Abstract
Origanum vulgare L. (oregano) is an aromatic plant with wide applications in the food and pharmaceutical industries. Cronobacter sakazakii, which has a high detection rate in powdered infant formula, adversely impacts susceptible individuals. Oregano essential oil (OEO) is a natural antibacterial agent that can be used to fight bacterial contamination. Here, OEO chemical compounds from eight oregano varieties were analyzed by gas chromatography–mass spectrometry and their antibacterial properties were assessed. The eight OEOs were clustered into two groups and were more diverse in group 2 than in group 1. Six compounds, including p-cymene, 3-thujene, γ-terpinene, thymol, carvacrol, and caryophyllene, were shared by eight OEOs. Among the eight oregano varieties, OEOs from O. vulgare sc2 had the strongest antibacterial activity against C. sakazaki, with the inhibition zone of 18.22mm. OEOs from O. vulgare jx, O. ‘Nvying’, O. vulgare ‘Ehuang’, and O. vulgare ssp. virens were also potent. Moreover, the antibacterial activity of OEOs was positively correlated with the relative content of thymol. As the main OEO antibacterial compound, thymol affected the normal growth and metabolism of C. sakazakii cells by destroying the bacterial membrane and decreasing the intracellular ATP concentration. Thus, in light of the antibacterial activity detected in the OEOs from the eight oregano varieties, this study provides a theoretical foundation for oregano cultivar management and development.
1. Introduction
Natural plant extracts, such as essential oils and organic acids, have a specific antibacterial effect [1]. Essential oil exerts a bacteriostatic effect on multiple targets in bacteria by disrupting the bacterial membrane and reducing the intracellular ATP concentration and intracellular pH value [2,3]; thus, developing antibiotic resistance is not easy. In addition, essential oil has been extensively studied for applications in food, hygiene, cosmetics, and medicine for its antibacterial properties [4,5,6].
Origanum vulgare L. (oregano), a perennial aromatic plant that belongs to the family Lamiaceae, is commonly used as a flavoring herb, feed additive, and garden ornamental, but the plant is also medicinal and has antibacterial, antioxidant, and anti-inflammatory properties [7]. Besides tremendous application potential in food preservation because of its rich active compounds, such as carvacrol and thymol [8], oregano has a specific bacteriostatic effect on Escherichia coli [9], Staphylococcus aureus [10], Listeria monocytogenes [11], Salmonella enteritidis [12], and Cronobacter sakazakii [13].
The oregano essential oil (OEO) content and quality from various germplasm resources are different. Even under the same growth environment, the chemical composition of OEOs from different populations has notable differences [14,15]. In addition to the different plant types, the composition of plant essential oils is affected by various factors, such as the geographical environment and harvest period, thereby affecting their antibacterial properties [16,17]. With the demand for oregano, artificial cultivation and cultivar development of oregano have become more crucial.
C. sakazakii, a Gram-negative bacterium and foodborne opportunistic pathogen, can remain active at a low temperature (4 °C) and is resistant to desiccation [18,19]. It is highly harmful to newborns, premature infants, and elderly people with a weakened immune system. For example, it can lead to necrotizing enterocolitis, sepsis, meningitis, cerebrospinal effusion, peritoneal effusion, and intracerebral infarction. Moreover, it has a high mortality rate in infants, up to 80% [20,21]. Patients may experience chronic neurological disease and developmental complications [19]. Therefore, the International Commission for the Microbiological Specifications of Foods has classified C. sakazakii as a “serious hazard for restricted populations, life-threatening or with severe chronic sequelae” [22].
External stimuli, such as drying, toxic substances, and antibiotics, can make bacteria enter a viable but non-cultivable state [23,24], which may be related to the tolerance of C. sakazakii. Although C. sakazakii was removed by heat and drying processes, it still had a high detection rate in foods such as powdered infant formula [25,26,27]. Cells in the viable but non-cultivable state can still be pathogenic, posing certain public health risks [24,25]. In addition, the abuse of antibiotics enhances bacterial resistance, as evidenced by the isolation of environmental strains resistant to tetracycline, penicillin, trimethoprim, and other antibacterial drugs [28]. Therefore, finding alternatives to antibiotics, solving the current bacteriostatic dilemma, and effectively killing bacteria have become research topics for food safety [29].
OEO is a potent substitute to antibiotics, and the cultivation of oregano has received much attention. Our previous study evaluated the antibacterial activity of OEO extracted from different parts of the same site [30]. In this study, the antibacterial properties of OEOs from eight oregano varieties were evaluated by comparing the diameter of the inhibition zone (DIZ), the minimum inhibitory concentration (MIC), and the minimum bactericidal concentration (MBC). In addition, the effects of thymol, the main antibacterial compound of OEO, on C. sakazakii cell membrane integrity and morphology, protein leakage concentration, and intracellular ATP concentration were examined.
2. Results and Discussion
2.1. OEO Compounds of Eight Oregano Varieties
Sixty-seven compounds were detected in OEOs from the eight oregano varieties by GC–MS (Table 1). The total proportion of detected compounds was 95.35–99.13%. Sabinene, p-cymene, γ-terpinene, methyl thymyl ether, thymol, carvacrol, and spathulenol, mainly terpenes, were largely present in OEOs. Ovsc1 and Ovsc2 were similar in their main compounds (Table 1). Thymol was the most abundant constituent of OEOs, with a relative percentage of 53.95%, which was consistent with previous results [31]. The main OEO compounds of Ovny, Oveh, and Ovvr were similar, and the top three compounds were thymol, γ-terpinene, and p-cymene separately. All OEO compounds were visualized using a heatmap (Figure 1A). According to the plant materials and the composition analysis results, habitats may have specific effects on OEO composition, in addition to various species, artificial cultivation, and selection factors [2,32,33].

Table 1.
Chemical composition of essential oils from eight oregano varieties.
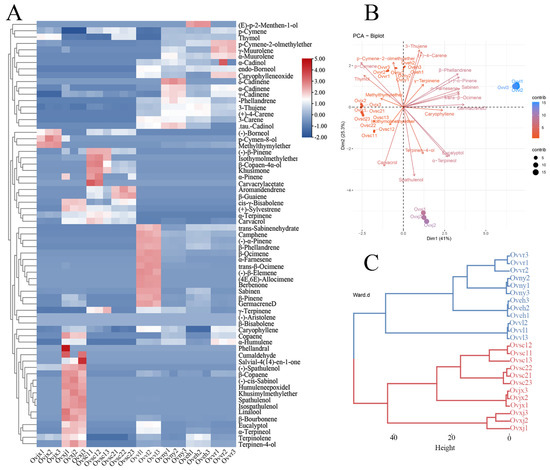
Figure 1.
Composition analysis of OEOs. The results are illustrated by a heatmap (A), PCA plot (B), and dendrogram (C).
2.2. Clustering and Diversification Analysis of OEO Composition
The principal component analysis is shown in Figure 1B. They were clustered into four categories according to their compounds, which can be observed from the nodes in the figure. In addition, carvacrol, γ-terpinene, and p-cymene had a negative correlation, which was determined by analyzing each vector (Figure 1B). In contrast, p-cymene and thymol had a positive correlation. The correlation between these compounds may be related to the anabolic pathway of essential oils in plants. Furthermore, based on the dendrogram results, all eight OEOs could be grouped into the following two categories: group 1 (red line) of plant material was collected from the wild, and group 2 (blue line) was precisely collected from Institute of Botany, Chinese Academy of Sciences (Figure 1C). According to OEO composition, the relative content of thymol was higher in wild oregano than in cultivated oregano, which was consistent with the findings of El Gendy et al. [34]. In future oregano cultivar development, the wild-type with high relative content of thymol in OEO can be considered parents to obtain valuable cultivars.
Comprehensive data analysis of 24 samples (Figure 1) showed that terpenes were the main OEO compounds. Furthermore, group 1 contained more monoterpenes, and group 2 contained more derivatives of terpenes. OEOs in group 1 were significantly different from those in group 2, in terms of composition, which may be an observable target in oregano breeding and one piece of evidence for oregano-related functional changes.
2.3. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) of Eight OEOs
All samples were divided into two groups according to the dendrogram results (Figure 1C). A supervised OPLS-DA statistical method was used to explore the compound clustering (Figure 2). The main characteristic compounds in group 1 were methyl thymyl ether, spathulenol, and α-terpinene. Group 2 was characterized by sabinen, caryophyllene, germacrene D, and (+)-4-carene. In the variable importance projection (VIP) diagram (Figure 2C), 3-thujene, (+)-4-carene, endo-borneol, α-terpinene, and α-phellandrene had higher VIP values. These may be the critical basis for classification. However, in this study, compounds with very low relative content were taken into consideration, which may have a particular impact on the VIP value of the compounds.
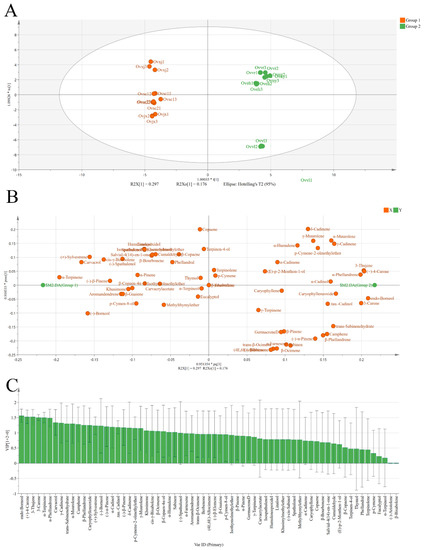
Figure 2.
Score plots (A), loading plot (B), and VIP values (C) from OPLS-DA based on the chemical profiles of eight OEOs.
2.4. Analysis of Common and Unique Compounds of Eight OEOs
Upset Venn and flower diagrams were drawn to visualize the composition dataset and further compare the compounds of the eight OEOs. Based on the Upset Venn diagram (Figure 3A), the number of compounds of the 8 OEOs ranged from 15 to 38. Except for Ovxj, the other compounds of oregano collected from the wild were relatively simple, with fewer than 20 compounds. However, the oregano composition in group 2 was relatively balanced, and it was more complex than that in group 1. There were six components shared by eight OEOs (Figure 3B), and only one of them was shared by oregano in group 1.
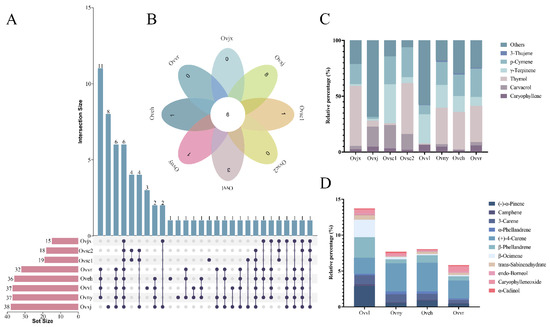
Figure 3.
Distribution of common and unique compounds. Amounts of OEO chemical compounds illustrated by an UpSet Venn diagram (A) and flower diagram (B). Stacked graph (C) of common compounds in all samples. Stacked graph (D) of shared compounds in all cultivated varieties.
According to the stacked graph of the standard compound relationships among the eight oregano varieties, the six compounds shared by all OEOs were 3-thujene, p-cymene, γ-terpinene, thymol, carvacrol, and caryophyllene (Figure 3C). Thymol accounted for the largest proportion in Ovjx and Ovsc2; p-cymene, γ-terpinene, and thymol accounted for equal importance in Ovny, Oveh, and Ovvr. These six compounds were similar in proportion in Ovvl and Ovxj. Overall, among the six compounds shared by the eight OEOs, thymol accounted for the largest proportion and varied the most. Eleven compounds were shared by oregano in group 2 (Figure 3D), which were similar in terms of the relative content. Furthermore, the stacked graph showed that the relative proportions of (+)-4-carene and β-phellandrene were high.
These results demonstrated that the OEO chemical compounds of both groups 1 and 2 were similar and the distribution of antibacterial compounds was uniform. Nevertheless, the relative percentages of these compounds were significantly different. Compared with group 1, the OEO compounds that belonged to group 2 were complex.
The OEO compounds in different varieties were remarkably similar to the genetic relationship in the parents selected for cultivation. They may also be related to the consistency of the habitats. Several studies have indicated that environmental factors, such as altitude, water availability, and pedo-climatic conditions, affect the proportion of compounds and qualitative chemical composition [35,36]. Perhaps because of the certain altitude and climatic conditions in Xinjiang, eight unique OEO compounds were detected in Ovxj.
2.5. Antibacterial Activity of OEOs
DIZ, MIC, and MBC were measured to evaluate the antibacterial properties of the eight OEOs (Figure 4). All eight OEOs had potent antibacterial activity, with 1–8 mg/mL of MIC and MBC values. DIZ of OEO was mostly above 10 mm, which was consistent with the high-efficiency inhibitory effect results of OEO on E. coli, S. aureus, L. monocytogenes, and other foodborne pathogens [37,38,39]. DMSO had no inhibitory effect on C. sakazakii, and the inhibition zone was almost absent (Figure 4C).
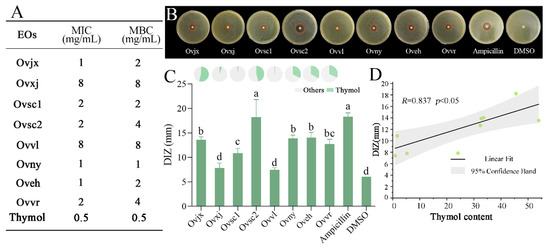
Figure 4.
Antibacterial activity of OEOs. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) (A), the diameter of inhibition zone (DIZ) images (B). Statistical data (histogram in C) for OEOs against C. sakazakii, and percentages of thymol in the eight OEOs (pie graph in C); Discs were 6 mm in diameter, and values are means ± standard deviations; different lowercase letters (a, b, c, d) above bars indicate significant differences (p < 0.05) (C), correlation between thymol content in OEOs and its antibacterial activity against C. sakazakii (D).
The DIZ results showed that the antibacterial activity of OEOs against C. sakazakii can be divided into the following three levels: (1) Ovsc2 was the strongest, reaching 18.22 mm; (2) Ovsc1, Ovjx, Ovny, and Oveh were moderate; and (3) Ovvl and Ovxj were the weakest, which showed no significant difference from DMSO. The antibacterial properties of OEOs from group 2 were relatively average, and most were at a medium level. In contrast, group 1 oregano had excellent or inferior antibacterial properties, which were relatively scattered, suggesting that the cultivation environment impacted the OEO composition, thereby affecting its antibacterial activity.
The MIC and MBC values of OEOs were relatively consistent with the trend of DIZ results (Figure 4A,B). Similar to the DIZ result, the OEOs of Ovvl and Ovxj had the highest MIC and MBC values, implying that their antibacterial ability was the weakest. However, the OEO of Ovny, which belonged to group 2, had the lowest MBC value. Figure 4 showed that the MIC and MBC values of group 2 OEOs were more potent than those of group 1. The different ductility of OEOs might affect their performance in the DIZ test, which might be the reason for the slight deviation from the DIZ results.
On the basis of the antibacterial status of OEOs and the proportional distribution of each compound, it was determined that thymol, one of the compounds shared by all OEOs (Figure 3C), was the most variable compound. In addition, DIZ of OEO was consistent with the variation in thymol concentration (Figure 4C). After studying the relation between the thymol content in OEOs and antibacterial activity, it was found that the greater the thymol concentration, the stronger the OEO antibacterial impact, and a substantial positive correlation was observed between the two variables (Figure 4D). The antibacterial activity of OEO is produced by the synergistic action of various chemical compounds; thus, it is not completely positively correlated with the relative content of thymol. Essential oil and its main antibacterial compounds do not easily produce drug resistance because of multiple targets. Therefore, they are ideal natural antibacterial agents to replace antibiotics [40]. However, clarifying the mechanism of action of the core antibacterial compounds is essential for the development of OEO as an alternative to antibiotics. Thus, the specific antibacterial activity and mechanism of thymol, the main compound in OEOs, require further study.
2.6. Antibacterial Activity of Thymol against C. sakazakii
CLSM was used to detect cell membrane damage (Figure 5A). With the increase in thymol concentration, the green fluorescence gradually decreased and the red fluorescence gradually increased. SEM can observe changes in cell morphology, which was direct evidence that thymol may disrupt bacterial structures. Bacteria in the control group had a smooth surface and a complete morphology (Figure 5B). When the concentration of thymol reached ½ MIC, the morphology of bacteria was basically complete. In Figure 5C–E, it can be observed that the protein leakage occurred at this time. However, C. sakazakii treated with ½ MIC of thymol could still maintain certain normal life activities compared with those treated with MIC of thymol.
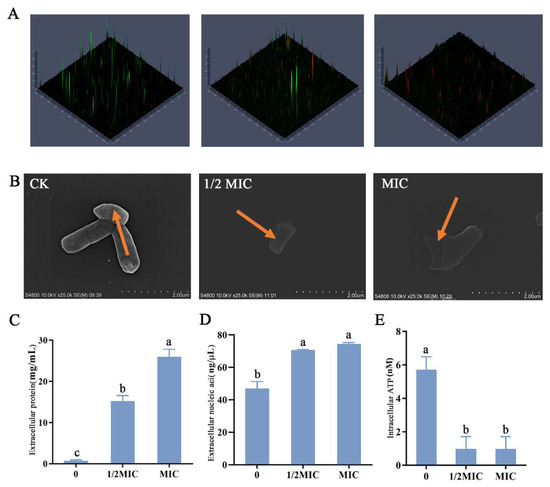
Figure 5.
Cell damage of C. sakazakii ATCC 29544. CLSM showed C. sakazakii viability treated with different concentrations of thymol. (A) Cells stained with propidium iodide (PI) are labeled red, whereas cells stained with SYTO9 are labeled green. (B) Scanning electron micrographs of C. sakazakii ATCC 29544 treated with different concentrations of thymol. (C) Extracellular protein concentration. (D) Extracellular nucleic acid concentration. (E) Intracellular ATP concentration. Different lowercase letters (a, b, c) above bars indicate significant differences (p < 0.05).
With the increase in thymol concentration, the bacterial surface appeared to fold, and when the treatment concentration reached the MIC value, the bacterial membrane was completely damaged. The concentration of extracellular proteins and nucleic acids significantly increased with thymol concentration (Figure 5C,D), indicating that the severity of bacterial membrane damage was gradual. This further substantiated that treatment with thymol disrupted bacterial membranes. In addition to changes in bacterial membranes, thymol severely impacted the normal life activities of C. sakazakii. Compared with the control, ATP concentration in bacteria significantly decreased after treatment, indicating that thymol treatment had a significant inhibitory effect on the normal life activities of C. sakazakii (Figure 5E).
Previous studies have shown that the antibacterial mechanism of carvacrol, an isomer of thymol, works mainly by disrupting bacterial membranes, affecting the leakage of bacterial intracellular components, and the normal metabolic activities of bacteria, resulting in decreased bacterial activity and even death [3,41]. In the present study, thymol was found to destroy bacterial membranes to produce antibacterial effects, indicating that the antibacterial mechanisms of thymol and carvacrol had certain similarities. Furthermore, it provided a specific theoretical basis for the similar application of different OEO compounds. Further studies will explore differences in the antibacterial mechanism between thymol and carvacrol at the molecular level.
3. Materials and Methods
3.1. Bacterial Strain
C. sakazakii ATCC 29544 was preserved in 25% glycerol at −80 °C at the Institute of Botany, Chinese Academy of Sciences. Before use, cells of the strain were taken out and cultured in Luria–Bertani (LB) broth at 37 °C with 180 rpm for 12 h.
3.2. Plant Material and Extraction of OEOs
Details of the plant materials used in this study are provided in Table 2.

Table 2.
Plant materials and collection sites.
The specimens were kept at the Chinese National Herbarium, Institute of Botany, Chinese Academy of Sciences, with the following accession numbers: Ovjx (PE 02347462), Ovxj (PE 02347461), Ovsc1 (PE 02347445), Ovsc2 (PE 02347444), Ovvl (PE 02347464), and Ovvr (PE 02347463). Ovny and Oveh are new varieties cultivated by our research group, with patent numbers 20210721 and 20210717. All plant materials were taken from aerial parts and dried at room temperature in the shade. Samples were then ground to a powder. The powdered samples (100 g) were mixed with 1000 mL of distilled water, and a steam distillation method with a Clevenger apparatus was used to extract the OEOs. After boiling, the extraction process was performed for 3 h. Extracted OEOs were dried using anhydrous sodium sulfate and stored in an amber bottle at 4 °C [30,31].
3.3. Analysis of OEO Composition
All OEO samples were filtered and diluted with n-hexane at a 1:200 ratio. The 7890A-7000B GC–MS (Agilent Technologies, Santa Clara, CA, USA), equipped with an HP-5MS column (30 m × 250 μm × 0.25 μm; Agilent Technologies), was used to perform gas chromatography–mass spectrometry (GC–MS), and the specific procedure was a modified method described previously [42]. The injector temperature was 250 °C. The temperature program was as follows: OEO sample (1 μL) was injected in the split mode at 20:1; the temperature was maintained at 60 °C for 4 min and was then increased linearly to 84 °C at a rate of 6 °C/min; the temperature was then increased to 102 °C at a rate of 4 °C/min, next increased to 156 °C at a rate of 6 °C/min, followed by increasing to 180 °C at 4 °C/min. Finally, the temperature was increased to 280 °C at a rate of 20 °C/min. The transfer line temperature was 280 °C, and helium was used as the carrier gas at a flow rate of 1.2 mL/min through the column. The MS conditions were as follows: ionization energy, 70 eV; electronic impact ion source temperature, 230 °C; quadrupole temperature, 150 °C; and mass range, 40–700 μL. Simultaneously, the relevant information for n-alkanes was determined, and the relative percentage of each compound was determined according to the peak area. The retention index (RI) value of each compound was calculated according to the RI value and C7-C40 data, and compared with the NIST 17 library to determine the specific compound of each OEO [43].
3.4. Antibacterial Activity of Extracted OEOs
3.4.1. Diameter of Inhibition Zone (DIZ) Measurement
The disc diffusion method, with some modifications, was used to compare the zone of inhibition of OEOs and to evaluate their antibacterial activity in one dimension. Then, 100 μL of C. sakazakii bacterial suspension (107 CFU/mL) was spread evenly on an LB agar plate, and sterilized special filter paper with a diameter of 6 mm was placed in the center of the plate, and 10 μL of 200 mg/mL OEO diluted with dimethylsulfoxide (DMSO) was carefully dropped onto the plate. The plates were incubated at 37 °C for 24 h, and the DIZ value was measured with a Vernier caliper (AIRAJ, Qingdao, China). Ampicillin (10 μg) was the positive control, and DMSO was the negative control.
3.4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
MIC and MBC of C. sakazakii were assessed according to our previous method [42]. OEOs were diluted with LB broth containing 107 CFU/mL at two-fold serial dilutions to obtain concentrations of 16, 8, 4, 2, 1, 0.5, 0.25, and 0.125 mg/mL. Bacterial suspensions without OEO were used as the control group. The samples were then incubated for 24 h at 37 °C. The lowest concentration of OEO at which the medium had no visible bacterial growth was considered the MIC value. After MIC determination, bacterial suspensions that contained an OEO (not less than MIC) were spread evenly on agar. The lowest concentration of OEO at which the medium excluded visible bacterial colonies was considered the MBC value. The MIC and MBC values of thymol were determined in the same way as OEOs.
3.5. Cell Membrane Integrity Assay
C. sakazakii suspensions (~107 CFU/mL) were treated for 4 h with or without thymol at final concentrations of 0, ½ MIC, and MIC. Then, the treated C. sakazakii cells were washed three times with phosphate-buffered saline (PBS) and stained with 5 µM SYTO9 and 15 µM propidium iodide (PI) (Molecular Probes, Invitrogen, France) in the dark for 15 min [44,45]. SYTO9 and PI were diluted with 0.85% saline. Next, cells were washed three times with PBS and resuspended in PBS before analysis. The cell membrane integrity was measured using a confocal laser scanning microscope (CLSM; Zeiss LSM 980 with Elyra7, Jena, Germany).
3.6. Scanning Electron Microscope (SEM) Observations of C. sakazakii
Changes in morphological characteristics of C. sakazakii after exposure to thymol were observed as described by Hao et al. [45], with some modifications. C. sakazakii ATCC 29544 (107 CFU/mL) was treated with thymol at final concentrations of 0, ½ MIC, and MIC at 37 °C for 4 h. The samples were then centrifuged (5000× g, 10 min, 4 °C) and washed three times with PBS. Sterile water containing 2.5% glutaraldehyde was used to fix cells for 6 h at 4 °C. Then, the C. sakazakii cells were dehydrated using a water–ethanol gradient (25%, 50%, 75%, 90%, 95%, 100%), with 10 min standing for each concentration. After dehydration and drying, the samples were fixed on the SEM support and sprayed with gold by ion sputtering. Finally, all samples were examined using a SEM (Regulus 8100, Hitachi Co., Ltd., Japan) [46].
3.7. Bacterial Extracellular Protein Assay
The extracellular protein concentration of the samples was detected with a micro BCA protein assay kit (C503061, Sangon Biotech, Shanghai, China). The standard curve assay solution was prepared according to the kit instructions. The bacterial solution treated with thymol at final concentrations of 0, ½ MIC, and MIC was centrifuged at 5000× g for 5 min, and the supernatant was diluted to a specific multiple. A microplate reader (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the standard and test solutions at 562 nm. Extracellular protein concentration of the samples was calculated according to the standard curve.
3.8. Bacterial Extracellular Nucleic Acid Assay
The treatment approach of C. sakazakii suspensions was identical to the pretreatment procedure of extracellular protein concentration measurement. After collecting the supernatant, the extracellular nucleic acid concentration was determined using a nucleic acid concentration detector (IMPLEN, Munich, Germany).
3.9. Bacterial Intracellular ATP Assay
To determine the ATP content of cells with different treatments, the residue, collected after centrifugation at 5000× g for 5 min, was detected using an ATP bioluminescence assay kit (S0026, Beyotime, Haimen, China), following the manufacturer’s instructions. A standard ATP concentration curve was used to determine the intracellular ATP concentration of C. sakazakii suspensions.
3.10. Statistical Analysis
All experiments were independently performed in triplicate. The difference in DIZ (p < 0.05 was considered significant) was determined using SPSS software (version 25.0; IBM, Endicott, NY, USA). The OEO compounds and their correlation with antibacterial activity were analyzed by heatmaps, principal component analysis, cluster analysis, UpSet Venn, and flower diagrams based on the R drawing platform. Correlations between DIZ and OEO compounds were analyzed using Origin 2021 (OriginLab, Northampton, MA, USA).
4. Conclusions
OEOs had potent antimicrobial activity against C. sakazakii. Through multidimensional exploration, oregano collected from different environments showed significant differences in OEO composition. Moreover, the relative content of thymol was positively correlated with OEO antibacterial activity, which implied that thymol was important to the biological activities of OEOs. However, the correlation was not always positive between them, which may be because of the synergistic and antagonistic effects among the various compounds. In addition, as the main antibacterial compound in these OEOs, thymol appeared to have some definitive mechanisms of action on C. sakazakii, including decreasing bacterial activity, disrupting the bacterial membrane, causing the leakage of bacterial proteins, and reducing the intracellular ATP concentration. This study evaluated the antibacterial properties of OEOs and provided evidence for the antibacterial activity of thymol. Moreover, it provided a theoretical foundation for oregano cultivar development from wild oregano resources for OEO with a high relative content of thymol.
Author Contributions
Conceptualization, X.G., Y.H. and W.Z.; methodology, X.G., Y.H. and L.S.; software, X.G. and Y.H.; validation, X.G., Y.H. and H.L.; investigation, H.L. and H.B.; resources, H.L., F.X. and H.B.; writing—original draft preparation, X.G.; writing—review and editing, L.S. and H.L.; visualization, X.G.; supervision, L.S.; project administration, H.L.; funding acquisition, L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA23080603).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Jing-Quan Li, Xiu-Ping Xu, and Yan Zhu from the Plant Science Facility of the Institute of Botany, Chinese Academy of Sciences, for their excellent technical assistance with laser confocal microscopy, scanning electron microscope and mass spectrum analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chauhan, R.; Azmi, W.; Bansal, S.; Goel, G. Multivariate analysis of adaptive response to ferulic acid and p-coumaric acid after physiological stresses in Cronobacter sakazakii. J. Appl. Microbiol. 2021, 131, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, M.; Zagorcheva, T.; Rusanova, M.; Rusanov, K.; Atanassov, I. Genetic and Flower Volatile Diversity in Natural Populations of Origanum vulgare subsp. hirtum (Link) Ietsw. in Bulgaria: Toward the Development of a Core Collection. Front. Plant. Sci. 2021, 12, 679023. [Google Scholar]
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Gong, G. Antibacterial mechanism of thymol against Enterobacter sakazakii. Food Control. 2020, 123, 107716. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.Y.; Mo, X.M.; Wang, H.S. Fabrication of scaffold based on gelatin and polycaprolactone (PCL) for wound dressing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102501. [Google Scholar] [CrossRef]
- Hao, R.Y.; Shah, B.R.; Sternisa, M.; Mozina, S.S.; Mraz, J. Development of essential oil-emulsion based coating and its preservative effects on common carp. LWT—Food Sci. Technol. 2022, 154, 112582. [Google Scholar] [CrossRef]
- Nagmetova, G.; Berthold-Pluta, A.; Garbowska, M.; Kurmanbayev, A.; Stasiak-Rozanska, L. Antibacterial Activity of Biocellulose with Oregano Essential Oil against Cronobacter Strains. Polymers 2020, 12, 1647. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.J.; Chen, B.C.; McClements, D.J. Improving the efficacy of essential oils as antimicrobials in foods: Mechanisms of action. Annu. Rev. Food Sci. Technol. 2019, 10, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zheng, K.X.; Lu, J.Q.; Zeng, D.D.; Xiang, Q.S.; Ma, Y.F. Antibacterial characteristics of oregano essential oil and its mechanisms against Escherichia coli O157:H7. J. Food Meas. Charact. 2022, 16, 2989–2998. [Google Scholar] [CrossRef]
- Oh, J.; Kim, H.; Beuchat, L.R.; Ryu, J.H. Inhibition of Staphylococcus aureus on a laboratory medium and black peppercorns by individual and combinations of essential oil vapors. Food Control. 2022, 132, 108487. [Google Scholar]
- Dogruyol, H.; Mol, S.; Cosansu, S. Increased thermal sensitivity of Listeria monocytogenes in sous-vide salmon by oregano essential oil and citric acid. Food Microbiol. 2020, 90, 103496. [Google Scholar] [CrossRef]
- Barbosa, L.N.; Alves, F.C.B.; Andrade, B.; Albano, M.; Rall, V.L.M.; Fernandes, A.A.H.; Buzalaf, M.A.R.; Leite, A.D.; de Pontes, L.G.; dos Santos, L.D.; et al. Proteomic analysis and antibacterial resistance mechanisms of Salmonella Enteritidis submitted to the inhibitory effect of Origanum vulgare essential oil, thymol and carvacrol. J. Proteom. 2020, 214, 103652. [Google Scholar] [CrossRef] [PubMed]
- Frankova, A.; Marounek, M.; Mozrova, V.; Weber, J.; Kloucek, P.; Lukesova, D. Antibacterial Activities of Plant-Derived Compounds and Essential Oils Toward Cronobacter sakazakii and Cronobacter malonaticus. Foodborne Pathog. Dis. 2014, 11, 795–797. [Google Scholar] [CrossRef] [PubMed]
- Falco, E.D.; Roscigno, G.; Landolfi, S.; Scandolera, E.; Senatore, F. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind. Crops Prod. 2014, 62, 242–249. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Salami, S.A.; Nazeri, V.; Maggi, F.; Craker, L. Essential oil profile of oregano (Origanum vulgare L.) populations grown under similar soil and climate conditions. Ind. Crops Prod. 2018, 119, 183–190. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Jordan, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef] [PubMed]
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Yan, Q.Q.; Condell, O.; Power, K.; Butler, F.; Tall, B.D.; Fanning, S. Cronobacter species (formerly known as Enterobacter sakazakii) in powdered infant formula: A review of our current understanding of the biology of this bacterium. J. Appl. Microbiol. 2012, 113, 1–15. [Google Scholar] [CrossRef]
- Feeney, A.; Kropp, K.A.; O’Connor, R.; Sleator, R.D. Cronobacter sakazakii: Stress survival and virulence potential in an opportunistic foodborne pathogen. Gut Microbes 2014, 5, 711–718. [Google Scholar] [CrossRef]
- Joseph, S.; Forsythe, S.J. Predominance of Cronobacter sakazakii Sequence Type 4 in Neonatal Infections. Emerg. Infect. Dis. 2011, 17, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.G.; Amerson-Brown, M.H.; Hulten, K.; Cameron, L.H.; Holzmann-Pazgal, G.; Edwards, M.S.; Foster, C.E. Two Cases of Cronobacter Sakazakii Meningitis in Infants The Importance Of Early Advanced Brain Imaging and Public Health Reporting. Pediatric Infect. Dis. J. 2021, 40, E346–E348. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in foods 7. In Microbiological Testing in Food Safety Management, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 345–353. [Google Scholar]
- Jameelah, M.; Dewanti-Hariyadi, R.; Nurjanah, S. Expression of rpoS, ompA and hfq genes of Cronobacter sakazakii strain Yrt2a during stress and viable but nonculturable state. Food Sci. Biotechnol. 2018, 27, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Shi, L.; Chen, X.; Chen, C.; Hong, Z.; Cao, Y.; Zhao, L. Survival strategy of Cronobacter sakazakii against ampicillin pressure: Induction of the viable but nonculturable state. Int. J. Food Microbiol. 2020, 334, 108819. [Google Scholar] [CrossRef]
- Niu, H.M.; Yang, M.; Qi, Y.H.; Liu, Y.T.; Wang, X.; Dong, Q.L. Heat shock in Cronobacter sakazakii induces direct protection and cross-protection against simulated gastric fluid stress. Food Microbiol. 2022, 103, 103948. [Google Scholar] [CrossRef]
- Fei, P.; Xing, M.; Feng, Y.G.; Liu, S.; Chang, Y.J.; Wang, Y.; Yu, Y.P.; Shi, E.C.; Zhang, Y.Q.; Bian, X.; et al. Occurrence, Molecular Characterization, and Antibiotic Resistance of Cronobacter sakazakii in Goat Milk-Based Infant Formula from Shaanxi Province, China. Foodborne Pathog. Dis. 2022, 19, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Parra-Flores, J.; Maury-Sintjago, E.; Rodriguez-Fernandez, A.; Acuna, S.; Cerda, F.; Aguirre, J.; Holy, O. Microbiological Quality of Powdered Infant Formula in Latin America. J. Food Prot. 2020, 83, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Hachler, H.; Stephan, R.; Lehner, A. Presence of AmpC Beta-Lactamases, CSA-1, CSA-2, CMA-1, and CMA-2 Conferring an Unusual Resistance Phenotype in Cronobacter sakazakii and Cronobacter malonaticus. Microb. Drug Resist. 2014, 20, 275–280. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 1128. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, J.; Zhang, W.; Sun, M.; Li, H.; Xia, F.; Cui, H.; Bai, H.; Shi, L. Analysis of the Chemical Profiles and Anti-S. aureus Activities of Essential Oils Extracted from Different Parts of Three Oregano Cultivars. Foods 2021, 10, 2328. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; El, H.S.; Noureddine, E.; Fatima, M.; Mahdi, C.; Abdellah, C. Phytochemical profiling of essential oils isolated using hydrodistillation and microwave methods and characterization of some nutrients in origanum compactum benth from central-northern Morocco. Biointerface Res. Appl. Chem. 2021, 11, 9358–9371. [Google Scholar]
- De Mastro, G.; Tarraf, W.; Verdini, L.; Brunetti, G.; Ruta, C. Essential oil diversity of Origanum vulgare L. populations from Southern Italy. Food Chem. 2017, 235, 1–6. [Google Scholar] [CrossRef]
- Tsitlakidou, P.; Papachristoforou, A.; Tasopoulos, N.; Matzara, A.; Hatzikamari, M.; Karamanoli, K.; Mourtzinos, I. Sensory analysis, volatile profiles and antimicrobial properties of Origanum vulgare L. essential oils. Flavour Fragr. J. 2022, 37, 43–51. [Google Scholar] [CrossRef]
- El Gendy, A.N.; Leonardi, M.; Mugnaini, L.; Bertelloni, F.; Ebani, V.V.; Nardoni, S.; Mancianti, F.; Hendawy, S.; Omer, E.; Pistelli, L. Chemical composition and antimicrobial activity of essential oil of wild and cultivated Origanum syriacum plants grown in Sinai, Egypt. Ind. Crops Prod. 2015, 67, 201–207. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Weglarz, Z.; Kosakowska, O.; Przybyl, J.L.; Pioro-Jabrucka, E.; Baczek, K. The Quality of Greek Oregano (O. vulgare L. subsp. hirtum (Link) Ietswaart) and Common Oregano (O. vulgare L. subsp. vulgare) Cultivated in the Temperate Climate of Central Europe. Foods 2020, 9, 1617. [Google Scholar] [CrossRef]
- Barbosa, I.d.M.; da Costa Medeiros, J.A.; Rima de Oliveira, K.A.; Gomes-Neto, N.J.; Tavares, J.F.; Magnani, M.; de Souza, E.L. Efficacy of the combined application of oregano and rosemary essential oils for the control of Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis in leafy vegetables. Food Control 2016, 59, 468–477. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Development of bioactive fish gelatin/chitosan nanoparticles composite films with antimicrobial properties. Food Chem. 2016, 194, 1266–1274. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; Oliveira, K.d.S.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of oregano essential oil and carvacrol on biofilms of Staphylococcus aureus from food-contact surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Cta, B.; Jc, A.; Yang, Z.A.; Ping, D.C.; Gh, C.; Lz, D.; Zz, A.; Dy, A. Exploring antimicrobial mechanism of essential oil of Amomum villosum Lour through metabolomics based on gas chromatography-mass spectrometry in methicillin-resistant Staphylococcus aureus. Microbiol. Res. 2020, 242, 126608. [Google Scholar]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Tsitsilin, A.; Li, J.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.; Linstrom, P.; Zenkevich, I. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, Y.; Wang, X. Ferulic Acid Inactivates Shigella flexneri through Cell Membrane Destructieon, Biofilm Retardation, and Altered Gene Expression. J. Agric. Food Chem. 2020, 68, 7121–7131. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, J.; Shi, L. A Carvacrol-Rich Essential Oil Extracted From Oregano (Origanum vulgare "Hot & Spicy") Exerts Potent Antibacterial Effects Against Staphylococcus aureus. Front. Microbiol. 2021, 12, 741861. [Google Scholar]
- Kang, J.; Liu, L.; Wu, X.; Sun, Y.; Liu, Z. Effect of thyme essential oil against Bacillus cereus planktonic growth and biofilm formation. Appl. Microbiol. Biotechnol. 2018, 102, 10209–10218. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).