Abstract
The insect nervous system is critical for its functional integrity. The cholinergic system, of which acetylcholinesterase (AChE) is a key enzyme, is essential to the Anopheles (consisting of major malaria vector species) nervous system. Furthermore, the nervous system is also the primary target site for insecticides used in malaria vector control programs. Insecticides, incorporated in insecticide-treated nets and used for indoor residual spraying, are a core intervention employed in malaria vector control. However, Anopheles resistance against these insecticides has grown rapidly. Due to this major setback, novel agents with potential activity against resistant Anopheles and/or capacity to overcome resistance against current WHO-approved insecticides are urgently needed. The essential oils have the potential to be natural sources of novel insecticides with potential to inhibit the Anopheles AChE target. In the current review, the scientific evidence highlights the ability of essential oils and specific essential oil constituents to serve as anticholinesterase insecticides. For this reason, the published data from scientific databases on the essential oils and essential oil constituents on anticholinesterase, ovicidal, larvicidal, pupicidal and adulticidal activities were analyzed. The identification of major constituents in active essential oils and their possible influence on the biological activity have also been critically evaluated. Furthermore, the toxicity to mammals as well as potential activity against the mammalian AChE target has also been reviewed. The importance of identifying novel potent insecticides from essential oils has been discussed, in relation to human safety and cost-effectiveness. Finally, the critical insights from this review can be used to inform future researchers towards potent and safe anticholinesterase insecticides for the management of Anopheles malaria vectors.
1. Introduction
Malaria is a devastating disease caused by a protozoan parasite, namely Plasmodium falciparum which is the major causative agent in the pathogenesis of this infectious disease [1,2,3]. Anopheles vectors are infected with malaria after they ingest blood from an infected human host. The female Anopheles vectors effectively bite the human hosts between dusk and dawn [3] and it is during this time that she ingests gametocytes. The Plasmodium gametocytes develop into an oocyst in the mosquito midgut, which then matures into sporozoites. The sporozoites are released into the hemolymph and migrate to the salivary glands [1,4]. This parasite developmental process within the vector takes approximately 11–16 days before the female mosquito is able to transmit the parasite to the next human host during a blood feeding. This means that a long lifespan of the Anopheles vector is required for the successful completion of the parasite development and reinfection of the human host. Vertebrate blood is needed every 2–3 days by the female mosquito for nutrition, as well as egg development. The eggs are oviposited into water and fertile eggs hatch into larvae a few days later. Larvae will develop into pupae and finally adults will emerge after a few days [3]. There are more than 400 Anopheles species of which about 30 are major malaria vectors. The African Anopheles vectors have both long lifespans and a higher preference for human feeding and, collectively, these account for the high malaria cases and mortality that is recorded in Africa [3,5]. Other factors, such as climate conditions and political and economic stability, also affect the intensity of transmission and enhance the problem [3].
The malaria vectors have long been controlled by using insecticides. Insecticide classes include organophosphates, carbamates, pyrethroids, organochlorines and neonicotinoids [6]. Larvicides including insect growth inhibitors as well as bacterial larvicides, such as Bacillus thuringiensis subspecies israelensis, Bacillus sphaericus and spinosyns from Saccharopolyspora species, have also gained popularity in mosquito control activities [6,7,8]. The implementation of large-scale larviciding is however challenging in Sub-Saharan Africa and these may be used as a complementary intervention [6,9,10,11]. The Anopheles vectors have developed substantial resistance against almost all current insecticides [12,13,14,15]. To compound the issue, the commercial development of insecticides through various and often complicated synthetic mechanisms is expensive and time-consuming [16,17]. We propose that the identification of potential insecticides from natural product resources, such as essential oils (EOs), is a relatively cost-effective and faster alternative. Target identification and the corresponding mechanism of action are critical components of the drug discovery process [18]. Acetylcholinesterase (AChE) is a validated target in the insect nervous system and inhibitors of this critical enzyme have been useful in the control of malaria vectors for over eighty years [6,19,20].
To facilitate future research in EOs and potential insecticidal activity through AChE inhibition, this review will first discuss malaria vector control systems and current challenges, and we will explore the insect nervous system with relevance to a specific neurotransmitter, acetylcholine (ACh), and provide an in-depth discussion on AChE function and inhibition. Finally, we identify the potential of various EOs and essential oil constituents (EOCs) as anticholinesterase agents against Anopheles vectors. This was achieved by collecting scientific data using keywords such as anticholinesterase/acetylcholinesterase inhibition, EOs, terpenoids/terpenes, Anopheles, larvicidal, pupicidal, adulticidal and insecticidal in the search engines of PubMed, Google Scholar, ScienceDirect, SciFinder, SCOPUS and Web of Science.
2. Malaria Vector Control
The early vector control strategies adopted vast activities to reduce larval populations, which included, amongst others, the drainage of breeding sites such as swamps or the application of copper (II) acetoarsenite (Paris green), a highly toxic inorganic compound to the breeding sites [21,22]. In addition, the screening of windows and doors to prevent vectors entering houses and the use of mosquito nets have been at the forefront in protecting people against mosquito bites [6]. The plant-based insecticides were the first preparations used historically. Pyrethrins extracted from the flowers of Chrysanthemum cinerariifolium and Chrysanthemum roseum were used against indoor Anopheles mosquitoes in the 19th century [23,24]. However, the structural modifications of the natural pyrethrins and the generation of first synthetic pyrethroids were first reported in the period 1924 to 1970 [25]. The discovery of an organochloride, namely, dichlorodiphenyltrichloroethane (DDT), was reported in 1939 [25,26]. DDT has been highly effective against malaria vectors. However, in recent times increasing safety concerns have seen it being replaced in many countries by newer insecticides with reduced toxicity profiles [27,28,29].
Currently, malaria vector control adopts an integrated vector management program through the use of insecticides targeting both the larval and adult stages [6,27]. This is achieved through two main interventions, namely, insecticide-treated mosquito nets and indoor residual spraying, with additional interventions including larviciding [6]. The insecticide-treated nets provide both a physical barrier and insecticidal activity against Anopheles vectors. Indoor residual spraying (IRS) on the other hand, provides host protection through the Anopheles insecticidal effect [3]. Pyrethroids, pyrethroid-PBO combinations and pyrroles are the only insecticide classes used for the insecticide-treated nets, as the latter insecticides pose a low toxicity risk to humans [30,31]. The pyrethroids used in IRS include deltamethrin, alpha-cypermethrin, etofenprox, lambda-cyhalothrin, bifenthrin and cyfluthrin, while the organochlorines include DDT. On the other hand, the organophosphates approved by WHO for IRS include malathion, fenitrothion, pirimiphos-methyl and the carbamates such as propoxur and bendiocarb [6,32,33,34].
2.1. Insecticide Resistance in Main African Malaria Vectors
Insecticide resistance has been reported in all of the main African malaria vectors and this resistance against WHO approved insecticidal agents is rapidly increasing in intensity and geographical distribution [5,35,36]. An overview of mosquito resistance has been highlighted below. To keep this brief, only a few examples will be provided to explain the extent of the problem mainly on the African continent. Insecticide resistance in the main vector species has been reported for pyrethroids [35,37,38,39,40,41], organochlorides [38,41,42,43], organophosphates [12,44] and carbamates [20,38,43,45].
Common insecticide resistance markers associated with pyrethroid and organochloride resistance include the L1014F and L1014S mutation of the voltage-gated sodium channel gene, known as knockdown resistance (kdr). These mutations shift activation voltage dependence of sodium channels stabilizing them in the closed state. This antagonizes the action of pyrethroids and organochlorines since these compounds bind to open sodium channels [25,41,46,47]. Apart from the kdr mutations, elevated metabolic enzymes, including P450 monooxygenases, glutathione-S-transferases and non-specific esterases, also convey high resistance to pyrethroids and organochlorides [13,39,40,44,48,49]. On the other hand, the resistance mechanism commonly conferring organophosphate and carbamate resistance is a single point polymorphism resulting from glycine conversion to a serine residue at position 119 (G119S; Torpedo californica AChE numbering) or more precisely, position 280 (G280S; Anopheles gambiae AChE numbering) in the AChE target [41,47,50,51]. Resistance mechanisms often prevent the intended biological activity of a specific insecticide; therefore, it is important to discuss modes of action of the major insecticide classes.
2.2. Modes of Action of Main Insecticides Used in Malaria Vector Control
Regardless of their small size, insects have a high surface area for the penetration and subsequent systemic distribution of an insecticide from contact exposure. Furthermore, the small size generates short pathways to the insect’s nervous system and as a result, most insecticides act on the insect’s nervous system [52]. Organochlorines act specifically on the peripheral nervous system, where they bind and stabilize the open voltage-gated sodium channels [25]. The stabilized open state of the sodium channels allows for continuous sodium influx and prolonged action potentials leading to spontaneous neuronal firings succeeded by muscle twitches and sustained body tremors [46]. In contrast to the organochlorines, pyrethroids act on both the peripheral and central nervous systems; however, they act in a similar manner to prevent the closing of the voltage-gated sodium channels, resulting in continuous neuronal discharges followed by paralysis [46]. Similarly, the organophosphates and carbamates also exert their effects on the central nervous system. However, these insecticide classes inhibit acetylcholinesterase, a principal enzyme in the insect nervous system, which leads to an increase in the neurotransmitter ACh levels in the synapse. This leads to enhanced ACh effects on the cholinergic receptors resulting in constant neurotransmission and neuronal hyperexcitation [53]. On the other hand, the neonicotinoids enhance cholinergic activity by acting as agonists on nicotinic acetylcholine receptors (nAChRs). Similarly, this disrupts neuronal transmission in the insect nervous system, causing paralysis and subsequent insect death [54]. Since the insect nervous system is the target site for all of the major insecticide classes, it is worth discussing it in more detail.
2.3. Insect Nervous System
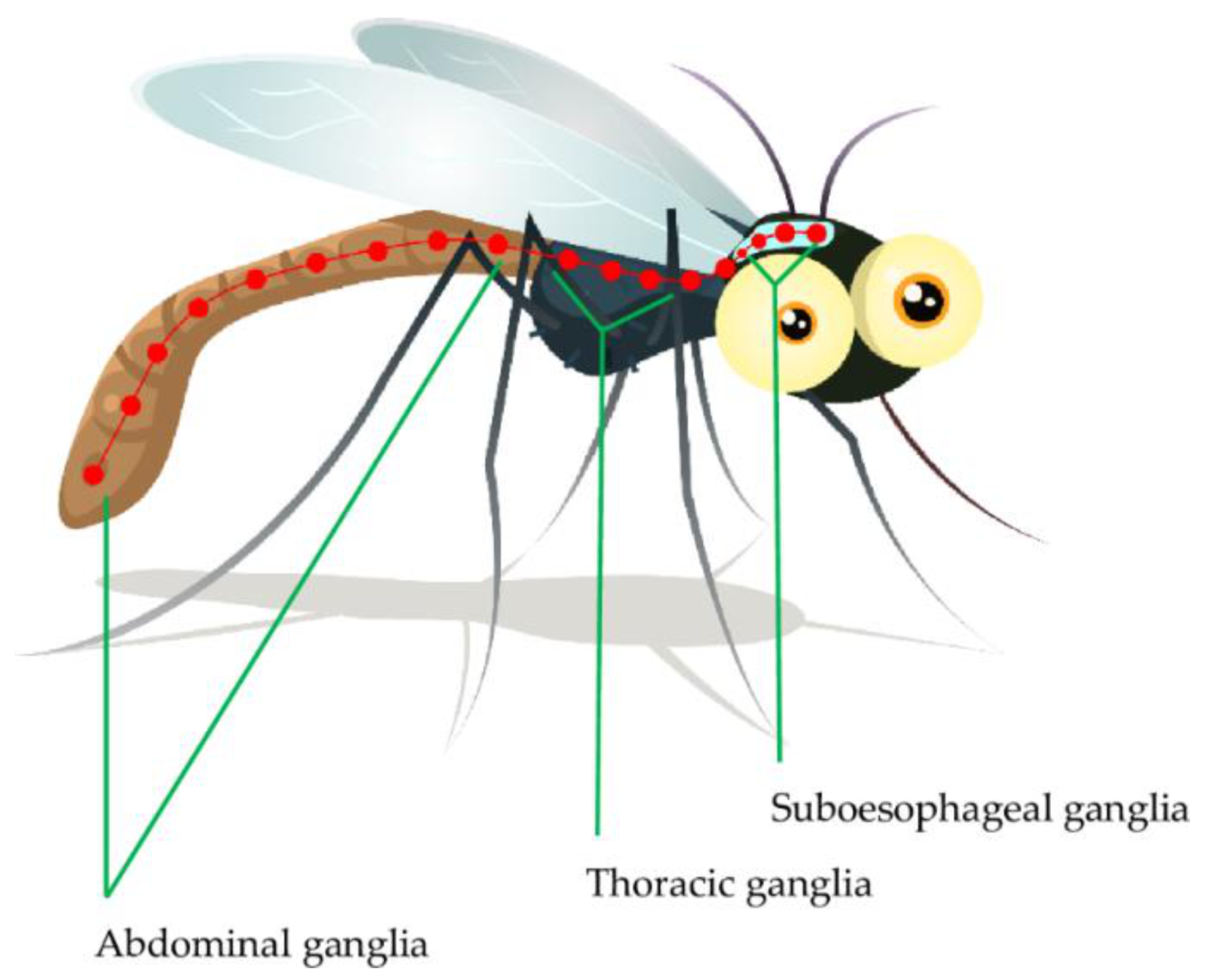
The insect nervous system is composed of central, visceral and peripheral nervous systems [55]. The insect central nervous system (CNS) is composed of the ventral nerve cord and brain connected to various ganglia including supra- and sub-esophageal ganglia, thoracic ganglia and abdominal ganglia (Figure 1). The sub-esophageal ganglion transmits impulses to the mouthparts and salivary glands. The insect brain is composed of three cephalic neuromeres, including the protocerebrum, deutocerebrum and tritocerebrum. The deutocerebrum carries out olfactory and sensory functions through the antennae; where the olfactory signal transduction is important in host identification and interaction by the insect. The tritocerebrum nerves innervate the ventral nerve cord and internal organs including the anterior digestive canal [55,56,57]. The insect’s peripheral nervous system, commonly referred to as a stomatogastric nervous system is composed of the peripheral ganglia complex and nerves that innervate visceral organs. This system mainly controls food intake and digestion. Generally, the insect CNS ganglia receive sensory impulses from the appendages and body cuticle after which the efferent signals are sent to the body muscles, internal organs and genitalia [58,59]. The protocerebrum controls the insect’s vision through compound eyes and ocelli. Most importantly, neurosecretory cells are located in the protocerebrum [59,60]. Most insecticides including the organophosphates and carbamates affect neurotransmitter secretion and action [61,62]. As a result, the insect neurotransmitters, more specifically ACh and its transmission cascade, will be discussed in more detail.
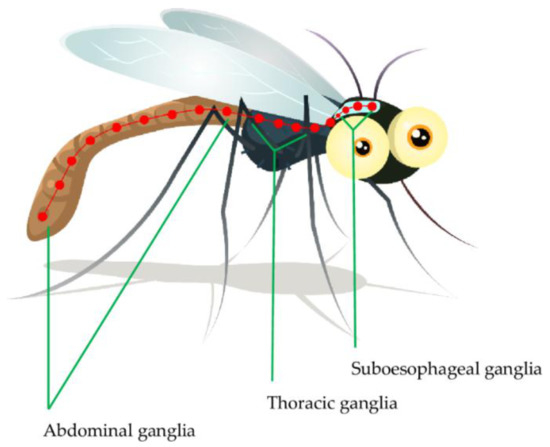
Figure 1.
The nervous system ganglia of Anopheles [55].
2.3.1. Insect Neurotransmitters
The protocerebrum of the insect brain has nerves that innervate the corpora cardiaca, an organ located posterior to the brain that is composed of the neurohemal and endocrine sections. This enables the corpora cardiaca to perform the important role of neurotransmitter storage and release [55]. Biogenic amines play an important role in the insect nervous system as neuromodulators, neurohormones and neurotransmitters. Moreover, biogenic amines are also a key role player in associative learning and memory for insects. Common neurotransmitters found in the insect CNS include biogenic amines such as dopamine, 5-hydroxy-tryptamine, octopamine, noradrenaline and ACh [58,63,64]. In addition, other neurotransmitters important in the insect nervous system are histamine, glutamate and gamma-aminobutyric acid (GABA). GABA is a main CNS inhibitory neurotransmitter, while glutamate plays an excitatory role both centrally and peripherally [55,65]. For example, the insect heart rate and contraction are regulated by glutamate and other neurotransmitters including ACh, norepinephrine, dopamine, serotonin and octopamine [66]. ACh is a key excitatory neurotransmitter in the insect nervous system [67].
2.3.2. Acetylcholine and Its Function in Insects
ACh is synthesized from acetyl-coenzyme A and choline in insect cholinergic neurons and stored in nerve terminals inside presynaptic vesicles from which they are released upon detecting the nerve impulse. This impulse activates voltage-gated calcium channels leading to the influx of calcium ions that then induce ACh release from vesicles via exocytosis [68]. After being released in the synapse, ACh exerts its actions by binding to the postsynaptic nAChRs [69]. ACh is the main excitatory neurotransmitter in insects and its binding to nAChRs and generation of excitatory action potential controls the rapid synaptic neurotransmission process [70]. The nAChRs are ligand-gated channels controlled by binding of ACh and its subsequent removal from the binding site. As long as ACh is bound on the postsynaptic nAChRs, the nerve impulses are transmitted. However, this is short-lived as ACh is rapidly hydrolyzed from the binding site by a specialized enzyme, namely AChE. This ensures that the action potentials are initiated at precise and accurate intervals for efficient neurotransmission [71,72].
2.3.3. Acetylcholinesterase and Its Function in Insects
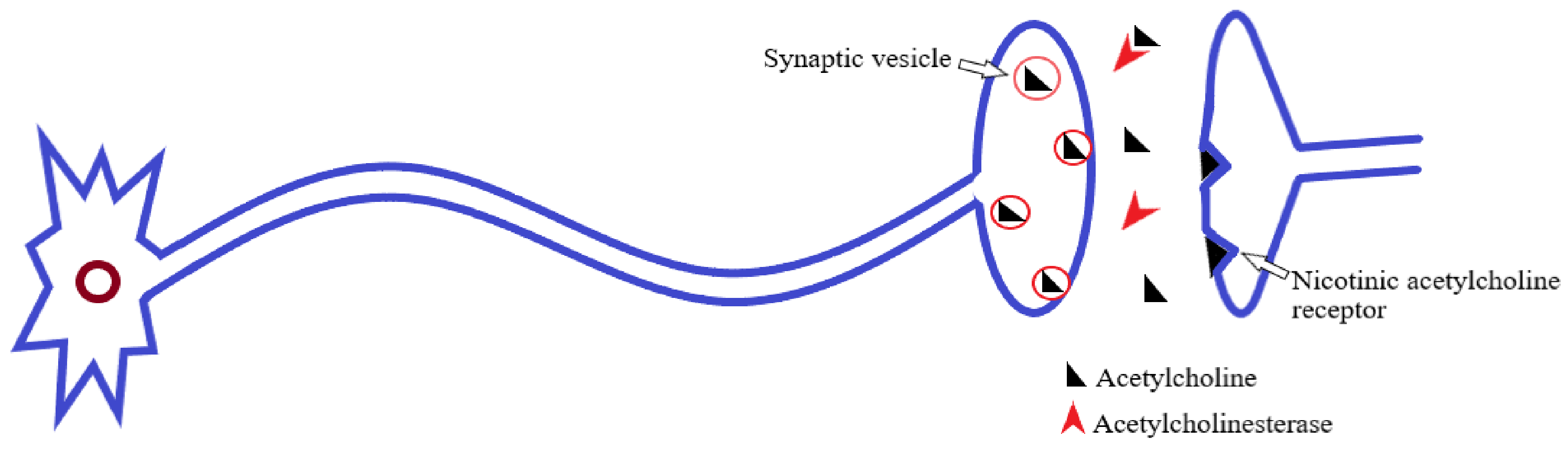
AChE belongs to the general family of cholinesterases; these are the specialized hydrolase enzymes that hydrolyze the choline ester bonds [73]. Therefore, AChE rapidly hydrolyzes the neurotransmitter ACh, to the resultant products, choline and acetate (Figure 2). Through this, it prevents constant nerve firings and maintains normal neuronal impulse transmission at cholinergic synapses and neuromuscular junctions [74,75].
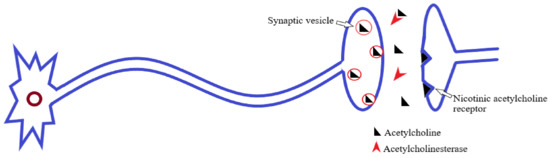
Figure 2.
Acetylcholine release, postsynaptic receptor binding, and hydrolysis by acetylcholinesterase [74].
Exclusively in insects, the cholinergic system is localized centrally and is absent at the neuromuscular junctions [76]. The cholinergic system is essential for the functioning of the insect nervous system and AChE is a key enzyme in this system [77]. The functional integrity of insects is maintained by its nervous system, and it is for this reason that most insecticides act on the insects’ nervous system [72,78].
2.4. Molecular Characterization of Acetylcholinesterase
There are apparent AChE structural differences between insects and mammals. These span from their distinct genomics, amino acid sequences to their active and peripheral anionic site conformations [76]. Recent biochemical studies have revealed critical differences between the Anopheles AChE and human AChE that could serve as potential drug targets for directed insecticide design.
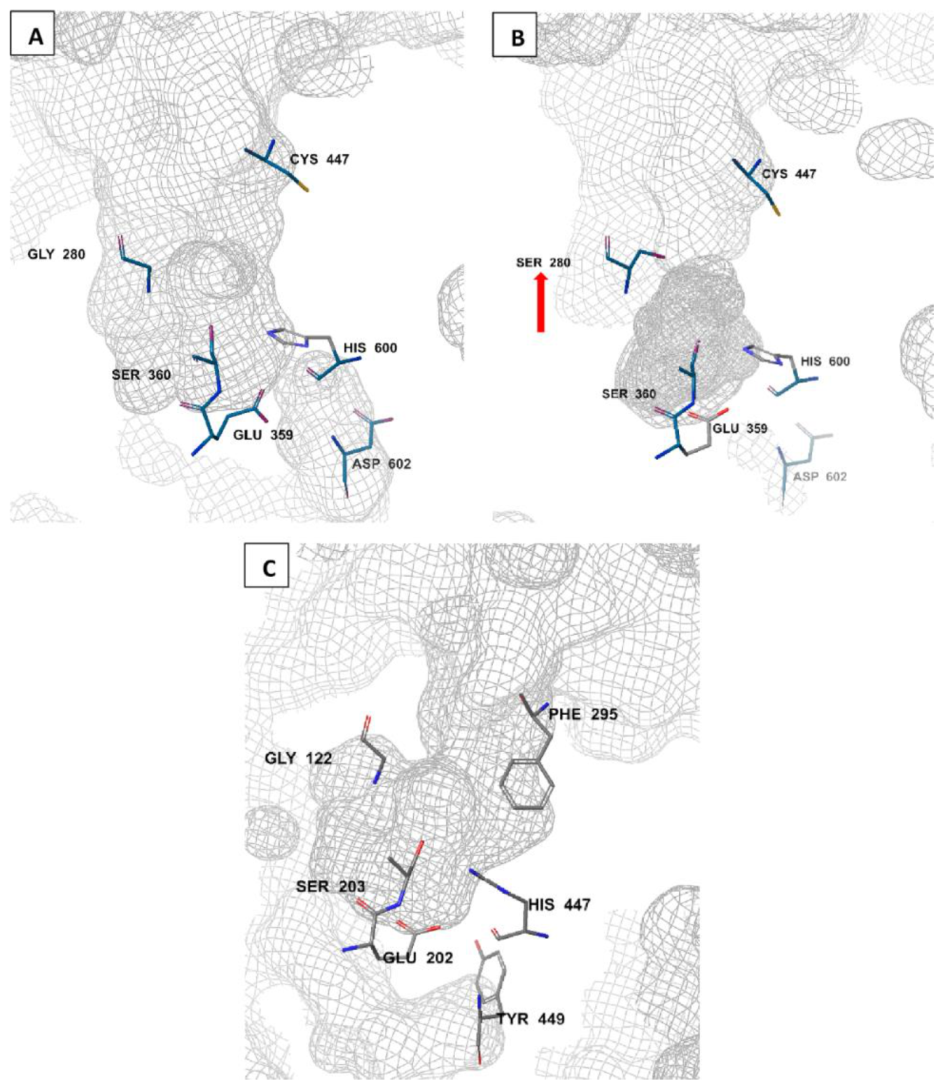
The amino acid sequence of Anopheles AChE is reported to be 48–49% identical to that of the human AChE [79,80]. Unlike humans where there is a single ace gene coding for AChE, mosquitoes have two ace genes, ace-1 and ace-2, coding for AChE1 and AChE2 enzymes, respectively [81,82]. These genes are crucial in all life stages of the mosquito, ranging from egg through to adult stages [83]. AChE1 is the main catalytic enzyme, while AChE2 is involved in non-catalytic activities such as reproduction. As a result, target site insensitivity on insect AChEs, such as G280S genotype, is linked to mutations in ace-1 but not ace-2 [51,62,81]. AChE is characterized by a deep and narrow active-site gorge (Figure 3). There are differences in these gorge structures between Anopheles and human AChEs and this may affect ligand binding and specificity [51,79,84]. Notably, a free cysteine residue (Cys447) is available at the entrance to the active site gorge of Anopheles AChE (Figure 3A,B), but not in human AChE. Instead, a human AChE has a bulky phenylalanine (Phe295) at the active site entrance (Figure 3C). Additionally, in Anopheles AChE, a smaller aspartic acid residue (Asp602; Figure 3A,B) replaces a larger tyrosine residue (Tyr449) at the base of the active site gorge [51]. Moreover, a conserved arginine residue (Arg339; not shown in order to maintain the catalytic side resolution) has also been identified in Anopheles AChE [85]. In addition, the displayed An. gambiae AChE catalytic site in Figure 3B shows a G280S mutated site (pointed).
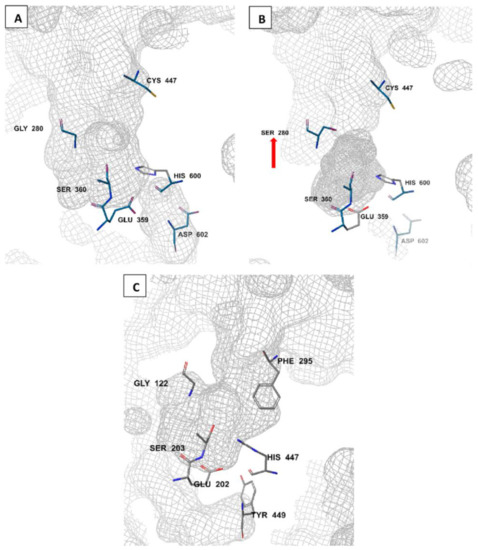
Figure 3.
Molecular comparison of An. gambiae wild-type (A) and resistant (B) AChE catalytic sites (PDB IDs: 5YDI and 6ARY, respectively) to the human AChE (PDB ID: 7E3H) (C). generated by Schrodinger’s Maestro 2018-2 software (New York, NY, USA). The G280S mutation is shown (red arrow) in the resistant Anopheles AChE phenotype (B) [51,75].
2.4.1. Acetylcholinesterase Inhibition in Anopheles
The catalytic site in Anopheles is characterized with a catalytic triad made of His-Ser-Glu (His600-Ser360-Glu359; Figure 3A) amino acid combination. The catalytic serine (Ser360; Figure 3A) is the target for covalent insecticides, including organophosphates and carbamates [61,62]. These insecticides establish a covalent bond with AChE through phosphorylation and carbamoylation, respectively [67]. The inhibition of AChE leads to ACh accumulation that eventually results in over-stimulation of postsynaptic cholinergic receptors [76]. This neuroexcitation causes rapid insect paralysis and death [72,76]. The Anopheles resistance to the anticholinesterase insecticide classes is usually caused by ace-1 G280S mutation (Figure 3B) and metabolic resistance resulting from the elevated levels of monooxygenases, glutathione-S-transferases and general esterases [62,86,87,88]. Given the widespread resistance that has largely rendered organophosphates and carbamates non-effective, there is an urgent need to identify novel anticholinesterase insecticides. AChE has proven to be a valid target in Anopheles vectors [79] and EOs have also shown to be the promising sources of novel insecticides [89,90,91]. Interestingly, the EOs have shown activity against resistant Anopheles species and a capability to synergize conventional insecticides including the pyrethroids [92,93]. Additionally, the EOs and their constituents inhibit the P450 monooxygenase and glutathione-S-transferase detoxification enzymes involved in multiple insecticide resistance including the pyrethroids, organophosphates and carbamates. The insect toxicity of pyrethroids, organophosphates and carbamates has been greatly enhanced by synergy with EOs including cedarwood oil, geranium oil, clove oil, patchouli oil, cinnamon oil, basil oil, oregano oil, purple nutsedge oil, thyme, coriander and galangal oil [94,95,96]. The identified individual EOCs capable of synergizing conventional insecticides, especially pyrethroids, include thymol, eugenol, carvacrol, geraniol and linalool [96,97].
Essential Oils and Constituents as Potential Anopheles AChE Inhibitors
EOs are hydrophobic secondary metabolites extracted from different aromatic plant parts by steam distillation, hydro-distillation, head-space analysis, solvent extraction or liquid carbon dioxide extraction [93,98]. Various terpenoids including sesquiterpenes, monoterpenes, diterpenes and phenylpropanoids are major phytoconstituents in EOs [99,100]. Many EOs and certain EOCs have exhibited significant anticholinesterase activity in in vitro studies. In insects, many EOs have been reported to exhibit neurotoxic effects characterized by rapid paralysis and death [98], which links them to AChE inhibition. Interestingly, most EOCs can only inhibit mammalian AChE very weakly. In addition, most EOs and EOCs are relatively non-toxic to mammals with very low potency for acute oral toxicity, whereby lethal doses for pure compounds range from 800 to 3000 mg/kg and more than 5000 mg/kg for EOs or EOCs incorporated in pharmaceutical formulations [93,101,102]. The EOs are natural resources and therefore, the identification of novel insecticides from such sources is relatively cost-effective. Production of insecticides from EOs can be relatively less expensive translating into low costs for the malaria endemic countries [103,104]. In addition, the discovery of insecticides is relatively rapid since the lead compounds are screened on the actual insect as opposed to pharmaceuticals intended for human use that undergo a lengthy translational science process from the in vitro to the in vivo studies including animal models and human clinical trials [105].
In vitro anticholinesterase activity is usually assessed with the Ellman assay, a globally accepted method to assess the AChE activity and potential inhibition thereof [106]. For such studies, the insect homogenate may be used as a crude enzyme source [107,108,109]. However, some studies use AChE from Electrophorus electricus (electric eel) to estimate insect AChE activity [110,111]. The electric eel and Anopheles AChEs are reported to have nearly the same backbone conformation [85]. For the purpose of this review, EOs or EOCs inhibiting AChE in the in vitro studies are only considered to have potential Anopheles anticholinesterase activity if they have shown Anopheles insecticidal activity in addition to the observed AChE inhibition. Furthermore, where available, the selectivity between human and Anopheles AChE targets, as well as human toxicity potential for the EOs and EOCs, are extensively discussed.
The EOs as Potential Anticholinesterase Insecticides
Several EOs have shown insecticidal activity against Anopheles species [93]. Given their high volatility, the EOs can reach their target through inhalation by the insect species, ingestion or contact [112]. The absorption of EOs is facilitated by the lipophilic character and low molecular weights of their active constituents [100,112]. This behavior is also important for the partitioning of EOs into the insect midgut plasma membrane [112,113].
Some of the EOs have not only shown the insecticidal effects, but also exhibited anticholinesterase activity indicating a potential to be anticholinesterase insecticides [102]. With increasing resistance against current insecticides, the potential use of EOs as alternative insecticides has been suggested [93]. Various EOs have shown potential to be active against resistant colonies including pyrethroid resistant Anopheles species, such as An. gambiae and An. stephensi [93,114,115]. Furthermore, the EOs and EOCs have shown potential to synergize with conventional insecticides and to inhibit those enzymes responsible for insecticide detoxification, which shows promise in overcoming resistance [88,92,94]. This study identified essential oils from 16 plant species with anticholinesterase potential and corresponding insecticidal capabilities (Table 1). Seven of these exhibited both anticholinesterase and insecticidal activities at IC50 and LC50 values less than 100 µg/mL, indicating high potencies [116]. These highly potent EOs with potential of being anticholinesterase insecticides include Hyptis suaveolens, Hyptis spicigera, Ocimum canum, Lantana camara, Ferulago carduchorum, Ferulago trifida, Salvia officinalis and Curcuma longa (Table 1); the latter EOs belong to the general plant families of Lamiaceae, Verbenaceae, Apiaceae and Zingiberaceae [93,117,118].

Table 1.
Anticholinesterase and insecticidal activities of EOs.
Notably, one EO could exhibit considerable differences in anticholinesterase and/or insecticidal activity (Table 1). This is probably due to variations in the identity or quantity of its specific phytoconstituents. Three Salvia officinalis EOs from different locations in Italy exhibited anticholinesterase activity at IC50 values of 47.68, 58.35 and 77.51 µg/mL [135]. While these EOs had a similar content of camphor (16.84%, 16.16% and 18.92%, respectively) as the main constituent, it was notable that the borneol content in the third EO (IC50: 77.51 µg/mL) was approximately half (2.34%) of that yielded from the former EOs (4.48% and 4.68%, respectively) [135]. Borneol has been previously shown to inhibit AChE [121]. Using similar experimental conditions and AChE enzyme concentrations, the Ocimum canum Sims. EOs from Burkina Faso inhibited AChE at the IC50 value of 0.21 µg/mL [110] and a relatively higher value of 36.16 µg/mL [133]. The latter EO had 59.9% content of 1,8-cineole as the main constituent, while the composition of the former and more active EO was not mentioned [110,133]. Mentha pulegium L. EO from Iran exhibited the larvicidal activity against An. stephensi with the LC50 value of 40.13 µg/mL [130], while that from Portugal attained the LC50 of 113.6 µg/mL against the same Anopheles species [132]. Mentha pulegium L. EO from Portugal had 61.4% pulegone and 20% menthone as the main constituents, while the phytochemical analysis was not reported for the Mentha species from Iran [130,132].
Major constituents in EOs are often responsible for the observed biological activity associated with such a sample [100,140,141]. For this reason, this review collected phytochemical data on the major components in the identified bioactive EOs (Table 2). Some of these major constituents possess anticholinesterase and insecticidal activity as displayed in Table 3. Common constituents in most EOs include α-pinene, β-pinene, p-cymene, γ-terpinene and β-caryophyllene. Certain EOCs such as terpinen-4-ol, 1,8-cineole, menthone, menthol, fenchone, γ-terpinene, (-)-bornyl acetate, linalool, citral and pulegone have been reported as competitive AChE inhibitors [98,142,143,144]. Additionally, common constituents in seven of the most active EOs, especially Hyptis suaveolens, Hyptis spicigera, Ocimum canum, Lantana camara, Ferulago carduchorum, Ferulago trifida and Salvia officinalis are α-pinene, β-pinene, β-caryophyllene and γ-terpinene (Table 2). The Curcuma longa EO has a unique composition profile of ar-turmerone, tumerone, curlone, α-curcumene and β-sesquiphellandrene [123]. The main constituent ar-turmerone has only attained anticholinesterase activity against human AChE with an IC50 value of >100 μg/mL (IC50: 191.1 ± 0.3 μg/mL), indicating a low potency [116]. Curcuma longa EO has been reported as possessing an anticholinesterase with an IC50 value of 34.70 ± 3.10 μg/mL. The Curcuma longa EO has also been reported to possess strong larvicidal activity with the LC50 range of 1.5 to 34 μg/mL [122,123], indicating that it may be selectively targeting the insect.

Table 2.
Major constituents of EOs with anticholinesterase insecticidal activity.

Table 3.
Anticholinesterase and insecticidal activities of EOCs.
The EOCs as Potential Anticholinesterase Insecticides
Some studies have isolated the active principles from EOs and demonstrated the potential of these to exert Anopheles anticholinesterase activity [98,156]. Moreover, some of these have also exerted Anopheles mortality in insecticide susceptibility assays [93,131,157]. Gnankiné and Bassolé (2017) reported that several constituents in EOs possess ovicidal, larvicidal and adulticidal effects against Anopheles species. In this study, 22 EOCs from various terpenoid classes such as sesquiterpene alcohols, sesquiterpene oxides, monoterpenoids and monoterpene alcohols, as well as phenylpropanoids, have been identified as possessing both anticholinesterase and insecticidal activity against Anopheles species (Table 3). Some of these EOCs, such as 1,8-cineole, α-pinene, camphor, linalool, borneol, (+)-3-δ-carene, γ-terpinene, caryophyllene oxide, p-cymene, (E)-anethole, terpinen-4-ol, pulegone and limonene, have shown low potency towards human AChE [98,102,158,159,160].
The α-pinene, estragole, carvacrol, (+)-δ-3-carene, eugenol and camphor were the most active in terms of both AChE inhibition and insecticidal activity (Table 3); thus, indicating that these EOCs show potential to serve as anticholinesterase insecticides. Interestingly, previous studies have shown that these EOCs have a lower activity against human AChE. For example, camphor and α-pinene could only inhibit AChE from human erythrocytes with IC50 values of >10 mM and 0.4–0.7 mM, respectively [159,161]. Similarly, an IC50 range of 0.2 to 0.3 mM of (+)-3-δ-carene was needed to inhibit bovine and human erythrocytes AChE [159,161]. These EOCs may therefore produce insecticides with high selectivity towards Anopheles AChE inhibition over the human target.
Monoterpenoids: The monoterpenoid α-pinene is the main constituent in many essential oils [93]. Orhan et al. (2008) reported that α-pinene, but not β-pinene, has anticholinesterase properties [162]. α-Pinene has shown anticholinesterase activity at an IC50 value as low as 22 μg/mL [163]; however, this was higher than its parent EOs from Hyptis suaveolens and Hyptis spicigera that obtained IC50 values between 0.5 and 6.5 μg/mL [110]. Apart from the fact that different Anopheles species were used, this suggests the possibility of synergism among the EOCs in such EOs. Due to their smaller molecular weights, more than one monoterpenoid can bind to the AChE catalytic and/or peripheral site and, usually, binding of one monoterpenoid facilitates binding of the other [102]. On the other hand, α-pinene exhibited larvicidal activity (LC50 of 32.1 μg/mL) against An. subpictus that was comparable to its anticholinesterase activity [93,163]. In another study, Wojtunik-Kulesza et al. (2017) reported a higher anticholinesterase IC50 value of 102.0 mM for α-pinene along with declaration that insolubility of the EOCs affected the accuracy of spectrophotometric measurements [164]. Generally, the observed variations were caused by the use of different AChE types and protein contents. For example, using 0.25 U/mL AChE, Farag et al. (2016) determined an IC50 of 0.337 µM for estragole; meanwhile, Lopez et al. (2015) doubled the AChE concentration and obtained an IC50 value of 12.6 mM [102,165]. The purity and stability of reagents and EOs or EOCs used in AChE activity assays may also affect the outcome [166,167]. Additionally, AChE activity is a pH-dependent reaction [168] and variations in pH across studies may cause potential differences in inhibition kinetics.
A major constituent in the EO of Echinophora lamondiana, (+)-δ-3-carene, resulted in comparable anticholinesterase (IC50 value: 36 µg/mL) and larvicidal (LC50 value of 42.9 µg/mL) activity against An. quadrimaculatus [169,170]. The latter larvicidal LC50 value of the EOC was observed to be similar to that obtained for the EO of the flowers (LC50: 46.9 µg/mL) with 61.9% (+)-δ-3-carene content, but higher than the EOC of the leaves (LC50: 26.2 µg/mL) with comparable (+)-δ-3-carene content (75.0%) [169]. Though less predominant than (+)-δ-3-carene in the Echinophora lamondiana EO, both terpinolene (2.7 to 3.3%) and α-phellandrene (12.8 to 20.3%) were reported to be even more potent larvicidal agents than (+)-δ-3-carene, with LC50 values of 20.9 and 15.6 µg/mL, respectively [169].
Camphor, a monoterpenoid ketone and the major component in the EOs of Ocimum africanum and Ocimum americanum, was also shown to exhibit low anticholinesterase activity (IC50 value of 21.43 µM) [165]. Moreover, camphor is known to non-competitively inhibit nAChRs [98,102,171]. Camphor is also active as a repellent against An. culicifacies, An. gambiae and An. funestus [172,173,174]. However, poor larvicidal activity by camphor was obtained with a LC50 value > 100 µg/mL [175].
Aazza et al. (2011) reported that the EO of Thymus vulgaris consists of 16% carvacrol. Interestingly, carvacrol is more than three times as active (IC50 value of 63.0 μg/mL) as its parent EO (IC50 value of 216.9 μg/mL) in AChE inhibition studies [121,139]. This indicates the potential effect of antagonistic interactions within the complex mixture of the Thymus vulgaris EO resulting in decreased activity. However, the Anopheles anticholinesterase potential of carvacrol (IC50 value of 63.0 μg/mL) is non-specific as its activity is comparable to that against AChE from bovine serum (IC50 value of 70.3 μg/mL) [139,162]. Stereochemistry in AChE inhibition also plays a role, whereby carvone (a monoterpenoid ketone) attained weak AChE inhibition (IC50 value of 830 μg/mL), in comparison to the structurally related phenolic monoterpenoid, carvacrol (IC50 value of 63.0 μg/mL) [93,139,164]. Carvone has previously been reported to be a potent non-competitive AChE inhibitor of mammal AChE [102,162].
Another phenolic monoterpene, thymol, is ineffective in inhibiting AChE, but is a positive allosteric modulator of the insect’s GABA-A receptors [118,164]. Thymol has also been reported to act at the octopamine receptors; this is where octopamine is an analogue of norepinephrine, functioning as a neurotransmitter, neuromodulator and neurohormone in insects [176]. Most studies have shown thymol to be as ineffective as its parent EO Thymus vulgaris against the AChE target [121,139].
Phenylpropanoids: Estragole is a phenylpropanoid isolated from the EOs of the Ocimum species. Along with strong anticholinesterase activity (IC50 value of 0.337 µM) [165], it has shown similarly potent larvicidal activity against An. stephensi (LC50 value of 11.01 μg/mL) and An. atroparvus (LC50 value of 15.7 μg/mL) [102,118]. Estragole was reported to be potentially selective for Anopheles, as it has shown no inhibition of mammal AChE [162].
Eugenol, an allyl chain-substituted guaiacol (phenol), is a major constituent (31.12%) in the Plectranthus barbatus EO, an EO with the LC50 value of 84.2 µg/mL, against An. subpictus larvae [177]. However, eugenol is three times more potent (LC50 value of 25.45 µg/mL) than this latter parent EO as a larvicide against An. subpictus [93]. Again, this suggests possible antagonism with other constituents. Eugenol and (E)-anethole (a phenylpropanoid) reportedly interacted in an antagonistic manner when tested for larvicidal activity [178]. Although eugenol had an IC50 value of 40.32 µg/mL for AChE inhibition, this inhibitory activity was however comparable to that attained against bovine erythrocytes AChE (42.44 ± 1.21 µg/mL) [165,179]. In contrast, Dohi et al. (2009) reported a much higher IC50 value for eugenol (480 µg/mL) against electric eel AChE [163]. Based on these two findings, eugenol has a potential nonselective AChE inhibition, and to be an antagonist at insect octopamine receptors [93].
While showing potent insecticidal activity, some EOs and EOCs did not show any evidence that their mode of action involved targeting AChE. Pulegone has a potent insecticidal activity against An. stephensi, however, it could not efficiently inhibit AChE [141,164]. In contrast, the epoxide form of pulegone, the pulegone-1,2-epoxide isolated from the Lippia steochadifolia EO, has be reported to be an insect neurotoxin, acting as an irreversible inhibitor of AChE [191]. In addition, the Citrus limon EO and it major EOC, limonene (99%) both possess weak AChE inhibition properties; whilst both are potent larvicides against An. gambiae and An. stephensi [120,121,184]. This suggests that a different mode of action may be responsible for their insecticidal activity. In Cimex cimicidae (bedbugs), limonene has been proposed as acting by destroying the wax layer of the insect respiratory system [192].
This review identified EOs and specific EOCs with anticholinesterase activity and indeed the capacity to cause Anopheles mortality. The EOs and EOCs spectrum of activity against Anopheles included ovicidal, larvicidal and adulticidal activities, where they were regarded as active if they obtained LC50 values less than 100 µg/mL [193]. The common Anopheles species involved in global malaria transmission that have been assessed include An. stephensi, An. gambiae, An. arabiensis, An. subpictus, An. anthropophagus, An. quadrimaculatus, An. dirus, An. cracens, An.labranchiae, An. sinensis and An. atroparvus [194,195]. Interestingly, almost all of these have already developed clinically significant resistance against current insecticides [194,196,197,198]. The commonly assessed An. Stephensi, that was susceptible to many of the EOs and EOCs, is predominant in Asia and recently invaded Africa [199,200]. This species is resistant to conventional AChE insecticides, including organophosphates and carbamates, and has also shown resistance to other insecticide classes such as pyrethroids and organochlorines [201,202]. The EO of Ferulago carduchorum has shown both larvicidal and anticholinesterase activity at LC50 and IC50 values around 12 µg/mL, which are extremely promising [126].
Interestingly, the EOs of Hyptis suaveolens, Hyptis spicigera, Ocimum canum and Lantana camara have shown activity against all three developmental stages of An. gambiae, a main African vector. Meanwhile, the EO of Mentha pulegium and Ocimum canum have been shown to target the larval stage of An. gambiae [93,131] and An. funestus (LC50 value of 91.2 µg/mL), respectively [93,195,203]. Of all the species, An. arabiensis is the main vector in Sub-Saharan Africa [48] against which the EOs of Schinus mole and Eucalyptus globulus have displayed larvicidal activity with LC50 values less than 100 µg/mL. However, the anticholinesterase activity of these latter EOs, as well as their major EOCs, α-phellandrene and eucalyptol, respectively, were in the millimolar range indicating that their larvicidal activity is not primarily through AChE inhibition. Both Schinus mole and Eucalyptus globulus EOs are non-toxic towards mammals and the Eucalyptus globulus EO is recommended for skin application as a repellent [93,204].
It is crucial to identify novel insecticides from EOs as most are relatively safe to mammals [93,118]. An EO of Foeniculum vulgare could only exhibit acute oral toxicity at the LD50 of 3120 mg/kg in the rat model [118]. This EO is active against An. stephensi and An. dirus with LD50 values between 20 and 35 µg/mL, thus indicating a high safety index for human use [93]. The EOCs, (E)-anethole, eugenol, limonene, thymol and γ-terpinene, displayed acute oral toxicity at the LD50 values ranging from 980 to 4600 mg/kg [118] where all attained insecticidal LC50 values less than 100 µg/mL (Table 3); thus, indicating their potential as bioinsecticides.
3. Conclusions and Future Perspective
The Anopheles vectors are responsible for malaria transmission across the world; additionally, most of these vectors have acquired resistance against current insecticide classes. Anopheles AChE is a valid target by conventional AChE inhibitors in the market. Due to the current resistance status towards anticholinesterase insecticides, organophosphates and carbamates, the identification of novel insecticides is critical. The EOs and EOCs have shown potential to serve as anticholinesterase insecticides against Anopheles vectors. This is afforded by the capability of some EOs and EOCs to exhibit both anticholinesterase and insecticidal activity. In this review, seven EOs from Hyptis suaveolens, Hyptis spicigera, Ocimum canum, Lantana camara, Ferulago carduchorum, Ferulago trifida, Salvia officinalis and Curcuma longa plant species which showed potent anticholinesterase and insecticidal activities against various Anopheles species were summarized. Along with these, six EOCs, namely, α-pinene, estragole, carvacrol, (+)-3-δ-carene, eugenol and camphor, were identified as being the most active against the Anopheles vector and AChE target. All of these, except for eugenol and carvacrol, have the potential to be selective towards Anopheles AChE. This scientific data review is important for informing future research towards novel anticholinesterase insecticides for malaria vector control. Future studies in this area should focus on the EOs and EOCs identified in this review for possible development into insecticidal agents. Active constituents should be identified from the EO complex mixtures and possible synergistic interaction taken into consideration. The EOs and the identified EOCs in this review require further assessment against various stages of the Anopheles life cycles and potential activity against insecticide resistant Anopheles vectors. Moreover, these promising EOs and EOCs should be assessed for possible activity against human AChE, toxicity against other aquatic lives, as well as for mammal toxicity.
In general, this review supports the future development of EOs as a potential source for novel insecticides with AChE inhibitory potential; including the specific EOs with potential anticholinesterase and insecticidal activities outlined in this review, belonging to the families of Lamiaceae, Verbenaceae, Apiaceae and Zingiberaceae. Meanwhile, the promising EOCs for development into novel anticholinesterase insecticides belong to broader classes of sesquiterpene alcohols, monoterpenoids, monoterpene alcohols and phenylpropanoids.
Author Contributions
Conceptualization, T.A.R. and R.L.v.Z.; literature search, T.A.R.; data curation, T.A.R.; writing–original draft preparation, T.A.R.; writing–review and editing, T.A.R., L.L.K., J.-L.P. and R.L.v.Z.; supervision, L.L.K., J.-L.P. and R.L.v.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by University of the Witwatersrand (PhD Merit Award and Staff Bursary offered to T.A.R.) as well as the Department of Science and Innovation (DSI)/National Research Foundation (NRF) Research Chairs Initiative Grant (UID: 64763) to LLK. RvZ acknowledges funding from the South African Medical Research Council for the Self-Initiated Research (SIR) Grant (RBZL021)–the research reported in this publication was supported by the South African Medical Research Council under a Self-Initiated Research Grant. The views and opinions expressed are those of the author(s) and do not necessarily represent the official views of the SA MRC.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available molecular datasets were analyzed in this review. The data can be found in Protein Data Bank (https://www.rcsb.org/ accessed on 1 September 2022): [PDB IDs: 5YDI, 6ARY and 7E3H].
Acknowledgments
The authors are indebted to the Wits Marketing Department that designed the graphic figures reported in this paper. TAR acknowledges the Centre for High Performance Computing (CHPC, Cape Town, South Africa)/Council for Scientific and Industrial Research (CSIR) for the molecular modelling software that was used to generate AChE catalytic site molecular comparisons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthr. 2021, 40, 1. [Google Scholar] [CrossRef] [PubMed]
- Crutcher, J.M.; Hoffman, S.L. Malaria. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- WHO. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 15 January 2022).
- Smith, R.C.; Jacobs-Lorena, M. Plasmodium–Mosquito Interactions: A tale of roadblocks and detours. Adv. Insect Physiol. 2010, 39, 119–149. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 21 March 2022).
- WHO. Guidelines for Malaria Vector Control. Available online: https://apps.who.int/iris/bitstream/handle/10665/310862/9789241550499-eng.pdf (accessed on 15 January 2022).
- Kirst, H.A. The spinosyn family of insecticides: Realizing the potential of natural products research. J. Antibiot. 2010, 63, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Nkondjio, C.; Sandjo, N.N.; Awono-Ambene, P.; Wondji, C.S. Implementing a larviciding efficacy or effectiveness control intervention against malaria vectors: Key parameters for success. Parasites Vectors 2018, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Dambach, P.; Traoré, I.; Kaiser, A.; Sié, A.; Sauerborn, R.; Becker, N. Challenges of implementing a large scale larviciding campaign against malaria in rural Burkina Faso–Lessons learned and recommendations derived from the EMIRA project. BMC Public Health 2016, 16, 1023. [Google Scholar] [CrossRef]
- Rubin, N.B.; Mboera, L.E.; Lesser, A.; Miranda, M.L.; Kramer, R. Process Evaluation of a Community-Based Microbial Larviciding Intervention for Malaria Control in Rural Tanzania. Int. J. Environ. Res. Public Health 2020, 17, 7309. [Google Scholar] [CrossRef]
- Choi, L.; Majambere, S.; Wilson, A.L. Larviciding to prevent malaria transmission. Cochrane Database Syst. Rev. 2019, 8, CD012736. [Google Scholar] [CrossRef]
- Munywoki, D.N.; Kokwaro, E.D.; Mwangangi, J.M.; Muturi, E.J.; Mbogo, C.M. Insecticide resistance status in Anopheles gambiae (s.l.) in coastal Kenya. Parasites Vectors 2021, 14, 207. [Google Scholar] [CrossRef]
- Muhammad, A.; Ibrahim, S.S.; Mukhtar, M.M.; Irving, H.; Abajue, M.C.; Edith, N.M.A.; Da’U, S.S.; Paine, M.J.I.; Wondji, C.S. High pyrethroid/DDT resistance in major malaria vector Anopheles coluzzii from Niger-Delta of Nigeria is probably driven by metabolic resistance mechanisms. PLoS ONE 2021, 16, e0247944. [Google Scholar] [CrossRef]
- Bamou, R.; Kopya, E.; Nkahe, L.D.; Menze, B.D.; Awono-Ambene, P.; Tchuinkam, T.; Njiokou, F.; Wondji, C.S.; Antonio-Nkondjio, C. Increased prevalence of insecticide resistance in Anopheles coluzzii populations in the city of Yaoundé, Cameroon and influence on pyrethroid-only treated bed net efficacy. Parasite 2021, 28, 8. [Google Scholar] [CrossRef]
- Keïta, M.; Sogoba, N.; Kané, F.; Traoré, B.; Zeukeng, F.; Coulibaly, B.; Sodio, A.B.; Traoré, S.F.; Djouaka, R.; Doumbia, S. Multiple Resistance Mechanisms to Pyrethroids Insecticides in Anopheles gambiae sensu lato Population from Mali, West Africa. J. Infect. Dis. 2021, 223, S81–S90. [Google Scholar] [CrossRef] [PubMed]
- Flannery, E.L.; Chatterjee, A.K.; Winzeler, E. Antimalarial drug discovery—Approaches and progress towards new medicines. Nat. Rev. Microbiol. 2013, 11, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Lees, R.; Praulins, G.; Davies, R.; Brown, F.; Parsons, G.; White, A.; Ranson, H.; Small, G.; Malone, D. A testing cascade to identify repurposed insecticides for next-generation vector control tools: Screening a panel of chemistries with novel modes of action against a malaria vector. Gates Open Res. 2019, 3, 1464. [Google Scholar] [CrossRef] [PubMed]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Ayad, H.; Georghiou, G.P. Resistance to Organophosphates and Carbamates in Anopheles albimanus Based on Reduced Sensitivity of Acetylcholinesterase. J. Econ. Èntomol. 1975, 68, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Aïzoun, N.; Aïkpon, R.; Gnanguenon, V.; Oussou, O.; Agossa, F.; Padonou, G.G.; Akogbéto, M. Status of organophosphate and carbamate resistance in Anopheles gambiae sensu lato from the south and north Benin, West Africa. Parasites Vectors 2013, 6, 274. [Google Scholar] [CrossRef]
- Maggio, F.; Sollod, B.L.; Tedford, H.W.; King, G.F. 5.7–Spider Toxins and their Potential for Insect Control. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 221–238. [Google Scholar] [CrossRef]
- Sur, S.N.; Sarkar, H. Paris Green as an Anopheline Larvicide. Indian Med. Gaz. 1929, 64, 376–378. [Google Scholar]
- Park Ross, G. Insecticide as a major measure in the control of malaria, being an account of the methods and organizations put into force in Natal and Zululand during the past six years. Q. Bull. Health Org. Leag. Nat. 1936, 5, 114–133. [Google Scholar]
- Oberemok, V.V.; Laikova, K.V.; Gninenko, Y.I.; Zaitsev, A.S.; Nyadar, P.M.; Adeyemi, T.A. A short history of insecticides. J. Plant Prot. Res. 2015, 55, 221–226. [Google Scholar] [CrossRef]
- Davies, T.G.E.; Field, L.; Usherwood, P.N.R.; Williamson, M.S. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life 2007, 59, 151–162. [Google Scholar] [CrossRef]
- Casida, J.E.; Quistad, G.B. Golden Age of Insecticide Research: Past, Present, or Future? Annu. Rev. Èntomol. 1998, 43, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M. Insecticide substitutes for DDT to control mosquitoes may be causes of several diseases. Environ. Sci. Pollut. Res. 2013, 20, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- Rogan, W.J.; Chen, A. Health risks and benefits of bis(4-chlorophenyl)-1,1,1-trichloroethane (DDT). Lancet 2005, 366, 763–773. [Google Scholar] [CrossRef]
- Attaran, A.; Maharaj, R.; Liroff, A.A.R. Ethical debate: Doctoring malaria, badly: The global campaign to ban DDT. BMJ 2000, 321, 1403–1405. [Google Scholar] [CrossRef]
- CDC. Insecticide-Treated Bed Nets. Available online: https://www.cdc.gov/malaria/malaria_worldwide/reduction/itn.html (accessed on 15 January 2022).
- Gleave, K.; Lissenden, N.; Chaplin, M.; Choi, L.; Ranson, H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane Database Syst. Rev. 2021, 2021, CD012776. [Google Scholar] [CrossRef]
- Tangena, J.-A.A.; Hendriks, C.M.J.; Devine, M.; Tammaro, M.; Trett, A.E.; Williams, I.; DePina, A.J.; Sisay, A.; Herizo, R.; Kafy, H.T.; et al. Indoor residual spraying for malaria control in sub-Saharan Africa 1997 to 2017: An adjusted retrospective analysis. Malar. J. 2020, 19, 150. [Google Scholar] [CrossRef]
- Syme, T.; Fongnikin, A.; Todjinou, D.; Govoetchan, R.; Gbegbo, M.; Rowland, M.; Akogbeto, M.; Ngufor, C. Which indoor residual spraying insecticide best complements standard pyrethroid long-lasting insecticidal nets for improved control of pyrethroid resistant malaria vectors? PLoS ONE 2021, 16, e0245804. [Google Scholar] [CrossRef]
- WHO. Indoor Residual Spraying: An Operational Manual for Indoor Residual Spraying (IRS) for Malaria Transmission Control and Elimination, 2nd ed.; WHO: Geneva, Switzerland, 2015; Available online: https://apps.who.int/iris/bitstream/handle/10665/177242/9789241508940_eng.pdf;sequence=1 (accessed on 15 January 2022).
- Ranson, H.; Lissenden, N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- WHO. World Malaria Report. 2021. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 7 December 2021).
- Brooke, B.; Kloke, G.; Hunt, R.; Koekemoer, L.; Tem, E.; Taylor, M.; Small, G.; Hemingway, J.; Coetzee, M. Bioassay and biochemical analyses of insecticide resistance in southern African Anopheles funestus (Diptera: Culicidae). Bull. Èntomol. Res. 2001, 91, 265–272. [Google Scholar] [CrossRef]
- Hunt, R.H.; Fuseini, G.; Knowles, S.; Stiles-Ocran, J.; Verster, R.; Kaiser, M.L.; Choi, K.S.; Koekemoer, L.L.; Coetzee, M. Insecticide resistance in malaria vector mosquitoes at four localities in Ghana, West Africa. Parasites Vectors 2011, 4, 107. [Google Scholar] [CrossRef]
- Hunt, R.H.; Brooke, B.D.; Pillay, C.; Koekemoer, L.L.; Coetzee, M. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Med. Vet. Èntomol. 2005, 19, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.; Edwardes, M.; Coetzee, M. Pyrethroid resistance in southern African Anopheles funestus extends to Likoma Island in Lake Malawi. Parasites Vectors 2010, 3, 122. [Google Scholar] [CrossRef] [PubMed]
- Gueye, O.K.; Tchouakui, M.; Dia, A.K.; Faye, M.B.; Ahmed, A.A.; Wondji, M.J.; Nguiffo, D.N.; Mugenzi, L.M.J.; Tripet, F.; Konaté, L.; et al. Insecticide Resistance Profiling of Anopheles coluzzii and Anopheles gambiae Populations in the Southern Senegal: Role of Target Sites and Metabolic Resistance Mechanisms. Genes 2020, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Koekemoer, L.L.; Spillings, B.L.; Christian, R.N.; Lo, T.-C.M.; Kaiser, M.L.; Norton, R.A.; Oliver, S.V.; Choi, K.S.; Brooke, B.D.; Hunt, R.H.; et al. Multiple Insecticide Resistance in Anopheles gambiae (Diptera: Culicidae) from Pointe Noire, Republic of the Congo. Vector-Borne Zoonotic Dis. 2011, 11, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Mzilahowa, T.; Chiumia, M.; Mbewe, R.B.; Uzalili, V.T.; Luka-Banda, M.; Kutengule, A.; Mathanga, D.P.; Ali, D.; Chiphwanya, J.; Zoya, J.; et al. Increasing insecticide resistance in Anopheles funestus and Anopheles arabiensis in Malawi, 2011–2015. Malar. J. 2016, 15, 563. [Google Scholar] [CrossRef] [PubMed]
- Hamid-Adiamoh, M.; Amambua-Ngwa, A.; Nwakanma, D.; D’Alessandro, U.; Awandare, G.A.; Afrane, Y.A. Insecticide resistance in indoor and outdoor-resting Anopheles gambiae in Northern Ghana. Malar. J. 2020, 19, 314. [Google Scholar] [CrossRef] [PubMed]
- Chanda, J.; Saili, K.; Phiri, F.; Stevenson, J.C.; Mwenda, M.; Chishimba, S.; Mulube, C.; Mambwe, B.; Lungu, C.; Earle, D.; et al. Pyrethroid and Carbamate Resistance in Anopheles funestus Giles along Lake Kariba in Southern Zambia. Am. J. Trop. Med. Hyg. 2020, 103, 90–97. [Google Scholar] [CrossRef]
- Silver, K.S.; Du, Y.; Nomura, Y.; Oliveira, E.E.; Salgado, V.L.; Zhorov, B.S.; Dong, K. Voltage-Gated Sodium Channels as Insecticide Targets. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 46, pp. 389–433. [Google Scholar] [CrossRef]
- Soma, D.D.; Zogo, B.M.; Somé, A.; Tchiekoi, B.N.; Hien, D.F.D.S.; Pooda, H.S.; Coulibaly, S.; Gnambani, J.E.; Ouari, A.; Mouline, K.; et al. Anopheles bionomics, insecticide resistance and malaria transmission in southwest Burkina Faso: A pre-intervention study. PLoS ONE 2020, 15, e0236920. [Google Scholar] [CrossRef]
- Brooke, B.D.; Robertson, L.; Kaiser, M.L.; Raswiswi, E.; Munhenga, G.; Venter, N.; Wood, O.R.; Koekemoer, L.L. Insecticide resistance in the malaria vector Anopheles arabiensis in Mamfene, KwaZulu-Natal. S. Afr. J. Sci. 2015, 111, 3. [Google Scholar] [CrossRef]
- Nardini, L.; Christian, R.N.; Coetzer, N.; Koekemoer, L.L. DDT and pyrethroid resistance in Anopheles arabiensis from South Africa. Parasites Vectors 2013, 6, 229. [Google Scholar] [CrossRef][Green Version]
- Elanga-Ndille, E.; Nouage, L.; Ndo, C.; Binyang, A.; Assatse, T.; Nguiffo-Nguete, D.; Djonabaye, D.; Irwing, H.; Tene-Fossog, B.; Wondji, C.S. The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation. Genes 2019, 10, 790. [Google Scholar] [CrossRef]
- Cheung, J.; Mahmood, A.; Kalathur, R.; Liu, L.; Carlier, P.R. Structure of the G119S Mutant Acetylcholinesterase of the Malaria Vector Anopheles gambiae Reveals Basis of Insecticide Resistance. Structure 2018, 26, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.T. The penetration of cuticle by insecticides. In Cuticle Techniques in Arthropods; Miller, T.A., Ed.; Springer: New York, NY, USA, 1980; pp. 367–400. [Google Scholar]
- English, B.A.; Webster, A.A. Chapter 132–Acetylcholinesterase and its Inhibitors. In Primer on the Autonomic Nervous System, 3rd ed.; Robertson, D., Biaggioni, I., Burnstock, G., Low, P.A., Paton, J.F.R., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 631–633. [Google Scholar]
- Simon-Delso, N.; Amaralrogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Osborne, R.H. Insect neurotransmission: Neurotransmitters and their receptors. Pharmacol. Ther. 1996, 69, 117–142. [Google Scholar] [CrossRef]
- Kanzok, S.M.; Zheng, L. The mosquito genome–A turning point? Trends Parasitol. 2003, 19, 329–331. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Schirmeier, S. Insect models of central nervous system energy metabolism and its links to behavior. Glia 2018, 66, 1160–1175. [Google Scholar] [CrossRef] [PubMed]
- Mancini, N.; Giurfa, M.; Sandoz, J.-C.; Avarguès-Weber, A. Aminergic neuromodulation of associative visual learning in harnessed honey bees. Neurobiol. Learn. Mem. 2018, 155, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Vleugels, R.; Zels, S.; Dillen, S.; Lenaerts, C.; Crabbé, K.; Spit, J.; Vanden Broeck, J. Receptors for Neuronal or Endocrine Signalling Molecules as Potential Targets for the Control of Insect Pests. In Advances in Insect Physiology; Cohen, E., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 46, pp. 167–303. [Google Scholar] [CrossRef]
- Ritzmann, R.E.; Quinn, R.D.; Willis, M.A.; Perry, C.E. Adaptive Control Responses to Behavioral Perturbation Based Upon the Insect, F08630-03-1-003; Case Western Reserve University: Cleveland, OH, USA, 2006. [Google Scholar] [CrossRef]
- Knutsson, S.; Engdahl, C.; Kumari, R.; Forsgren, N.; Lindgren, C.; Kindahl, T.; Kitur, S.; Wachira, L.; Kamau, L.; Ekström, F.J.; et al. Noncovalent Inhibitors of Mosquito Acetylcholinesterase 1 with Resistance-Breaking Potency. J. Med. Chem. 2018, 61, 10545–10557. [Google Scholar] [CrossRef] [PubMed]
- Alout, H.; Berthomieu, A.; Hadjivassilis, A.; Weill, M. A new amino-acid substitution in acetylcholinesterase 1 confers insecticide resistance to Culex pipiens mosquitoes from Cyprus. Insect Biochem. Mol. Biol. 2007, 37, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B.; Donnellan, J. Concentrations of some putative neurotransmitters in the CNS of quick-frozen insects. Insect Biochem. 1982, 12, 623–638. [Google Scholar] [CrossRef]
- Avarguès-Weber, A.; Giurfa, M. Conceptual learning by miniature brains. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131907. [Google Scholar] [CrossRef]
- Wafford, K.; Sattelle, D. Effects of amino acid neurotransmitter candidates on an identified insect motoneurone. Neurosci. Lett. 1986, 63, 135–140. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Estévez-Lao, T.Y.; Mirzai, H.E. The neurotransmitters serotonin and glutamate accelerate the heart rate of the mosquito Anopheles gambiae. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 49–57. [Google Scholar] [CrossRef]
- Yousafi, Q.; Sarfaraz, A.; Saad Khan, M.; Saleem, S.; Shahzad, U.; Abbas Khan, A.; Sadiq, M.; Ditta Abid, A.; Sohail Shahzad, M.; ul Hassan, N. In silico annotation of unreviewed acetylcholinesterase (AChE) in some lepidopteran insect pest species reveals the causes of insecticide resistance. Saudi J. Biol. Sci. 2021, 28, 2197–2209. [Google Scholar] [CrossRef] [PubMed]
- Zaagsma, J.; Meurs, H. Acetylcholine. In Encyclopedia of Respiratory Medicine; Laurent, G.J., Shapiro, S.D., Eds.; Academic Press: Oxford, UK, 2006; pp. 1–5. [Google Scholar]
- Kao, P.N.; Karlin, A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J. Biol. Chem. 1986, 261, 8085–8088. [Google Scholar] [CrossRef]
- Thany, S.H.; Tricoire-Leignel, H. Emerging Pharmacological Properties of Cholinergic Synaptic Transmission: Comparison between Mammalian and Insect Synaptic and Extrasynaptic Nicotinic Receptors. Curr. Neuropharmacol. 2011, 9, 706–714. [Google Scholar] [CrossRef]
- Jones, A.K.; Sattelle, D.B. Diversity of Insect Nicotinic Acetylcholine Receptor Subunits. In Insect Nicotinic Acetylcholine Receptors; Thany, S.H., Ed.; Springer: New York, NY, USA, 2010; pp. 25–43. [Google Scholar] [CrossRef]
- Scharf, M.E. Neurological effects of insecticides and the insect nervous system. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2596–2607. [Google Scholar]
- Lionetto, M.G.; Caricato, R.; Calisi, A.; Giordano, M.E.; Schettino, T. Acetylcholinesterase as a Biomarker in Environmental and Occupational Medicine: New Insights and Future Perspectives. BioMed Res. Int. 2013, 2013, 321213. [Google Scholar] [CrossRef]
- Thapa, S.; Lv, M.; Xu, H. Acetylcholinesterase: A Primary Target for Drugs and Insecticides. Mini-Rev. Med. Chem. 2017, 17, 1665–1676. [Google Scholar] [CrossRef]
- Rants’O, T.A.; van der Westhuizen, C.J.; van Zyl, R.L. Optimization of covalent docking for organophosphates interaction with Anopheles acetylcholinesterase. J. Mol. Graph. Model. 2022, 110, 108054. [Google Scholar] [CrossRef] [PubMed]
- Casida, J.E.; Durkin, K.A. Anticholinesterase insecticide retrospective. Chem. Interact. 2013, 203, 221–225. [Google Scholar] [CrossRef]
- Fournier, D.; Bride, J.M.; Hoffmann, F.; Karch, F. Acetylcholinesterase. Two types of modifications confer resistance to insecticide. J. Biol. Chem. 1992, 267, 14270–14274. [Google Scholar] [CrossRef]
- Engdahl, C.; Knutsson, S.; Ekström, F.; Linusson, A. Discovery of Selective Inhibitors Targeting Acetylcholinesterase 1 from Disease-Transmitting Mosquitoes. J. Med. Chem. 2016, 59, 9409–9421. [Google Scholar] [CrossRef]
- Carlier, P.R.; Anderson, T.D.; Wong, D.M.; Hsu, D.C.; Hartsel, J.; Ma, M.; Wong, E.A.; Choudhury, R.; Lam, P.C.-H.; Totrov, M.M.; et al. Towards a species-selective acetylcholinesterase inhibitor to control the mosquito vector of malaria, Anopheles gambiae. Chem. Interact. 2008, 175, 368–375. [Google Scholar] [CrossRef]
- Engdahl, C.; Knutsson, S.; Fredriksson, S.Å.; Linusson, A.; Bucht, G.; Ekström, F. Acetylcholinesterases from the Disease Vectors Aedes aegypti and Anopheles gambiae: Functional Characterization and Comparisons with Vertebrate Orthologues. PLoS ONE 2015, 10, e0138598. [Google Scholar] [CrossRef]
- Weill, M.; Fort, P.; Berthomieu, A.; Dubois, M.P.; Pasteur, N.; Raymond, M. A novel acetylcholinesterase gene in mosquitoes codes for the insecticide target and is non–homologous to the ace gene Drosophila. Proc. R. Soc. B Biol. Sci. 2002, 269, 2007–2016. [Google Scholar] [CrossRef]
- Hoffmann, F.; Fournier, D.; Spierer, P. Minigene rescues acetylcholinesterase lethal mutations in Drosophila melanogaster. J. Mol. Biol. 1992, 223, 17–22. [Google Scholar] [CrossRef]
- Lu, Y.; Park, Y.; Gao, X.; Zhang, X.; Yao, J.; Pang, Y.-P.; Jiang, H.; Zhu, K.Y. Cholinergic and non-cholinergic functions of two acetylcholinesterase genes revealed by gene-silencing in Tribolium castaneum. Sci. Rep. 2012, 2, 288. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.-P. Novel Acetylcholinesterase Target Site for Malaria Mosquito Control. PLoS ONE 2006, 1, e58. [Google Scholar] [CrossRef]
- Nabeshima, T.; Mori, A.; Kozaki, T.; Iwata, Y.; Hidoh, O.; Harada, S.; Kasai, S.; Severson, D.W.; Kono, Y.; Tomita, T. An amino acid substitution attributable to insecticide-insensitivity of acetylcholinesterase in a Japanese encephalitis vector mosquito, Culex tritaeniorhynchus. Biochem. Biophys. Res. Commun. 2004, 313, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Weill, M.; Lutfalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Comparative genomics: Insecticide resistance in mosquito vectors. Nature 2003, 423, 136–137. [Google Scholar] [CrossRef]
- Aïkpon, R.; Sèzonlin, M.; Ossè, R.; Akogbéto, M. Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin. Parasites Vectors 2014, 7, 568. [Google Scholar] [CrossRef]
- Norris, E.J.; Gross, A.D.; Dunphy, B.M.; Bessette, S.; Bartholomay, L.; Coats, J.R. Comparison of the Insecticidal Characteristics of Commercially Available Plant Essential Oils Against Aedes aegypti and Anopheles gambiae (Diptera: Culicidae). J. Med. Èntomol. 2015, 52, 993–1002. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Ponsankar, A.; Thanigaivel, A.; Edwin, E.-S.; Selin-Rani, S.; Chellappandian, M.; Pradeepa, V.; Lija-Escaline, J.; Kalaivani, K.; et al. Comparative analysis of mosquito (Diptera: Culicidae: Aedes aegypti Liston) responses to the insecticide Temephos and plant derived essential oil derived from Piper betle L. Ecotoxicol. Environ. Saf. 2017, 139, 439–446. [Google Scholar] [CrossRef]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef]
- Chansang, A.; Champakaew, D.; Junkum, A.; Jitpakdi, A.; Amornlerdpison, D.; Aldred, A.K.; Riyong, D.; Wannasan, A.; Intirach, J.; Muangmoon, R.; et al. Synergy in the adulticidal efficacy of essential oils for the improvement of permethrin toxicity against Aedes aegypti L. (Diptera: Culicidae). Parasites Vectors 2018, 11, 417. [Google Scholar] [CrossRef]
- Gnankiné, O.; Bassolé, I.H.N. Essential Oils as an Alternative to Pyrethroids’ Resistance against Anopheles Species Complex Giles (Diptera: Culicidae). Molecules 2017, 22, 1321. [Google Scholar] [CrossRef]
- Norris, E.; Johnson, J.; Gross, A.; Bartholomay, L.; Coats, J. Plant Essential Oils Enhance Diverse Pyrethroids against Multiple Strains of Mosquitoes and Inhibit Detoxification Enzyme Processes. Insects 2018, 9, 132. [Google Scholar] [CrossRef]
- Norris, E.J.; Gross, A.D.; Bartholomay, L.C.; Coats, J.R. Plant essential oils synergize various pyrethroid insecticides and antagonize malathion in Aedes aegypti. Med. Vet. Èntomol. 2019, 33, 453–466. [Google Scholar] [CrossRef]
- Gaire, S.; Zheng, W.; Scharf, M.E.; Gondhalekar, A.D. Plant essential oil constituents enhance deltamethrin toxicity in a resistant population of bed bugs (Cimex lectularius L.) by inhibiting cytochrome P450 enzymes. Pestic. Biochem. Physiol. 2021, 175, 104829. [Google Scholar] [CrossRef]
- Gaire, S.; Scharf, M.E.; Gondhalekar, A.D. Synergistic Toxicity Interactions between Plant Essential Oil Components against the Common Bed Bug (Cimex lectularius L.). Insects 2020, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- López, M.D.; Campoy, F.J.; Pascual-Villalobos, M.J.; Muñoz-Delgado, E.; Vidal, C.J. Acetylcholinesterase activity of electric eel is increased or decreased by selected monoterpenoids and phenylpropanoids in a concentration-dependent manner. Chem. Biol. Interact. 2015, 229, 36–43. [Google Scholar] [CrossRef]
- Breman, J.G.; Egan, A.; Keusch, G.T. The intolerable burden of malaria: A new look at the numbers. Am. J. Trop. Med. Hyg. 2001, 64, 4–5. [Google Scholar] [CrossRef]
- Alonso, S.; Chaccour, C.J.; Elobolobo, E.; Nacima, A.; Candrinho, B.; Saifodine, A.; Saute, F.; Robertson, M.; Zulliger, R. The economic burden of malaria on households and the health system in a high transmission district of Mozambique. Malar. J. 2019, 18, 360. [Google Scholar] [CrossRef]
- Wing, K.D. Pharmaceutical technologies with potential application to insecticide discovery. Pest Manag. Sci. 2021, 77, 3617–3625. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Brogdon, W.G.; Barber, A.M. Microplate assay of acetylcholinesterase inhibition kinetics in single-mosquito homogenates. Pestic. Biochem. Physiol. 1987, 29, 252–259. [Google Scholar] [CrossRef]
- Alout, H.; Labbe, P.; Berthomieu, A.; Djogbenou, S.L.; Leonetti, J.-P.; Fort, P.; Weill, M. Novel AChE Inhibitors for Sustainable Insecticide Resistance Management. PLoS ONE 2012, 7, e47125. [Google Scholar] [CrossRef] [PubMed]
- Akiner, M.M. Malathion and Propoxur Resistance in Turkish Populations of the Anopheles maculipennis Meigen (Diptera: Culicidae) and Relation to the Insensitive Acetylcholinesterase. Türk. Parazitol. Derg. 2014, 38, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Wangrawa, D.; Badolo, A.; Guelbéogo, W.; Kiendrebeogo, M.; Nébié, R.; Sagnon, N.; Sanon, A. Biological activities of four essential oils against Anopheles gambiae in Burkina Faso and their in vitro inhibition of acetylcholinesterase. Int. J. Biol. Chem. Sci. 2015, 9, 793–802. [Google Scholar] [CrossRef]
- Phrompittayarat, W.; Hongratanaworakit, T.; Tadtong, K.S.; Sareedenchai, V.; Ingkaninan, K. Survey of Acetylcholinesterase Inhibitory Activity in Essential Oils from Aromatic Plants. Open Conf. Proc. J. 2013, 4, 84. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Terra, W.R.; Costa, R.H.; Ferreira, C. Plasma membranes from insect midgut cells. An. Acad. Bras. Ciênc. 2006, 78, 255–269. [Google Scholar] [CrossRef]
- Bossou, A.D.; Mangelinckx, S.; Yedomonhan, H.; Boko, P.M.; Akogbeto, M.C.; De Kimpe, N.; Avlessi, F.; Sohounhloue, D.C.K. Chemical composition and insecticidal activity of plant essential oils from Benin against Anopheles gambiae (Giles). Parasites Vectors 2013, 6, 337. [Google Scholar] [CrossRef]
- Manimaran, A.; Cruz, M.M.J.J.; Muthu, C.; Vincent, S.; Ignacimuthu, S. Larvicidal and knockdown effects of some essential oils against Culex quinquefasciatus Say, Aedes aegypti (L.) and Anopheles stephensi (Liston). Adv. Biosci. Biotechnol. 2012, 3, 855–862. [Google Scholar] [CrossRef][Green Version]
- Fujiwara, M.; Yagi, N.; Miyazawa, M. Acetylcholinesterase Inhibitory Activity of Volatile Oil from Peltophorum dasyrachis Kurz ex Bakar (Yellow Batai) and Bisabolane-Type Sesquiterpenoids. J. Agric. Food Chem. 2010, 58, 2824–2829. [Google Scholar] [CrossRef]
- Rojas, L.B.; Visbal, T.; Morillo, M.; de Rojas, Y.E.C.; Arzola, J.C.; Usubillaga, A. The Volatile Constituents of Salvia leucantha. Nat. Prod. Commun. 2010, 5, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Maggi, F.; Bonacucina, G.; Pavela, R.; Boukouvala, M.C.; Kavallieratos, N.G.; Canale, A.; Romano, D.; Desneux, N.; Wilke, A.B.; et al. Apiaceae essential oils and their constituents as insecticides against mosquitoes—A review. Ind. Crop. Prod. 2021, 171, 113892. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Arraouadi, S.; Noumi, E.; Aouadi, K.; Adnan, M.; Khan, M.; Kadri, A.; Snoussi, M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules 2021, 26, 3625. [Google Scholar] [CrossRef] [PubMed]
- Akono, P.N.; Dongmo, P.M.J.; Tonga, C.; Kouotou, S.; Kekeunou, S.; Magne, G.T.; Lehman, L.G.; Menut, C. Larvicidal activity of essential oils from pericarps of ripe Citrus fruits cultivated in Cameroon on pyrethroids sensitive and resistant strains of Anopheles gambiae Giles, 1902. J. Entomol. Zool. Stud. 2015, 3, 334–339. [Google Scholar]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and Antiacetylcholinesterase Activities of Some Commercial Essential Oils and Their Major Compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Kitphati, W.; Wattanakamolkul, K.; Lomarat, P.; Phanthong, P.; Anantachoke, N.; Nukoolkarn, V.; Thirapanmethee, K.; Bunyapraphatsara, N. Anticholinesterase activity of essential oils and their constituents from Thai medicinal plants in human neuroblastoma SK-N-SH cells. JAASP 2012, 1, 51–60. [Google Scholar]
- Intirach, J.; Junkum, A.; Tuetun, B.; Choochote, W.; Chaithong, U.; Jitpakdi, A.; Riyong, D.; Champakaew, D.; Pitasawat, B. Chemical Constituents and Combined Larvicidal Effects of Selected Essential Oils against Anopheles cracens (Diptera: Culicidae). Psyche J. Èntomol. 2012, 2012, 591616. [Google Scholar] [CrossRef]
- Ali, A.; Wang, Y.-H.; Khan, I.A. Larvicidal and Biting Deterrent Activity of Essential Oils of Curcuma longa, Ar-turmerone, and Curcuminoids Against Aedes aegypti and Anopheles quadrimaculatus (Culicidae: Diptera). J. Med. Èntomol. 2015, 52, 979–986. [Google Scholar] [CrossRef]
- Chauhan, N.; Malik, A.; Sharma, S.; Dhiman, R.C. Larvicidal potential of essential oils against Musca domestica and Anopheles stephensi. Parasitol. Res. 2016, 115, 2223–2231. [Google Scholar] [CrossRef]
- Golfakhrabadi, F.; Khanavi, M.; Ostad, S.N.; Saeidnia, S.; Vatandoost, H.; Abai, M.R.; Hafizi, M.; Yousefbeyk, F.; Rad, Y.R.; Baghenegadian, A.; et al. Biological Activities and Composition of Ferulago carduchorum Essential Oil. J. Arthropod-Borne Dis. 2014, 9, 104–115. [Google Scholar]
- Tavakoli, S.; Vatandoost, H.; Zeidabadinezhad, R.; Hajiaghaee, R.; Hadjiakhoondi, A.; Abai, M.R.; Yassa, N. Gas Chromatography, GC/Mass Analysis and Bioactivity of Essential Oil from Aerial Parts of Ferulago trifida: Antimicrobial, Antioxidant, AChE Inhibitory, General Toxicity, MTT Assay and Larvicidal Activities. J. Arthropod-Borne Dis. 2017, 11, 414–426. [Google Scholar] [PubMed]
- Sedaghat, M.; Dehkordi, A.S.; Abai, M.; Khanavi, M.; Mohtarami, F.; Abadi, Y.S.; Rafi, F.; Vatandoost, H. Larvicidal Activity of Essential Oils of Apiaceae Plants against Malaria Vector, Anopheles stephensi. Iran. J. Arthropod-Borne Dis. 2011, 5, 51–59. [Google Scholar] [PubMed]
- Wangrawa, D.W.; Badolo, A.; Ilboudo, Z.; Guelbeogo, W.M.; Kiendrébeogo, M.; Nébié, R.C.H.; Sagnon, N.; Sanon, A. Insecticidal Activity of Local Plants Essential Oils Against Laboratory and Field Strains of Anopheles gambiae s. l. (Diptera: Culicidae) From Burkina Faso. J. Econ. Èntomol. 2018, 111, 2844–2853. [Google Scholar] [CrossRef]
- Kazempour, S.; Shayeghi, M.; Abai, M.; Vatandoost, H.; Pirmohammadi, M. Larvicidal activities of essential oils of indigenous medicinal plants, Mentha pulegium L., Satureja hortensis L., and Thymus vulgaris L. against malaria vector, Anopheles stephensi. S. Afr. J. Bot. 2021, 139, 38–41. [Google Scholar] [CrossRef]
- Benabdallah, A.; Boumendjel, M.; Aissi, O.; Rahmoune, C.; Boussaid, M.; Messaoud, C. Chemical composition, antioxidant activity and acetylcholinesterase inhibitory of wild Mentha species from northeastern Algeria. S. Afr. J. Bot. 2018, 116, 131–139. [Google Scholar] [CrossRef]
- Rocha, D.; Novo, M.; Matos, O.; Figueiredo, A.; Delgado, M.; Cabral, M.; Liberato, M.; Moiteiro, C. Potential of Mentha pulegium for mosquito control. J. Agric. Sci. 2015, 38, 155–165. [Google Scholar]
- Kiendrebeogo, M.; Coulibaly, A.Y.; Nebie, R.C.H.; Zeba, B.; Lamien, C.E.; Lamien-Meda, A.; Nacoulma, O.G. Antiacetylcholinesterase and antioxidant activity of essential oils from six medicinal plants from Burkina Faso. Rev. Bras. Farm. 2011, 21, 63–69. [Google Scholar] [CrossRef]
- Villalta, G.; Salinas, M.; Calva, J.; Bec, N.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE inhibitory activity, chemical composition, and enantiomeric content of the essential oil from Salvia leucantha Cav. Collected in Ecuador. Plants 2021, 10, 1169. [Google Scholar] [CrossRef]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: A Comparative Chemical and Biological Study of Its Essential Oil in the Mediterranean Context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef]
- Aboalhaija, A.; Amro, R.; Abaza, I.; Khalil, E.; Al-Aboudi, A.; Abu-Zarga, M.; Afifi, F. Schinus molle L. Collected from Jordan and Turkey: Essential Oil Composition and Anticholinesterase Activity. J. Essent. Oil Bear. Plants 2019, 22, 704–716. [Google Scholar] [CrossRef]
- Massebo, F.; Tadesse, M.; Bekele, T.; Balkew, M.; Gebre-Michael, T. Evaluation on larvicidal effects of essential oils of some local plants against Anopheles arabiensis Patton and Aedes aegypti Linnaeus (Diptera, Culicidae) in Ethiopia. Afr. J. Biotechnol. 2009, 8, 4183–4188. [Google Scholar]
- El-Akhal, F.; Guemmouh, R.; Maniar, S.; Taghzouti, K.; El Ouali Lalami, A. Larvicidal activity of essential oils of Thymus vulgaris and Origanum majorana (Lamiaceae) against of the malaria vector Anopheles labranchiae (diptera: Culicidae). Int. J. Pharm. Pharm. Sci. 2016, 8, 372–376. [Google Scholar]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Nakatsu, T.; Lupo, A.T., Jr.; Chinn, J.W., Jr.; Kang, R.K. Biological Activity of Essential Oils and Their Constituents. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 21, pp. 571–631. [Google Scholar] [CrossRef]
- Pandey, S.K.; Upadhyay, S.; Tripathi, A.K. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi. Parasitol. Res. 2009, 105, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.; Cleary, B.; Walsh, J.J.; Gilmer, J.F. Inhibition of acetylcholinesterase by Tea Tree oil. J. Pharm. Pharmacol. 2004, 56, 375–379. [Google Scholar] [CrossRef]
- Lee, S.-E.; Lee, B.-H.; Choi, W.-S.; Park, B.-S.; Kim, J.-G.; Campbell, B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Almas, I.; Innocent, E.; Machumi, F.; Kisinza, W. Chemical composition of essential oils from Eucalyptus globulus and Eucalyptus maculata grown in Tanzania. Sci. Afr. 2021, 12, e00758. [Google Scholar] [CrossRef]
- Song, A.; Wang, Y.; Liu, Y. Study on the chemical constituents of the essential oil of the leaves of Eucalyptus globulus Labill from China. Asian J. Tradit. Med. 2009, 4, 134–140. [Google Scholar]
- García-Jiménez, N.; Péerez-Alonso, M.J.; Velasco-Negueruela, A. Chemical Composition of Fennel Oil, Foeniculum vulgare Miller, from Spain. J. Essent. Oil Res. 2000, 12, 159–162. [Google Scholar] [CrossRef]
- Kini, F.; Kam, B.; Aycard, J.-P.; Gaydou, E.M.; Bombarda, I. Chemical Composition of the Essential Oil of Hyptis spicigera Lam. from Burkina Faso. J. Essent. Oil Res. 1993, 5, 219–221. [Google Scholar] [CrossRef]
- Ladan, Z.; Amupitan, J.; Oyewale, O.; Okonkwo, E.; Ladan, E.; Odjobo, B.; Habila, N. Chemical composition and biological activity of the volatile oils of Hyptis spicigera against Trypanosoma brucei brucei, (Tbb) found in northern Nigeria. Afr. J. Pure Appl. Chem. 2011, 5, 53–58. [Google Scholar]
- Azeez, G.O.; Lawal, I.A.; Muriana, M.; Adeniji, S.T.; Hammed, T. Chemical composition of Hyptis suaveolens grown in Saki, south western Nigerian, for its resource recovery. Int. J. Dev. Res. 2014, 4, 1765–1767. [Google Scholar]
- Ahmed, M.; Scora, R.W.; Ting, I.P. Composition of Leaf Oil of Hyptis suaveolens (L.) Poit. J. Essent. Oil Res. 1994, 6, 571–575. [Google Scholar] [CrossRef]
- Sousa, E.O.; Barreto, F.S.S.; Rodrigues, F.F.; Campos, A.R.; Costa, J.G. Chemical composition of the essential oils of Lantana camara L. and Lantana montevidensis Briq. and their synergistic antibiotic effects on aminoglycosides. J. Essent. Oil Res. 2012, 24, 447–452. [Google Scholar] [CrossRef]
- Kurade, N.P.; Jaitak, V.; Kaul, V.K.; Sharma, O.P. Chemical composition and antibacterial activity of essential oils of Lantana camara, Ageratum houstonianum and Eupatorium adenophorum. Pharm. Biol. 2010, 48, 539–544. [Google Scholar] [CrossRef]
- Da Silva, V.D.; Almeida-Souza, F.; Teles, A.M.; Neto, P.A.; Mondego-Oliveira, R.; Filho, N.E.M.; Taniwaki, N.N.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Filho, V.E.M. Chemical composition of Ocimum canum Sims. essential oil and the antimicrobial, antiprotozoal and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Ind. Crop. Prod. 2018, 119, 201–208. [Google Scholar] [CrossRef]
- Do Nascimento, V.H.N.; dos Santos Lima, C.; Paixão, J.T.C.; da Silva Freitas, J.J.; Kietzer, K.S. Antioxidant effects of açaí seed (Euterpe oleracea) in anorexia-cachexia syndrome induced by Walker-256 tumor. Acta Cir. Bras. 2016, 31, 597–601. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Mathivanan, T.; Elumalai, K.; Krishnappa, K.; Anandan, A. Mosquito larvicidal, ovicidal, and repellent properties of botanical extracts against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2011, 109, 353–367. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro Inhibition of Human Erythrocyte Acetylcholinesterase by Salvia lavandulaefolia Essential Oil and Constituent Terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yamafuji, C. Inhibition of Acetylcholinesterase Activity by Bicyclic Monoterpenoids. J. Agric. Food Chem. 2005, 53, 1765–1768. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of Acetylcholinesterase Activity by Monoterpenoids with a p-Menthane Skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res. 2004, 18, 315–324. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Kan, Y.; Şener, B. Activity of Essential Oils and Individual Components against Acetyland Butyrylcholinesterase. Z. Nat. C 2008, 63, 547–553. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase Inhibitory Activity and Chemical Composition of Commercial Essential Oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, Ł. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Nat. C 2016, 71, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Zhu, J.; Dhimitruka, A.I.; Pei, D. 5-(2-Aminoethyl)dithio-2-nitrobenzoate as a More Base-Stable Alternative to Ellman’s Reagent. Org. Lett. 2004, 6, 3809–3812. [Google Scholar] [CrossRef]
- Komersová, A.; Kovářová, M.; Komers, K.; Lochař, V.; Čegan, A. Why is the hydrolytic activity of acetylcholinesterase pH dependent? Kinetic study of acetylcholine and acetylthiocholine hydrolysis catalyzed by acetylcholinesterase from electric eel. Z. Nat. C 2018, 73, 345–351. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential Oils of Echinophora lamondiana (Apiales: Umbelliferae): A Relationship Between Chemical Profile and Biting Deterrence and Larvicidal Activity Against Mosquitoes (Diptera: Culicidae). J. Med. Èntomol. 2015, 52, 93–100. [Google Scholar] [CrossRef]
- Peng, X.; Yang, X.; Gu, H.; Yang, L.; Gao, H. Essential oil extraction from fresh needles of Pinus pumila (Pall.) Regel using a solvent-free microwave-assisted methodology and an evaluation of acetylcholinesterase inhibition activity in vitro compared to that of its main components. Ind. Crop. Prod. 2021, 167, 113549. [Google Scholar] [CrossRef]
- Park, T.-J.; Seo, H.-K.; Kang, B.-J.; Kim, K.-T. Noncompetitive inhibition by camphor of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2001, 61, 787–793. [Google Scholar] [CrossRef]
- Ansari, M.A.; Razdan, R.K. Relative efficacy of various oils in repelling mosquitoes. Indian J. Malariol. 1995, 32, 104–111. [Google Scholar] [PubMed]
- Seyoum, A.; Pålsson, K.; Kung’A, S.; Kabiru, E.; Lwande, W.; Killeen, G.; Hassanali, A.; Knots, B. Traditional use of mosquito-repellent plants in western Kenya and their evaluation in semi-field experimental huts against Anopheles gambiae: Ethnobotanical studies and application by thermal expulsion and direct burning. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 225–231. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Zhu, L.; Tian, Y. Chemical composition and larvicidal activity of essential oil of Artemisia gilvescens against Anopheles anthropophagus. Parasitol. Res. 2012, 112, 1137–1142. [Google Scholar] [CrossRef]
- Boudko, D.; Donly, B.; Stevens, B.; Harvey, W. Amino Acid and Neurotransmitter Transporters. In Comprehensive Molecular Insect Science; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 255–307. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef]
- Chang, K.-S.; Shin, E.-H.; Yoo, D.-H.; Ahn, Y.-J. Enhanced Toxicity of Binary Mixtures of Bacillus thuringiensis subsp. israelensis and Three Essential Oil Major Constituents to Wild Anopheles sinensis (Diptera: Culicidae) and Aedes albopictus (Diptera: Culicidae). J. Med. Èntomol. 2014, 51, 804–810. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Dalai, M.K.; Bhadra, S.; Chaudhary, S.K.; Bandyopadhyay, A. Anti-cholinesterase activity of the standardized extract of Syzygium aromaticum L. Pharmacogn. Mag. 2014, 10, S276–S282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tian, Y.-J. Chemical composition and larvicidal effects of essential oil of Blumea martiniana against Anopheles anthropophagus. Asian Pac. J. Trop. Med. 2011, 4, 371–374. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Yilmaz, S.V.; Özdemir, Ö.; Koca, M.; Pınar, N.M.; Demirci, B.; Yıldırım, K.; Sytar, O.; Turkez, H.; Baser, K.H.C. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae). J. Essent. Oil Res. 2020, 32, 512–525. [Google Scholar] [CrossRef]
- Osanloo, M.; Sedaghat, M.M.; Esmaeili, F.; Amani, A. Larvicidal activity of essential oil of Syzygium aromaticum (Clove) in comparison with its major constituent, eugenol, against Anopheles stephensi. J. Arthropod-Borne Dis. 2018, 12, 361–369. [Google Scholar]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae). Fitoterapia 2009, 80, 297–300. [Google Scholar] [CrossRef]
- Zarrad, K.; Ben Hamouda, A.; Chaieb, I.; Laarif, A.; Mediouni-Ben Jemâa, J. Chemical composition, fumigant and anti-acetylcholinesterase activity of the Tunisian Citrus aurantium L. essential oils. Ind. Crop. Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Perumalsamy, H.; Wang, M.; Shu, S.; Ahn, Y.-J. Larvicidal activity of Magnolia denudate seed hydrodistillate constituents and related compounds and liquid formulations towards two susceptible and two wild mosquito species. Pest Manag. Sci. 2016, 72, 897–906. [Google Scholar] [CrossRef]
- Arruda, M.; Viana, H.; Rainha, N.; Neng, N.R.; Rosa, J.S.; Nogueira, J.M.F.; do Carmo Barreto, M. Anti-acetylcholinesterase and Antioxidant Activity of Essential Oils from Hedychium gardnerianum Sheppard ex Ker-Gawl. Molecules 2012, 17, 3082–3092. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.; Benelli, G. Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res. Vet. Sci. 2016, 104, 77–82. [Google Scholar] [CrossRef]
- Govindarajan, M.; Sivakumar, R.; Rajeswary, M.; Yogalakshmi, K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp. Parasitol. 2013, 134, 7–11. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- Grundy, D.L.; Still, C.C. Inhibition of acetylcholinesterases by pulegone-1,2-epoxide. Pestic. Biochem. Physiol. 1985, 23, 383–388. [Google Scholar] [CrossRef]
- Mursiti, S.; Lestari, N.A.; Febriana, Z.; Rosanti, Y.M.; Ningsih, T.W. The Activity of D-Limonene from Sweet Orange Peel (Citrus Sinensis L.) Exctract as a Natural Insecticide Controller of Bedbugs (Cimex cimicidae). Orient. J. Chem. 2019, 35, 1420–1425. [Google Scholar] [CrossRef]
- Dias, C.N.; Moraes, D.F.C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicides: Review. Parasitol. Res. 2014, 113, 565–592. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.B.; Santos, J.M.M.; Martins, A.J. Mutations in the voltage-gated sodium channel gene of anophelines and their association with resistance to pyrethroids–A review. Parasites Vectors 2014, 7, 450. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Kabaria, C.W.; et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2010, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Vantaux, A.; Riehle, M.M.; Piv, E.; Farley, E.J.; Chy, S.; Kim, S.; Corbett, A.G.; Fehrman, R.L.; Pepey, A.; Eiglmeier, K.; et al. Anopheles ecology, genetics and malaria transmission in northern Cambodia. Sci. Rep. 2021, 11, 6458. [Google Scholar] [CrossRef] [PubMed]
- Faraj, C.; Adlaoui, E.; Elkohli, M.; Herrak, T.; Ameur, B.; Chandre, F. Review of Temephos Discriminating Concentration for Monitoring the Susceptibility of Anopheles labranchiae (Falleroni, 1926), Malaria Vector in Morocco. Malar. Res. Treat. 2010, 2010, 126085. [Google Scholar] [CrossRef]
- Xu, T.; Zhong, D.; Tang, L.; Chang, X.; Fu, F.; Yan, G.; Zheng, B. Anopheles sinensis mosquito insecticide resistance: Comparison of three mosquito sample collection and preparation methods and mosquito age in resistance measurements. Parasites Vectors 2014, 7, 54. [Google Scholar] [CrossRef]
- Balkew, M.; Mumba, P.; Dengela, D.; Yohannes, G.; Getachew, D.; Yared, S.; Chibsa, S.; Murphy, M.; George, K.; Lopez, K.; et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasites Vectors 2020, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.; Pironon, S.; Massey, N.; Longbottom, J.; Hemingway, J.; Moyes, C.; Willis, K. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. USA 2020, 117, 24900–24908. [Google Scholar] [CrossRef] [PubMed]
- Yared, S.; Gebressielasie, A.; Damodaran, L.; Bonnell, V.; Lopez, K.; Janies, D.; Carter, T.E. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar. J. 2020, 19, 180. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.; Hanafi-Bojd, A.A.; Sedaghat, M.M.; Zaim, M.; Hemingway, J. Evolution of insecticide resistance and its mechanisms in Anopheles stephensi in the WHO Eastern Mediterranean Region. Malar. J. 2020, 19, 258. [Google Scholar] [CrossRef] [PubMed]
- Amenya, D.A.; Naguran, R.; Lo, T.-C.M.; Ranson, H.; Spillings, B.L.; Wood, O.R.; Brooke, B.; Coetzee, M.; Koekemoer, L.L. Over expression of a Cytochrome P450 (CYP6P9) in a Major African Malaria Vector, Anopheles Funestus, Resistant to Pyrethroids. Insect Mol. Biol. 2008, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).