Alternative Extraction and Downstream Purification Processes for Anthocyanins
Abstract
:1. Introduction
2. Alternative Solvents
2.1. Deep Eutectic Solvents
2.2. Anthocyanins Extraction Using DES
3. Compressed Fluid-Based Extraction Techniques
3.1. Supercritical Carbon Dioxide Extraction
3.2. Pressurized Liquid Extraction
3.3. Anthocyanin Extraction and Separation Using Compressed Fluids
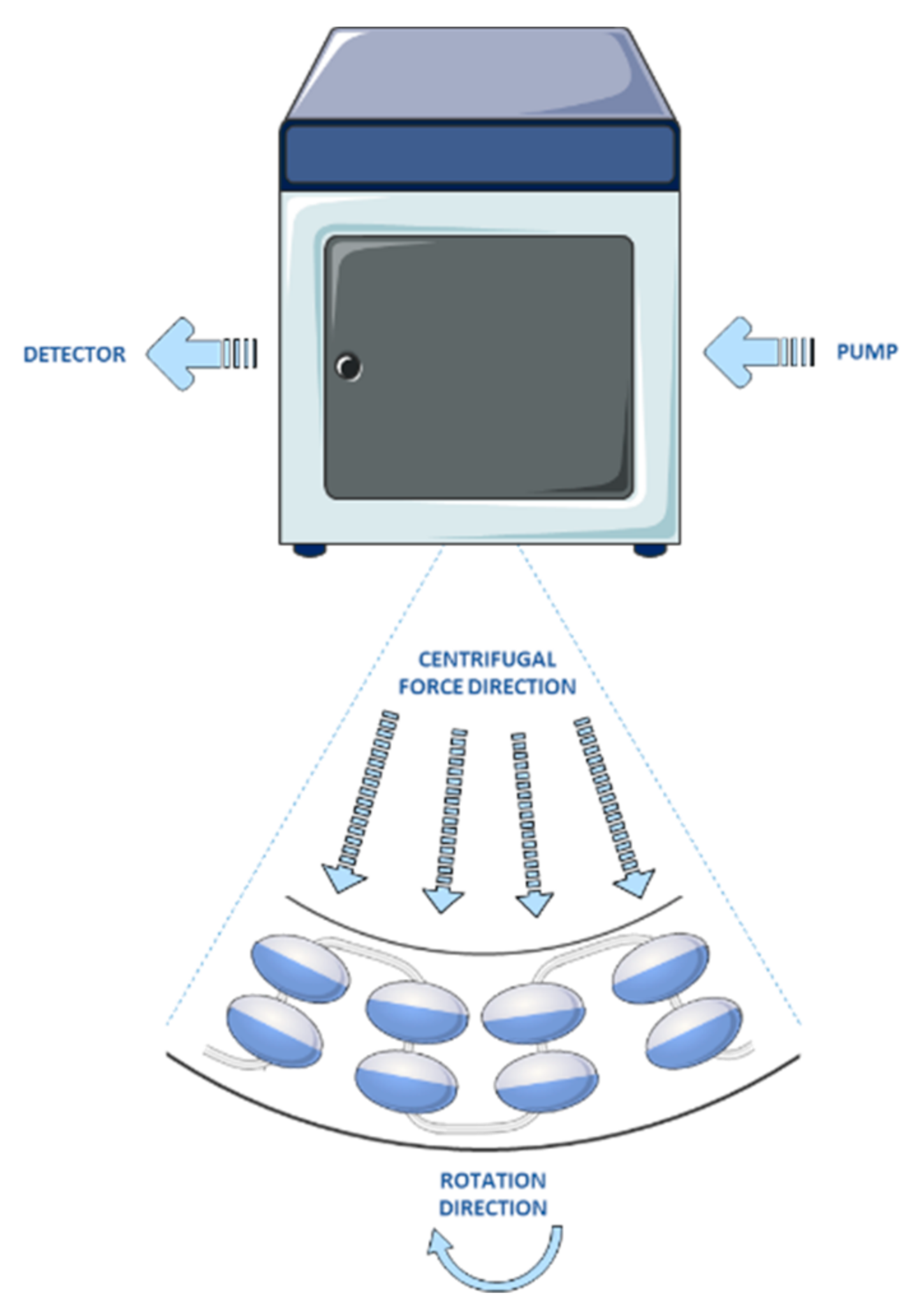
4. Counter-Current Chromatography
Anthocyanin Separation Using Counter-Current Chromatography
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Brouillard, R. Chemical Structure of Anthocyanins; Academic Press Inc.: New York, NY, USA, 1992. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A.; Speers, R.A. Anthocyanins as food colorants—A review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, J.; Fernandes, I.; Faria, A.; Oliveira, J.; Fernandes, A.; de Freitas, V.; Mateus, N. Antioxidant properties of anthocyanidins, anthocyanidin-3-glucosides and respective portisins. Food Chem. 2010, 119, 518–523. [Google Scholar] [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Moore, J.; Cheng, Z.; Luther, M.; Rao, J.N.; Wang, J.Y.; Yu, L.L. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. J. Agric. Food Chem. 2006, 54, 3773–3778. [Google Scholar] [CrossRef] [PubMed]
- Renis, M.; Calandra, L.; Scifo, C.; Tomasello, B.; Cardile, V.; Vanella, L.; Bei, R.; La Fauci, L.; Galvano, F. Response of cell cycle/stress-related protein expression and DNA damage upon treatment of CaCo2 cells with anthocyanins. Br. J. Nutr. 2008, 100, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Sen, C.K.; Bagchi, M.; Atalay, M. Anti-angiogenic, antioxidant, and anti-carcinogenic properties of a novel anthocyanin-rich berry extract formula. Biochemistry 2004, 69, 75–80. [Google Scholar] [CrossRef]
- Olsson, M.; Gustavsson, K.-E.; Andersson, S.; Nilsson, A.; Duan, R.-D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2005, 52, 7264–7271. [Google Scholar] [CrossRef]
- Singletary, K.W.; Jung, K.J.; Giusti, M. Anthocyanin-rich grape extract blocks breast cell DNA damage. J. Med. Food 2007, 10, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, R. Chapter 1—Chemical structure of anthocyanins. In Anthocyanins as Food Colors; Markakis, P., Ed.; Academic Press: Cambridge, MA, USA, 1982; pp. 1–40. [Google Scholar]
- Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Álvarez, J.A.; Ayuso, J.; Barbero, G.F. Extraction of anthocyanins and total phenolic compounds from açai (Euterpe oleracea mart.) using an experimental design methodology. Part 1: Pressurized liquid extraction. Agronomy 2020, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 68, 240–248. [Google Scholar] [CrossRef]
- Revilla, E.; Ryan, J.-M.; Martín-Ortega, G. Comparison of several procedures used for the extraction of anthocyanins from red grapes. J. Agric. Food Chem. 1998, 46, 4592–4597. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Da Porto, C.; Natolino, A.; Decorti, D. Extraction of proanthocyanidins from grape marc by supercritical fluid extraction using CO2 as solvent and ethanol–water mixture as co-solvent. J. Supercrit. Fluids 2014, 87, 59–64. [Google Scholar] [CrossRef]
- Saldaña, M.D.A.; Martinez, E.R.; Sekhon, J.K.; Vo, H. The effect of different pressurized fluids on the extraction of anthocyanins and total phenolics from cranberry pomace. J. Supercrit. Fluids 2021, 175, 105279. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of acoustic frequency and power density on the aqueous ultrasonic-assisted extraction of grape pomace (Vitis vinifera L.)—A response surface approach. Ultrason. Sonochem. 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Pedroza, M.A.; Amendola, D.; Maggi, L.; Zalacain, A.; De Faveri, D.M.; Spigno, G. Microwave-assisted extraction of phenolic compounds from dried waste grape skins. Int. J. Food Eng. 2015, 11, 359–370. [Google Scholar] [CrossRef]
- Dai, Y.; Rozema, E.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J. Chromatogr. A 2016, 1434, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Li, S.; Zhang, S. Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers. Green Chem. 2005, 7, 606–608. [Google Scholar] [CrossRef]
- Nieto de Castro, C.; Vieira, S.; Francą, J.M.P.; Lourenco, M.; Santos, F.; Murshed, S.M.S.; Goodrich, P.; Hardacre, C. Ionic Liquids—New Aspects for the Future; IntechOpen: London, UK, 2013; Volume 7. [Google Scholar]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Rogers, R.D. Ionic liquids. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 218–225. [Google Scholar]
- Ruß, C.; König, B. Low melting mixtures in organic synthesis—An alternative to ionic liquids? Green Chem. 2012, 14, 2969–2982. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction techniques with deep eutectic solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Ramón, D.J.; Guillena, G. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chainsElectronic supplementary information (ESI) available: Plot of conductivity vs. temperature for the ionic liquid formed from zinc chloride and choline chloride (2:1). Chem. Commun. 2001, 19, 2010–2011. [Google Scholar]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chemistry 2007, 13, 6495–6501. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.B.; Jha, D.; Kumar, R.; Marriyappan Sivagnanam, B. Ternary hydrophobic deep eutectic solvents for carbon dioxide absorption. Int. J. Greenh. Gas Control 2020, 92, 102839. [Google Scholar] [CrossRef]
- Ci, Y.-H.; Yu, F.; Zhou, C.-X.; Mo, H.-E.; Li, Z.-Y.; Ma, Y.-Q.; Zang, L.-H. New ternary deep eutectic solvents for effective wheat straw deconstruction into its high-value utilization under near-neutral conditions. Green Chem. 2020, 22, 8713–8720. [Google Scholar] [CrossRef]
- Zhong, F.-Y.; Peng, H.-L.; Tao, D.-J.; Wu, P.-K.; Fan, J.-P.; Huang, K. Phenol-based ternary deep eutectic solvents for highly efficient and reversible absorption of NH3. ACS Sustain. Chem. Eng. 2019, 7, 3258–3266. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. Chemphyschem 2006, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Row, K.H. Application of novel ternary deep eutectic solvents as a functional monomer in molecularly imprinted polymers for purification of levofloxacin. J. Chromatogr. B 2017, 1068, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Chen, Y.-A.; Xing, Y.-J. Synthesis and characterization of novel ternary deep eutectic solvents. Chin. Chem. Lett. 2014, 25, 104–106. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Benvenutti, L.; del Pilar Sanchez-Camargo, A.; Zielinski, A.; Ferreira, S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Pimentel-Mora, S.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109646. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Esclapez, M.D.; García-Pérez, J.V.; Mulet, A.; Cárcel, J.A. Ultrasound-assisted extraction of natural products. Food Eng. Rev. 2011, 3, 108. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Pekel, A.G.; Bilgin, M.; Makris, D.P.; Şahin, S. Citric acid-based deep eutectic solvent for the anthocyanin recovery from Hibiscus sabdariffa through microwave-assisted extraction. Biomass Convers. Biorefin. 2020, 1–10. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep eutectic solvents and nonconventional technologies for blueberry-peel extraction: Kinetics, anthocyanin stability, and antiproliferative activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Bi, Y.; Chi, X.; Zhang, R.; Lu, Y.; Wang, Z.; Dong, Q.; Ding, C.; Yang, R.; Jiang, L. Highly efficient extraction of mulberry anthocyanins in deep eutectic solvents: Insights of degradation kinetics and stability evaluation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Kou, P.; Wan, N.; Wang, L.-T.; Pan, H.-Y.; Jiao, J.; Zhao, C.-J.; Liu, Z.-G.; Wang, X.-Q.; Fu, Y.-J. A sustainable and efficient preparation process of anthocyanins from blue honeysuckle fruit and comprehensive bioactivity assessment. J. Taiwan Inst. Chem. Eng. 2020, 116, 3–10. [Google Scholar] [CrossRef]
- Saponea, V.; Ciccib, A.; Franceschic, D.; Vincenzic, S.; Bravia, M. Antioxidant extraction and bioactivity preservation from winery by-products by natural deep eutectic solvents (NaDES). Chem. Eng. 2020, 79, 157–162. [Google Scholar]
- Aslan Türker, D.; Doğan, M. Application of deep eutectic solvents as a green and biodegradable media for extraction of anthocyanin from black carrots. LWT 2021, 138, 110775. [Google Scholar] [CrossRef]
- Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-assisted extraction of anthocyanins using natural deep eutectic solvents and their incorporation in edible films. Molecules 2021, 26, 984. [Google Scholar] [CrossRef]
- Guo, N.; Ping, K.; Jiang, Y.-W.; Wang, L.-T.; Niu, L.-J.; Liu, Z.-M.; Fu, Y.-J. Natural deep eutectic solvents couple with integrative extraction technique as an effective approach for mulberry anthocyanin extraction. Food Chem. 2019, 296, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Kou, P.; Kang, Y.-F.; Wang, L.-T.; Niu, L.-J.; Xiao, Y.; Guo, N.; Cui, Q.; Li, Y.-Y.; Fu, Y.-J. An integrated strategy for production of four anthocyanin compounds from Ribes nigrum L. by deep eutectic solvents and flash chromatography. J. Ind. Eng. Chem. 2019, 80, 614–625. [Google Scholar] [CrossRef]
- De Silva, D.T.; Pauletto, R.; de Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; de Bona da Silva, C.; Dal Pont Morisso, F.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Li, L.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multi-stability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Oktaviyanti, N.; Kartini, K.; Mun’im, A. Application and optimization of ultrasound-assisted deep eutectic solvent for the extraction of new skin-lightening cosmetic materials from Ixora javanica flower. Heliyon 2019, 5, e02950. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Tan, J.; Li, Q.; Tang, J.; Cai, X. Optimization ultrasound-assisted deep eutectic solvent extraction of anthocyanins from raspberry using response surface methodology coupled with genetic algorithm. Foods 2020, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Koca, I.; Aldawoud, T.; Galanakis, C.M. Recovery and stabilization of anthocyanins and phenolic antioxidants of roselle (Hibiscus sabdariffa L.) with hydrophilic deep eutectic solvents. Molecules 2020, 25, 3715. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Bentley, J.; Olsen, E.K.; Moore, J.P.; Farrant, J.M. The phenolic profile extracted from the desiccation-tolerant medicinal shrub Myrothamnus flabellifolia using Natural Deep Eutectic Solvents varies according to the solvation conditions. Phytochemistry 2020, 173, 112323. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef]
- Souza, H.K.S.; Mateus, N.; de Freitas, V.; Gonçalves, M.P.; Cruz, L. Chemical/color stability and rheological properties of cyanidin-3-glucoside in deep eutectic solvents as a gateway to design task-specific bioactive compounds. ACS Sustain. Chem. Eng. 2020, 8, 16184–16196. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Yammine, S.; Brianceau, S.; Manteau, S.; Turk, M.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M. Extraction and purification of high added value compounds from by-products of the winemaking chain using alternative/nonconventional processes/technologies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Machado, B.A.S.; Pereira, C.G.; Nunes, S.B.; Padilha, F.F.; Umsza-Guez, M.A. Supercritical fluid extraction using CO2: Main applications and future perspectives. Sep. Sci. Technol. 2013, 48, 2741–2760. [Google Scholar] [CrossRef]
- Chai, Y.H.; Yusup, S.; Kadir, W.N.A.; Wong, C.Y.; Rosli, S.S.; Ruslan, M.S.H.; Chin, B.L.F.; Yiin, C.L. Valorization of tropical biomass waste by supercritical fluid extraction technology. Sustainability 2021, 13, 233. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Parat, M.-O.; Shaw, P.N.; Falconer, J.R. Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: A review. Molecules 2017, 22, 1186. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Ongkowijoyo, P.; Luna-Vital, D.A.; Gonzalez de Mejia, E. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chem. 2018, 250, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Junior, M.R.M.; Leite, A.V.; Dragano, N.R.V. Supercritical fluid extraction and stabilization of phenolic compounds from natural sources—Review (Supercritical extraction and stabilization of phenolic compounds). Open Chem. Eng. J. 2010, 4, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green extraction methods for polyphenols from plant matrices and their byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Navas, M.J.; Jiménez-Moreno, A.M.; Bueno, J.M.; Sáez-Plaza, P.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part IV: Extraction of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 313–342. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Sticher, O. Natural product isolation. Nat. Prod. Rep. 2008, 25, 517–554. [Google Scholar] [CrossRef]
- Farooq, S.; Shah, M.A.; Siddiqui, M.W.; Dar, B.N.; Mir, S.A.; Ali, A. Recent trends in extraction techniques of anthocyanins from plant materials. J. Food Meas. Charact. 2020, 14, 3508–3519. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Lisiane de Marsillac, T.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Fomo, G.; Madzimbamuto, T.N.; Ojumu, T.V. Applications of nonconventional green extraction technologies in process industries: Challenges, limitations and perspectives. Sustainability 2020, 12, 5244. [Google Scholar] [CrossRef]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Ghafoor, K.; Park, J.; Choi, Y.-H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Jiao, G.; Kermanshahi pour, A. Extraction of anthocyanins from haskap berry pulp using supercritical carbon dioxide: Influence of co-solvent composition and pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
- Eliasson, L.; Labrosse, L.; Ahrné, L. Effect of drying technique and particle size of bilberry press cake on the extraction efficiency of anthocyanins by pressurized carbon dioxide extraction. LWT 2017, 85, 510–516. [Google Scholar] [CrossRef]
- Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E. A screening analysis of the high-pressure extraction of anthocyanins from red grape pomace with carbon dioxide and cosolvent. Eng. Life Sci. 2003, 3, 38–42. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B.; Manikandan, S. Modeling and optimization of supercritical fluid extraction of anthocyanin and phenolic compounds from Syzygium cumini fruit pulp. J. Food Sci. Technol. 2014, 51, 1938–1946. [Google Scholar] [CrossRef] [Green Version]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.; de Sousa, H.C. Effect of solvent (CO2/ethanol/H2O) on the fractionated enhanced solvent extraction of anthocyanins from elderberry pomace. J. Supercrit. Fluids 2010, 54, 145–152. [Google Scholar] [CrossRef]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef] [Green Version]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical Solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef]
- Muangrat, R.; Williams, P.T.; Saengcharoenrat, P. Subcritical solvent extraction of total anthocyanins from dried purple waxy corn: Influence of process conditions. J. Food Process. Preserv. 2017, 41, e13252. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, K.; King, J.W.; Monrad, J.K.; Howard, L.R.; Zhang, D. Pressurized solvent extraction of flavonoids from grape pomace utilizing organic acid additives. Ital. J. Food Sci. 2011, 23, 90–105. [Google Scholar]
- Koyu, H.; Kazan, A.; Ozturk, T.K.; Yesil-Celiktas, O.; Haznedaroglu, M.Z. Optimizing subcritical water extraction of Morus nigra L. fruits for maximization of tyrosinase inhibitory activity. J. Supercrit. Fluids 2017, 127, 15–22. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R.S. Sensory and nutritive qualities of food subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Modifiers based on natural deep eutectic mixtures to enhance anthocyanins isolation from grape pomace by pressurized hot water extraction. LWT 2021, 149, 111889. [Google Scholar] [CrossRef]
- Del Pilar Garcia-Mendoza, M.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Junior, M.R.M.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16. [Google Scholar] [CrossRef]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Martínez de la Ossa, E.J. Green extraction of antioxidants from different varieties of red grape pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Sainz Martinez, A.; Kornpointner, C.; Haselmair-Gosch, C.; Mikulic-Petkovsek, M.; Schröder, K.; Halbwirth, H. Dynamic streamlined extraction of iridoids, anthocyanins and lipids from haskap berries. LWT 2021, 138, 110633. [Google Scholar] [CrossRef]
- Serra, A.T.; Matias, A.A.; Almeida, A.P.C.; Bronze, M.R.; Alves, P.M.; de Sousa, H.C.; Duarte, C.M.M. Processing cherries (Prunus avium) using supercritical fluid technology. Part 2. Evaluation of SCF extracts as promising natural chemotherapeutical agents. J. Supercrit. Fluids 2011, 55, 1007–1013. [Google Scholar] [CrossRef]
- Kühn, S.; Temelli, F. Recovery of bioactive compounds from cranberry pomace using ternary mixtures of CO2+ethanol+water. J. Supercrit. Fluids 2017, 130, 147. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Monroy, Y.M.; Rodrigues, R.A.; Sartoratto, A.; Cabral, F. Extraction of bioactive compounds from cob and pericarp of purple corn (Zea mays L.) by sequential extraction in fixed bed extractor using supercritical CO2, ethanol, and water as solvents. J. Supercrit. Fluids 2016, 107, 250–259. [Google Scholar] [CrossRef]
- Tamkutė, L.; Liepuoniūtė, R.; Pukalskienė, M.; Venskutonis, P.R. Recovery of valuable lipophilic and polyphenolic fractions from cranberry pomace by consecutive supercritical CO2 and pressurized liquid extraction. J. Supercrit. Fluids 2020, 159, 104755. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Berthod, A.; Faure, K. Separations with a liquid stationary phase: Countercurrent chromatography or centrifugal partition chromatography. In Analytical Separation Science; Wiley-VCH: Weinheim, Germany, 2015; Volume 4, Chapter 3; pp. 1177–1206. [Google Scholar]
- Quirino, J.P.; Alejandro, F.M.; Bissember, A.C. Towards cleaner downstream processing of biomass waste chemical products by liquid chromatography: A review and recommendations. J. Clean. Prod. 2020, 253, 119937. [Google Scholar] [CrossRef]
- Costa, F.; Leitão, G.G. Strategies of solvent system selection for the isolation of flavonoids by countercurrent chromatography. J. Sep. Sci. 2010, 33, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Origin and evolution of the coil planet centrifuge: A personal reflection of my 40 years of CCC research and development. Sep. Purif. Rev. 2005, 34, 131–154. [Google Scholar] [CrossRef]
- Ito, Y.; Sandlin, J.; Bowers, W.G. High-speed preparative counter-current chromatography with a coil planet centrifuge. J. Chromatogr. A 1982, 244, 247–258. [Google Scholar] [CrossRef]
- Murayama, W.; Kobayashi, T.; Kosuge, Y.; Yano, H.; Nunogaki, Y.; Nunogaki, K. A new centrifugal counter-current chromatograph and its application. J. Chromatogr. A 1982, 239, 643–649. [Google Scholar] [CrossRef]
- Marchal, L.; Legrand, J.; Foucault, A. Centrifugal partition chromatography: A survey of its history, and our recent advances in the field. Chem. Rec. 2003, 3, 133–143. [Google Scholar] [CrossRef]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [Green Version]
- Bojczuk, M.; Żyżelewicz, D.; Hodurek, P. Centrifugal partition chromatography—A review of recent applications and some classic references. J. Sep. Sci. 2017, 40, 1597–1609. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K. Developments in the application of counter-current chromatography to plant analysis. J. Chromatogr. A 2006, 1112, 181–194. [Google Scholar] [CrossRef]
- Morley, R.; Minceva, M. Operating mode selection for the separation of intermediately-eluting components with countercurrent and centrifugal partition chromatography. J. Chromatogr. A 2019, 1594, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Y.; Ignatova, S.; Hewitson, P.; Di, D.-L. An overview of recent progress in elution mode of counter current chromatography. Trends Anal. Chem. 2016, 77, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Skalicka-Woźniak, K.; Garrard, I. A comprehensive classification of solvent systems used for natural product purifications in countercurrent and centrifugal partition chromatography. Nat. Prod. Rep. 2015, 32, 1556–1561. [Google Scholar] [CrossRef]
- Thiébaut, D.; Rosset, R. Hydrodynamic and hydrostatic high-speed countercurrent chromatography and its coupling with various kinds of detectors: Application to biochemical separations. J. Chromatogr. A 1992, 626, 41–52. [Google Scholar] [CrossRef]
- Hu, R.; Pan, Y. Recent trends in counter-current chromatography. Trends Anal. Chem. 2012, 40, 15–27. [Google Scholar] [CrossRef]
- Foucault, A.P. Enantioseparations in counter-current chromatography and centrifugal partition chromatography. J. Chromatogr. A 2001, 906, 365–378. [Google Scholar] [CrossRef]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent Chromatography in Analytical Chemistry (IUPAC Technical Report). Chem. Int.—Newsmag. IUPAC 2009, 31, 24–25. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Morley, R.; Minceva, M. Operating mode and parameter selection in liquid-liquid chromatography. J. Chromatogr. A 2020, 1617, 460479. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tong, S.; Zhang, K.; Yan, J. Recent progress in separation prediction of counter-current chromatography. J. Sep. Sci. 2021, 44, 6–16. [Google Scholar] [CrossRef]
- Winterhalter, P. Application of countercurrent chromatography (CCC) to the analysis of natural pigments. Trends Food Sci. Technol. 2007, 18, 507–513. [Google Scholar] [CrossRef]
- Yin, L.; Li, Y.; Lu, B.; Jia, Y.; Peng, J. Trends in counter-current chromatography: Applications to natural products purification. Sep. Purif. Rev. 2010, 39, 33–62. [Google Scholar] [CrossRef]
- Leitão, G.G.; Costa, F. Gradient elution in countercurrent chromatography. Planta Med. 2015, 81, 1592–1596. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Lila, M.A.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food Chem. 2012, 131, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renault, J.H.; Thepenier, P.; Zeches-Hanrot, M.; Foucault, A.P. Separation of the two major anthocyanins from champagne vintage by-products by gradient elution centrifugal partition chromatography. J. Liq. Chromatogr. 1995, 18, 1663–1670. [Google Scholar] [CrossRef]
- Renault, J.-H.; Thépenier, P.; Zéches-Hanrot, M.; Le Men-Olivier, L.; Durand, A.; Foucault, A.; Margraff, R. Preparative separation of anthocyanins by gradient elution centrifugal partition chromatography. J. Chromatogr. A 1997, 763, 345–352. [Google Scholar] [CrossRef]
- Degenhardt, A.; Knapp, H.; Winterhalter, P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J. Agric. Food Chem. 2000, 48, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Yousof, N.; Xynos, N.; Dina, E.; Omar, M.; Keong, C.Y.; Wasiman, M.I.; Aligiannis, N. Comparison of standard elution and displacement modes in centrifugal partition chromatography for an efficient purification of four anthocyanins from Hibiscus sabdariffa L. J. Pharm. Pharmacol. 2018, 6, 701–711. [Google Scholar]
- Ruiz, A.; Mardones, C.; Vergara, C.; von Baer, D.; Gómez-Alonso, S.; Gómez, M.V.; Hermosín-Gutiérrez, I. Isolation and structural elucidation of anthocyanidin 3,7-β-O-diglucosides and caffeoyl-glucaric acids from calafate berries. J. Agric. Food Chem. 2014, 62, 6918–6925. [Google Scholar] [CrossRef]
- Paissoni, M.A.; Waffo-Teguo, P.; Ma, W.; Jourdes, M.; Rolle, L.; Teissedre, P.L. Chemical and sensorial investigation of in-mouth sensory properties of grape anthocyanins. Sci. Rep. 2018, 8, 17098. [Google Scholar] [CrossRef]
- Lima, Á.S.; de Oliveira, B.S.; Shabudin, S.V.; Almeida, M.; Freire, M.G.; Bica, K. Purification of anthocyanins from grape pomace by centrifugal partition chromatography. J. Mol. Liq. 2021, 326, 115324. [Google Scholar] [CrossRef]

| Source of Anthocyanins | DES | Preparation and Auxiliary Extraction Techniques | Anthocyanin Content (mg/g) | Ref. | ||
|---|---|---|---|---|---|---|
| Composition | Molar Ratio | Water (%) | ||||
| Frozen blueberry peels | ChCl:LA | 1:1 | 22 | Stirring and heating at 50 °C for 2 h (Microwave and ultrasound-assisted) | 23.59 | [51] |
| Grape skin | ChCl:Oa | 1:1 | 25 | Stirring and heating at 80 °C for 2–6 h (Ultrasonic processor-assisted) | ~12 | [52] |
| Hibiscus sabdariffa L. calyces | ChCl:Oa | 1:1 | 25 | Heating at 80 °C (Microwave-assisted) | 3.76 ± 0.03 (delphinidin-3-sambubioside) 3.60 ± 0.03 (cyanidin-3-sambubioside) | [46] |
| Cranberry pomace | Glucose:LA | 1:5 | 20 | Stirring and heating at 50 °C for 30 min (Ultrasonic processor-assisted) | 1.54 | [53] |
| Jabuticaba pomace | ChCl:Pro | 1:2 | - | Heating at 80 °C for 21 min | ~2.8 (expressed as Monomeric Anthocyanin Pigment) | [45] |
| Mulberry fruits | ChCl:LA | 1:2 | 3.19 | Stirring and heating at ≤100 °C followed by drying in a vacuum oven at 45 °C | 4.24 ± 0.20 | [54] |
| Blue honey-suckle fruits | ChCl:LA | 1:2 | 20 | Stirring and heating at 80 °C | 5 | [55] |
| Red wine by products | ChCl:TA | 1:2 | 44 | Stirring and heating at 90 °C | 3.33 | [56] |
| Black carrots | ChCl:CA | 1:1 | - | Stirring and heating at 80 °C for 2–6 h (Ultrasound-assisted) | 6 | [57] |
| L. chequen (Molina) A. Gray | Glu:LA | 1:8 | - | Storage at 80 °C for 2 h followed by lyophilization for 18–24 h until a homogeneous and viscous liquid was obtained. (Ultrasound-assisted) | 3.30 | [58] |
| Wine lees | ChCl:MA | - | 34.5 | Stirring and heating at 80 °C for 120–360 min (Ultrasound-assisted) | 6.55 | [47] |
| Mulberry fruits | ChCl:CA | 1:1 | 30 | Stirring and heating at 80 °C (High-speed homogenization and cavitation-burst assisted) | ~5.50 | [59] |
| Blackcurrant | ChCl:LA | 1:2 | 20 | Stirring and heating at 80 °C (Microwave assisted) | ~2.0 | [60] |
| Blueberry | ChCl:gly:CA | 0.5:2:0.5 | 25 | Stirring and heating at 80 °C for 30 min | 2.30 | [61] |
| Blueberry pomace | ChCl:Oa | 1:1 | 30 | Stirring and heating at 80 °C (pulse-ultrasonication assisted extraction) | ~25 | [62] |
| Ixora javanica flower | ChCl:Eg | 1:2 | - | Stirring and heating at 50 °C for 30 min (Ultrasound-assisted) | ~13 | [63] |
| Raspberry | ChCl:1,4-butanediol | 1:3 | 29 | Stirring and heating at 80 °C (Ultrasound-assisted) | 1.378 ± 0.009 | [64] |
| Source of Anthocyanin | Technique | Solvent | Modifier | Temperature (°C) | Pressure (bar) | Flow Rate | Time | Anthocyanin Content/Yield | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Grape (Vitis labrusca B.) peel | SFE | CO2 | 6–7% Ethanol | 45–46 | 160–165 | 2 mL/min | 30 min | 1.2 mg/mL | [92] |
| Indian blackberry (Syzygium cumini L.) | SFE | CO2 | Ethanol | 50 | 162 | 2 g/min | n.d. | 231.3 ± 0.8 mg/100 g | [96] |
| Haskap berry pulp | SFE | CO2 | 5.4 g Water/3.2 g berry pulp paste | 65 | 450 | 10 mL/min | 15 min static, 20 min dynamic | 52.7% anthocyanins yield | [93] |
| Blackberry residues | LE | Ethanol | - | 60 | 75 | 3.80 mL/min | 30 min | 1.40 ± 0.02 mg cyanidin-3-O-glucoside equivalent/g fresh residue | [99] |
| PLE | Ethanol/water (50% v/v) | - | 80 | 75 | 3.35 mL/min | 30 min | 1.39 ± 0.02 mg cyanidin-3-O-glucoside equivalent/g fresh residue | ||
| Jabuticaba skins | PLE | Ethanol | - | 80 | 50 | Static | 9 min | 2.4 ± 0.5 mg cyanidin-3-O-glucoside/g dry material | [100] |
| Red Grape Pomace | PLE | Ethanol (50–70% v/v) | - | 100 | 68 | Static | n.d. | 497 ± 13 mg/100 g dry weight | [101] |
| Purple waxy corn (Zea mays L.) | PLE | Ethanol (50–75% v/v) | - | 100 | n.d. | Static | 15 min | 991 to 1552 µg cyanidin-O-3-glucoside/g dry weight | [102] |
| Cranberry pomace | PLE | Ethanol | - | 40 | 50 | 5 mL/min | 10 min | 6.3–7.8 mg cyanidin-3-O-glucoside equivalent | [20] |
| Grape pomace | PLE | Ethanol/water (80% v/v) | - | 80 | 103 | Static | 1 min | 1028 mg/100 g dry weight | [103] |
| Morus nigra L. fruits | PLE | Water | - | 60 | 150 | 2 mL/min | 60 min | 9.4 mg cyanidin-3-O-glucoside equivalent/g extract | [104] |
| Red grape skin | PLE | Water | Sodium metabisulfite (1400 µg/mL) | 100 | n.d. | Static | 40 s | 59.3 ± 0.7 mg/g dry weight | [105] |
| Grape pomace | PLE | Water | Choline chloride and oxalic acid (30% w/w) | 60 | n.d. | Static | 2 cycles of 10 min | 11.2 ± 1.4 mg/L | [106] |
| SFE vs. PLE | |||||||||
| Juçara residues | SFE | CO2 | 10% Ethanol/water (50% v/v, pH 2) | 60 | 200 | 2.08 × 10−4 kg/s | 7 min static, 39 min dynamic | 22 mg cyanidin-3-O-rutinoside equivalent/g dry residue | [107] |
| PLE | Water (pH 2) | - | 40 | 100 | 1.5 mL/min | n.d. | 9.7 mg cyanidin-3-O-rutinoside equivalent/g dry residue | ||
| Blueberry residues | SFE | CO2 | 10% Ethanol/water (50% v/v) | 40 | 200 | 10 mL/min | n.d. | 1071 ± 64 mg/100 g | [98] |
| PLE | Ethanol/water (50% v/v, pH 2) | - | 40 | 200 | 10 mL/min | n.d. | 254.0 ± 0.6 mg/100 g | ||
| Red Grape Pomace | SFE | CO2 | 20% Ethanol | 55 | 100 | 25 g/min | 3 h | 116 ± 2 mg malvin chloride/g dry extract | [108] |
| PLE | Ethanol/water (50% v/v) | - | 120 | 100 | 5 g/min | 3 h | 742 ± 42 mg malvin chloride/g dry extract | ||
| Integrated process | |||||||||
| Haskap berries | 1st step: SFE | CO2 | 1% Ethanol | 50 | 300 | 2 mL/min | 30 min | Lipophilic fraction | [109] |
| 2nd step: SFE | CO2 | 20% Ethanol | 50 | 300 | 2 mL/min | 1 h static, 1 h dynamic | Terpenoids fraction | ||
| 3rd step: SFE | CO2 | 50% Ethanol | 50 | 300 | 2 mL/min | 1 h static, 1 h dynamic | Anthocyanins fraction | ||
| Sweet cherry (Prunus avium) | 1st step: SFE | CO2 | - | 50 | 250 | n.d. | 15 min static, 1 h dynamic | Lipophilic fraction | [110] |
| 2nd step: PLE | 100% Ethanol | - | 50 | 250 | n.d. | 1.5 h | 0.99 ± 0.05 mg cyanidin-3-O-glucoside/g | ||
| Elderberry pomace | 1st step: SFE | CO2 | 40 | 210 | 12.3 ± 1.4 × 10−5 kg/s | 15 min static, 40 min dynamic | Lipophilic fraction | [97] | |
| 2nd step: SFE | CO2 | CO2/ethanol/water (80:1:19) and (60:8:32) | 40 | 210 | 7.2 ± 0.4 × 10−5 kg/s | 45 min dynamic | 12.0–13.3% total anthocyanins | ||
| Bilberry (Vaccinium myrtillus) | 1st step: SFE | CO2 | 6% Ethanol/water (70% v/v) | 45 | 250 | 8 kg/h | 1 h | [111] | |
| 2nd step: SFE | CO2 | 6% Ethanol/water (50% v/v) | 45 | 250 | 6 kg/h | 1 h | |||
| 3rd step: SFE | 9% Ethanol/water (10% v/v) | 45 | 250 | 6 kg/h | 3 h | 0.62 ± 0.05 mg/g dw anthocyanins | |||
| Cranberry pomace | 1st step: SFE | CO2 | - | 50 | 400 | 1 L/min | 180 min | Lipophilic fraction | [112] |
| 2nd step: SFE | CO2 | CO2/ethanol/water (0.312:0.048:0.640) | 50 | 400 | 1 L/min | 420 min | 84.6 ± 2.3% Total anthocyanin | ||
| Purple corn (Zea mays L.) | 1st step: SFE | CO2 | - | 50 | 400 | 1.6 g/min | 90–100 min | Lipophilic fraction | [113] |
| 2nd step: PLE | Ethanol | - | 50 | 400 | 0.5 mL/min | 150–220 min | 63.8–63.1 mg/g total monomeric anthocyanins | ||
| 3rd step: PLE | 50 | 400 | 0.5 mL/min | 150–220 min | 41.3–54.6 mg/g total monomeric anthocyanins | ||||
| Cranberry pomace | 1st step: SFE | CO2 | - | 53 | 424 | n.d. | 158 min | Lipophilic fraction | [114] |
| 2nd step: PLE | Ethanol | - | 70 | 103 | Static | 3 cycles, 10 min | 9.0 ± 1.1 mg/g extract 416 ± 51 mg/100 g dry weight | ||
| Grap marc | 1st step: PLE | Ethanol/water (50% w/w, pH 2) | - | 40 | 100 | 5 g/min | 40 min | 10.0 mg malvidin-3-O-glucoside/g of dried grape marc | [115] |
| 2nd step: PLE | Ethanol/water (50% w/w) | - | 100 | 100 | 5 g/min | 40 min | Phenolic fraction |
| Source of Anthocyanin | Solvent System | Column Capacity (mL) | Flow Rate (mL/min) | Rotation Speed (rpm) | Ref. |
|---|---|---|---|---|---|
| Champagne vintage by-products | EtOAc:BuOH:W, 0.8% TFA | 240 | 3 | 1300–1500 | [140] |
| Stationary phase—5:5:90 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Champagne vintage by-products | EtOAc:BuOH:W, 0.2% TFA | 5470 | 60 | 1140 | [141] |
| Stationary phase—5:5:90 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Blackcurrant (Ribes nigrum L.) | EtOAc:BuOH:W, 0.2% TFA | 230 | 3 | 1400 | [141] |
| Stationary phase—5:5:90 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Calafate berry (Berberis microphylla G. Forst) | EtOAc:BuOH:W, 0.1% TFA | 200 | 5 | n.d. | [144] |
| Stationary phase—4:5:91 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Grape skin (Vitis vinifera L.) | EtOAc:BuOH:W, 0.1% TFA | 200 | 3 | 1000 | [145] |
| Stationary phase—5:5:90 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Grape skin (Vitis vinifera L.) | EtOAc:BuOH:W, 0.1% TFA | 1000 | 15 | 1000 | [145] |
| Stationary phase—5:5:90 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Maqui Berry (Aristotelia chilensis) | EtOAc:BuOH:W, 0.1% TFA | 1000 | n.d. | n.d. | [139] |
| 2:3:5 | |||||
| Ascending | |||||
| Hibiscus sabdariffa L. | EtOAc:BuOH:W, 0.5% TFA | 200 | 10 | 1500 | [143] |
| Stationary phase—5:5:90 | |||||
| Initial mobile phase—77:15:8 | |||||
| Final mobile phase—40:46:14 | |||||
| Ascending | |||||
| Hibiscus sabdariffa L. | EtOAc:BuOH:W | 200 | 10 | 1500 | [143] |
| Stationary phase—5:5:90, 20 mM NaOH (pH~10) | |||||
| Initial mobile phase—77:15:8, 16 mM TFA (pH~2) | |||||
| Final mobile phase—40:46:14, 16 mM TFA (pH~2) | |||||
| Ascending, pH-zone refining | |||||
| Hibiscus sabdariffa L. | EtOAc:BuOH:W | 200 | 10 | 1500 | [143] |
| Stationary phase—5:5:90, quaternary ammonium salt (Aliquat 336™) | |||||
| Initial mobile phase—77:15:8, NaI | |||||
| Final mobile phase—40:46:14, NaI | |||||
| Ascending, strong-on exchange | |||||
| Grape pomace | ACN:[2HEA]HSO4]:W | 50 | 1.5 | 2500 | [146] |
| 5:1:4 | |||||
| Ascending |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.N.; Borges, A.; Matias, A.A.; Bronze, M.R.; Oliveira, J. Alternative Extraction and Downstream Purification Processes for Anthocyanins. Molecules 2022, 27, 368. https://doi.org/10.3390/molecules27020368
Nunes AN, Borges A, Matias AA, Bronze MR, Oliveira J. Alternative Extraction and Downstream Purification Processes for Anthocyanins. Molecules. 2022; 27(2):368. https://doi.org/10.3390/molecules27020368
Chicago/Turabian StyleNunes, Ana N., Alexandra Borges, Ana A. Matias, Maria Rosário Bronze, and Joana Oliveira. 2022. "Alternative Extraction and Downstream Purification Processes for Anthocyanins" Molecules 27, no. 2: 368. https://doi.org/10.3390/molecules27020368







