chem. A Eur. J. 2007, 13, 8240–8247. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. MOPAC: A Semiempirical Molecular Orbital Program. J. Comput. Aided. Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Soltani, A.R.; Baei, M.T. A DFT Study on Structure and Electronic Properties of BN Nanostructures Adsorbed with Dopamine. Computation 2019, 7, 61. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Kudur Jayaprakash, G.; Pandith, A.; Karaoglanli, A.C.; Uzun, O. Electrocatalytic Investigation by Improving the Charge Kinetics between Carbon Electrodes and Dopamine Using Bio-Synthesized CuO Nanoparticles. Catalysts 2022, 12, 994. [Google Scholar] [CrossRef]
- Rossi-Fernández, A.C.; Meier, L.A.; Castellani, N.J. Neutral and Zwitterionic Dopamine Species Adsorbed on Silver Surfaces: A DFT Investigation of Interaction Mechanism. Int. J. Quantum Chem. 2019, 119, e25817. [Google Scholar] [CrossRef]








| Electrode | EA/cm2 | jN (μA cm−2) [a] | jph (μA cm−2) [b] | Response Time [c]/s | Rct (kΩ) [d] | ||
|---|---|---|---|---|---|---|---|
| Visible Light | Dark | Visible Light | Dark | ||||
| ITO | 0.44 | 0.30 | 0.20 | ~0.1 | * | * | * |
| ITO/FeVO(I) | 0.62 | 15.60 | 0.05 | 15.55 | 0.41 | 119 | 5525 |
| ITO/FeVO(II) | 0.50 | 7.80 | 0.10 | 7.70 | 0.51 | 257 | 2561 |
| ITO/FeVO(III) | 0.73 | 0.70 | 0.05 | 0.65 | 0.88 | 2160 | 2225 |
| Analytical Parameters | Condition | |
|---|---|---|
| Dark | Visible Light | |
| Linear range/µmol L−1 | 1.27–31.64; 31.64–75.93 | 1.27–31.64; 31.64–75.93 |
| Sensitivity/µA cm−2 µmol−1 L | 0.029 | 0.113 |
| Correlation coefficient (R2) 1 | 0.9982 | 0.9987 |
| Limit of detection/µmol L−1 | 0.76 | 1.71 |
| Limit of quantification/µmol L−1 | 2.52 | 5.69 |
| Analytical Parameters | Condition | |
|---|---|---|
| Dark | Visible Light | |
| Linear range/µmol L−1 | 1.21–30.32; 30.32–72.77 | 1.21–30.32; 30.32–72.77 |
| Sensitivity/µA cm−2 µmol−1 L | 0.028 | 0.123 |
| Correlation coefficient (R2) 1 | 0.9922 | 0.9985 |
| Limit of detection/µmol L−1 | 0.33 | 0.34 |
| Limit of quantification/µmol L−1 | 1.10 | 1.12 |
| Sensor | Technique | Linear Range/µmol L−1 | Limit of Detection/µmol L−1 | Reference |
|---|---|---|---|---|
| CdSe/TiO2 | PEC-CHRO | 0.05–20.0 | 16.70 | [36] |
| CuTsPc/TiO2 | PEC-CHRO | 4–810 | 0.50 | [56] |
| FeOOH + BiVO4 | PEC-CHRO | 0.2–40; 40–1400 | 0.09 | [24] |
| GO + Fe3O4 | ELE-DPV | 1–10 | 0.48 | [17] |
| Fe3O4 | ELE-SWV | 5–50 | 7.10 | [20] |
| Cu + GO | ELE-DPV | 1–100 | 0.41 | [21] |
| GO/WO3 | ELE-CHRO | 0.3–1245 | 0.31 | [22] |
| ITO/FeVO(I) | PEC-DPV | 1.27–31.64 | 1.71 | This work |
| PEC-CHRO | 1.21–30.32 | 0.34 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, L.P.; da Silva Pelissari, M.R.; da Silva, P.R.C.; Batagin-Neto, A.; Medeiros, R.A.; Dias, M.A.; Dall’Antonia, L.H. Visible Light Photoelectrochemical Sensor for Dopamine: Determination Using Iron Vanadate Modified Electrode. Molecules 2022, 27, 6410. https://doi.org/10.3390/molecules27196410
Camargo LP, da Silva Pelissari MR, da Silva PRC, Batagin-Neto A, Medeiros RA, Dias MA, Dall’Antonia LH. Visible Light Photoelectrochemical Sensor for Dopamine: Determination Using Iron Vanadate Modified Electrode. Molecules. 2022; 27(19):6410. https://doi.org/10.3390/molecules27196410
Chicago/Turabian StyleCamargo, Luan Pereira, Marcelo Rodrigues da Silva Pelissari, Paulo Rogério Catarini da Silva, Augusto Batagin-Neto, Roberta Antigo Medeiros, Marcos Antônio Dias, and Luiz Henrique Dall’Antonia. 2022. "Visible Light Photoelectrochemical Sensor for Dopamine: Determination Using Iron Vanadate Modified Electrode" Molecules 27, no. 19: 6410. https://doi.org/10.3390/molecules27196410
APA StyleCamargo, L. P., da Silva Pelissari, M. R., da Silva, P. R. C., Batagin-Neto, A., Medeiros, R. A., Dias, M. A., & Dall’Antonia, L. H. (2022). Visible Light Photoelectrochemical Sensor for Dopamine: Determination Using Iron Vanadate Modified Electrode. Molecules, 27(19), 6410. https://doi.org/10.3390/molecules27196410