Preparation of Biocomposites with Natural Reinforcements: The Effect of Native Starch and Sugarcane Bagasse Fibers
Abstract
:1. Introduction
2. Results
2.1. Structure of the Reinforcements
2.2. Composite Properties
2.3. Reinforcement
2.4. Deformation and Failure
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ulonska, K.; König, A.; Klatt, M.; Mitsos, A.; Viell, J. Optimization of Multiproduct Biorefinery Processes under Consideration of Biomass Supply Chain Management and Market Developments. Ind. Eng. Chem. Res. 2018, 57, 6980–6991. [Google Scholar] [CrossRef]
- Miller, S.A. Sustainable Polymers: Opportunities for the Next Decade. ACS Macro Lett. 2013, 2, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Tomadoni, B.; Casalongué, C.; Alvarez, V.A. Biopolymer-Based Hydrogels for Agriculture Applications: Swelling Behavior and Slow Release of Agrochemicals. In Polymers for Agri-Food Applications; Gutiérrez, T.J., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 99–125. [Google Scholar]
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Gigante, V.; Seggiani, M.; Cinelli, P.; Signori, F.; Vania, A.; Navarini, L.; Amato, G.; Lazzeri, A. Utilization of coffee silverskin in the production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biopolymer-based thermoplastic biocomposites for food contact applications. Compos. Part A Appl. Sci. Manuf. 2021, 140, 106172. [Google Scholar] [CrossRef]
- Niaounakis, M. 6-Automotive Applications. In Biopolymers: Applications and Trends; William Andrew Publishing: Oxford, UK, 2015; pp. 257–289. [Google Scholar]
- Verma, D.; Dogra, V.; Chaudhary, A.K.; Mordia, R. 5-Advanced biopolymer-based composites: Construction and structural applications. In Sustainable Biopolymer Composites; Verma, D., Sharma, M., Goh, K.L., Jain, S., Sharma, H., Eds.; Woodhead Publishing: Sawston, UK, 2022; pp. 113–128. [Google Scholar]
- Alam, M.A.; Sapuan, S.M.; Ya, H.H.; Hussain, P.B.; Azeem, M.; Ilyas, R.A. Chapter 1-Application of biocomposites in automotive components: A review. In Biocomposite and Synthetic Composites for Automotive Applications; Sapuan, S.M., Ilyas, R.A., Eds.; Woodhead Publishing: Duxford, UK, 2021; pp. 1–17. [Google Scholar]
- Rusu, D.; Boyer, S.A.E.; Lacrampe, M.F.; Krawczak, P. Bioplastics and vegetal fiber reinforced bioplastics for automotive applications. In Handbook of Bioplastics and Biocomposites Engineering Applications; Pilla, S., Ed.; Scrivener Publishing LLC.: Beverly, MA, USA, 2011; pp. 397–449. [Google Scholar]
- Ivar do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef]
- Cui, L.; Imre, B.; Tátraaljai, D.; Pukánszky, B. Physical ageing of poly(lactic acid): Factors and consequences for practice. Polymer 2020, 186, 122014. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Mallegni, N.; Rizzo, S.; Cinelli, P.; Lazzeri, A. Improved impact properties in poly(lactic acid) (PLA) blends containing cellulose acetate (CA) prepared by reactive extrusion. Materials 2019, 12, 270. [Google Scholar] [CrossRef] [Green Version]
- Hassouna, F.; Raquez, J.-M.; Addiego, F.; Toniazzo, V.; Dubois, P.; Ruch, D. New development on plasticized poly(lactide): Chemical grafting of citrate on PLA by reactive extrusion. Eur. Polym. J. 2012, 48, 404–415. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Arrieta, M.P.; López-Martínez, J.; Samper, M.D. Improvement of PLA film ductility by plasticization with epoxidized karanja oil. Polym. Degrad. Stab. 2020, 179, 109259. [Google Scholar] [CrossRef]
- Boyacioglu, S.; Kodal, M.; Ozkoc, G. A comprehensive study on shape memory behavior of PEG plasticized PLA/TPU bio-blends. Eur. Polym. J. 2020, 122, 109372. [Google Scholar] [CrossRef]
- Li, X.; Hegyesi, N.; Zhang, Y.; Mao, Z.; Feng, X.; Wang, B.; Pukánszky, B.; Sui, X. Poly(lactic acid)/lignin blends prepared with the Pickering emulsion template method. Eur. Polym. J. 2019, 110, 378–384. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Bhat, A.H.; Ahmad, F. Polymer blend of PLA/PHBV based bionanocomposites reinforced with nanocrystalline cellulose for potential application as packaging material. Carbohydr. Polym. 2017, 157, 1323–1332. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Dorgan, J.R.; Schilling, K. Water transport in polylactic acid (PLA), PLA/polycaprolactone copolymers, and PLA/polyethylene glycol blends. J. Environ. Polym. Degrad. 1997, 5, 125–136. [Google Scholar] [CrossRef]
- Gao, H.; Hu, S.; Su, F.; Zhang, J.; Tang, G. Mechanical, thermal, and biodegradability properties of PLA/modified starch blends. Polym. Compos. 2011, 32, 2093–2100. [Google Scholar] [CrossRef]
- Yao, M.; Deng, H.; Mai, F.; Wang, K.; Zhang, Q.; Chen, F.; Fu, Q. Modification of poly(lactic acid)/poly(propylene carbonate) blends through melt compounding with maleic anhydride. Express Polym. Lett. 2011, 5, 937–949. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Franciszczak, P.; Meljon, A. High performance hybrid PP and PLA biocomposites reinforced with short man-made cellulose fibres and softwood flour. Compos. Part A Appl. Sci. Manuf. 2015, 74, 132–139. [Google Scholar] [CrossRef]
- Csikós, Á.; Faludi, G.; Domján, A.; Renner, K.; Móczó, J.; Pukánszky, B. Modification of interfacial adhesion with a functionalized polymer in PLA/wood composites. Eur. Polym. J. 2015, 68, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Bartos, A.; Nagy, K.; Anggono, J.; Purwaningsih, H.; Móczó, J.; Pukánszky, B. Biobased PLA/sugarcane bagasse fiber composites: Effect of fiber characteristics and interfacial adhesion on properties. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106273. [Google Scholar] [CrossRef]
- Lila, M.K.; Shukla, K.; Komal, U.K.; Singh, I. Accelerated thermal ageing behaviour of bagasse fibers reinforced poly(lactic acid) based biocomposites. Compos. Part B Eng. 2019, 156, 121–127. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Wood-fiber-reinforced poly(lactic acid) composites: Evaluation of the physicomechanical and morphological properties. J. Appl. Polym. Sci. 2006, 102, 4856–4869. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Bax, B.; Müssig, J. Impact and tensile properties of PLA/Cordenka and PLA/flax composites. Compos. Sci. Technol. 2008, 68, 1601–1607. [Google Scholar] [CrossRef]
- Oksman, K.; Skrifvars, M.; Selin, J.-F. Natural fibres as reinforcement in polylactic acid (PLA) composites. Compos. Sci. Technol. 2003, 63, 1317–1324. [Google Scholar] [CrossRef]
- Yusuf, M.; Shabbir, M.; Mohammad, F. Natural Colorants: Historical, Processing and Sustainable Prospects. Nat. Prod. Bioprospecting 2017, 7, 123–145. [Google Scholar] [CrossRef]
- Van Den Oever, M.J.A.; Boeriu, C.G.; Blaauw, R.; Van Haveren, J. Colorants based on renewable resources and food-grade colorants for application in thermoplastics. J. Appl. Polym. Sci. 2004, 92, 2961–2969. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Kmiotek, M.; Bieliński, D.; Piotrowska, M. Propolis as an antidegradant and biocidal agent for natural rubber. J. Appl. Polym. Sci. 2018, 135, 45911. [Google Scholar] [CrossRef]
- Liu, L.; Qian, M.; Song, P.; Huang, G.; Yu, Y.; Fu, S. Fabrication of Green Lignin-based Flame Retardants for Enhancing the Thermal and Fire Retardancy Properties of Polypropylene/Wood Composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Strandberg, C.; Albertsson, A.C. Process efficiency and long-term performance of α-tocopherol in film-blown linear low-density polyethylene. J. Appl. Polym. Sci. 2005, 98, 2427–2439. [Google Scholar] [CrossRef]
- Dabbaghi, A.; Jahandideh, A.; Kabiri, K.; Ramazani, A.; Zohuriaan-Mehr, M.J. The synthesis and incorporation of a star-shaped bio-based modifier in the acrylic acid based superabsorbent: A strategy to enhance the absorbency under load. Polym. Plast. Technol. Mater. 2019, 58, 1678–1690. [Google Scholar] [CrossRef]
- Wypych, G. 3-Impact Modifiers. In Databook of Impact Modifiers; ChemTec Publishing: Scarborough, ON, Canada, 2022; pp. 17–448. [Google Scholar]
- Carvalho, A.J.F. Starch: Major sources, properties and applications as thermoplastic materials. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 129–152. [Google Scholar]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-based biodegradable materials: Challenges and opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glass transition behaviour of concentrated barley starch-glycerol-water mixtures, a model for thermoplastic starch. Carbohydr. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Li, H.B.; Huneault, M.A. Comparison of Sorbitol and Glycerol as Plasticizers for Thermoplastic Starch in TPS/PLA Blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Wokadala, O.C.; Emmambux, N.M.; Ray, S.S. Inducing PLA/starch compatibility through butyl-etherification of waxy and high amylose starch. Carbohydr. Polym. 2014, 112, 216–224. [Google Scholar] [CrossRef]
- Yang, X.; Finne-Wistrand, A.; Hakkarainen, M. Improved dispersion of grafted starch granules leads to lower water resistance for starch-g-PLA/PLA composites. Compos. Sci. Technol. 2013, 86, 149–156. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Sun, X. Mechanical Properties of Poly(lactic acid)/Starch Composites Compatibilized by Maleic Anhydride. Biomacromolecules 2004, 5, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, H.P. Fillers and reinforcing materials in plastics-physicochemical aspects for the processor. Kunstst.-Ger. Plast. 1983, 73, 511–515. [Google Scholar]
- Bledzki, A.K.; Gassan, J. Composites reinforced with cellulose based fibres. Prog. Polym. Sci. 1999, 24, 221–274. [Google Scholar] [CrossRef]
- Bartos, A.; Anggono, J.; Farkas, Á.E.; Kun, D.; Soetaredjo, F.E.; Móczó, J.; Purwaningsih, H.; Pukánszky, B. Alkali treatment of lignocellulosic fibers extracted from sugarcane bagasse: Composition, structure, properties. Polym. Test. 2020, 88, 106549. [Google Scholar] [CrossRef]
- Bartos, A.; Utomo, B.P.; Kanyar, B.; Anggono, J.; Soetaredjo, F.E.; Móczó, J.; Pukánszky, B. Reinforcement of polypropylene with alkali-treated sugarcane bagasse fibers: Mechanism and consequences. Compos. Sci. Technol. 2020, 200, 108428. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Sunthornvarabhas, J.; Park, J.-W.; Lee, J.-H.; Kim, H.-J.; Piyachomkwan, K.; Sriroth, K.; Cho, D. Tensile properties of kenaf fiber and corn husk flour reinforced poly(lactic acid) hybrid bio-composites: Role of aspect ratio of natural fibers. Compos. Part B Eng. 2014, 56, 232–237. [Google Scholar] [CrossRef]
- Pukánszky, B.; Belina, K.; Rockenbauer, A.; Maurer, F.H.J. Effect of nucleation, filler anisotropy and orientation on the properties of PP composites. Composites 1994, 25, 205–214. [Google Scholar] [CrossRef]
- Rowell, R.M. Natural fibres: Types and properties. In Properties and Performance of Natural-Fibre Composites; Pickering, K.L., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2008; pp. 3–66. [Google Scholar]
- Schroeter, J.; Hobelsberger, M. On the Mechanical Properties of Native Starch Granules. Starch 1992, 44, 247–252. [Google Scholar] [CrossRef]
- Dányádi, L.; Renner, K.; Szabó, Z.; Nagy, G.; Móczó, J.; Pukánszky, B. Wood flour filled PP composites: Adhesion, deformation, failure. Polym. Adv. Technol. 2006, 17, 967–974. [Google Scholar] [CrossRef]
- Faludi, G.; Dora, G.; Imre, B.; Renner, K.; Móczó, J.; Pukánszky, B. PLA/lignocellulosic fiber composites: Particle characteristics, interfacial adhesion, and failure mechanism. J. Appl. Polym. Sci. 2014, 131, 39902. [Google Scholar] [CrossRef]
- Várdai, R.; Ferdinánd, M.; Lummerstorfer, T.; Pretschuh, C.; Jerabek, M.; Gahleitner, M.; Faludi, G.; Móczó, J.; Pukánszky, B. Effect of various organic fibers on the stiffness, strength and impact resistance of polypropylene; a comparison. Polym. Int. 2021, 70, 145–153. [Google Scholar] [CrossRef]
- Várdai, R.; Lummerstorfer, T.; Pretschuh, C.; Jerabek, M.; Gahleitner, M.; Pukánszky, B.; Renner, K. Impact modification of PP/wood composites: A new approach using hybrid fibers. Express Polym. Lett. 2019, 13, 223–234. [Google Scholar] [CrossRef]
- Pukánszky, B. Influence of interface interaction on the ultimate tensile properties of polymer composites. Composites 1990, 21, 255–262. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction; John Wiley & Son: New York, NY, USA, 2018. [Google Scholar]
- Ferdinánd, M.; Várdai, R.; Móczó, J.; Pukánszky, B. Deformation and Failure Mechanism of Particulate Filled and Short Fiber Reinforced Thermoplastics: Detection and Analysis by Acoustic Emission Testing. Polymers 2021, 13, 3931. [Google Scholar] [CrossRef]
- Pukánszky, B.; Vörös, G. Mechanism of interfacial interactions in particulate filled composites. Compos. Interfaces 1993, 1, 411–427. [Google Scholar] [CrossRef]
- Soest, P.J.V. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. Assoc. Off. Agric. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
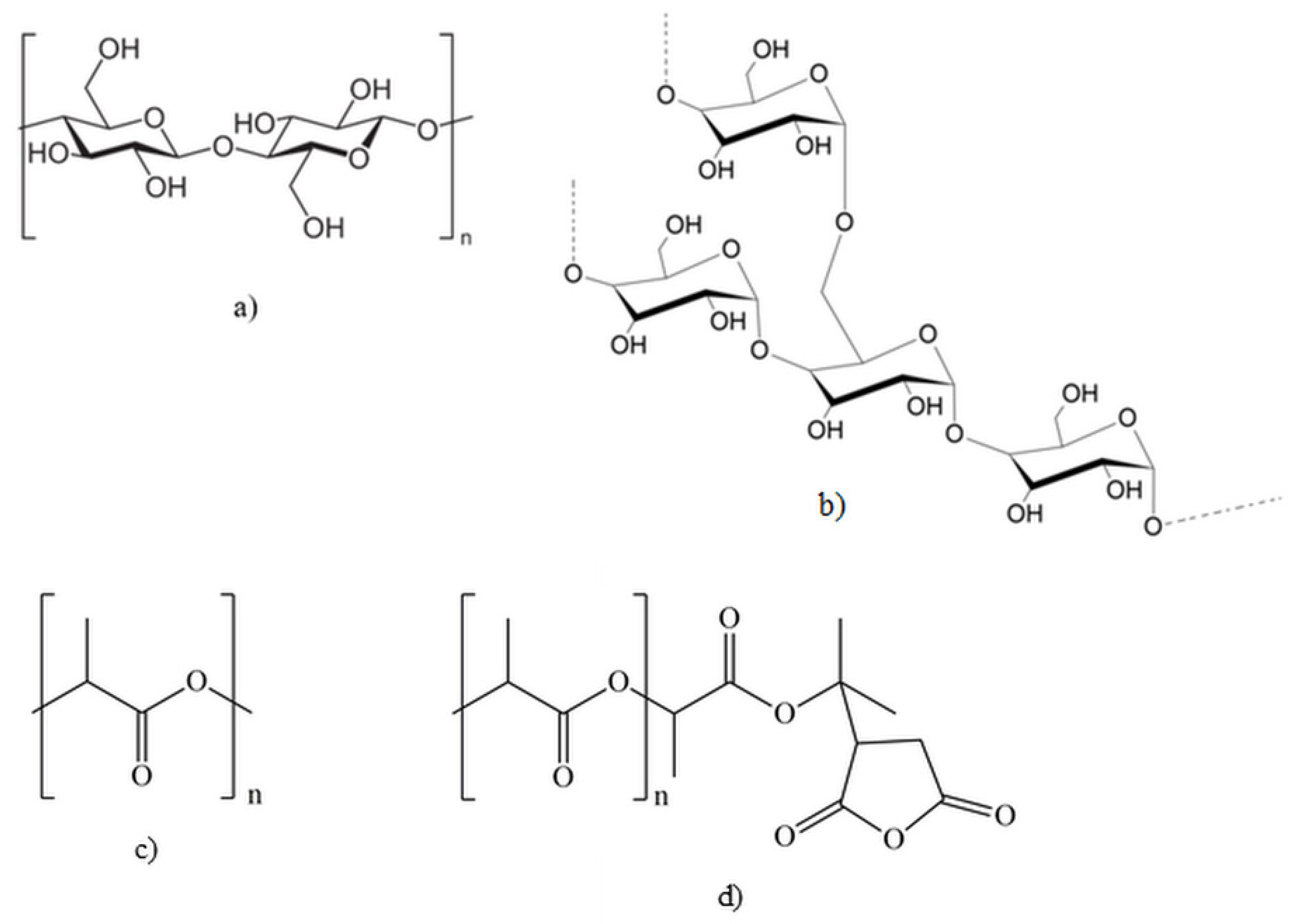
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.
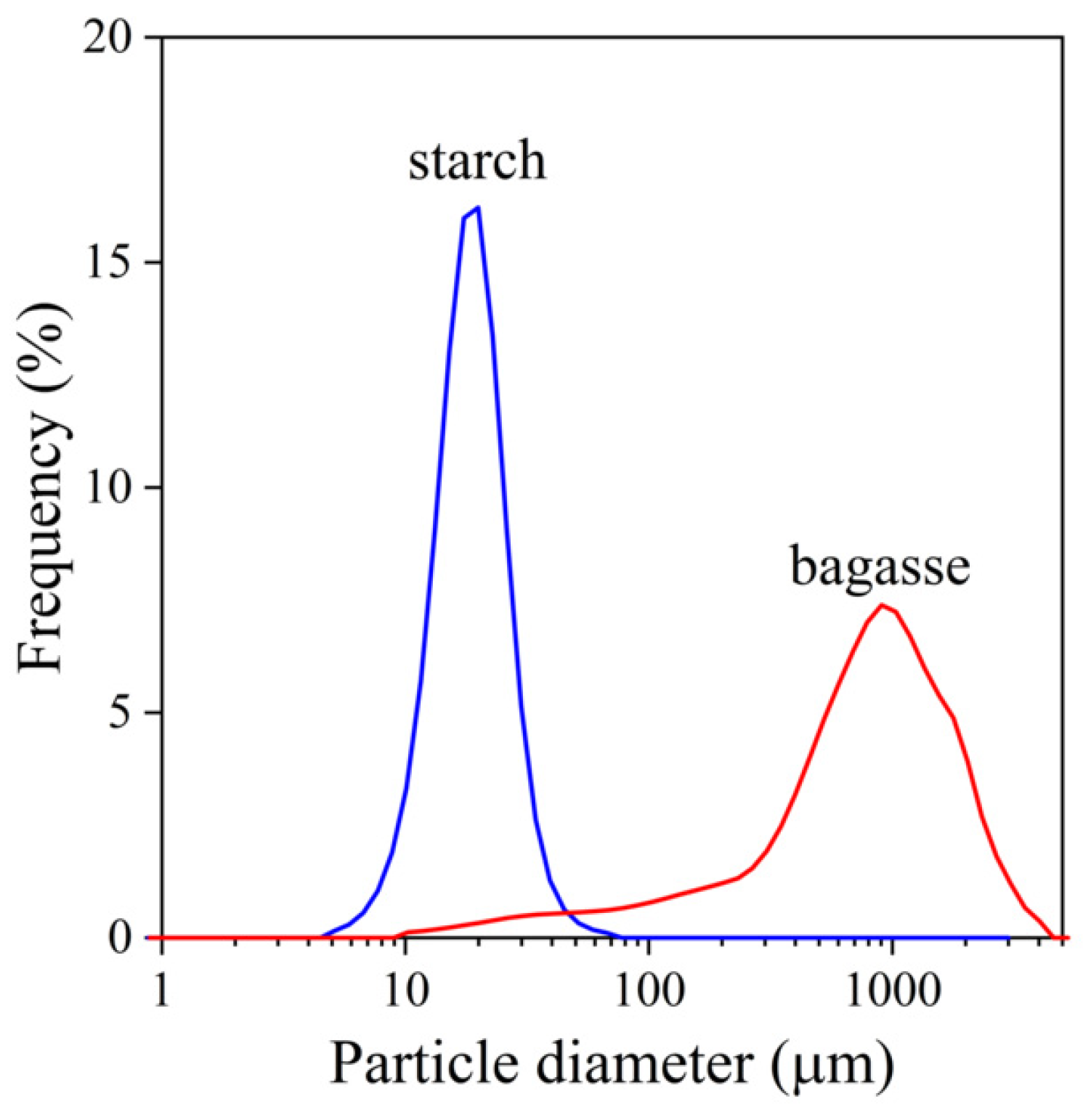
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.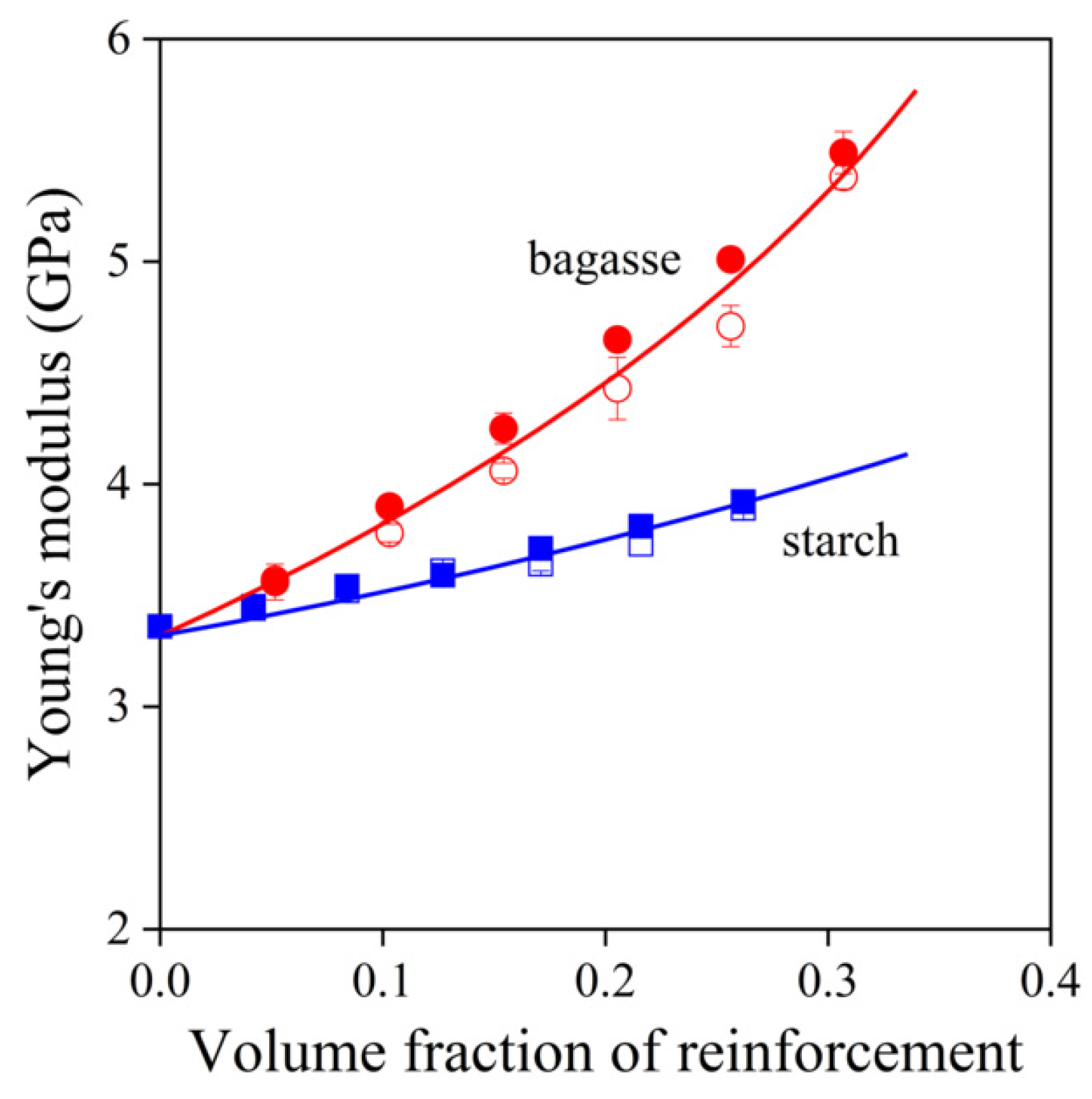
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.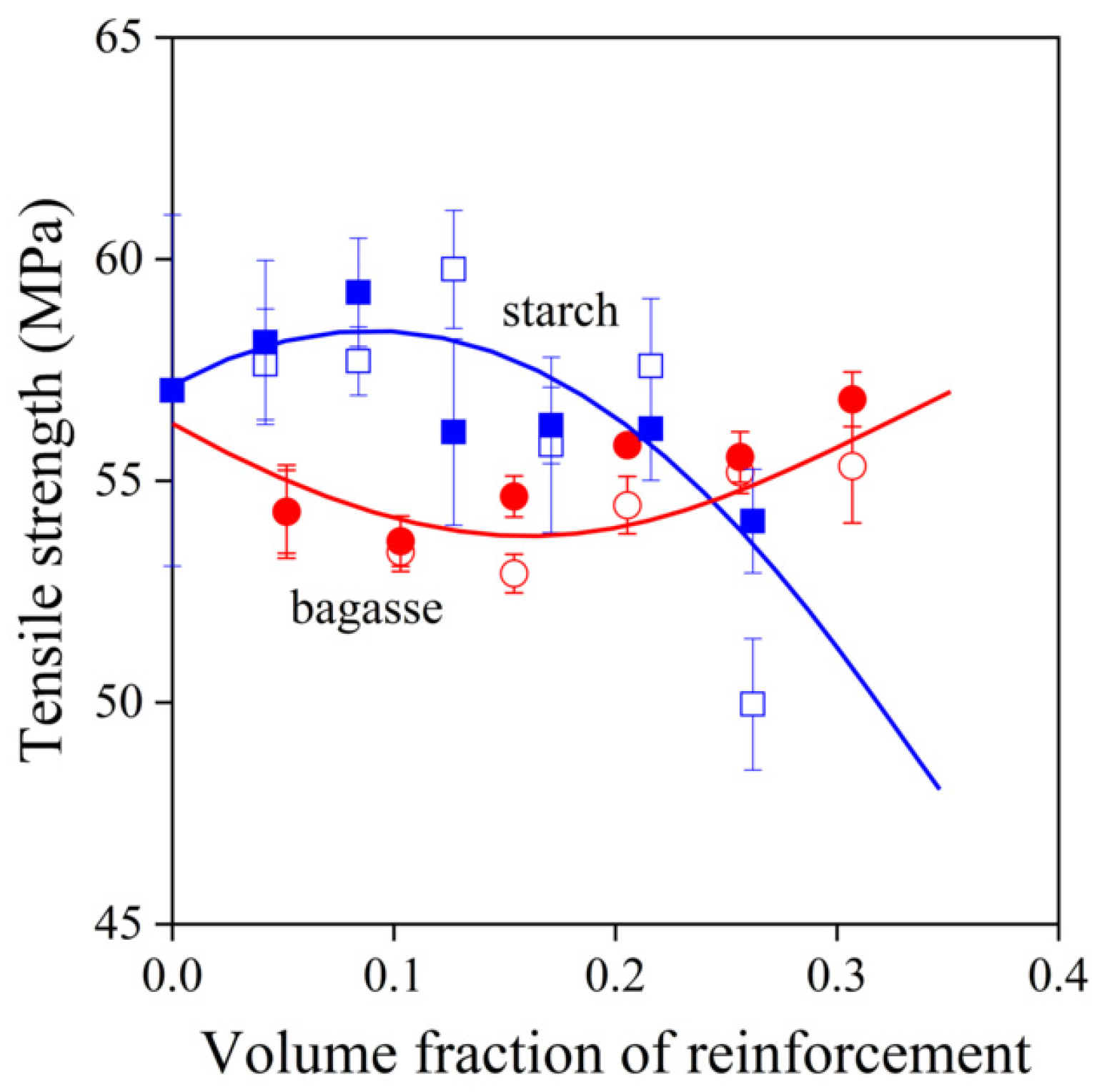
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.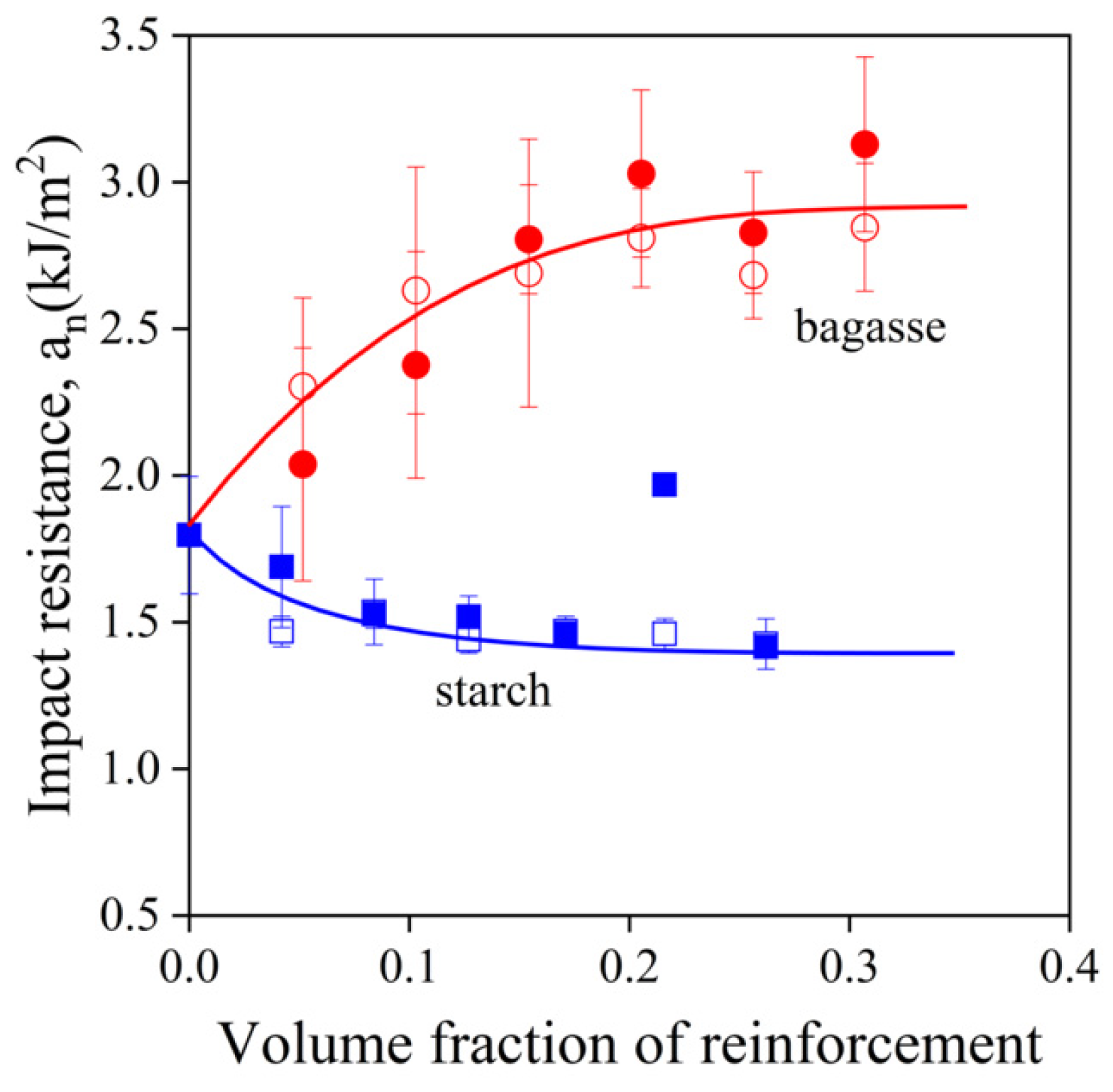
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.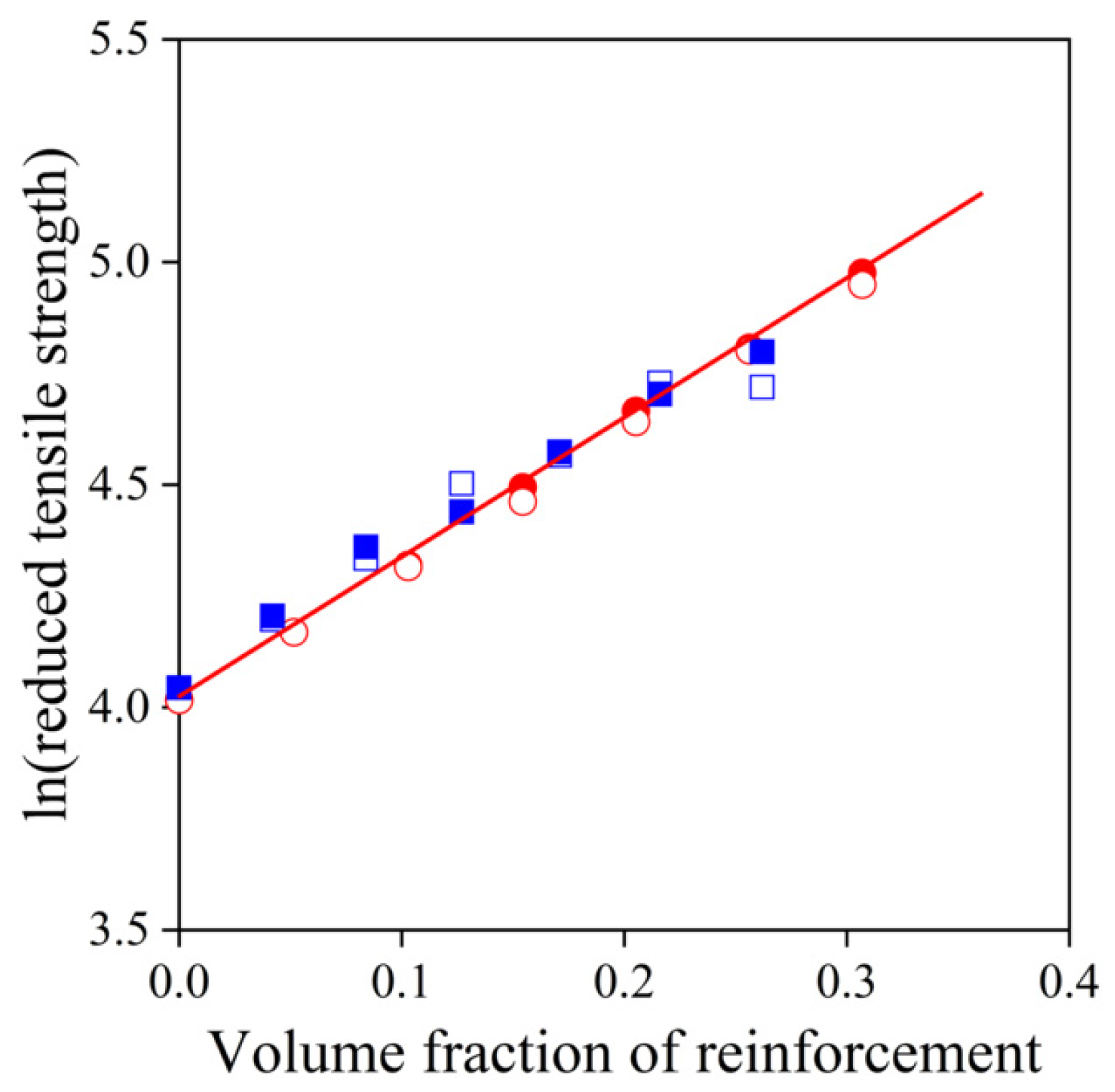
 ) individual acoustic events, full lines: stress vs. deformation (left axis, red) and cumulative number of signal vs. deformation (right axis, navy) correlations: (a) starch, (b) sugarcane bagasse fiber.
) individual acoustic events, full lines: stress vs. deformation (left axis, red) and cumulative number of signal vs. deformation (right axis, navy) correlations: (a) starch, (b) sugarcane bagasse fiber.
 ) individual acoustic events, full lines: stress vs. deformation (left axis, red) and cumulative number of signal vs. deformation (right axis, navy) correlations: (a) starch, (b) sugarcane bagasse fiber.
) individual acoustic events, full lines: stress vs. deformation (left axis, red) and cumulative number of signal vs. deformation (right axis, navy) correlations: (a) starch, (b) sugarcane bagasse fiber.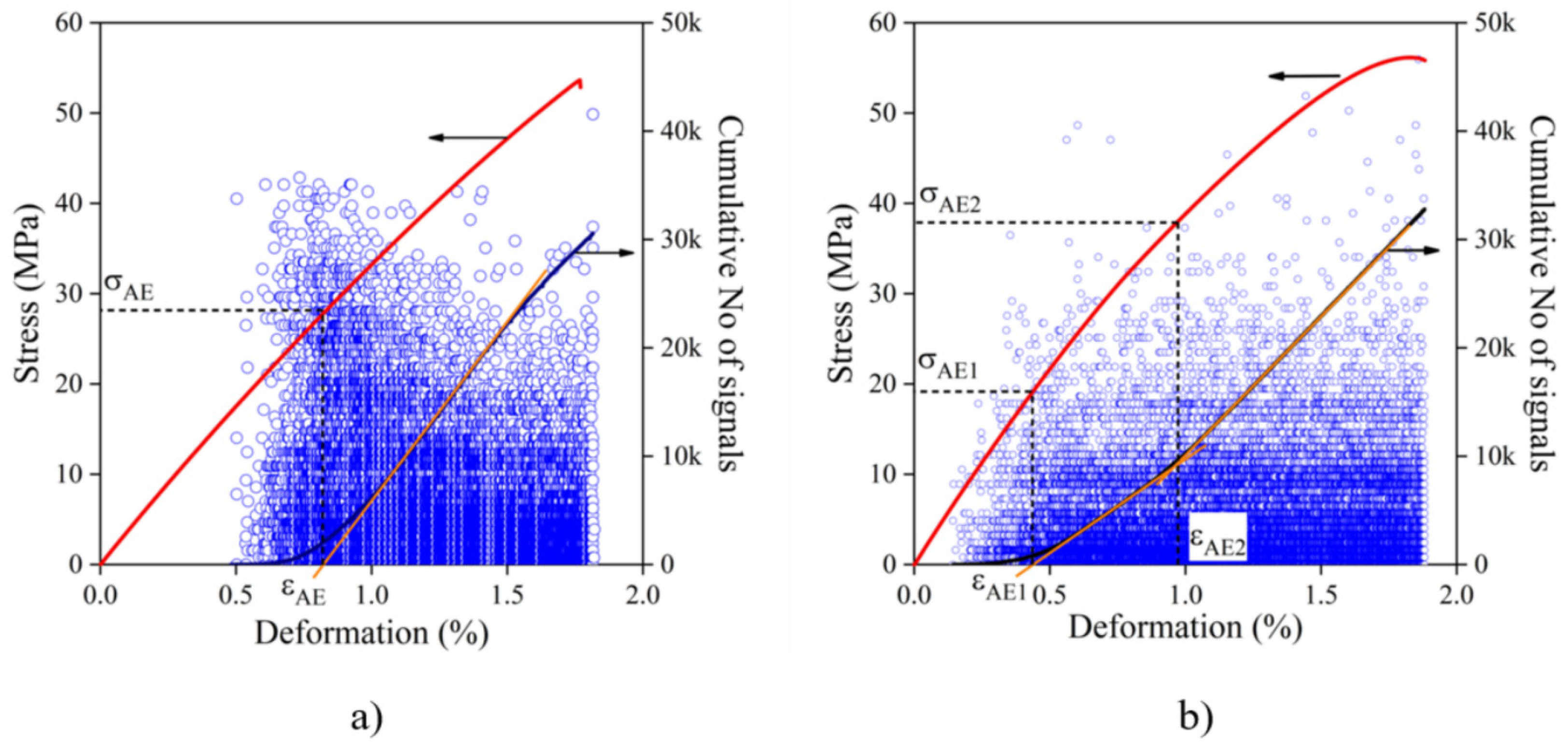
 ,
,  ) sugarcane bagasse fibers, σAE1 (
) sugarcane bagasse fibers, σAE1 ( ,
,  ) starch, σAE, (
) starch, σAE, ( ,
,  ) bagasse, σAE2; empty symbols without MAPLA, full symbols with MAPLA.
) bagasse, σAE2; empty symbols without MAPLA, full symbols with MAPLA.
 ,
,  ) sugarcane bagasse fibers, σAE1 (
) sugarcane bagasse fibers, σAE1 ( ,
,  ) starch, σAE, (
) starch, σAE, ( ,
,  ) bagasse, σAE2; empty symbols without MAPLA, full symbols with MAPLA.
) bagasse, σAE2; empty symbols without MAPLA, full symbols with MAPLA.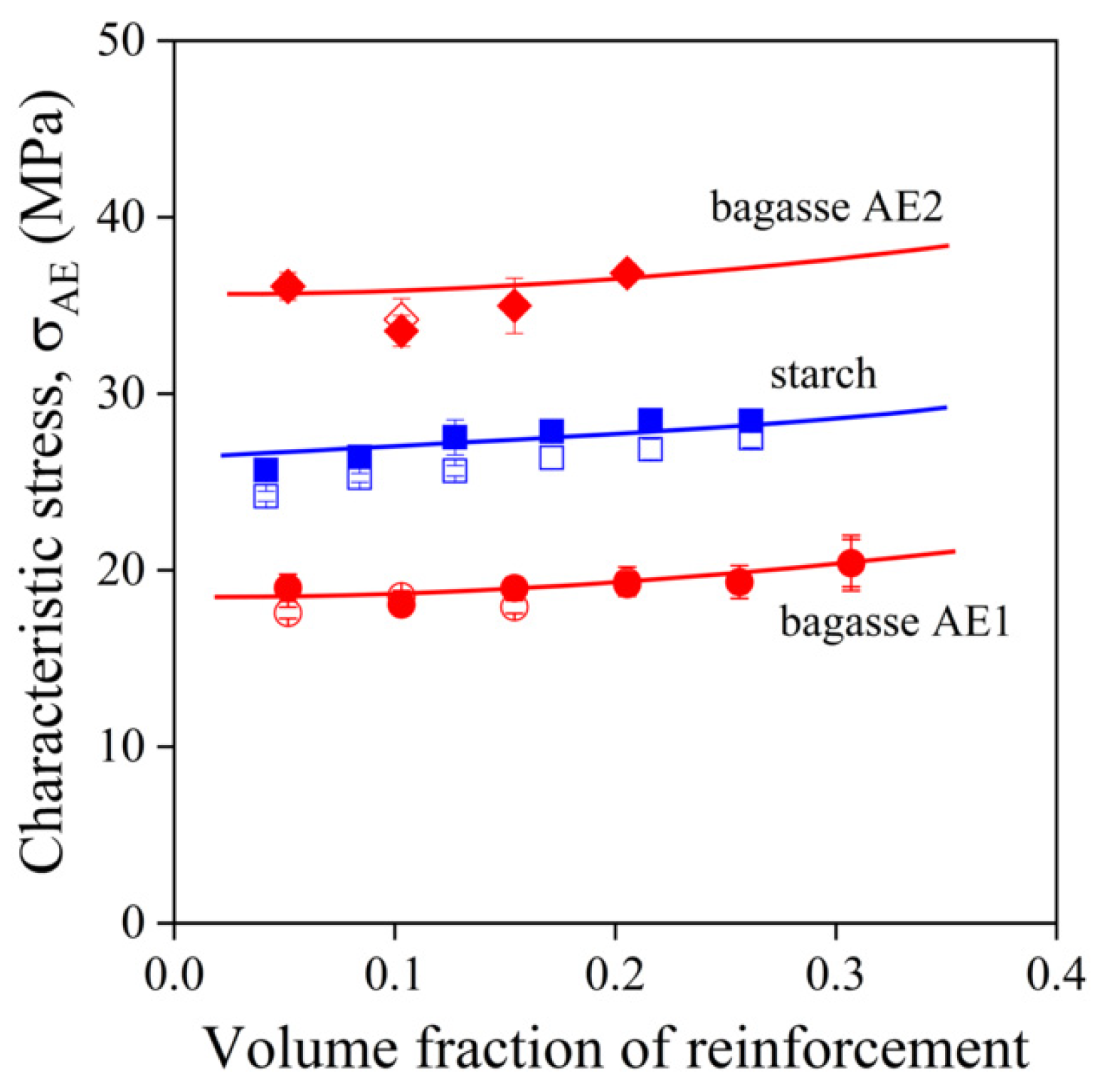
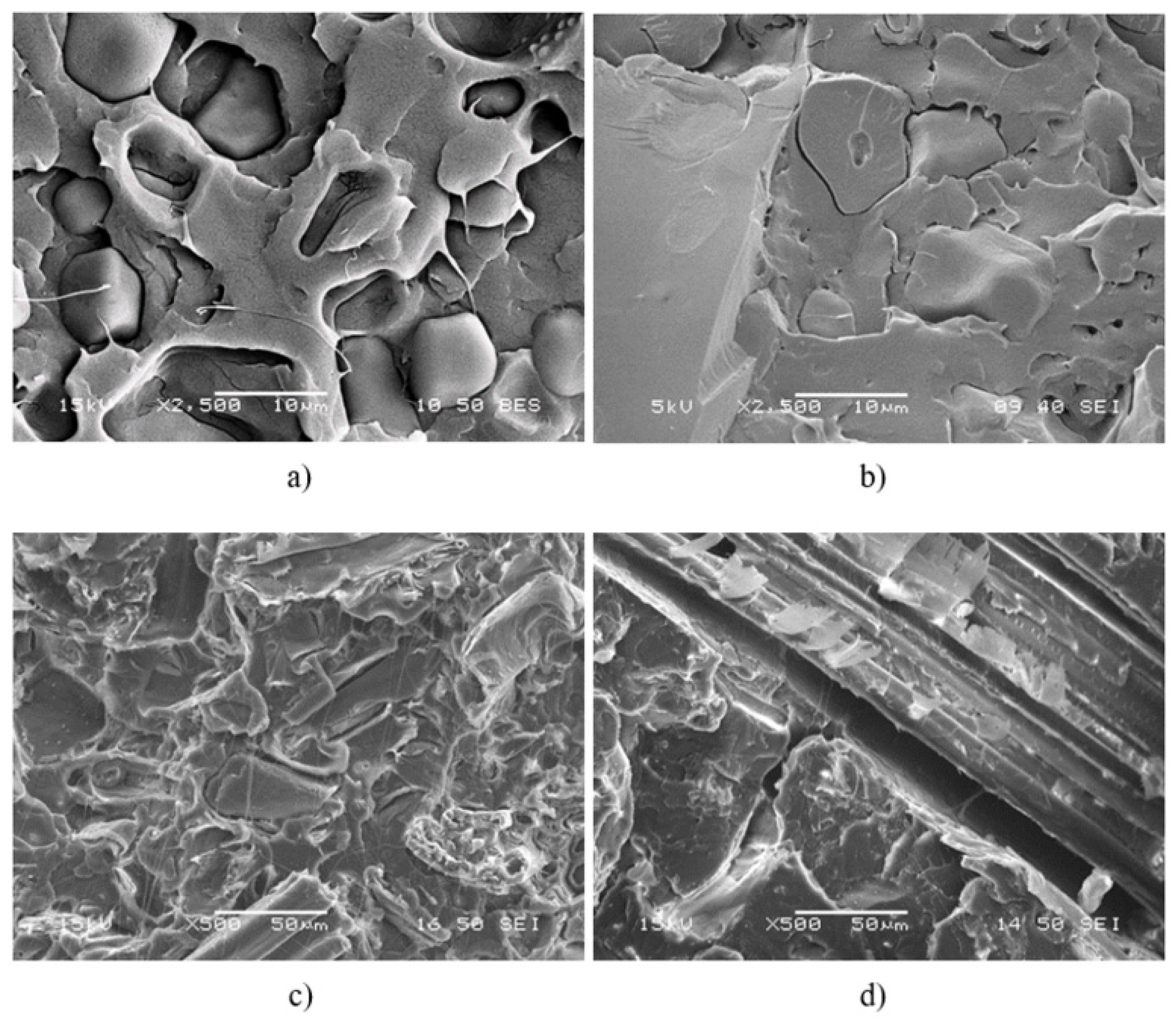
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.
 ,
,  ) sugarcane bagasse fibers, (
) sugarcane bagasse fibers, ( ,
,  ) starch; empty symbols without MAPLA, full symbols with MAPLA.
) starch; empty symbols without MAPLA, full symbols with MAPLA.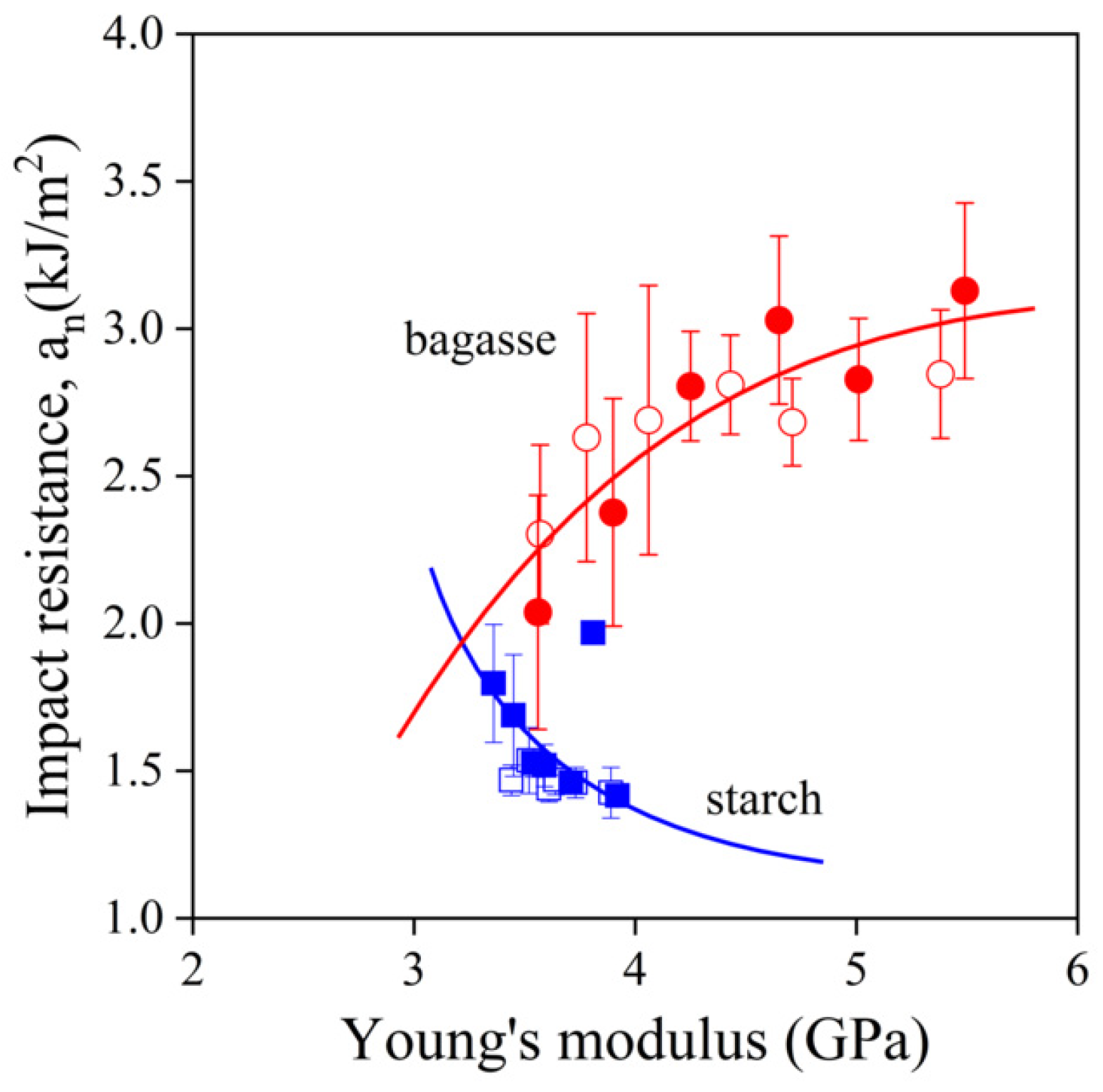
| Reinforcement | Coupling | σT0 (MPa) | Parameter B | R2 a |
|---|---|---|---|---|
| Starch | – | 58.2 | 3.11 ± 0.16 | 0.9896 |
| + | 59.2 | 2.84 ± 0.12 | 0.9910 | |
| Bagasse | – | 55.0 | 3.07 ± 0.04 | 0.9992 |
| + | 55.1 | 3.14 ± 0.03 | 0.9994 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Józó, M.; Várdai, R.; Bartos, A.; Móczó, J.; Pukánszky, B. Preparation of Biocomposites with Natural Reinforcements: The Effect of Native Starch and Sugarcane Bagasse Fibers. Molecules 2022, 27, 6423. https://doi.org/10.3390/molecules27196423
Józó M, Várdai R, Bartos A, Móczó J, Pukánszky B. Preparation of Biocomposites with Natural Reinforcements: The Effect of Native Starch and Sugarcane Bagasse Fibers. Molecules. 2022; 27(19):6423. https://doi.org/10.3390/molecules27196423
Chicago/Turabian StyleJózó, Muriel, Róbert Várdai, András Bartos, János Móczó, and Béla Pukánszky. 2022. "Preparation of Biocomposites with Natural Reinforcements: The Effect of Native Starch and Sugarcane Bagasse Fibers" Molecules 27, no. 19: 6423. https://doi.org/10.3390/molecules27196423






