Selenium: An Antioxidant with a Critical Role in Anti-Aging
Abstract
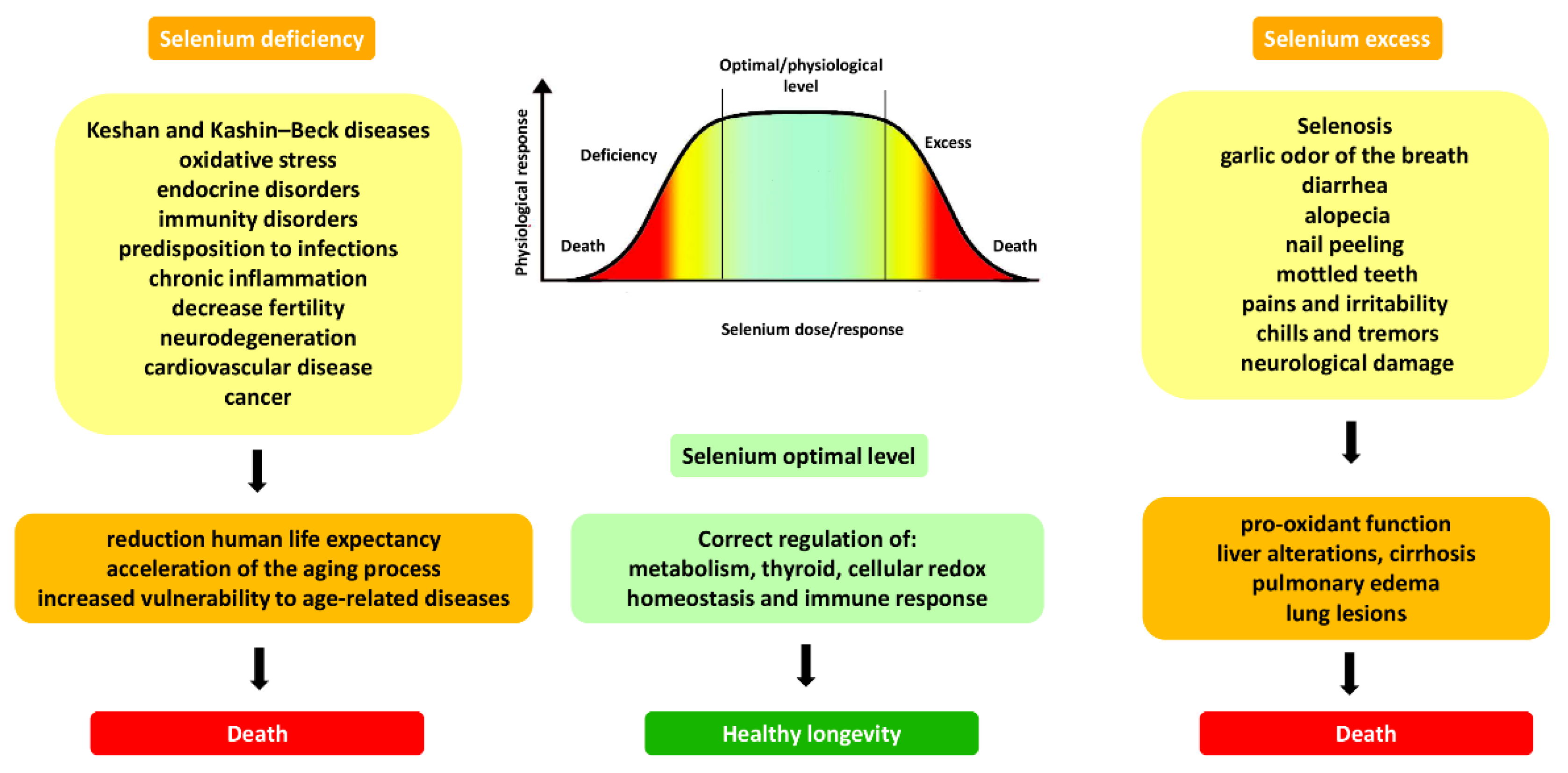
:1. Introduction
2. The Role of Se in the Prevention and Treatment of Health Disorders
2.1. Oxidative Stress, Inflammation, and Immunity
2.2. Infections
2.3. Endocrine System Disorders
2.4. Cancer
2.5. Intoxication
3. The Role of Se in Longevity and Age-Related Disorders
4. Sources of Se in the Human Diet
5. Nanoformulations of Se in Age-Related Disorders
6. Determination of Se in Foods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rusu, M.; Fizesan, I.; Vlase, L.; Popa, D.-S. Antioxidants in Age-Related Diseases and Anti-Aging Strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef]
- Leiter, O.; Zhuo, Z.; Rust, R.; Wasielewska, J.M.; Grönnert, L.; Kowal, S.; Overall, R.W.; Adusumilli, V.S.; Blackmore, D.G.; Southon, A.; et al. Selenium mediates exercise-induced adult neurogenesis and reverses learning deficits induced by hippocampal injury and aging. Cell Metab. 2022, 34, 408–423.e8. [Google Scholar] [CrossRef] [PubMed]
- Afanas’ev, I.B. Free radical mechanisms of aging processes under physiological conditions. Biogerontology 2005, 6, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, H. A Review on Free Radicals and Antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koo, N.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Gasmi, A.; Chirumbolo, S.; Peana, M.; Mujawdiya, P.K.; Dadar, M.; Menzel, A.; Bjørklund, G. Biomarkers of Senescence during Aging as Possible Warnings to Use Preventive Measures. Curr. Med. Chem. 2021, 28, 1471–1488. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef]
- Shanaida, M.; Adamiv, S.; Yaremchuk, O.; Ivanusa, I. Pharmacological study of the polyphenol-containing phytosubstance obtained from the anise hyssop herb. PharmacologyOnLine 2021, 2, 105–112. [Google Scholar]
- Gons’kyi Ia, I.; Korda, M.M.; Klishch, I.M. Status of the free radical oxidation and antioxidant system in rats with toxic liver damage; effect of tocopherol and dimethylsulfoxide. Ukr. Biokhimicheskii Zhurnal 1991, 63, 112–116. [Google Scholar]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M.; et al. Polyphenols in Metabolic Diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef]
- Ćurko-Cofek, B. Micronutrients in Ageing and Longevity. In Nutrition, Food and Diet in Ageing and Longevity; Rattan, S.I.S., Kaur, G., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 63–83. [Google Scholar] [CrossRef]
- Antonyak, H.; Iskra, R.; Panas, N.; Lysiuk, R. Selenium. In Trace Elements and Minerals in Health and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 63–98. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Malavolta, M.; Muti, E.; Costarelli, L.; Cipriano, C.; Piacenza, F.; Tesei, S.; Giacconi, R.; Lattanzio, F. Zinc, metallothioneins and longevity: Interrelationships with niacin and selenium. Curr. Pharm. Des. 2008, 14, 2719–2732. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Arnér, E.S.J. Common modifications of selenocysteine in selenoproteins. Essays Biochem. 2020, 64, 45–53. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195. [Google Scholar] [CrossRef]
- Kieliszek, M.; Blazejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124–7128. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Porres, J.; Cheng, W.H. Selenium, Selenoproteins, and Age-Related Disorders. Bioact. Food Diet. Interv. Aging Popul. 2013, 227–239. [Google Scholar] [CrossRef]
- Marciel, M.; Hoffmann, P. Molecular Mechanisms by Which Selenoprotein K Regulates Immunity and Cancer. Biol. Trace Elem. Res. 2019, 192, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Dietary Antioxidants and Related Compounds. In Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, I.; Hartmann, T.; Brustbauer, R.; Minear, G.; Dam, K. Selenium levels in patients with autoimmune thyroiditis and controls in lower Austria. Horm. Metab. Res. 2014, 46, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Thiry, C.; Ruttens, A.; De Temmerman, L.; Schneider, Y.-J.; Pussemier, L. Current knowledge in species-related bioavailability of selenium in food. Food Chem. 2012, 130, 767–784. [Google Scholar] [CrossRef]
- Brozmanová, J.; Mániková, D.; Vlčková, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Huang, L.; Shi, Y.; Lu, F.; Zheng, H.; Liu, X.; Gong, B.; Yang, J.; Lin, Y.; Cheng, J.; Ma, S.; et al. Association study of polymorphisms in selenoprotein genes and Kashin-Beck disease and serum selenium/iodine concentration in a Tibetan population. PLoS ONE 2013, 8, e71411. [Google Scholar] [CrossRef]
- Joshi, T.; Durgapal, S.; Juyal, V.; Jantwal, A.; Rana, M.; Kumar, A. Chapter4.15—Selenium. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 461–474. [Google Scholar] [CrossRef]
- Prabhu, K.S.; Lei, X.G. Selenium. Adv. Nutr. 2016, 7, 415–417. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M. Chapter Eleven—Selenium. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 96, pp. 417–429. [Google Scholar] [CrossRef]
- Savarino, L.; Granchi, D.; Ciapetti, G.; Cenni, E.; Ravaglia, G.; Forti, P.; Maioli, F.; Mattioli, R. Serum concentrations of zinc and selenium in elderly people: Results in healthy nonagenarians/centenarians. Exp. Gerontol. 2001, 36, 327–339. [Google Scholar] [CrossRef]
- Akbaraly, N.T.; Arnaud, J.; Hininger-Favier, I.; Gourlet, V.; Roussel, A.M.; Berr, C. Selenium and mortality in the elderly: Results from the EVA study. Clin. Chem. 2005, 51, 2117–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, A.M.; Lasheras, C. Life-quality indicators in elderly people are influenced by selenium status. Aging Clin. Exp. Res. 2007, 19, 10–15. [Google Scholar] [CrossRef]
- Alehagen, U.; Opstad, T.B.; Alexander, J.; Larsson, A.; Aaseth, J. Impact of Selenium on Biomarkers and Clinical Aspects Related to Ageing. A Review. Biomolecules 2021, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, M.; Sergeant, C.; Garnier, N.; Moretto, P.; Llabador, Y.; Simonoff, G.; Conri, C. Antioxidant Status (Selenium, Vitamins A and E) and Aging. In Free Radicals and Aging; Springer: Berlin/Heidelberg, Germany, 1992; pp. 368–397. [Google Scholar]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef]
- Zeng, R.; Farooq, M.U.; Zhang, G.; Tang, Z.; Zheng, T.; Su, Y.; Hussain, S.; Liang, Y.; Ye, X.; Jia, X.; et al. Dissecting the Potential of Selenoproteins Extracted from Selenium-Enriched Rice on Physiological, Biochemical and Anti-Ageing Effects In Vivo. Biol. Trace Elem. Res. 2020, 196, 119–130. [Google Scholar] [CrossRef]
- Lv, J.; Ai, P.; Lei, S.; Zhou, F.; Chen, S.; Zhang, Y. Selenium levels and skin diseases: Systematic review and meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126548. [Google Scholar] [CrossRef]
- Ma, C.; Hoffmann, P.R. Selenoproteins as regulators of T cell proliferation, differentiation, and metabolism. Semin. Cell Dev. Biol. 2021, 115, 54–61. [Google Scholar] [CrossRef]
- Espaladori, M.C.; Diniz, J.M.B.; de Brito, L.C.N.; Tavares, W.L.F.; Kawai, T.; Vieira, L.Q.; Sobrinho, A.P.R. Selenium intracanal dressing: Effects on the periapical immune response. Clin. Oral Investig. 2021, 25, 2951–2958. [Google Scholar] [CrossRef]
- Ceyhan, D.; Guzel, K.G.U.; Cig, B. The protective role of selenium against dental amalgam-induced intracellular oxidative toxicity through the TRPV1 channel in DBTRG glioblastoma cells. J. Appl. Oral Sci. 2021, 29, e20200414. [Google Scholar] [CrossRef]
- Klapcinska, B.; Derejczyk, J.; Wieczorowska-Tobis, K.; Sobczak, A.; Sadowska-Krepa, E.; Danch, A. Antioxidant defense in centenarians (a preliminary study). Acta Biochim. Pol. 2000, 47, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodpoor, A.; Hamishehkar, H.; Shadvar, K.; Ostadi, Z.; Sanaie, S.; Saghaleini, S.H.; Nader, N.D. The Effect of Intravenous Selenium on Oxidative Stress in Critically Ill Patients with Acute Respiratory Distress Syndrome. Immunol. Investig. 2019, 48, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Misra, S.; Prabhu, K.S. Selenium and selenoproteins in prostanoid metabolism and immunity. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 484–516. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, N.; Dinand, V.; Rao, S.; Gupta, P.; Toteja, G.; Kalra, M.; Yadav, S.P.; Sachdeva, A. Antioxidant Levels at Diagnosis in Childhood Acute Lymphoblastic Leukemia. Indian J. Pediatr. 2012, 80, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.A.; Yoo, M.H.; Shrimali, R.K.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Role of selenium-containing proteins in T-cell and macrophage function. Proc. Nutr. Soc. 2010, 69, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Varlamova, E. Participation of selenoproteins localized in the ER in the processes occurring in this organelle and in the regulation of carcinogenesis-associated processes. J. Trace Elem. Med. Biol. 2018, 48, 172–180. [Google Scholar] [CrossRef]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2012, 16, 705–743. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Saad, R.; Taylor, E.W.; Rayman, M.P. Selenium and selenoproteins in viral infection with potential relevance to COVID-19. Redox Biol. 2020, 37, 101715. [Google Scholar] [CrossRef]
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2021, 26, 256–263. [Google Scholar] [CrossRef]
- Kamwesiga, J.; Mutabazi, V.; Kayumba, J.; Tayari, J.C.; Uwimbabazi, J.C.; Batanage, G.; Uwera, G.; Baziruwiha, M.; Ntizimira, C.; Murebwayire, A.; et al. Effect of selenium supplementation on CD4+ T-cell recovery, viral suppression and morbidity of HIV-infected patients in Rwanda: A randomized controlled trial. AIDS 2015, 29, 1045–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Luo, W.; Liang, B. Circulating trace elements status in COVID-19 disease: A meta-analysis. Front. Nutr. 2022, 9, 982032. [Google Scholar] [CrossRef] [PubMed]
- Fakhrolmobasheri, M.; Mazaheri-Tehrani, S.; Kieliszek, M.; Zeinalian, M.; Abbasi, M.; Karimi, F.; Mozafari, A.M. COVID-19 and Selenium Deficiency: A Systematic Review. Biol. Trace Elem. Res. 2022, 200, 3945–3956. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.R.; Mantle, D. COVID-19, Coenzyme Q10 and Selenium. Adv. Exp. Med. Biol. 2021, 1327, 161–168. [Google Scholar] [CrossRef]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked Selenium Nanoparticles for Antibacterial and Anticancer Treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Han, H.W.; Patel, K.D.; Kwak, J.H.; Jun, S.K.; Jang, T.S.; Lee, S.H.; Knowles, J.C.; Kim, H.W.; Lee, H.H.; Lee, J.H. Selenium Nanoparticles as Candidates for Antibacterial Substitutes and Supplements against Multidrug-Resistant Bacteria. Biomolecules 2021, 11, 1028. [Google Scholar] [CrossRef]
- Tran, P.; Hamood, A.; Mosley, T.; Gray, T.; Jarvis, C.; Webster, D.; Amaechi, B.; Enos, T.; Reid, T. Organo-selenium-containing dental sealant inhibits bacterial biofilm. J. Dent. Res. 2013, 92, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.; Kopel, J.; Ray, C.; Reed, J.; Reid, T.W. Organo-selenium containing dental sealant inhibits biofilm formation by oral bacteria. Dent. Mater. 2022, 38, 848–857. [Google Scholar] [CrossRef]
- Seguya, A.; Mowafy, M.; Gaballah, A.; Zaher, A. Chlorhexidine versus organoselenium for inhibition of S. mutans biofilm, an in vitro study. BMC Oral Health 2022, 22, 14. [Google Scholar] [CrossRef]
- Davis, C.D.; Brooks, L.; Calisi, C.; Bennett, B.J.; McElroy, D.M. Beneficial effect of selenium supplementation during murine infection with Trypanosoma cruzi. J. Parasitol. 1998, 84, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef] [Green Version]
- Méplan, C. Trace elements and ageing, a genomic perspective using selenium as an example. J. Trace Elem. Med. Biol. 2011, 25 (Suppl. 1), S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Shreenath, A.P.; Ameer, M.A.; Dooley, J. Selenium Deficiency; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schomburg, L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat. Rev. Endocrinol. 2011, 8, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef]
- Ambroziak, U.; Hybsier, S.; Shahnazaryan, U.; Krasnodebska-Kiljanska, M.; Rijntjes, E.; Bartoszewicz, Z.; Bednarczuk, T.; Schomburg, L. Severe selenium deficits in pregnant women irrespective of autoimmune thyroid disease in an area with marginal selenium intake. J. Trace Elem. Med. Biol. 2017, 44, 186–191. [Google Scholar] [CrossRef]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87 (Suppl. 1), S168–S177. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 441–448. [Google Scholar] [CrossRef]
- Mistry, H.D.; Broughton Pipkin, F.; Redman, C.W.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for Selenium in Metabolic Homeostasis and Human Reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Ujiie, S.; Kikuchi, H. The Relation between Serum Selenium Value and Cancer in Miyagi, Japan: 5-Year Follow up Study. Tohoku J. Exp. Med. 2002, 196, 99–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varlamova, E.; Turovsky, E.; Blinova, E. Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawska, A.; Bielawski, K. Selenium as a Bioactive Micronutrient in the Human Diet and Its Cancer Chemopreventive Activity. Nutrients 2021, 13, 1649. [Google Scholar] [CrossRef]
- Varlamova, E.; Turovsky, E. The main cytotoxic effects of methylseleninic acid on various cancer cells. Int. J. Mol. Sci. 2021, 22, 6614. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weekley, C.; Harris, H. Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2013, 42, 8870–8894. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Liu, L.; Tao, W.; Sun, W.; Pei, X.; Xiao, Z.; Jin, Y.; Wang, M. Prooxidation and Cytotoxicity of Selenium Nanoparticles at Nonlethal Level in Sprague-Dawley Rats and Buffalo Rat Liver Cells. Oxidative Med. Cell Longev. 2020, 2020, 7680276. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, D. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2012, 5, 299–304. [Google Scholar] [CrossRef]
- Daragó, A.; Klimczak, M.; Stragierowicz, J.; Jobczyk, M.; Kilanowicz, A. Age-Related Changes in Zinc, Copper and Selenium Levels in the Human Prostate. Nutrients 2021, 13, 1403. [Google Scholar] [CrossRef]
- Luo, H.; Wang, F.; Bai, Y.; Chen, T.; Zheng, W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf. B 2012, 94, 304–308. [Google Scholar] [CrossRef]
- Chen, Q.; Lin, L.S.; Chen, L.; Lin, J.; Ding, Y.; Bao, X.D.; Wu, J.F.; Lin, L.K.; Yan, L.J.; Wang, R.; et al. Relationship between selenium and the risk for oral cancer: A case-control study. Zhonghua Liuxingbingxue Zazhi 2019, 40, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Mampilly, M.O.; Ravindran, N.; Parambil, M.S.; Nilesh, K.; Jayagopalan, P.; Dhamali, D. Assessment of Serum Selenium and Ceruloplasmin in Potentially Malignant Disorders and Oral Cancer. J. Pharm. Bioallied Sci. 2021, 13, S989–S992. [Google Scholar] [CrossRef] [PubMed]
- Bleys, J.; Navas-Acien, A.; Stranges, S.; Menke, A.; Miller, E.R.; Guallar, E. Serum selenium and serum lipids in US adults. Am. J. Clin. Nutr. 2008, 88, 416–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielczykowska, M.; Kocot, J.; Pazdzior, M.; Musik, I. Selenium—A fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018, 27, 245–255. [Google Scholar] [CrossRef]
- Limaye, A.; Yu, R.C.; Chou, C.C.; Liu, J.R.; Cheng, K.C. Protective and Detoxifying Effects Conferred by Dietary Selenium and Curcumin against AFB1-Mediated Toxicity in Livestock: A Review. Toxins 2018, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G. Selenium as an antidote in the treatment of mercury intoxication. Biometals 2015, 28, 605–614. [Google Scholar] [CrossRef]
- Bjørklund, G.; Rahaman, M.S.; Shanaida, M.; Lysiuk, R.; Oliynyk, P.; Lenchyk, L.; Chirumbolo, S.; Chasapis, C.T.; Peana, M. Natural Dietary Compounds in the Treatment of Arsenic Toxicity. Molecules 2022, 27, 4871. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Adaptive dysfunction of selenoproteins from the perspective of the triage theory: Why modest selenium deficiency may increase risk of diseases of aging. FASEB J. 2011, 25, 1793–1814. [Google Scholar] [CrossRef] [Green Version]
- Robberecht, H.; De Bruyne, T.; Davioud-Charvet, E.; Mackrill, J.; Hermans, N. Selenium Status in Elderly People: Longevity and Age-Related Diseases. Curr. Pharm. Des. 2019, 25, 1694–1706. [Google Scholar] [CrossRef]
- Jiang, X.R.; Macey, M.G.; Lin, H.X.; Newland, A.C. The anti-leukaemic effects and the mechanism of sodium selenite. Leuk. Res. 1992, 16, 347–352. [Google Scholar] [CrossRef]
- Kondrat’eva, A.V.; Kovan’ko, E.G.; Liutinskiĭ, S.I.; Ivanov, S.D. Effect of selenium on reactions of elderly rats affected by low doses of radiochemical exposures. Adv. Gerontol. 2004, 15, 91–95. [Google Scholar] [PubMed]
- Martin-Romero, F.J.; Kryukov, G.V.; Lobanov, A.V.; Carlson, B.A.; Lee, B.J.; Gladyshev, V.N.; Hatfield, D.L. Selenium metabolism in Drosophila: Selenoproteins, selenoprotein mRNA expression, fertility, and mortality. J. Biol. Chem. 2001, 276, 29798–29804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, X.; Bai, L.; Li, Z. Effects of sodium selenite on the activity of GSH-Px and the life-span of Drosophila. Wei Sheng Yan Jiu 2000, 29, 166–167. [Google Scholar] [PubMed]
- Steinbrenner, H.; Klotz, L.O. Selenium and zinc: “antioxidants” for healthy aging? Z. Gerontol. Geriatr. 2020, 53, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Solovyev, N.; Drobyshev, E.; Bjørklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein P, and Alzheimer’s disease: Is there a link? Free Radic. Biol. Med. 2018, 127, 124–133. [Google Scholar] [CrossRef]
- Wei, K.; Guo, C.; Zhu, J.; Wei, Y.; Wu, M.; Huang, X.; Zhang, M.; Li, J.; Wang, X.; Wang, Y.; et al. The Whitening, Moisturizing, Anti-aging Activities, and Skincare Evaluation of Selenium-Enriched Mung Bean Fermentation Broth. Front. Nutr. 2022, 9, 837168. [Google Scholar] [CrossRef]
- Jackson, M.L. Selenium: Geochemical distribution and associations with human heart and cancer death rates and longevity in China and the United States. Biol. Trace Elem. Res. 1988, 15, 13–21. [Google Scholar] [CrossRef]
- Beck, J.; Ferrucci, L.; Sun, K.; Walston, J.; Fried, L.P.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Low serum selenium concentrations are associated with poor grip strength among older women living in the community. Biofactors 2007, 29, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.L.; Semba, R.D.; Walston, J.; Ferrucci, L.; Cappola, A.R.; Ricks, M.O.; Xue, Q.L.; Fried, L.P. Low serum selenium and total carotenoids predict mortality among older women living in the community: The women’s health and aging studies. J. Nutr. 2006, 136, 172–176. [Google Scholar] [CrossRef] [Green Version]
- Al-Mubarak, A.A.; van der Meer, P.; Bomer, N. Selenium, Selenoproteins, and Heart Failure: Current Knowledge and Future Perspective. Curr. Heart Fail. Rep. 2021, 18, 122–131. [Google Scholar] [CrossRef]
- Hao, Z.; Liu, Y.; Li, Y.; Song, W.; Yu, J.; Li, H.; Wang, W. Association between Longevity and Element Levels in Food and Drinking Water of Typical Chinese Longevity Area. J. Nutr. Health Aging 2016, 20, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rosenberg, M.; Hou, L.; Hu, M. Relationships among Environment, Climate, and Longevity in China. Int. J. Environ. Res. Public Health 2017, 14, 1195. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Wang, W.; Krafft, T.; Li, Y.; Zhang, F.; Yuan, F. Effects of several environmental factors on longevity and health of the human population of Zhongxiang, Hubei, China. Biol. Trace Elem. Res. 2011, 143, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Yin, Z.X.; Qian, H.Z.; Zhai, Y.; Liu, Y.Z.; Xu, J.W.; Zeng, Y. A study on chronic diseases and other related health indicators of centenarians in longevity areas in China. Zhonghua Yu Fang Yi Xue Za Zhi 2010, 44, 101–107. [Google Scholar]
- Foster, H.D.; Zhang, L. Longevity and selenium deficiency: Evidence from the People’s Republic of China. Sci. Total Environ. 1995, 170, 133–139. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Liu, W.; Fan, C.; Zheng, W.; Wong, Y.S.; Chen, T. Selective cellular uptake and induction of apoptosis of cancer-targeted selenium nanoparticles. Biomaterials 2013, 34, 7106–7116. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Deiana, M.; Pasella, S.; Baralla, A.; Occhineri, P.; Mura, I.; Madeddu, R.; Muresu, E.; Sotgia, S.; Zinellu, A.; et al. Metals in plasma of nonagenarians and centenarians living in a key area of longevity. Exp. Gerontol. 2014, 60, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Li, J.; Yu, R.; Cong, X.; Huang, D.; Li, Y.; Chen, S.; Zhu, S. Selenium Speciation in Selenium-Enriched Plant Foods. Food Anal. Methods 2022, 15, 1377–1389. [Google Scholar] [CrossRef]
- Lei, X.G.; Combs, G.F.; Sunde, R.A.; Caton, J.S.; Arthington, J.D.; Vatamaniuk, M.Z. Dietary Selenium Across Species. Annu. Rev. Nutr. 2022, 42, 337–375. [Google Scholar] [CrossRef]
- Finley, J.W. Selenium accumulation in plant foods. Nutr. Rev. 2005, 63 Pt 1, 196–202. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W. Reduction of cancer risk by consumption of selenium-enriched plants: Enrichment of broccoli with selenium increases the anticarcinogenic properties of broccoli. J. Med. Food 2003, 6, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Hewitt, K.L.; Chen, R.K.; Lill, R.E.; Hedderley, D.I.; Herath, T.D.; Matich, A.J.; McKenzie, M.J. Consumption of selenium-enriched broccoli increases cytokine production in human peripheral blood mononuclear cells stimulated ex vivo, a preliminary human intervention study. Mol. Nutr. Food Res. 2014, 58, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Goltyaev, M.; Mal’tseva, V.N.; Novoselov, V.I.; Varlamova, E. Expression patterns of er-resident selenoproteins in er- stress conditions caused by methylseleninic acid in different human cancer cells. Gene 2020, 755, 144884. [Google Scholar] [CrossRef] [PubMed]
- Ulewicz-Magulska, B.; Wesolowski, M. A chemometric approach to distribution of selenium in medicinal plants cultivated in poland. J. Med. Food 2013, 16, 460–466. [Google Scholar] [CrossRef]
- Huang, S.; Yang, W.; Huang, G. Preparation and activities of selenium polysaccharide from plant such as Grifola frondosa. Carbohydr. Polym. 2020, 242, 116409. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium Deficiency and Toxicity in the Environment; Elsevier—Academic Press: London, UK, 2005; pp. 373–415. [Google Scholar]
- Zhang, X.; He, H.; Xiang, J.; Yin, H.; Hou, T. Selenium-Containing Proteins/Peptides from Plants: A Review on the Structures and Functions. J. Agric. Food Chem. 2020, 68, 15061–15073. [Google Scholar] [CrossRef]
- Diowksz, A.; Pęczkowska, B.; Wŀodarczyk, M.; Ambroziak, W. Bacteria/Yeast and Plant Biomass Enriched in Se via Bioconversion Process as a Source of Se Supplementation in Food. In Progress in Biotechnology; Bielecki, S., Tramper, J., Polak, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 17, pp. 295–300. [Google Scholar] [CrossRef]
- Arnault, I.; Auger, J. Seleno-compounds in garlic and onion. J. Chromatogr. A 2006, 1112, 23–30. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Selenium yeast: Composition, quality, analysis, and safety. Pure Appl. Chem. 2006, 78, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J.; et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium nanoparticles: Enhanced nutrition and beyond. Crit. Rev. Food Sci. Nutr. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alhasan, R.; Kharma, A.; Leroy, P.; Jacob, C.; Gaucher, C. Selenium Donors at the Junction of Inflammatory Diseases. Curr. Pharm. Des. 2019, 25, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Yan, R.; Li, R.; Zhang, X. Selenium as a pleiotropic agent for medical discovery and drug delivery. Int. J. Nanomed. 2018, 13, 7473–7490. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.; Hong, B.; Hong, Z.; Sun, J.; Wang, C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in D-galactose-induced aging mice. J. Nanobiotechnol. 2017, 15, 92. [Google Scholar] [CrossRef]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2022, 29. [Google Scholar] [CrossRef]
- Sarkar, B.; Bhattacharjee, S.; Daware, A.; Tribedi, P.; Krishnani, K.K.; Minhas, P.S. Selenium Nanoparticles for Stress-Resilient Fish and Livestock. Nanoscale Res. Lett. 2015, 10, 371. [Google Scholar] [CrossRef]
- El-Kazaz, S.E.; Abo-Samaha, M.I.; Hafez, M.H.; El-Shobokshy, S.A.; Wirtu, G. Dietary supplementation of nano-selenium improves reproductive performance, sexual behavior and deposition of selenium in the testis and ovary of Japanese quail. J. Adv. Vet. Anim. Res. 2020, 7, 597–607. [Google Scholar] [CrossRef]
- Kumar, G.S.; Kulkarni, A.; Khurana, A.; Kaur, J.; Tikoo, K. Selenium nanoparticles involve HSP-70 and SIRT1 in preventing the progression of type 1 diabetic nephropathy. Chem. Biol. Interact. 2014, 223, 125–133. [Google Scholar] [CrossRef]
- Du, P.C.; Tu, Z.C.; Wang, H.; Hu, Y.M. Mechanism of Selenium Nanoparticles Inhibiting Advanced Glycation End Products. J. Agric. Food Chem. 2020, 68, 10586–10595. [Google Scholar] [CrossRef]
- Al-Otaibi, A.M.; Al-Gebaly, A.S.; Almeer, R.; Albasher, G.; Al-Qahtani, W.S.; Abdel Moneim, A.E. Potential of green-synthesized selenium nanoparticles using apigenin in human breast cancer MCF-7 cells. Environ. Sci. Pollut. Res. Int. 2022, 29, 47539–47548. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Kaur, L.; Sohal, H.S.; Malhi, D.S.; Garg, S.; Thakur, D. Application of Selenium Nanoparticles in Localized Drug Targeting for Cancer Therapy. Anticancer Agents Med. Chem. 2022, 22, 2715–2725. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, T.T. Elemental Selenium at Nano Size (Nano-Se) as a Potential Chemopreventive Agent with Reduced Risk of Selenium Toxicity: Comparison with Se-Methylselenocysteine in Mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+-Dependent Pro-Apoptotic Action of Selenium Nanoparticles, Mediated by Activation of Cx43 Hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef]
- Vahidi, H.; Barabadi, H.; Muthupandian, S. Emerging Selenium Nanoparticles to Combat Cancer: A Systematic Review. J. Clust. Sci. 2020, 31, 301–309. [Google Scholar] [CrossRef]
- Thavarajah, D.; Vandenberg, A.; George, G.; Pickering, I. Chemical Form of Selenium in Naturally Selenium-Rich Lentils (Lens culinaris L.) from Saskatchewan. J. Agric. Food Chem. 2007, 55, 7337–7341. [Google Scholar] [CrossRef]
- Ranjit, M.; Dash, K.; Dheram, K. UV-photolysis assisted digestion of food samples for the determination of selenium by electrothermal atomic absorption spectrometry (ETAAS). Food Chem. 2007, 105, 260–265. [Google Scholar] [CrossRef]
- Gilbert-Lopez, B.; Dernovics, M.; Moreno-Gonzalez, D.; Molina-Diaz, A.; Garcia-Reyes, J.F. Detection of over 100 selenium metabolites in selenized yeast by liquid chromatography electrospray time-of-flight mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1060, 84–90. [Google Scholar] [CrossRef]
- Rievaj, M.; Culková, E.; Šandorová, D.; Lukáčová-Chomisteková, Z.; Bellová, R.; Durdiak, J.; Tomčík, P. Electroanalytical Techniques for the Detection of Selenium as a Biologically and Environmentally Significant Analyte—A Short Review. Molecules 2021, 26, 1768. [Google Scholar] [CrossRef]
- Otilia, B.; Nartiță, R.; Rogobete, A.; Negrea, A.; Stroescu, R.; Teofana-Otilia, B.; Ilie, C.; Marginean, O. Spectrophotometric Determination of Selenium Through Triiodide Anion. Clin. Lab. 2017, 63, 887–899. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. https://doi.org/10.3390/molecules27196613
Bjørklund G, Shanaida M, Lysiuk R, Antonyak H, Klishch I, Shanaida V, Peana M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules. 2022; 27(19):6613. https://doi.org/10.3390/molecules27196613
Chicago/Turabian StyleBjørklund, Geir, Mariia Shanaida, Roman Lysiuk, Halyna Antonyak, Ivan Klishch, Volodymyr Shanaida, and Massimiliano Peana. 2022. "Selenium: An Antioxidant with a Critical Role in Anti-Aging" Molecules 27, no. 19: 6613. https://doi.org/10.3390/molecules27196613









