Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic
Abstract
1. Introduction
2. Results
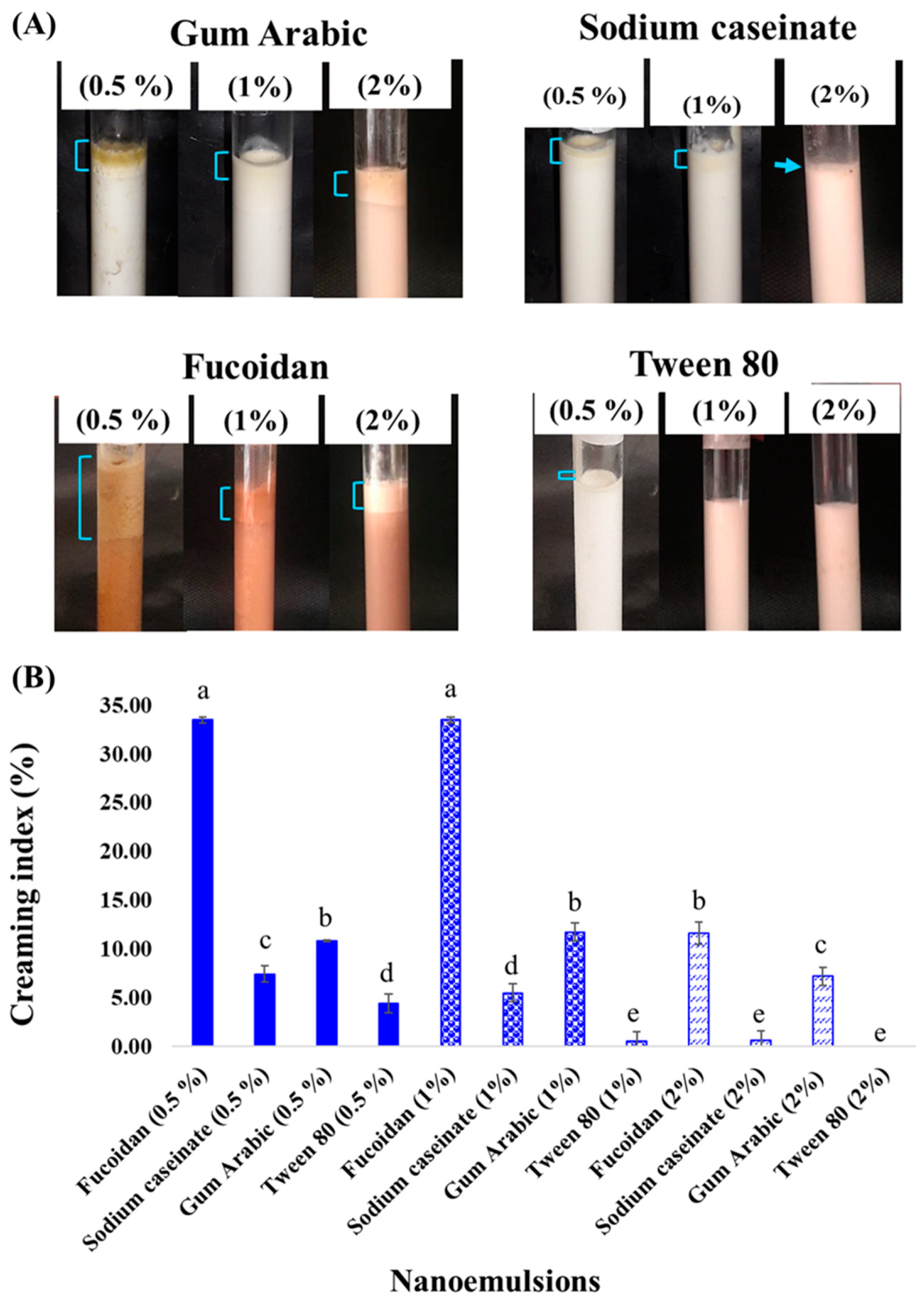
2.1. Emulsion Stability and Creaming Index
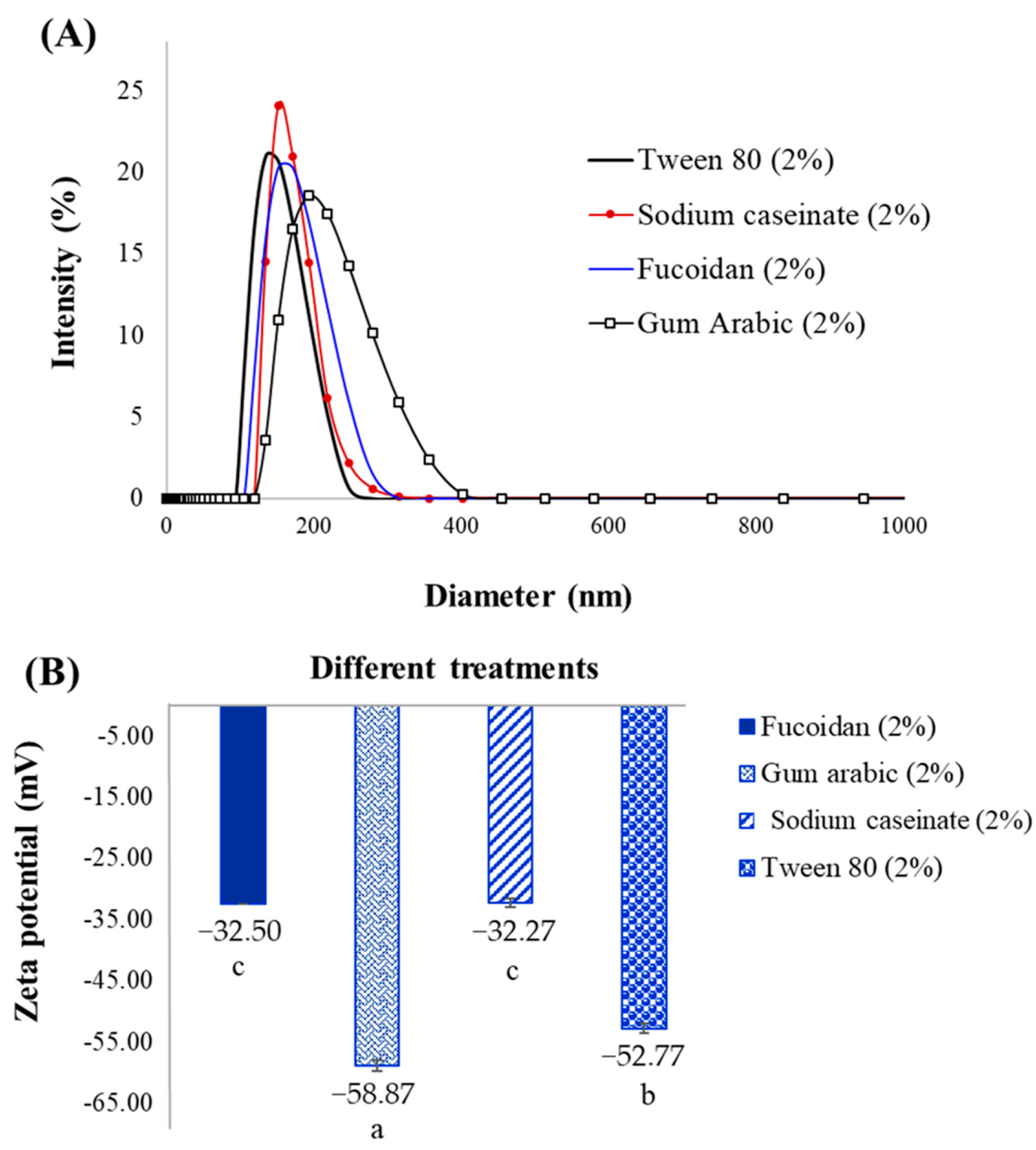
2.2. Particle Size and PDI
2.3. Zeta Potential
2.4. Encapsulation Efficiency (EE)
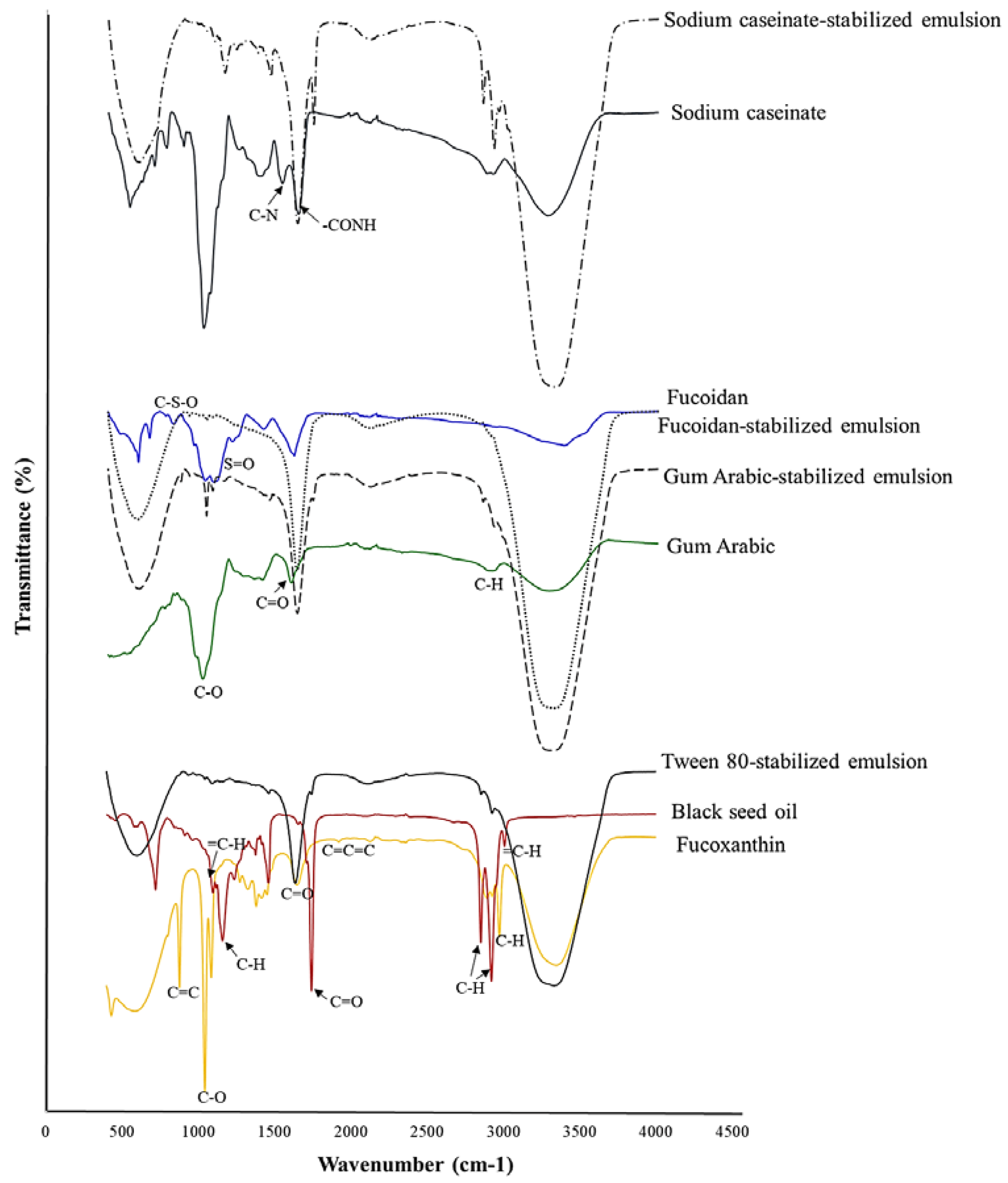
2.5. FTIR Spectra
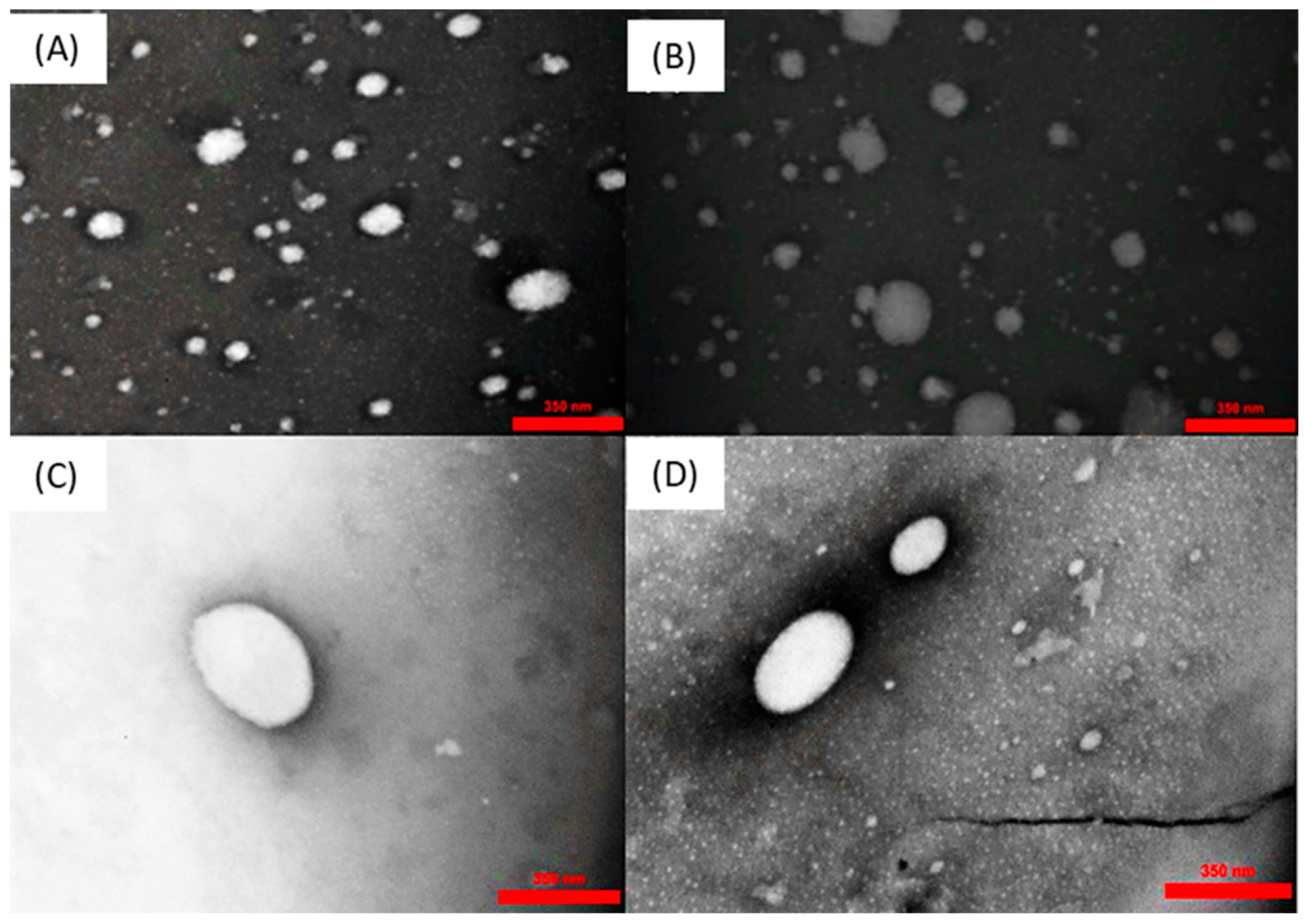
2.6. TEM
2.7. In Vitro Release
3. Materials and Methods
3.1. Extraction of Fucoxanthin
3.2. Extraction of Fucoidan
3.3. Nanoemulsions Preparation
3.4. Droplet Size and Zeta-Potential Measurement
3.5. Emulsion Stability and Creaming Index
3.6. Encapsulation Efficiency (EE)
3.7. Fourier-Transform Infrared (FT-IR) Spectroscopy
3.8. Transmission Electron Microscopy Analysis
3.9. In Vitro Release
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Fazaeli, M. Encapsulation of fucoxanthin in binary matrices of porous starch and halloysite. Food Hydrocoll. 2020, 100, 105458. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Nakamura, Y.; Yu, C.; Qi, H. Fucoxanthin activities motivate its nano/micro-encapsulation for food or nutraceutical application: A review. Food Funct. 2020, 11, 9338–9358. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Kim, S.-M.; Pan, C.-H.; Chung, D. Characterization of fucoxanthin-loaded microspheres composed of cetyl palmitate-based solid lipid core and fish gelatin–gum arabic coacervate shell. Food Res. Int. 2013, 50, 31–37. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Soni, M.; Maurya, A.; Das, S.; Prasad, J.; Yadav, A.; Singh, V.K.; Singh, B.K.; Dubey, N.K.; Dwivedy, A.K. Nanoencapsulation strategies for improving nutritional functionality, safety and delivery of plant-based foods: Recent updates and future opportunities. Plant Nano Biol. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Gonçalves, R.F.; Martins, J.T.; Abrunhosa, L.; Vicente, A.A.; Pinheiro, A.C. Nanoemulsions for enhancement of curcumin bioavailability and their safety evaluation: Effect of emulsifier type. Nanomaterials 2021, 11, 815. [Google Scholar] [CrossRef] [PubMed]
- Sari, T.; Mann, B.; Kumar, R.; Singh, R.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Kumar, D.L.; Sarkar, P. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018, 16, 59–70. [Google Scholar] [CrossRef]
- Huang, Z.; Xu, L.; Zhu, X.; Hu, J.; Peng, H.; Zeng, Z.; Xiong, H. Stability and bioaccessibility of fucoxanthin in nanoemulsions prepared from pinolenic acid-contained structured lipid. Int. J. Food Eng. 2017, 13, 1–14. [Google Scholar] [CrossRef]
- Noviendri, D.; Jaswir, I.; Taher, M.; Mohamed, F.; Salleh, H.M.; Noorbatcha, I.A.; Octavianti, F.; Lestari, W.; Hendri, R.; Ahmad, H. Fabrication of fucoxanthin-loaded microsphere (F-LM) by two steps double-emulsion solvent evaporation method and characterization of fucoxanthin before and after microencapsulation. J. Oleo Sci. 2016, 65, 641–653. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Sun, Q.; Um, B.H.; Park, Y.; McClements, D.J. In vitro and in vivo study of fucoxanthin bioavailability from nanoemulsion-based delivery systems: Impact of lipid carrier type. J. Funct. Foods 2015, 17, 293–304. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, D.; Kim, M.; Gu, M.-Y.; Kim, S.-M.; Pan, C.-H.; Kim, G.-H.; Chung, D. Effects of temperature, light, and pH on the stability of fucoxanthin in an oil-in-water emulsion. Food Chem. 2019, 291, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; McClements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Nielsen, C.K.; Kjems, J.; Mygind, T.; Snabe, T.; Meyer, R.L. Effects of Tween 80 on growth and biofilm formation in laboratory media. Front. Microbiol. 2016, 7, 1878. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E encapsulation within oil-in-water emulsions: Impact of emulsifier type on physicochemical stability and bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef]
- Chang, Y.; Hu, Y.; McClements, D.J. Competitive adsorption and displacement of anionic polysaccharides (fucoidan and gum arabic) on the surface of protein-coated lipid droplets. Food Hydrocoll. 2016, 52, 820–826. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Rezaei, M.; Tabarsa, M.; Rittà, M.; Donalisio, M.; Mariatti, F.; You, S.; Lembo, D.; Cravotto, G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2019, 124, 131–137. [Google Scholar] [CrossRef]
- Wang, B.; Tian, H.; Xiang, D. Stabilizing the oil-in-water emulsions using the mixtures of Dendrobium officinale polysaccharides and gum arabic or propylene glycol alginate. Molecules 2020, 25, 759. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidans: Downstream processes and recent applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of fucoidan utilization in the development of pharmaceutical dosage forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Fazaeli, M. Double encapsulation of fucoxanthin using porous starch through sequential coating modification with maltodextrin and gum Arabic. Food Sci. Nutr. 2020, 8, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Han, Y.; Li, Q.; Wu, L.; Zhang, J.; Zhong, X.; Xie, J.; Shao, S.; Zhang, Y. Emulsifying properties of polysaccharide conjugates prepared from chin-brick tea. J. Agric. Food Chem. 2019, 67, 10165–10173. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Khalid, N.; Tan, T.B.; Zhao, Y.; Neves, M.A.; Kobayashi, I.; Nakajima, M. Comparison of ergocalciferol nanodispersions prepared using modified lecithin and sodium caseinate: Insights of formulation, stability and bioaccessibility. J. Funct. Foods 2017, 38, 28–35. [Google Scholar] [CrossRef]
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf. B Biointerfaces 2018, 168, 163–168. [Google Scholar] [CrossRef]
- Salminen, H.; Bischoff, S.; Weiss, J. Impact of concentration ratio on the formation and stability of emulsions stabilized by Quillaja saponin–sodium caseinate mixtures. Food Biophys. 2019, 14, 109–119. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.-C.; Deng, Y.-Y.; Dong, H.; Zhang, Y.; Tang, X.-J.; Li, P.; Liu, G.; Zhang, M.-W. Comparison of the effects of different food-grade emulsifiers on the properties and stability of a casein-maltodextrin-soybean oil compound emulsion. Molecules 2020, 25, 458. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Koo, C.K.; Chung, C.; Fu, J.-T.R.; Sher, A.; Rousset, P.; McClements, D.J. Impact of sodium caseinate, soy lecithin and carrageenan on functionality of oil-in-water emulsions. Food Res. Int. 2019, 123, 779–789. [Google Scholar] [CrossRef]
- Bai, L.; Liu, F.; Xu, X.; Huan, S.; Gu, J.; McClements, D.J. Impact of polysaccharide molecular characteristics on viscosity enhancement and depletion flocculation. J. Food Eng. 2017, 207, 35–45. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-grade nanoemulsions: Preparation, stability and application in encapsulation of bioactive compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zou, L.; McClements, D.J.; Liu, W. One-step preparation of high internal phase emulsions using natural edible Pickering stabilizers: Gliadin nanoparticles/gum Arabic. Food Hydrocoll. 2020, 100, 105381. [Google Scholar] [CrossRef]
- Gasa-Falcon, A.; Arranz, E.; Odriozola-Serrano, I.; Martín-Belloso, O.; Giblin, L. Delivery of β-carotene to the in vitro intestinal barrier using nanoemulsions with lecithin or sodium caseinate as emulsifiers. LWT 2021, 135, 110059. [Google Scholar] [CrossRef]
- O’Dwyer, S.P.; O’Beirne, D.; Eidhin, D.N.; O’Kennedy, B.T. Effects of sodium caseinate concentration and storage conditions on the oxidative stability of oil-in-water emulsions. Food Chem. 2013, 138, 1145–1152. [Google Scholar] [CrossRef]
- Saravana, P.S.; Shanmugapriya, K.; Gereniu, C.R.N.; Chae, S.-J.; Kang, H.W.; Woo, H.-C.; Chun, B.-S. Ultrasound-mediated fucoxanthin rich oil nanoemulsions stabilized by κ-carrageenan: Process optimization, bio-accessibility and cytotoxicity. Ultrason. Sonochem. 2019, 55, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Dai, J.; Zhong, Y.; Zhang, D.; Liu, L.; Wang, L.; Huang, Y.; Chen, P.; Zhou, Z.; Chen, X. Caffeic acid phenethyl ester loaded in nano-targeted delivery system with casein: Physicochemical characterization, in vitro release, and binding mechanisms. LWT 2021, 150, 111938. [Google Scholar] [CrossRef]
- Ruengdech, A.; Siripatrawan, U. Improving encapsulating efficiency, stability, and antioxidant activity of catechin nanoemulsion using foam mat freeze-drying: The effect of wall material types and concentrations. LWT 2022, 162, 113478. [Google Scholar] [CrossRef]
- Jamshidi, A.; Shabanpour, B.; Pourashouri, P.; Raeisi, M. Using WPC-inulin-fucoidan complexes for encapsulation of fish protein hydrolysate and fish oil in W1/O/W2 emulsion: Characterization and nutritional quality. Food Res. Int. 2018, 114, 240–250. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.-k.; Ling, J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Ma, Y.; Liu, Y.; Wang, S.; Guo, Z.; Li, J.; Wang, Y.; Tan, B.; Wei, Y. Fabricating hydrophilic fatty acid-protein particles to encapsulate fucoxanthin: Fatty acid screening, structural characterization, and thermal stability analysis. Food Chem. 2022, 382, 132311. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Ma, Y.; Liu, Y.; Liu, G.; Wang, Y.; Tan, B. Dietary fatty acid-mediated protein encapsulation simultaneously improving the water-solubility, storage stability, and oral absorption of astaxanthin. Food Hydrocoll. 2022, 123, 107152. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Amin, H.H.; Aziz, S.B.; Sha, A.M.; Hassan, S.; Abdul Aziz, J.M.; Rahman, H.S. Structural characterization, antimicrobial activity, and in vitro cytotoxicity effect of black seed oil. Evid.-Based Complementary Altern. Med. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Prieto, P.; Silva-Castro, I.; Ramos-Silva, M.; Martín-Ramos, P.; Martín-Gil, J.; Hernández-Navarro, S. Vibrational Analysis and Thermal Behavior of Salvia Hispanica, Nigella Sativa and Papaver Somniferum Seeds. Pharmacogn J. 2017, 9, 157–162. [Google Scholar] [CrossRef]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and biological characterization of fucoidan from Fucus vesiculosus purified by dye affinity chromatography. Mar. Drugs 2016, 14, 79. [Google Scholar] [CrossRef]
- Chale-Dzul, J.; Moo-Puc, R.; Robledo, D.; Freile-Pelegrín, Y. Hepatoprotective effect of the fucoidan from the brown seaweed Turbinaria tricostata. J. Appl. Phycol. 2015, 27, 2123–2135. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Li, R.-Y. Preparation and characterization of antioxidant nanoparticles composed of chitosan and fucoidan for antibiotics delivery. Mar. Drugs 2014, 12, 4379–4398. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, L.; Tong, M.; Lu, Y.; Ouyang, X.-K.; Ling, J. Encapsulation of curcumin using fucoidan stabilized zein nanoparticles: Preparation, characterization, and in vitro release performance. J. Mol. Liq. 2021, 329, 115586. [Google Scholar] [CrossRef]
- Li, Y.; Dou, X.; Pang, J.; Liang, M.; Feng, C.; Kong, M.; Liu, Y.; Cheng, X.; Wang, Y.; Chen, X. Improvement of fucoxanthin oral efficacy via vehicles based on gum Arabic, gelatin and alginate hydrogel: Delivery system for oral efficacy enhancement of functional food ingredients. J. Funct. Foods 2019, 63, 103573. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, J.; Tiwari, A.; Bajpai, A.K. Designing casein-coated iron oxide nanostructures (CCIONPs) as superparamagnetic core–shell carriers for magnetic drug targeting. Prog. Biomater. 2015, 4, 39–53. [Google Scholar] [CrossRef]
- Szyk-Warszyńska, L.; Raszka, K.; Warszyński, P. Interactions of casein and polypeptides in multilayer films studied by FTIR and molecular dynamics. Polymers 2019, 11, 920. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Sun, X.; Wang, S.; Wang, J.; Zhu, J.; Wang, D.; Zhao, L. Stability, bioactivity, and bioaccessibility of fucoxanthin in zein-caseinate composite nanoparticles fabricated at neutral pH by antisolvent precipitation. Food Hydrocoll. 2018, 84, 379–388. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Sohag, A.A.M.; Hossain, M.T.; Zahan, M.S.; Uddin, M.J.; Hannan, M.A.; Moon, I.S.; Choi, J.-S. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Mar. Drugs 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-j.; Xu, S.; Wang, H.-m.; Ling, Y.; Dong, J.; Xia, R.-d.; Sun, X.-h. Nanoparticles: Oral delivery for protein and peptide drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Q.; McClements, D.J.; Li, B.; Liu, S.; Li, Y. Development of Salt-and Gastric-Resistant Whey Protein Isolate Stabilized Emulsions in the Presence of Cinnamaldehyde and Application in Salad Dressing. Foods 2021, 10, 1868. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Han, Y.; Li, F.; Li, Z.; McClements, D.J.; He, L.; Decker, E.A.; Xing, B.; Xiao, H. Impact of protein-nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: Not just simple protein corona effects. NanoImpact 2019, 13, 37–43. [Google Scholar] [CrossRef]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Industrial optimization of fucoidan extraction from Sargassum sp. and its potential antioxidant and emulsifying activities. Food Hydrocoll. 2016, 54, 77–88. [Google Scholar] [CrossRef]
- Richa, R.; Choudhury, A.R. Exploration of polysaccharide based nanoemulsions for stabilization and entrapment of curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. [Google Scholar] [CrossRef]
- Sharma, D.; Satapathy, B.K. Understanding release kinetics and collapse proof suture retention response of curcumin loaded electrospun mats based on aliphatic polyesters and their blends. J. Mech. Behav. Biomed. Mater. 2021, 120, 104556. [Google Scholar] [CrossRef]

| Emulsifiers | Initial Average Particle Size (nm) | PDI |
|---|---|---|
| Fucoidan (1%) | 143.67 ± 3.35 Bb | 0.31 ± 0.01 Bab |
| Gum Arabic (1%) | 148.00 ± 4.75 Bb | 0.42 ± 0.10 Aa |
| Sodium caseinate (1%) | 161.80 ± 1.95 Ba | 0.41 ± 0.04 Aab |
| Tween 80 (1%) | 128.53 ± 1.80 Bc | 0.27 ± 0.11 Ab |
| Fucoidan (2%) | 126.73 ± 2.68 Bc | 0.36 ± 0.03 Aa |
| Gum Arabic (2%) | 127.50 ± 2.62 Bc | 0.34 ± 0.08 Aa |
| Sodium caseinate (2%) | 113.43 ± 1.29 Bd | 0.33 ± 0.06 Aa |
| Tween 80 (2%) | 113.27 ± 0.45 Bd | 0.29 ± 0.03 Aa |
| Emulsifiers | Encapsulation Efficiency (%) |
|---|---|
| Fucoidan (1%) | 72.57 ± 0.11 Bc |
| Sodium caseinate (1%) | 85.01 ± 0.09 Bb |
| Gum Arabic (1%) | 59.34 ± 0.12 Bd |
| Tween 80 (1%) | 89.22 ± 0.16 Ba |
| Fucoidan (2%) | 79.32 ± 0.09 Ac |
| Sodium caseinate (2%) | 88.51± 0.11 Ab |
| Gum Arabic (2%) | 60.34 ± 0.13 Ad |
| Tween 80 (2%) | 92.15 ± 0.15 Aa |
| Nanoemulsions | Kinetic Parameters | ||
|---|---|---|---|
| n | k | R2 | |
| Fucoidan | 0.498 | 0.201 | 0.988 |
| Gum Arabic | 0.508 | 0.179 | 0.977 |
| Sodium caseinate | 0.499 | 0.215 | 0.980 |
| Tween 80 | 0.497 | 0.243 | 0.992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliyaei, N.; Moosavi-Nasab, M.; Tanideh, N. Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic. Molecules 2022, 27, 6713. https://doi.org/10.3390/molecules27196713
Oliyaei N, Moosavi-Nasab M, Tanideh N. Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic. Molecules. 2022; 27(19):6713. https://doi.org/10.3390/molecules27196713
Chicago/Turabian StyleOliyaei, Najmeh, Marzieh Moosavi-Nasab, and Nader Tanideh. 2022. "Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic" Molecules 27, no. 19: 6713. https://doi.org/10.3390/molecules27196713
APA StyleOliyaei, N., Moosavi-Nasab, M., & Tanideh, N. (2022). Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic. Molecules, 27(19), 6713. https://doi.org/10.3390/molecules27196713







