Au-TiO2/Ti Hybrid Coating as a Liquid and Gas Diffusion Layer with Improved Performance and Stability in Proton Exchange Membrane Water Electrolyzer
Abstract
:1. Introduction
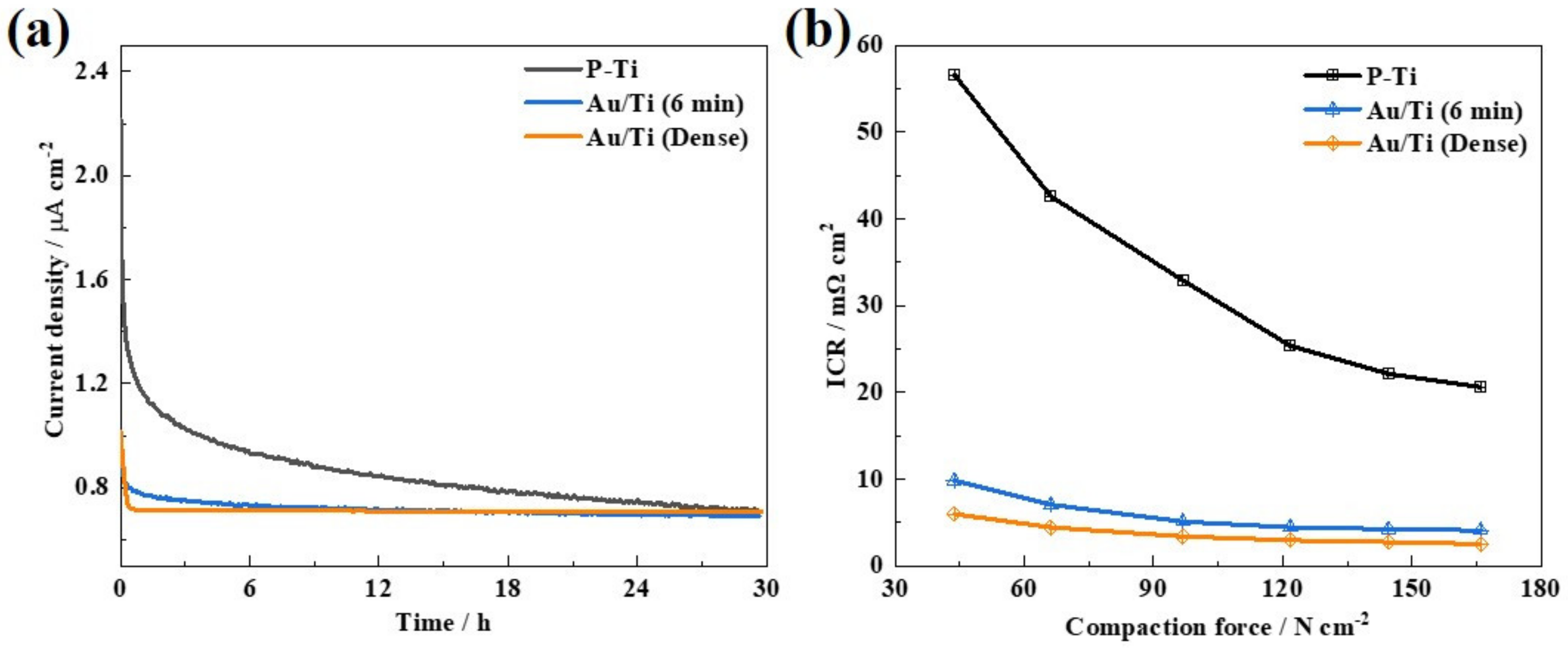
2. Results and Discussions
3. Experimental
3.1. Materials
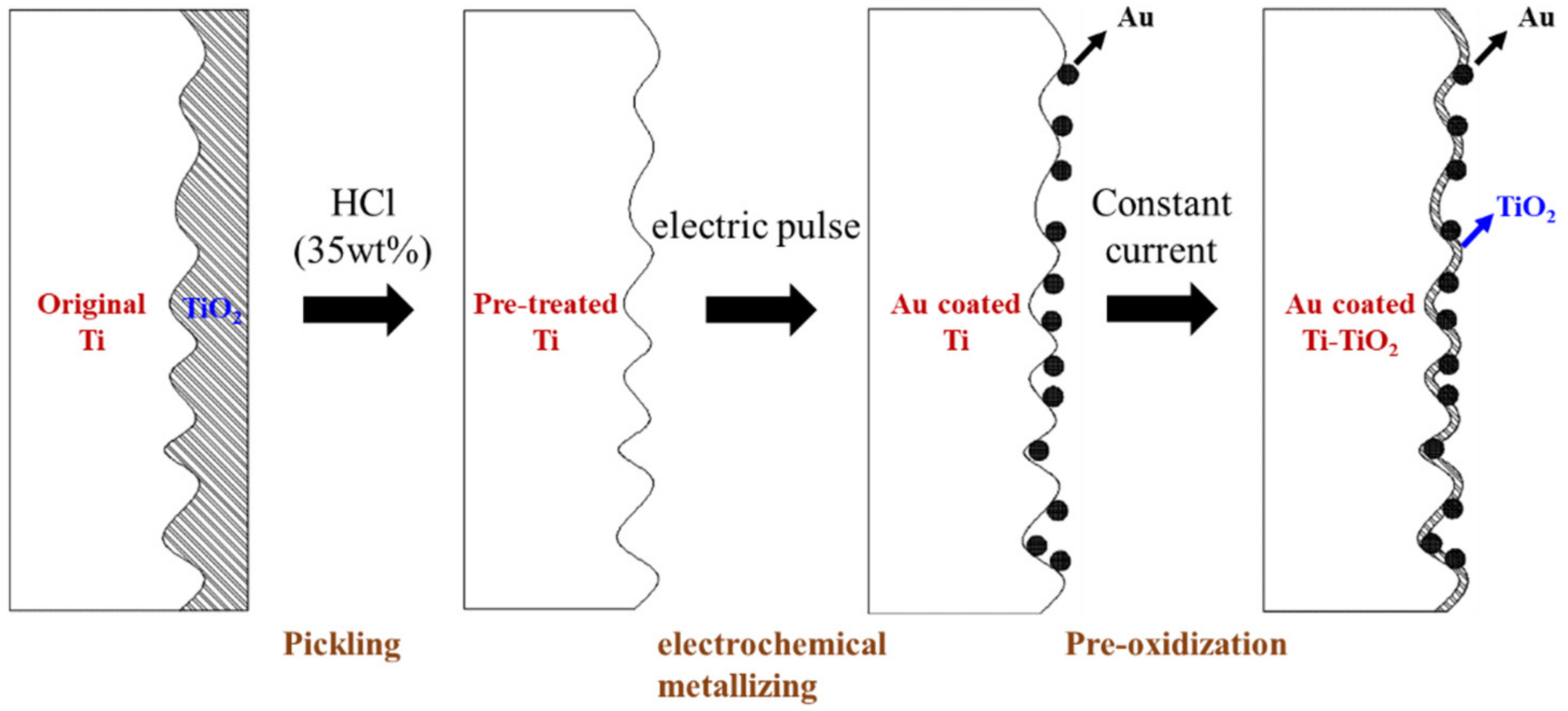
3.2. Pickling Pre-Treatment and Electrochemical Metallizing
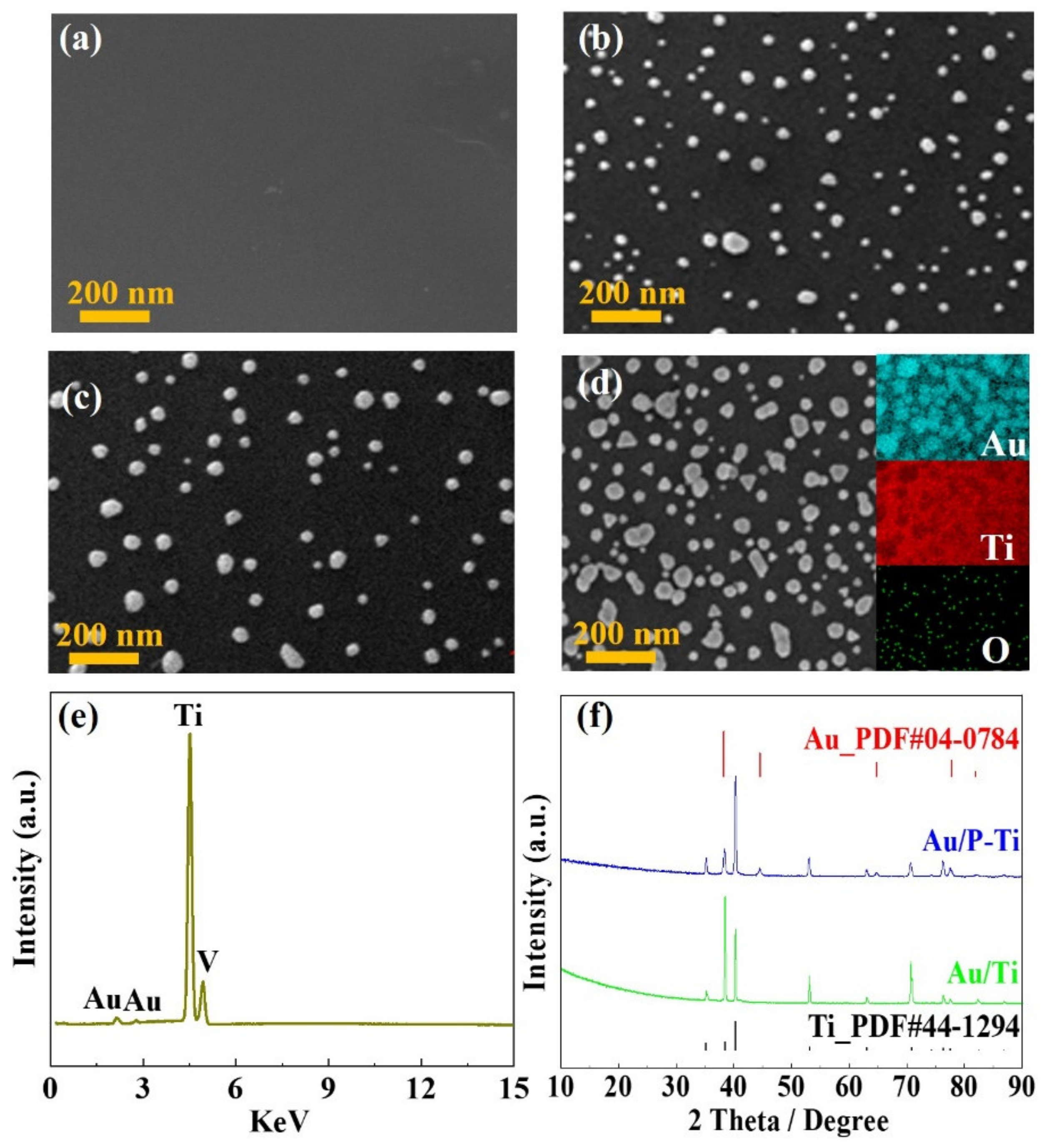
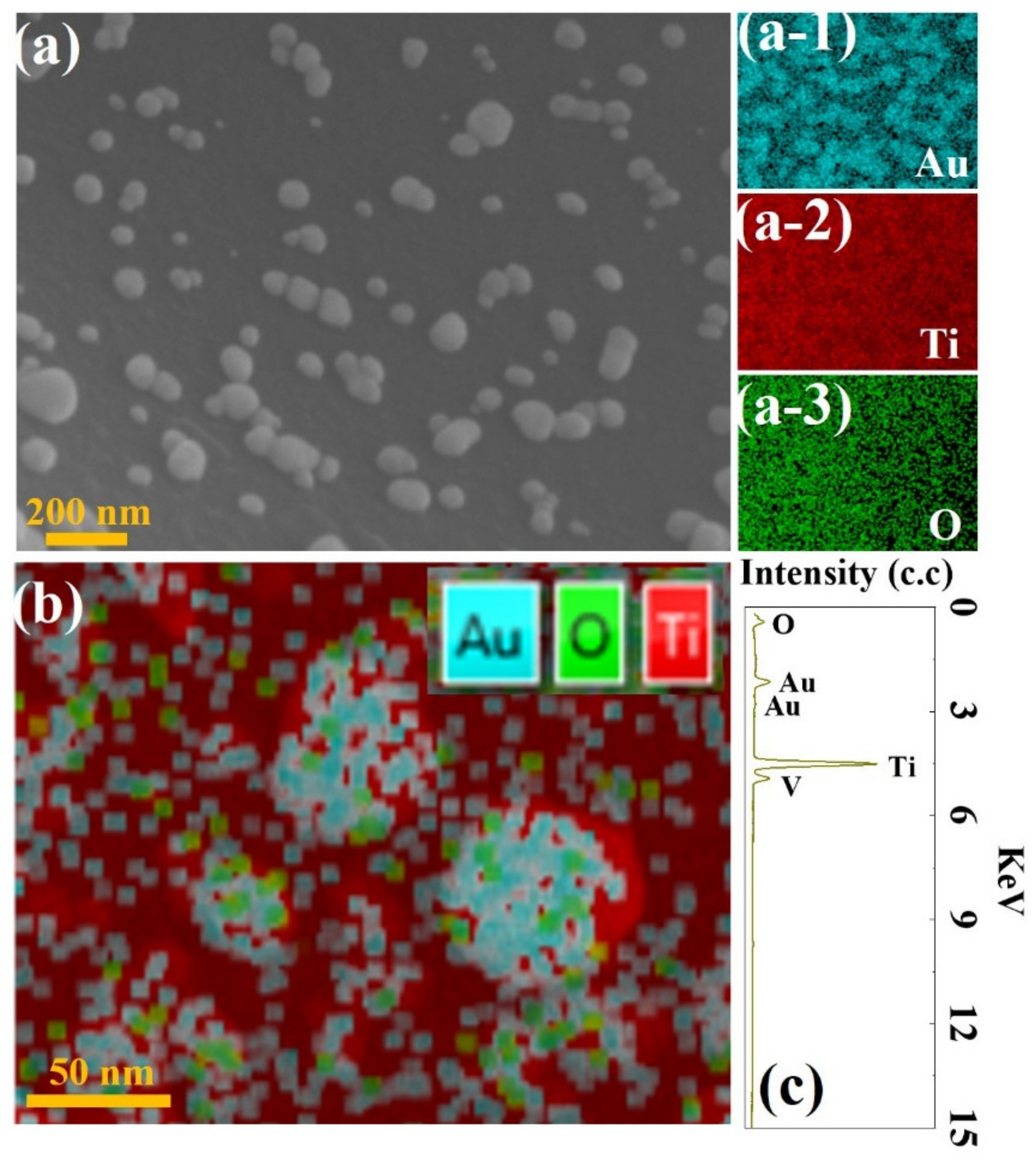
3.3. Microstructure and Composition Analysis
3.4. Electrochemical Measurements
3.5. ICR Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, G.; Hou, F.; Peng, S.; Wang, X.; Fang, B. Synthesis, physical properties and electrocatalytic performance of nickel phosphides for hydrogen evolution reaction of water electrolysis. Nanomaterials 2022, 12, 2935. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, B.; Wang, Z.; Wang, C.; Liu, Z.; Liu, F.; Wang, W.; Alfantazi, A.; Wang, D.; Wilkinson, D. MoS2 nanosheets: A designed structure with high active site density for the hydrogen evolution reaction. ACS Catal. 2013, 3, 2101–2107. [Google Scholar] [CrossRef]
- Kim, J.; Fang, B.; Song, M.; Yu, J. Topological transformation of thioether-bridged organosilicas into nanostructured functional materials. Chem. Mater. 2012, 24, 2256–2264. [Google Scholar] [CrossRef]
- Lu, L.; Zou, S.; Fang, B. The critical impacts of ligands on heterogeneous nanocatalysis: A review. ACS Catal. 2021, 11, 6020–6258. [Google Scholar] [CrossRef]
- Xing, Y.; Fang, B.; Bonakdarpour, A.; Zhang, S.; Wilkinson, D. Facile fabrication of mesoporous carbon nanofibers with unique hierarchical nanoarchitecture for electrochemical hydrogen storage. Int. J. Hydrog. Energy 2014, 39, 7859–7867. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-free synthesis of noble metal nanocatalysts for electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Li, X.; Fang, B. Emerging polymeric carbon nitride Z-scheme systems for photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.; Fang, B.; Yang, H. Z-scheme systems: From fundamental principles to characterization, synthesis, and photocatalytic fuel-conversion applications. Phys. Rep. 2022, 983, 1–41. [Google Scholar] [CrossRef]
- Liao, G.; Tao, X.; Fang, B. An innovative synthesis strategy for highly efficient and defects-switchable hydrogenated TiO2 photocatalysts. Matter 2022, 5, 377–379. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Fang, B. Donor-acceptor organic semiconductor heterojunction nanoparticles for efficient photocatalytic H2 evolution. Matter 2022, 5, 1635–1637. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.-Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrog. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Suen, N.; Hung, S.; Quan, Q.; Zhang, N.; Xu, Y.; Chen, H. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Teuku, H.; Alshami, I.; Goh, J.; Masdar, M.; Loh, K. Review on bipolar plates for low-temperature polymer electrolyte membrane water electrolyzer. Int. J. Energy Res. 2021, 45, 20583–20600. [Google Scholar] [CrossRef]
- Song, J.; Wei, C.; Huang, Z.; Liu, C.; Zeng, L.; Wang, X.; Xu, Z.J. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 2020, 49, 2196–2214. [Google Scholar] [CrossRef]
- Ursúa, A.; Gandía, L.; Sanchis, P. Hydrogen production from water electrolysis: Current status and future trends. Proc. IEEE 2012, 100, 410–426. [Google Scholar] [CrossRef]
- Huang, M.; Liu, G.; Wang, D. Synthesis and performance of multilayered titanium mesh oxygen evolution anode in polymer exchange membrane water electrolysis. Nonferrous Met. Sci. Eng. 2016, 7, 1–5. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, J.; Li, Y.; Wei, B.; Zhang, N.; Li, Y.; Bondarchuk, O.; Miao, H.; Araujo, A.; Wang, Z.; et al. Ultrafine oxygen-defective iridium oxide nanoclusters for efficient and durable water oxidation at high current densities in acidic media. J. Mater. Chem. A 2020, 8, 24743–24751. [Google Scholar] [CrossRef]
- Wu, S.; Yang, W.; Yan, H.; Zou, X.; Cao, Z.; Li, H.; Shi, M.; Chen, H. A review of modified metal bipolar plates for proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2021, 46, 8672–8701. [Google Scholar] [CrossRef]
- Toops, T.; Brady, M.; Zhang, F.Y.; Meyer, H.M., III; Ayers, K.; Roemer, A.; Dalton, L. Evaluation of nitrided titanium separator plates for proton exchange membrane electrolyzer cells. J. Power Sources 2014, 272, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Yang, J.; Liu, X.; Wang, D.; Yang, Z.; Liu, G.; Wang, X. Development and evaluation of nitride coated titanium bipolar plates for PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 1144–1154. [Google Scholar] [CrossRef]
- Choe, C.; Choi, H.; Hong, W.; Lee, J. Tantalum nitride coated AISI 316L as bipolar plate for polymer electrolyte membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 405–411. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. Electrochemical oxidation behavior of titanium nitride based electrocatalysts under PEM fuel cell conditions. Electrochim. Acta 2010, 55, 9024–9034. [Google Scholar] [CrossRef]
- LaConti, A.; Griffith, A.; Cropley, C.; Kosek, J. Titanium Carbide Pipolar Plate for Electrochemical Devices. U.S. Patent 6,083,641, 2000. [Google Scholar]
- Zhang, D.; Duan, L.; Guo, L.; Wang, Z.; Zhao, J.; Tuan, W.H.; Niihara, K. TiN-coated titanium as the bipolar plate for PEMFC by multi-arc ion plating. Int. J. Hydrogen Energy 2011, 36, 9155–9161. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Peng, S.; Wang, X.; Fang, B. Process and challenges of stainless steel based bipolar plates for proton exchange membrane fuel cells. Int. J. Miner. Metall. Mater. 2022, 29, 1099–1119. [Google Scholar] [CrossRef]
- Jin, N.; Yang, Y.; Luo, X.; Xia, Z. Development of CVD Ti-containing films. Prog. Mater. Sci. 2013, 58, 1490–1533. [Google Scholar] [CrossRef]
- Wang, S.; Peng, J.; Lui, W.; Zhang, J. Performance of the gold-plated titanium bipolar plates for the light weight PEM fuel cells. J. Power Sources 2006, 162, 486–491. [Google Scholar] [CrossRef]
- Rojas, N.; Sánchez-Molina, M.; Sevilla, G.; Amores, E.; Almandoz, E.; Esparza, J.; Vivas, M.R.C.; Colominas, C. Coated stainless steels evaluation for bipolar plates in PEM water electrolysis conditions. Int. J. Hydrogen Energy 2021, 46, 25929–25943. [Google Scholar] [CrossRef]
- Stiber, S.; Balzer, H.; Wierhake, A.; Wirkert, F.J.; Roth, J.; Rost, U.; Brodmann, M.; Lee, J.K.; Bazylak, A.; Waiblinger, W.; et al. Porous transport layers for proton exchange membrane electrolysis under extreme conditions of current censity, temperature, and pressure. Adv. Energy Mater. 2021, 11, 2100630. [Google Scholar] [CrossRef]
- Kumagai, M.; Myung, S.; Asaishi, R.; Sun, Y.; Yashiro, H. Nanosized TiN–SBR hybrid coating of stainless steel as bipolar plates for polymer electrolyte membrane fuel cells. Electrochim. Acta 2008, 54, 574–581. [Google Scholar] [CrossRef]
- Cui, J.; Xu, J.; Xiu, H.; Wang, H.; Li, J.; Yang, J. Graphene-dominated hybrid coatings with highly compacted structure on stainless steel bipolar plates. ACS Appl. Mater. Interfaces 2022, 14, 37059–37067. [Google Scholar] [CrossRef] [PubMed]
- Sadeghian, Z.; Hadidi, M.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Wang, F.; Alazemi, M.; Dutta, I.; Blunk, R.; Angelopoulos, A. Layer by layer assembly of hybrid nanoparticle coatings for proton exchange membrane fuel cell bipolar plates. J. Power Sources 2010, 195, 7054–7060. [Google Scholar] [CrossRef]
- Siva, T.; Rajkumar, S.; Muralidharan, S.; Sathiynarayanan, S. Bipolar properties of coatings to enhance the corrosion protection performance. Prog. Org. Coat. 2019, 137, 105379. [Google Scholar] [CrossRef]
- Quayum, M.; Ye, S.; Uosaki, K. Mechanism for nucleation and growth of electrochemical palladium deposition on an Au(111) electrode. J. Electroanal. Chem. 2002, 520, 126–132. [Google Scholar] [CrossRef]
- Ye, W.; Kou, H.; Liu, Q.; Yan, J.; Zhou, F.; Wang, C. Electrochemical deposition of Au–Pt alloy particles with cauliflower-like microstructures for electrocatalytic methanol oxidation. Int. J. Hydrogen Energy 2012, 37, 4088–4097. [Google Scholar] [CrossRef]
- Callen, B.; Lowenberg, B.; Lugowski, S.; Sodhi, R.; Davies, J. Nitric acid passivation of Ti6Al4V reduces thickness of surface oxide layer and increases trace element release. J. Biomed. Mater. Res. 1995, 29, 279–290. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Wang, D.; Zhang, H.; Shan, D.; Peng, S.; Shen, G.; Wang, L.; Wang, X. Modifying Ti-based gas diffusion layer passivation for polymer electrolyte membrane water electrolysis via electrochemical nitridation. ACS Appl. Mater. Interfaces 2022, 14, 15728–15735. [Google Scholar] [CrossRef]
- Wakayama, H.; Yamazaki, K. Low-Cost Bipolar Plates of Ti4O7-Coated Ti for water electrolysis with polymer electrolyte membranes. ACS Omega 2021, 6, 4161–4166. [Google Scholar] [CrossRef] [PubMed]
- DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components|Department of Energy. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 21 September 2022).
- Stein, T.; Ein-Eli, Y. Challenges and perspectives of metal-based proton exchange membrane’s pipolar plates: Exploring durability and longevity. Energy Technol. 2020, 8, 2000007. [Google Scholar] [CrossRef]


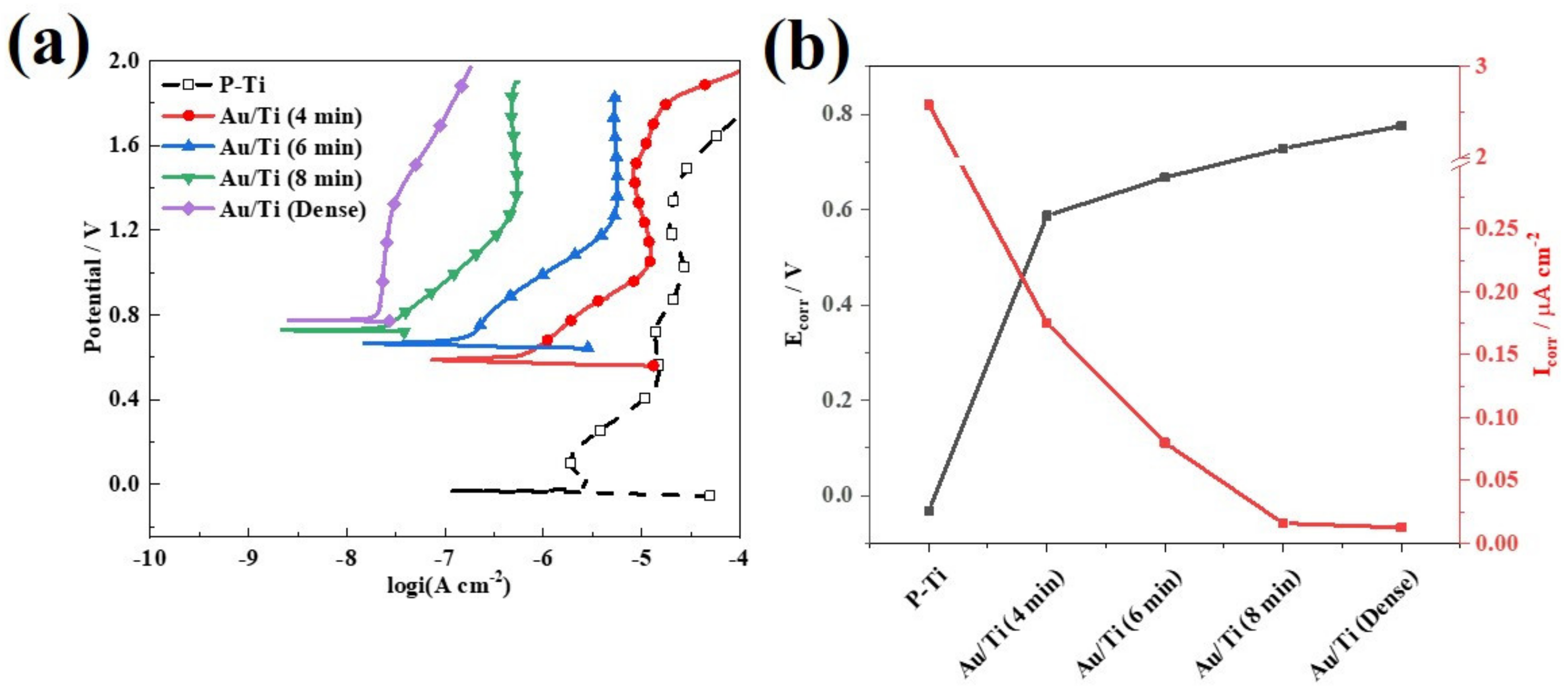
| Specimens | Corrosion Resistance | ICR at 140 N cm−2 | |||
|---|---|---|---|---|---|
| Ecorr (mV) | Icorr (μA cm−2) | I+1.8 V 30 h (μA cm−2) | ICRBefore (mΩ cm2) | ICRAfter (mΩ cm2) | |
| P-Ti | −0.032 | 2.580 | 0.718 | 19.7 | 22.1 |
| Au/Ti (4 min) | 0.587 | 0.175 | - | 5.2 | - |
| Au/Ti (6 min) | 0.667 | 0.080 | 0.689 | 3.5 | 4.2 |
| Au/Ti (8 min) | 0.728 | 0.016 | - | 2.8 | - |
| Au/Ti (dense) | 0.775 | 0.013 | 0.709 | 2.3 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Peng, S.; Hou, F.; Fang, B.; Wang, X. Au-TiO2/Ti Hybrid Coating as a Liquid and Gas Diffusion Layer with Improved Performance and Stability in Proton Exchange Membrane Water Electrolyzer. Molecules 2022, 27, 6644. https://doi.org/10.3390/molecules27196644
Liu G, Peng S, Hou F, Fang B, Wang X. Au-TiO2/Ti Hybrid Coating as a Liquid and Gas Diffusion Layer with Improved Performance and Stability in Proton Exchange Membrane Water Electrolyzer. Molecules. 2022; 27(19):6644. https://doi.org/10.3390/molecules27196644
Chicago/Turabian StyleLiu, Gaoyang, Shanlong Peng, Faguo Hou, Baizeng Fang, and Xindong Wang. 2022. "Au-TiO2/Ti Hybrid Coating as a Liquid and Gas Diffusion Layer with Improved Performance and Stability in Proton Exchange Membrane Water Electrolyzer" Molecules 27, no. 19: 6644. https://doi.org/10.3390/molecules27196644






