Optimization of a Sustainable Protocol for the Extraction of Anthocyanins as Textile Dyes from Plant Materials
Abstract
:1. Introduction
2. Results
2.1. Definition of an Extraction Protocol for Red Chicory
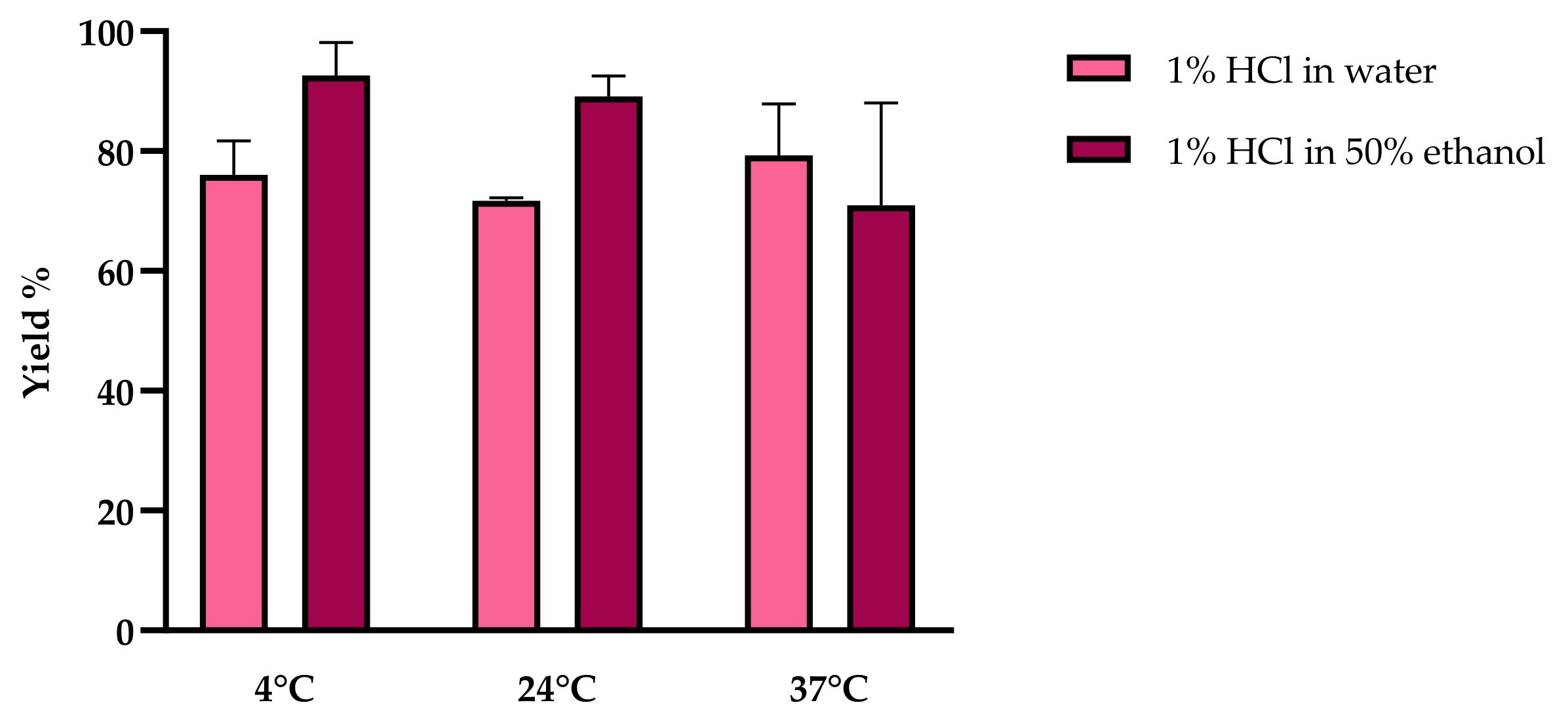
2.1.1. Gold-Standard Extraction
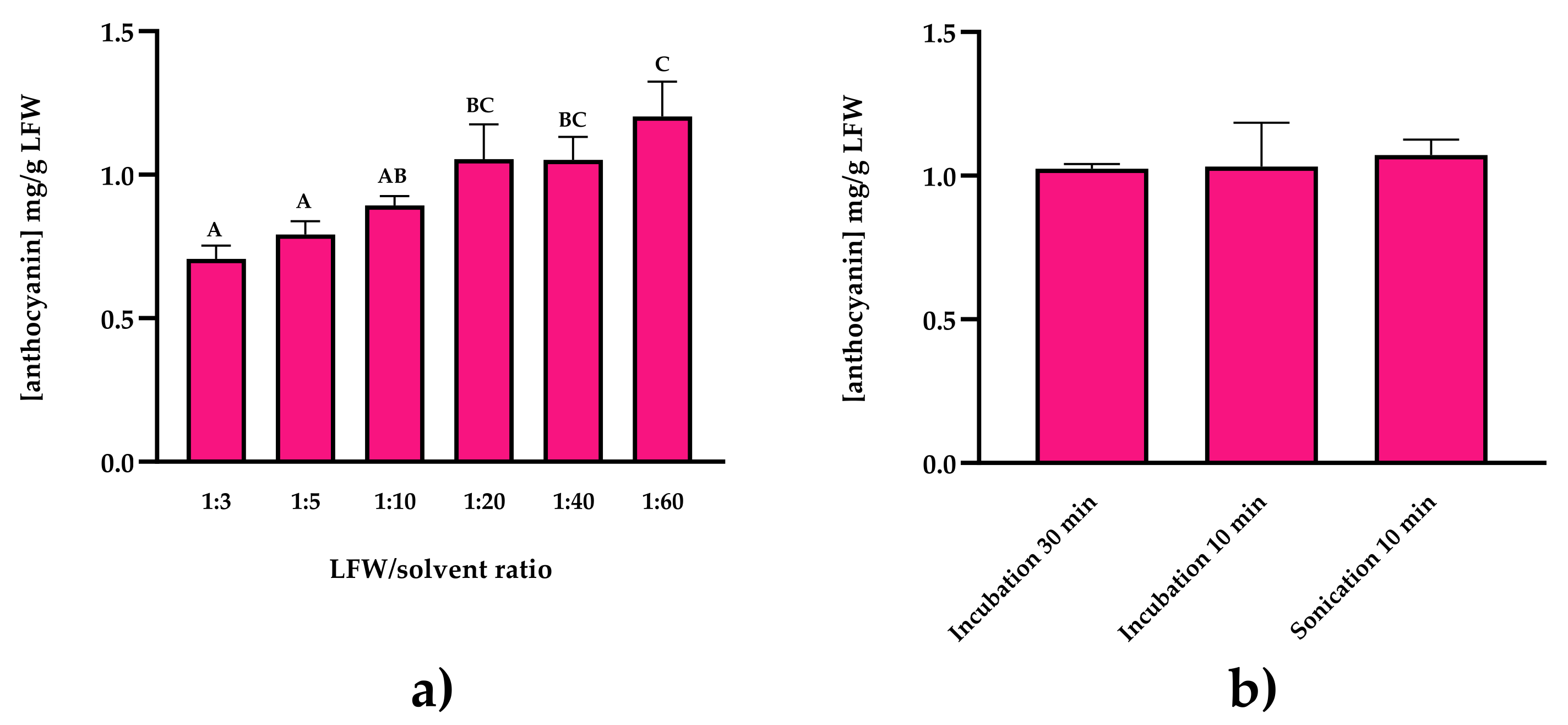
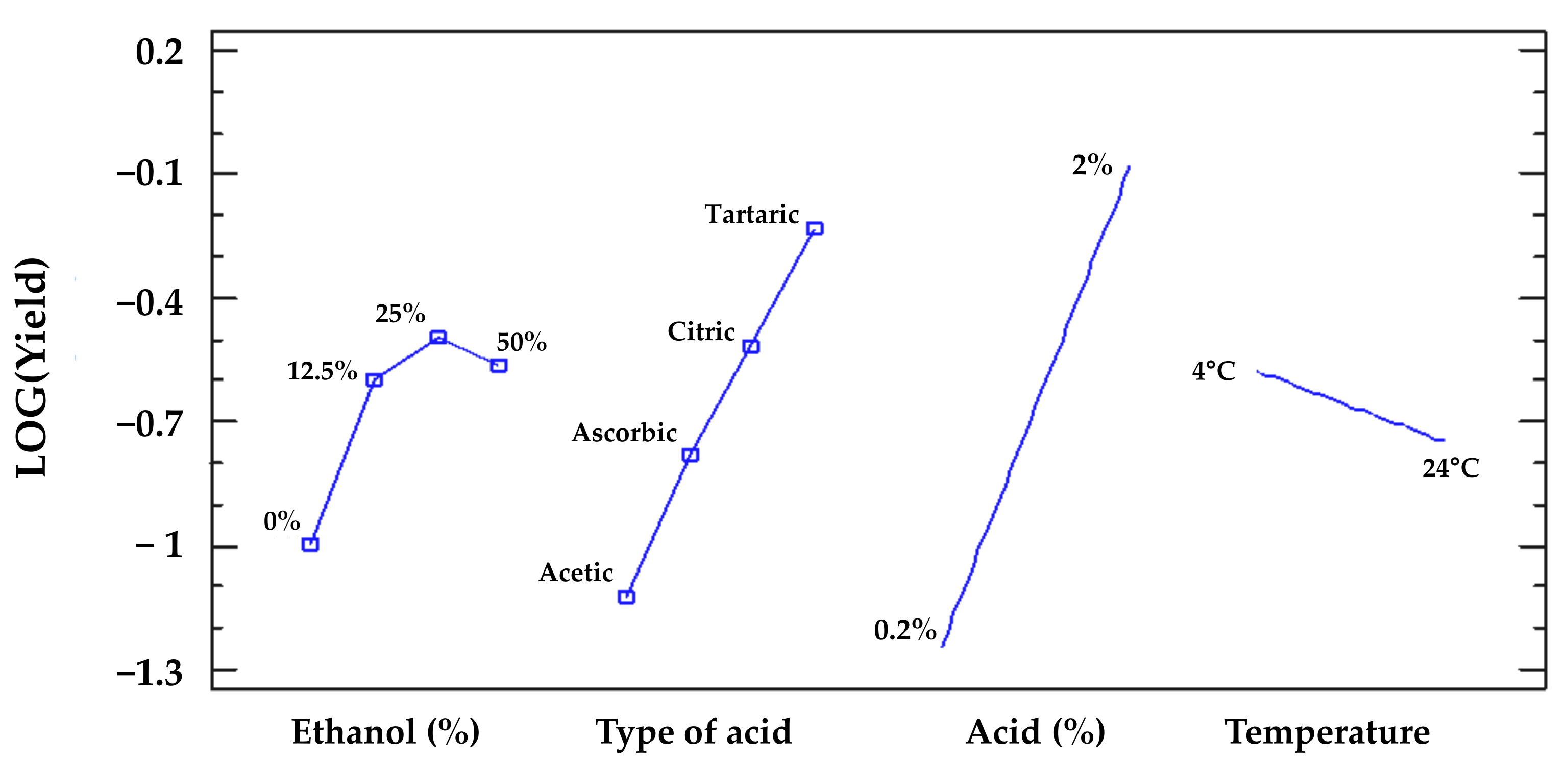
2.1.2. Establishment of the Sustainable Extraction Protocol
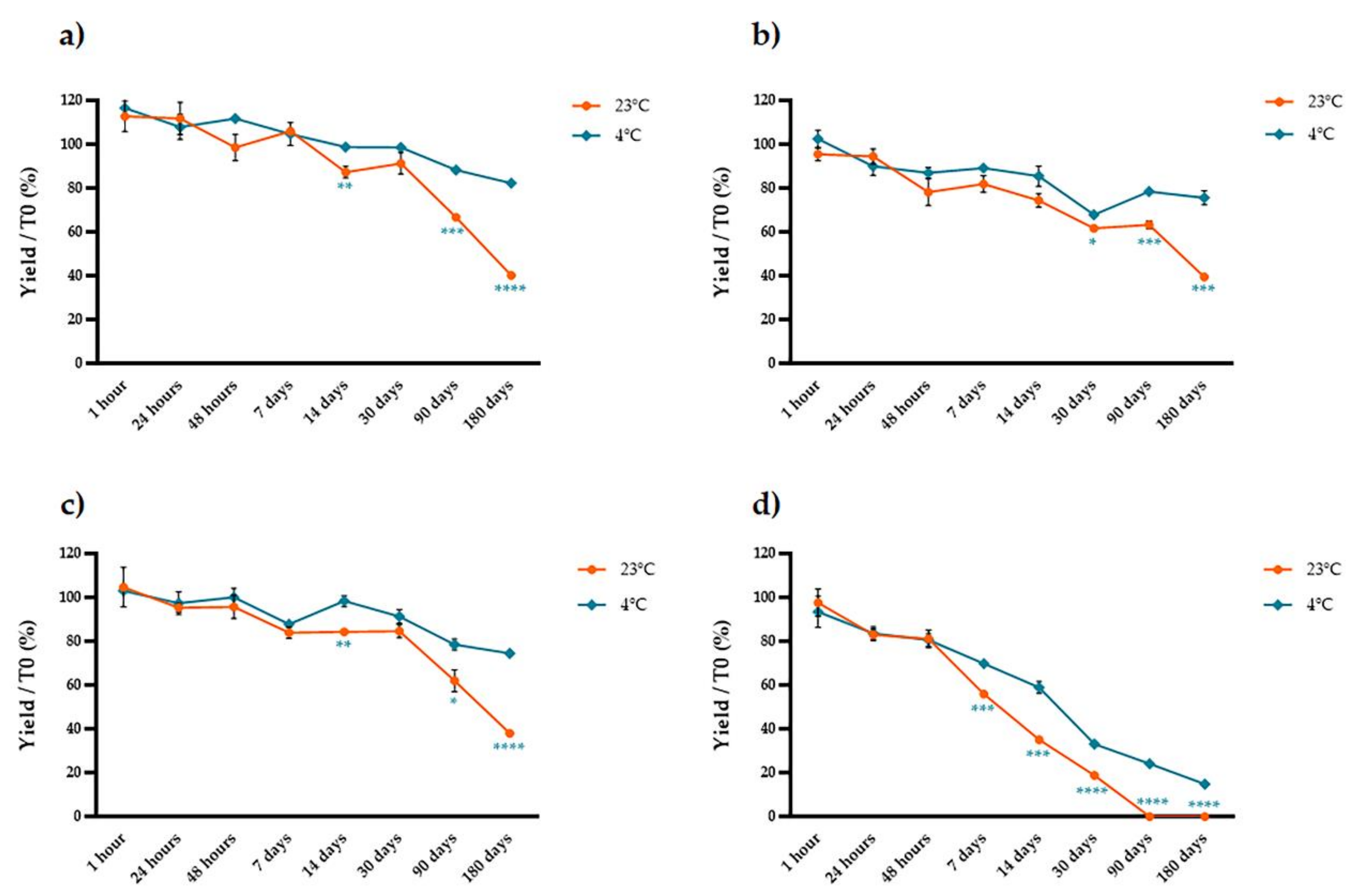
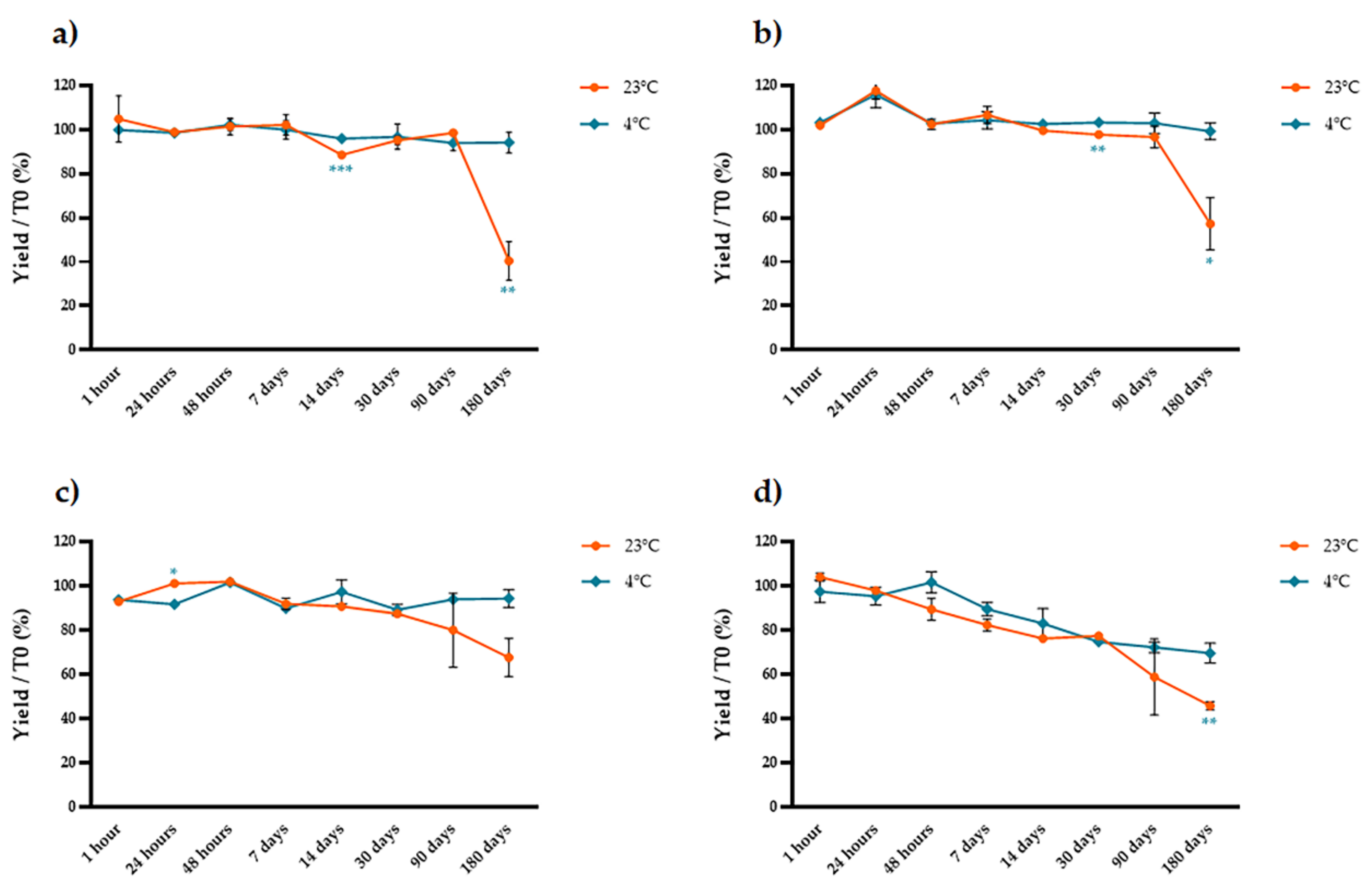
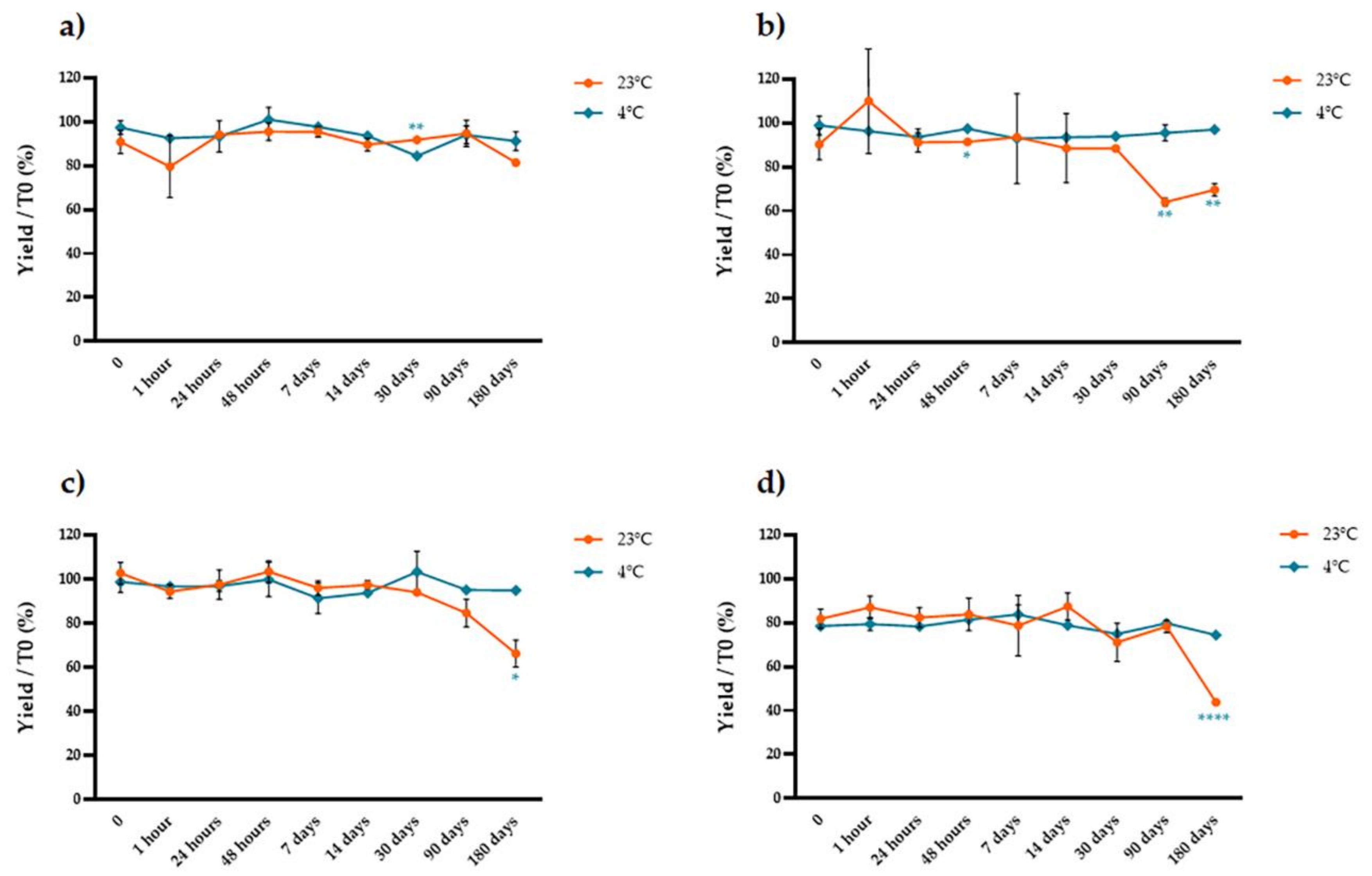
2.2. Stability of Extracted Anthocyanins over Time
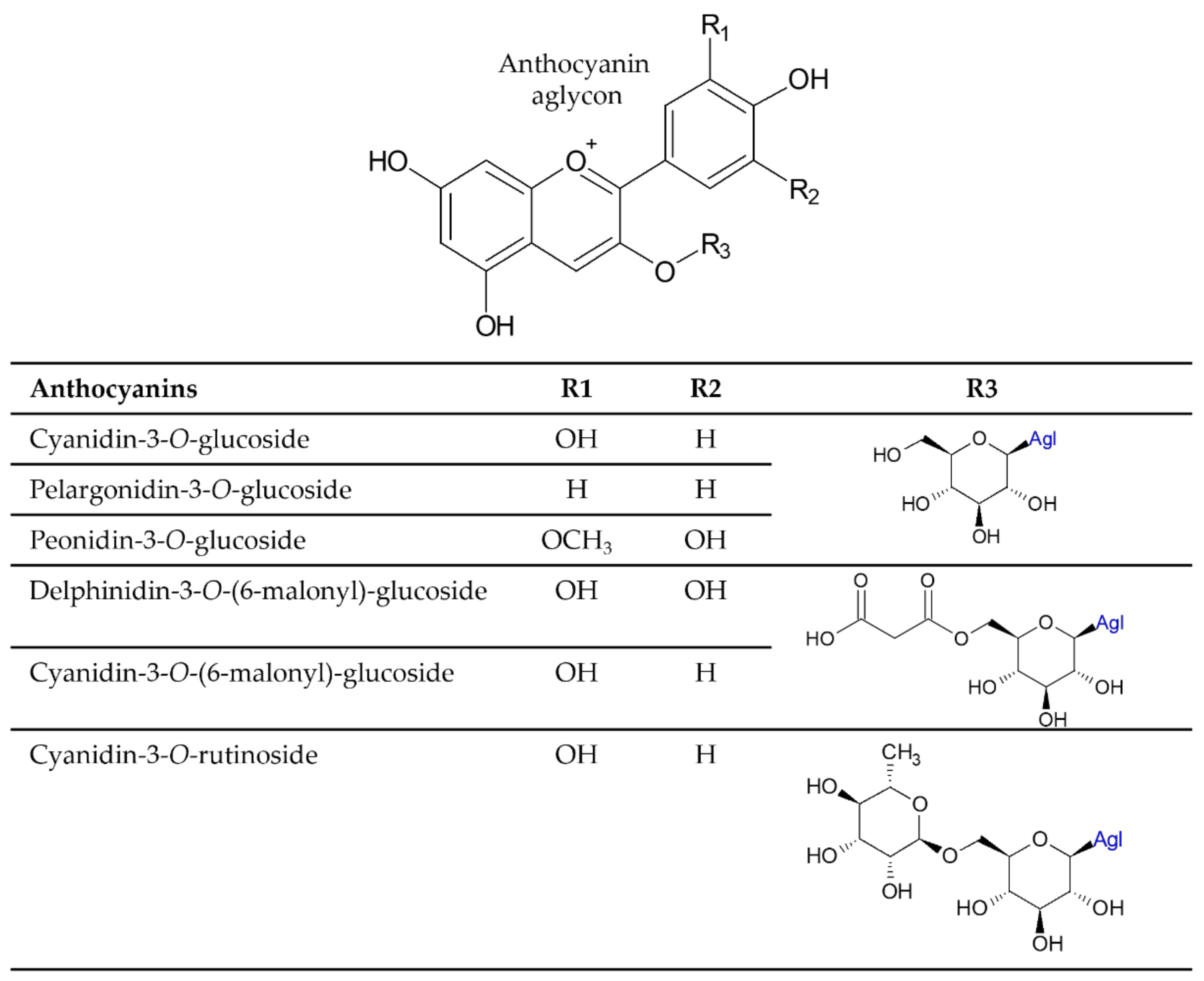
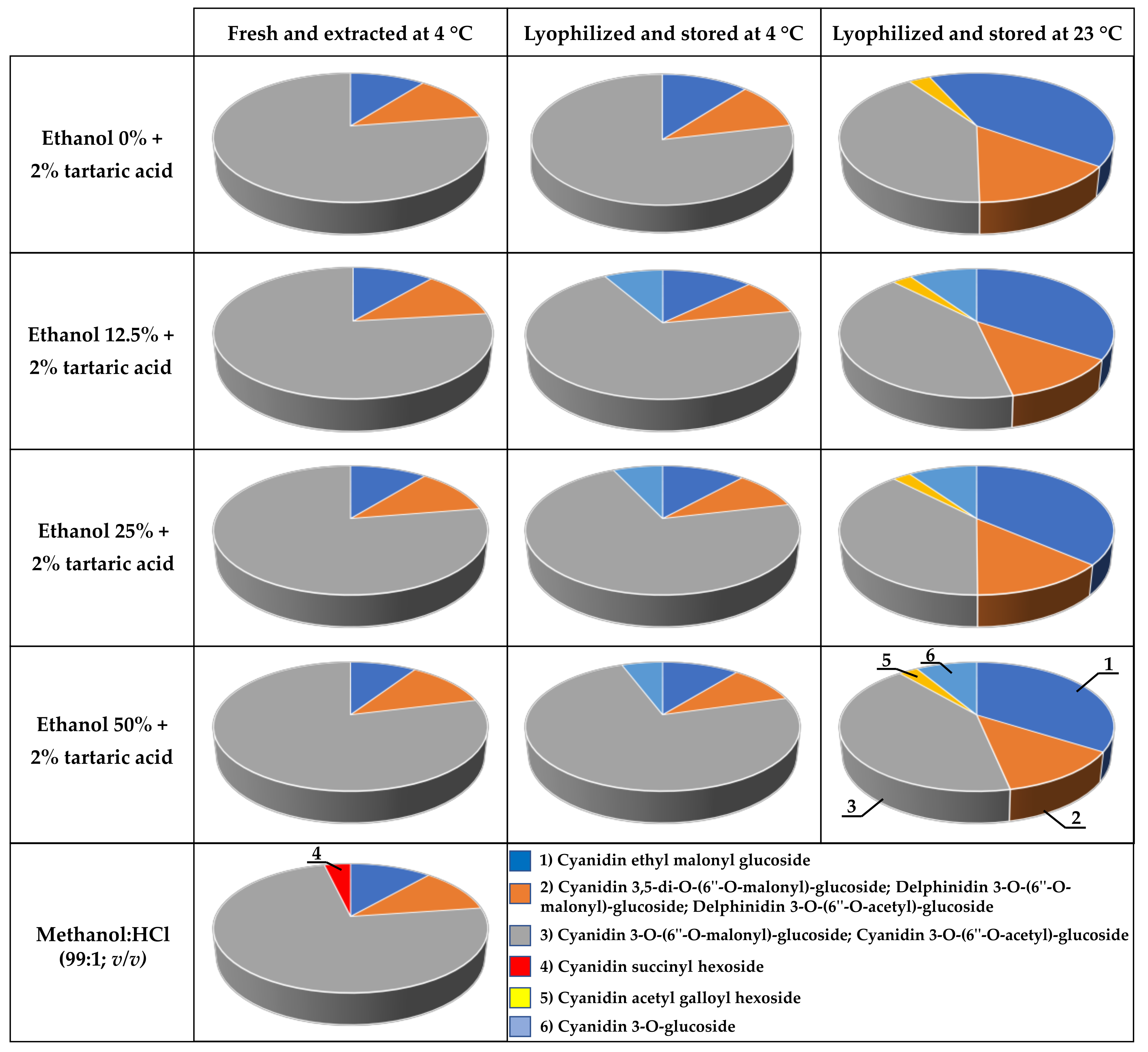
2.3. Molecular Characterization of the Extracted Dyes
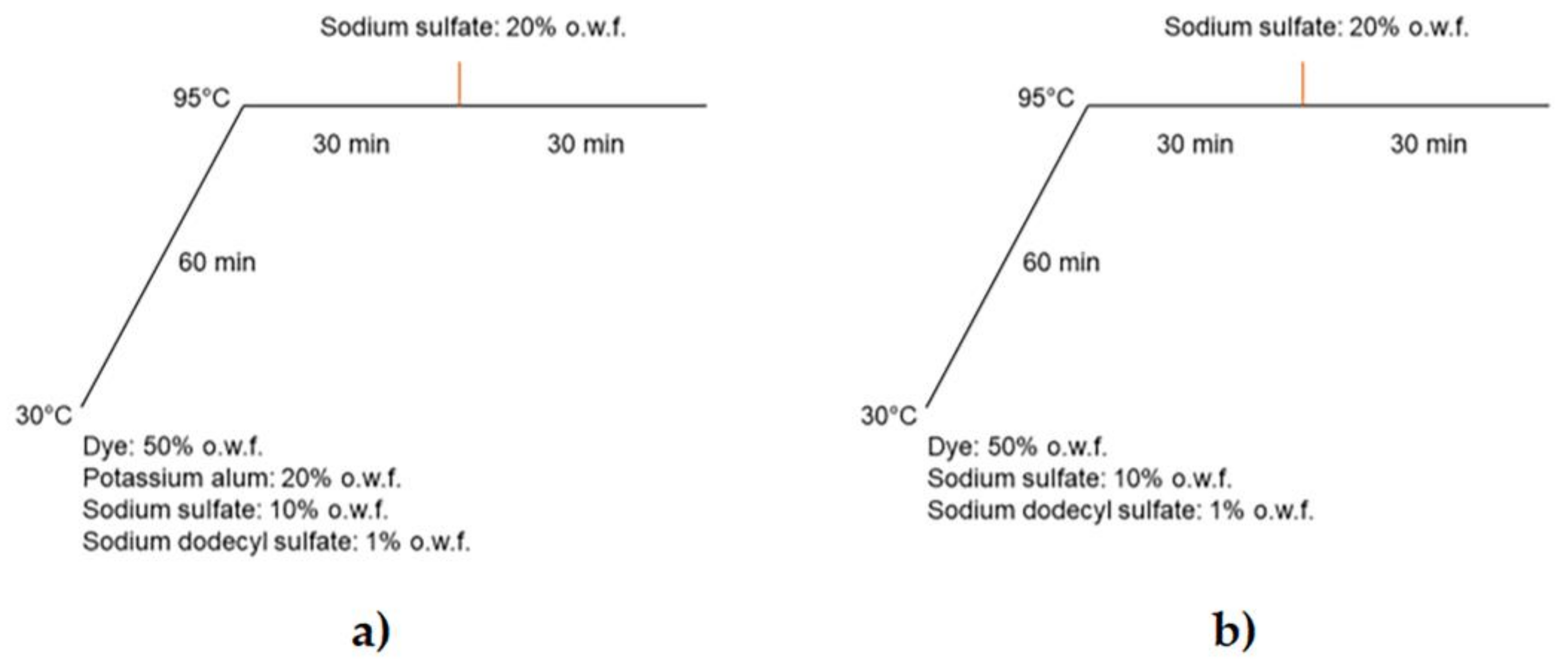
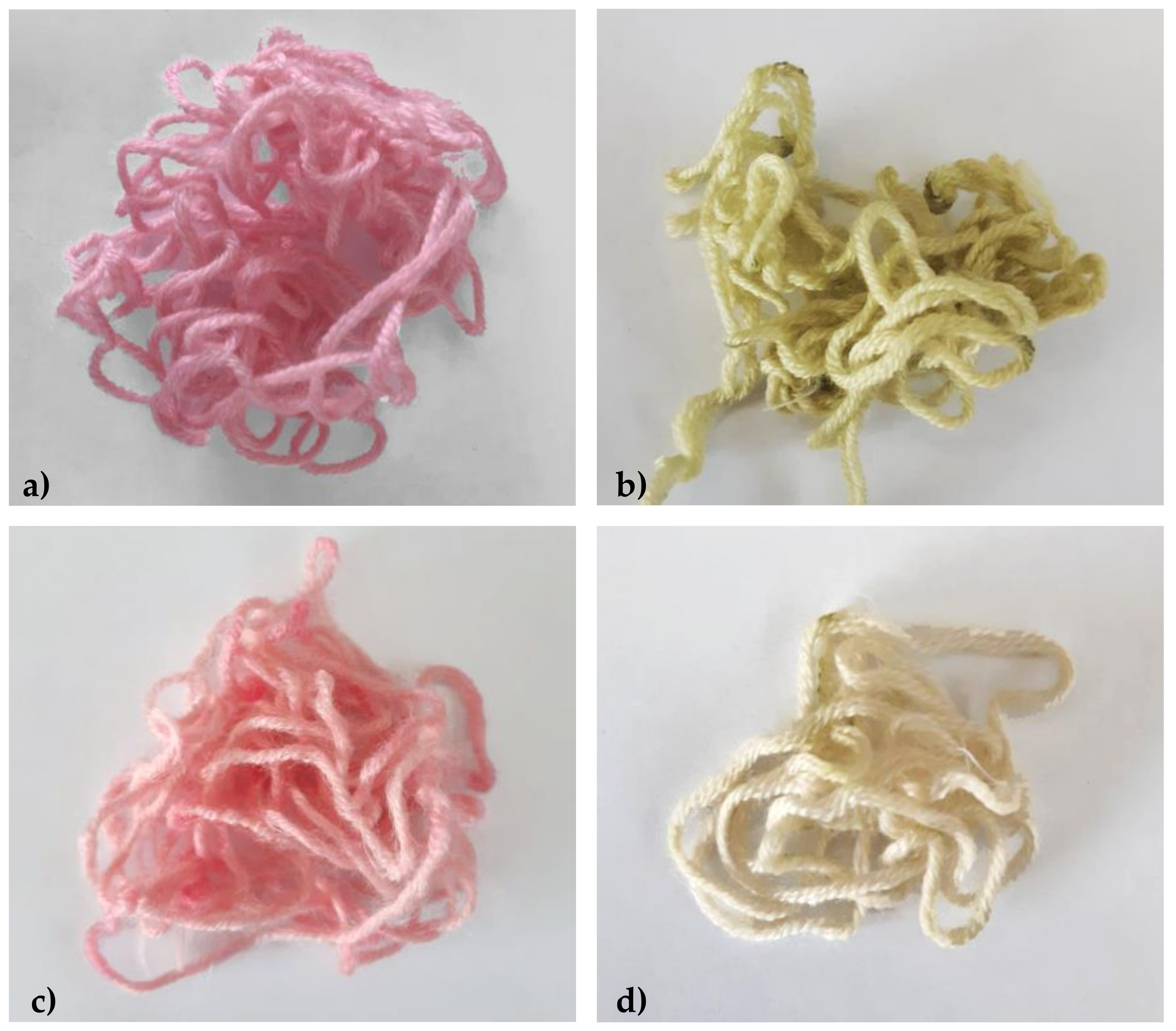
2.4. Dyeing Properties of Extracted Anthocyanins
2.5. Colour Fastness
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Gold-Standard Extraction of Polyphenols from Plant Material
4.3. Quantification of Anthocyanins
4.4. Design-of-Experiments
4.5. Stability of Extracted Anthocyanins
4.6. LC-MS Analysis and Data Processing
4.7. Wool Dyeing with Anthocyanins
4.8. Evaluation of Dyeing Process Kinetics and Textile Fastness
4.8.1. Dyeing Kinetic Parameters
4.8.2. Washing Fastness
4.8.3. Acid and Alkaline Perspiration Fastness
4.8.4. Light Fastness
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Leme, D.M.; de Oliveira, G.A.R.; Meireles, G.; dos Santos, T.C.; Zanoni, M.V.B.; de Oliveira, D.P. Genotoxicological assessment of two reactive dyes extracted from cotton fibres using artificial sweat. Toxicol. Vitr. 2014, 28, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Sanjeeda, I.; Taiyaba, A.N. Natural dyes: Their sources and ecofriendly use as textile materials. J. Environ. Res. Dev. 2014, 8, 683–688. [Google Scholar]
- Lee, Y.-H.; Hwang, E.-K.; Baek, Y.-M.; Kim, H.-D. Deodorizing function and antibacterial activity of fabrics dyed with gallnut (Galla chinensis) extract. Text. Res. J. 2015, 85, 1045–1054. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. J. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionisation tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, A.A.; Piroski, C.S.; Daniel, T.G.; Cruz, T.M.; Escher, G.B.; Vieira do Carmo, M.A.; Azevedo, L.; Marques, M.B.; Granato, D.; Rosso, N.D. Red chicory (Cichorium intybus) extract rich in anthocyanins: Chemical stability, antioxidant activity, and antiproliferative activity in vitro. J. Food Sci. 2019, 84, 990–1001. [Google Scholar] [CrossRef]
- Innocenti, M.; Gallori, S.; Giaccherini, C.; Ieri, F.; Vincieri, F.F.; Mulinacci, N. Evaluation of the phenolic content in the aerial parts of different varieties of Cichorium intybus L. J. Agric. Food Chem. 2005, 53, 6497–6502. [Google Scholar] [CrossRef] [PubMed]
- Lavelli, V. Antioxidant activity of minimally processed red chicory (Cichorium intybus L.) evaluated in xanthine oxidase-, myeloperoxidase-, and diaphorase-catalyzed reactions. J. Agric. Food Chem. 2008, 56, 7194–7200. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, N.M.; Stanković, M.S. Variability of secondary metabolites of the species Cichorium intybus L. from different habitats. Plants 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayodé, A.P.; Nout, M.R.; Linnemann, A.R.; Hounhouigan, J.D.; Berghofer, E.; Siebenhandl-Ehn, S. Uncommonly high levels of 3-deoxyanthocyanidins and antioxidant capacity in the leaf sheaths of dye sorghum. J. Agric. Food Chem. 2011, 59, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Laleh, G.; Frydoonfar, H.; Heidary, R.; Jameei, R.; Zare, S. The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pak. J. Nutr. 2006, 5, 90–92. [Google Scholar]
- Osorio, L.L.D.R.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.-A. The chemical reactivity of anthocyanins and its consequences in food science and nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [Green Version]
- Grigoras, C.G.; Destandau, E.; Zubrzycki, S.; Elfakir, C. Sweet cherries anthocyanins: An environmental friendly extraction and purification method. Sep. Purif. Technol. 2012, 100, 51–58. [Google Scholar] [CrossRef]
- Salamon, I.; Grulova, D.; Hancianu, M.; Cioanca, O. Optimization of lyophilization technology for purification and stabilization of anthocyanins from elderberry fruits. In Proceedings of the I International Symposium on Elderberry 1061, Columbia, MO, USA, 9–14 June 2013; pp. 245–252. [Google Scholar]
- Tseng, K.-C.; Chang, H.-M.; Wu, J.S.-B. Degradation kinetics of anthocyanin in ethanolic solutions. J. Food Process. Preserv. 2006, 30, 503–514. [Google Scholar] [CrossRef]
- Papetti, A.; Maietta, M.; Corana, F.; Marrubini, G.; Gazzani, G. Polyphenolic profile of green/red spotted Italian Cichorium intybus salads by RP-HPLC-PDA-ESI-MSn. J. Food Compost. Anal. 2017, 63, 189–197. [Google Scholar] [CrossRef]
- Lante, A.; Nardi, T.; Zocca, F.; Giacomini, A.; Corich, V. Evaluation of red chicory extract as a natural antioxidant by pure lipid oxidation and yeast oxidative stress response as model systems. J. Agric. Food Chem. 2011, 59, 5318–5324. [Google Scholar] [CrossRef]
- Rommel, A.; Wrolstad, R.; Heatherbell, D. Blackberry juice and wine: Processing and storage effects on anthocyanin composition, color and appearance. J. Food Sci. 1992, 57, 385–391. [Google Scholar] [CrossRef]
- Rose, P.M.; Cantrill, V.; Benohoud, M.; Tidder, A.; Rayner, C.M.; Blackburn, R.S. Application of anthocyanins from blackcurrant (Ribes nigrum L.) fruit waste as renewable hair dyes. J. Agric. Food Chem. 2018, 66, 6790–6798. [Google Scholar] [CrossRef] [PubMed]
- Toffali, K.; Ceoldo, S.; Stocchero, M.; Levi, M.; Guzzo, F. Carrot-specific features of the phenylpropanoid pathway identified by feeding cultured cells with defined intermediates. Plant Sci. 2013, 209, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Commisso, M.; Bianconi, M.; Di Carlo, F.; Poletti, S.; Bulgarini, A.; Munari, F.; Negri, S.; Stocchero, M.; Ceoldo, S.; Avesani, L. Multi-approach metabolomics analysis and artificial simplified phytocomplexes reveal cultivar-dependent synergy between polyphenols and ascorbic acid in fruits of the sweet cherry (Prunus avium L.). PLoS ONE 2017, 12, e0180889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commisso, M.; Negri, S.; Bianconi, M.; Gambini, S.; Avesani, S.; Ceoldo, S.; Avesani, L.; Guzzo, F. Untargeted and targeted metabolomics and tryptophan decarboxylase in vivo characterization provide novel insight on the development of kiwifruits (Actinidia deliciosa). Int. J. Mol. Sci. 2019, 20, 897. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| % Ethanol | % Acid | pH | |||
|---|---|---|---|---|---|
| Acetic Acid | Ascorbic Acid | Citric Acid | Tartaric Acid | ||
| 0% | 0.20% | 2.85 | 2.67 | 2.27 | 2.12 |
| 2% | 2.27 | 2.1 | 1.68 | 1.6 | |
| 12.50% | 0.20% | 2.84 | 2.7 | 2.27 | 2.22 |
| 2% | 2.4 | 2.1 | 1.75 | 1.68 | |
| 25% | 0.20% | 2.96 | 2.82 | 2.45 | 2.25 |
| 2% | 2.38 | 2.3 | 1.93 | 1.77 | |
| 50% | 0.20% | 3.35 | 3.23 | 2.75 | 2.67 |
| 2% | 2.65 | 2.65 | 2.14 | 2.01 | |
| Peak No. 1 | RT (Min) * | Ion Mode * | Exp. Mass (m/z) * | Theor. Mass (m/z) * | Error (ppm) | Formula | MS/MS | Putative Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 15.68 | Pos | 449.1083 | 449.1084 | −0.2 | C21H21O11 | 287.0562 | Cyanidin 3-O-glucoside |
| 2 | 18.09 | Pos | 783.162 | 783.162 | 0 | C33H35O22 | 535.1083; 287.0560 | Cyanidin 3,5-di-O-(6″-O-malonyl)-glucoside |
| 2 | 18.09 | Pos | 551.103 | 551.1037 | −1.3 | C24H23O15 | 303.0509 | Delphinidin 3-O-(6″-O-malonyl)-glucoside |
| 2 | 18.09 | Neg | 505.0985 | 505.0982 | 0.6 | C23H23O13 | 300.0271 | Delphinidin 3-O-(6″-O-acetyl)-glucoside |
| 3 | 19.5 | Pos | 535.1091 | 535.1088 | 0.6 | C24H23O14 | 287.0561 | Cyanidin 3-O-(6″-O-malonyl)-glucoside |
| 3 | 19.5 | Neg | 489.1042 | 489.1033 | 1.8 | C23H23O12 | 284.033 | Cyanidin 3-O-(6″-O-acetyl)-glucoside |
| 4 | 21.1 | Pos | 549.1238 | 549.1244 | −1.1 | C25H25O14 | 287.0564 | Cyanidin succinyl hexoside |
| 5 | 20.1 | Pos | 643.1303 | 643.1299 | 0.6 | C30H27O16 | 287.0553 | Cyanidin derivative 1 |
| 6 | 22.44 | Pos | 563.1401 | 563.1401 | 0 | C26H27O14 | 287.0559 | Cyanidin derivative 2 |
| Samples | Pseudo-First-Order Model | Pseudo-Second-Order Model | |||
|---|---|---|---|---|---|
| R2 | k1 (/min) | R2 | qe (mg/g) | k2 (g/mg min) | |
| WoolP_1 | 0.934 | −0.0032 | 0.991 | 0.48 | 0.0019 |
| WoolP_2 | 0.959 | −0.0035 | 0.994 | 0.44 | 0.0022 |
| WOOL_P1 | Washing Fastness | Acid Perspiration Fastness | Alkaline Perspiration Fastness | Light Fastness |
|---|---|---|---|---|
| Sample fading | 1 | 2/3 | 1 | 1/2 |
| Staining on wool | 5 | 5 | 5 | |
| Staining on acrylic | 5 | 5 | 5 | |
| Staining on polyester | 5 | 5 | 5 | |
| Staining on polyamide | 5 | 4 | 4/5 | |
| Staining on cotton | 4/5 | 4 | 4 | |
| Staining on acetate | 5 | 5 | 5 | |
| WOOL_G1 | Washing fastness | Acid perspiration fastness | Alkaline perspiration fastness | Lightfastness |
| Sample fading | 4 | 1 | 3/4 | 2 |
| Staining on wool | 5 | 5 | 5 | |
| Staining on acrylic | 5 | 5 | 5 | |
| Staining on polyester | 5 | 5 | 5 | |
| Staining on polyamide | 5 | 5 | 5 | |
| Staining on cotton | 5 | 4 | 5 | |
| Staining on acetate | 5 | 5 | 5 | |
| WOOL_P2 | Washing fastness | Acid perspiration fastness | Alkaline perspiration fastness | Lightfastness |
| Sample fading | 1 | 1 | 1 | 2 |
| Staining on wool | 5 | 5 | 5 | |
| Staining on acrylic | 5 | 5 | 5 | |
| Staining on polyester | 5 | 5 | 5 | |
| Staining on polyamide | 5 | 5 | 4/5 | |
| Staining on cotton | 4/5 | 4/5 | 4/5 | |
| Staining on acetate | 5 | 5 | 5 | |
| WOOL_G2 | Washing fastness | Acid perspiration fastness | Alkaline perspiration fastness | Lightfastness |
| Sample fading | 4 | 2 | 4 | 2 |
| Staining on wool | 5 | 5 | 5 | |
| Staining on acrylic | 5 | 5 | 5 | |
| Staining on polyester | 5 | 5 | 5 | |
| Staining on polyamide | 5 | 5 | 5 | |
| Staining on cotton | 4/5 | 4/5 | 5 | |
| Staining on acetate | 5 | 5 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gecchele, E.; Negri, S.; Cauzzi, A.; Cuccurullo, A.; Commisso, M.; Patrucco, A.; Anceschi, A.; Zaffani, G.; Avesani, L. Optimization of a Sustainable Protocol for the Extraction of Anthocyanins as Textile Dyes from Plant Materials. Molecules 2021, 26, 6775. https://doi.org/10.3390/molecules26226775
Gecchele E, Negri S, Cauzzi A, Cuccurullo A, Commisso M, Patrucco A, Anceschi A, Zaffani G, Avesani L. Optimization of a Sustainable Protocol for the Extraction of Anthocyanins as Textile Dyes from Plant Materials. Molecules. 2021; 26(22):6775. https://doi.org/10.3390/molecules26226775
Chicago/Turabian StyleGecchele, Elisa, Stefano Negri, Anna Cauzzi, Anna Cuccurullo, Mauro Commisso, Alessia Patrucco, Anastasia Anceschi, Giorgio Zaffani, and Linda Avesani. 2021. "Optimization of a Sustainable Protocol for the Extraction of Anthocyanins as Textile Dyes from Plant Materials" Molecules 26, no. 22: 6775. https://doi.org/10.3390/molecules26226775






