Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption
Abstract
:1. Introduction
2. Results and Discussion
2.1. Process Parameters Optimization for SSA and Yield
Parameters Optimization for SSA and Yield of the BC
2.2. BCs Characterization
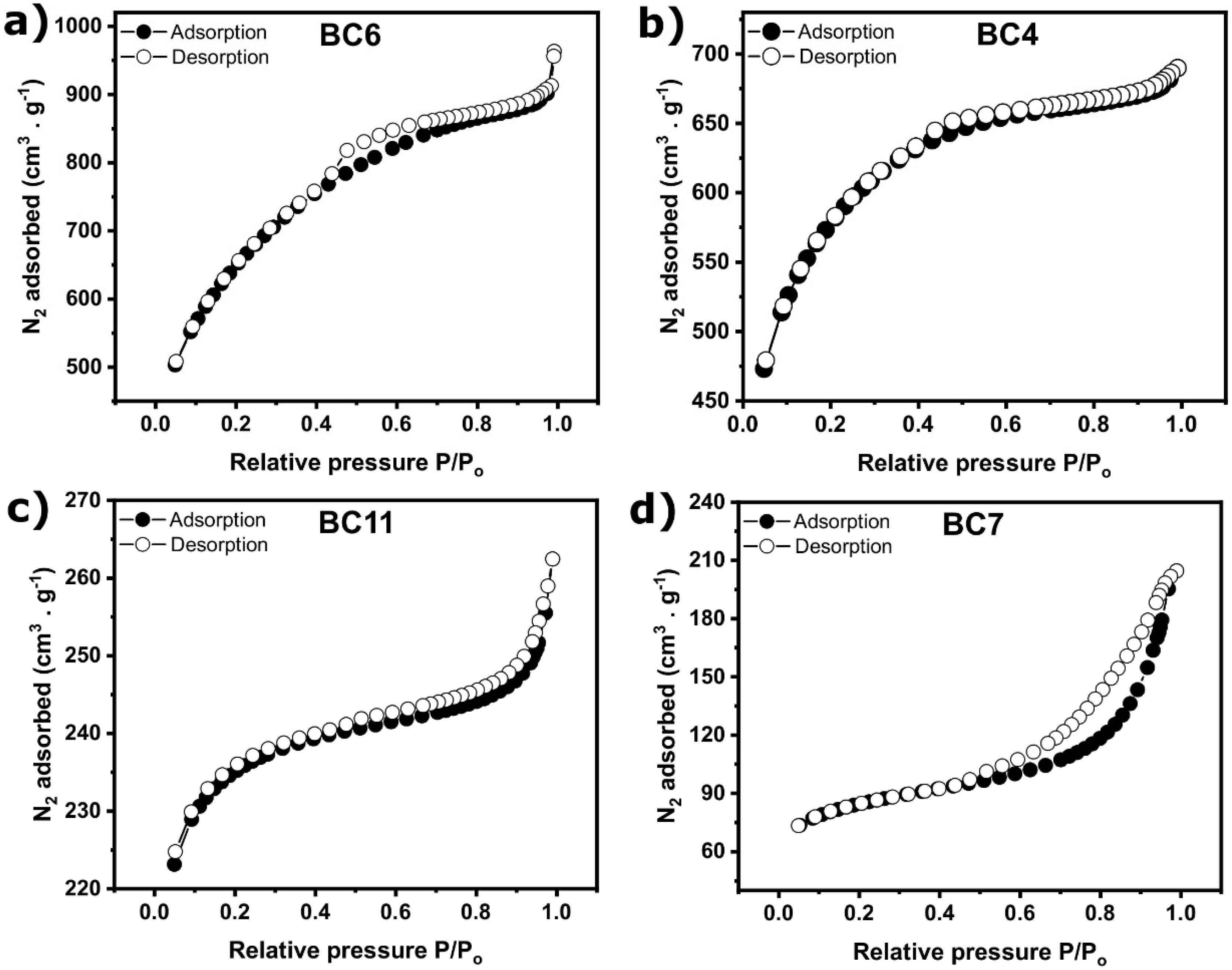
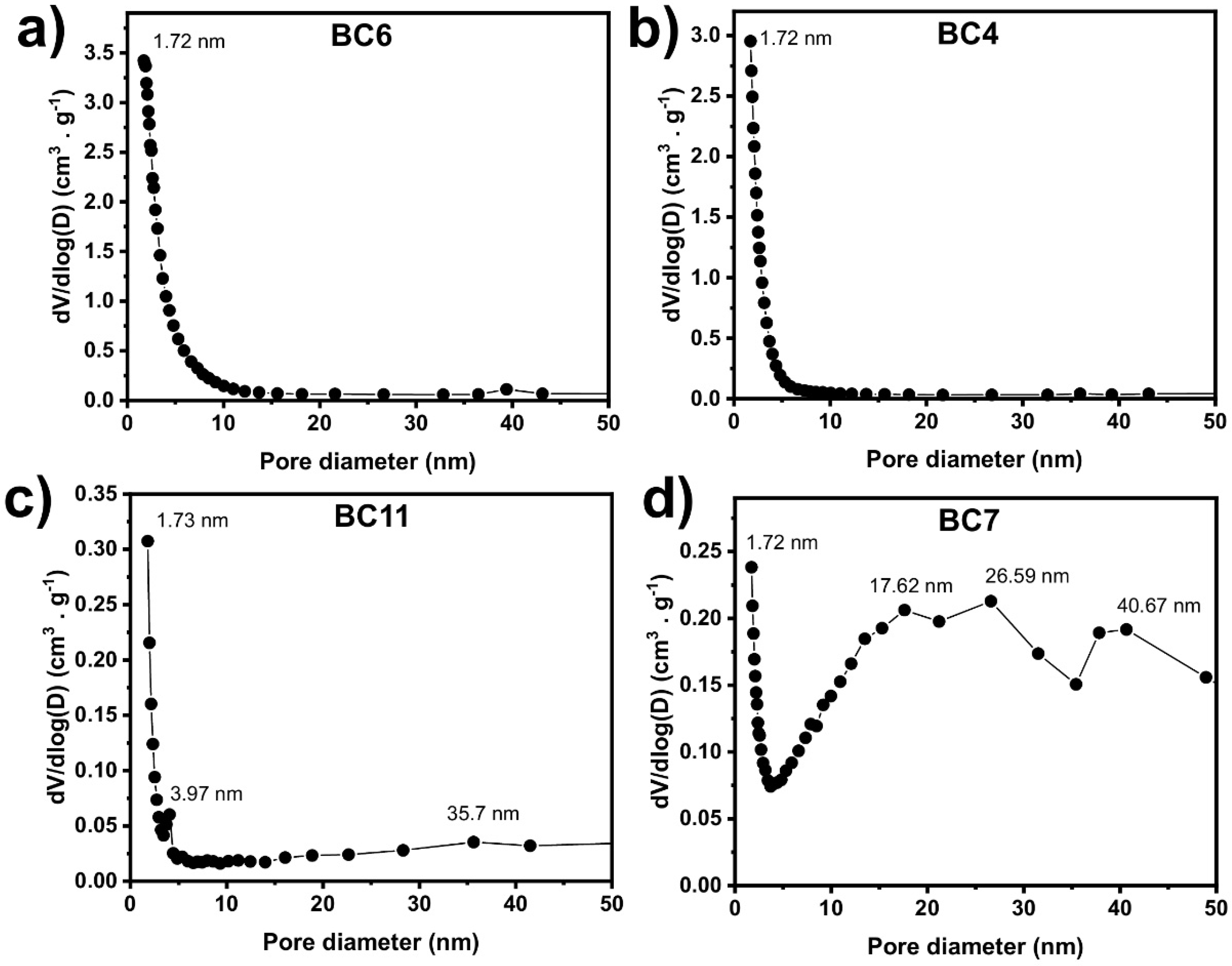
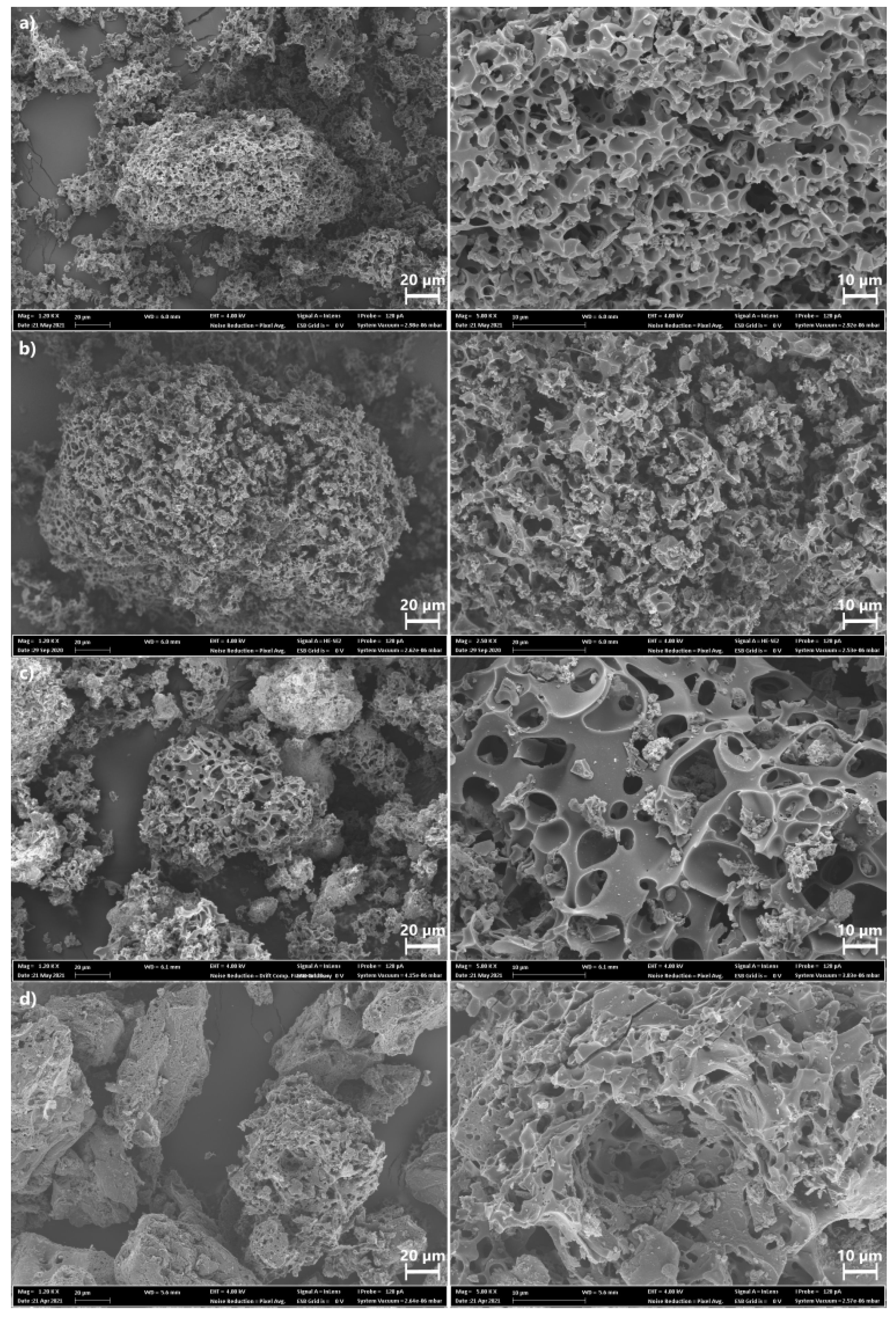
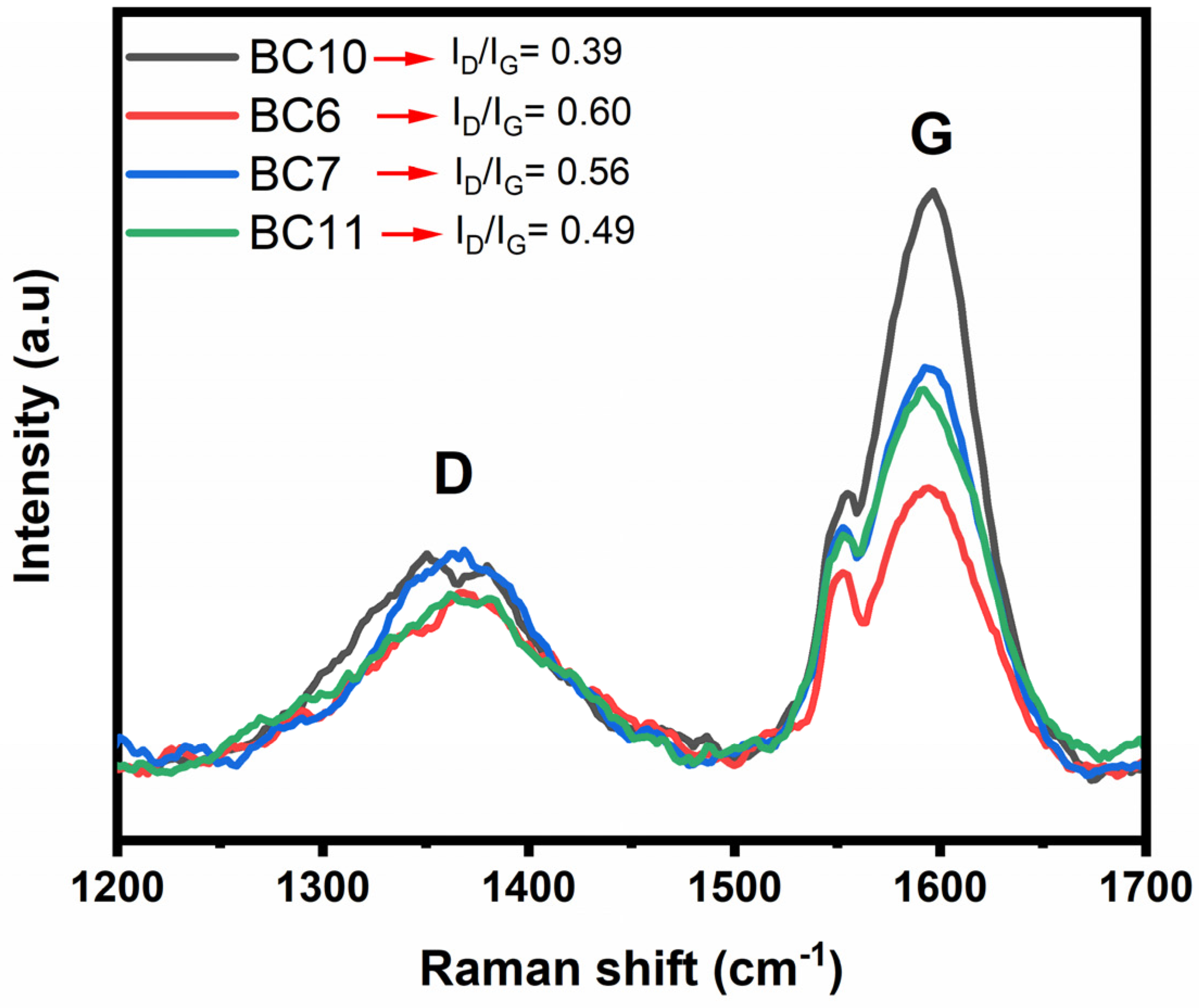
2.2.1. Textural Properties and Morphology of BCs
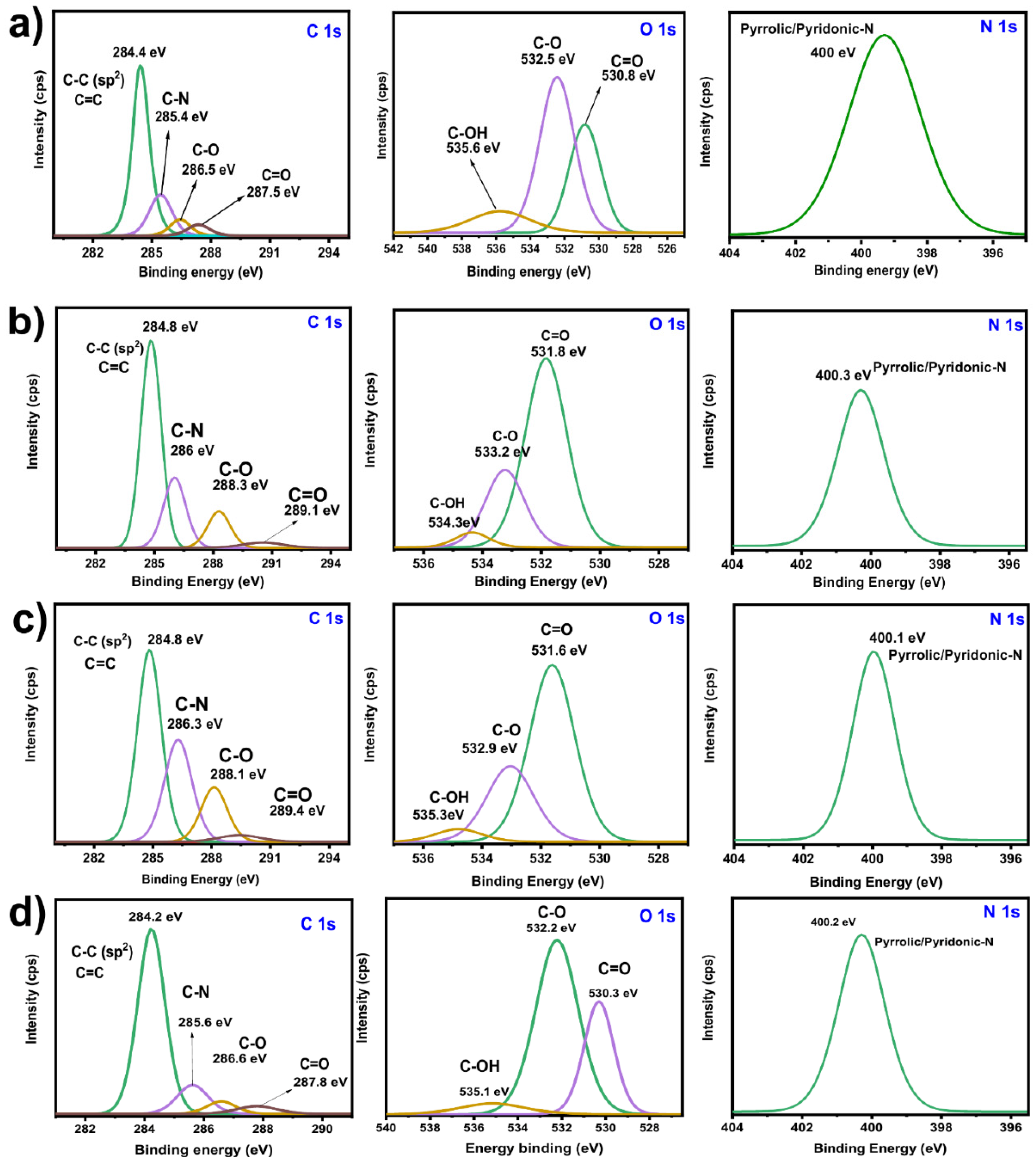
2.2.2. Chemical Characterization
2.3. Evans Blue Removal
2.3.1. Effect of pH and Point of Zero Charge
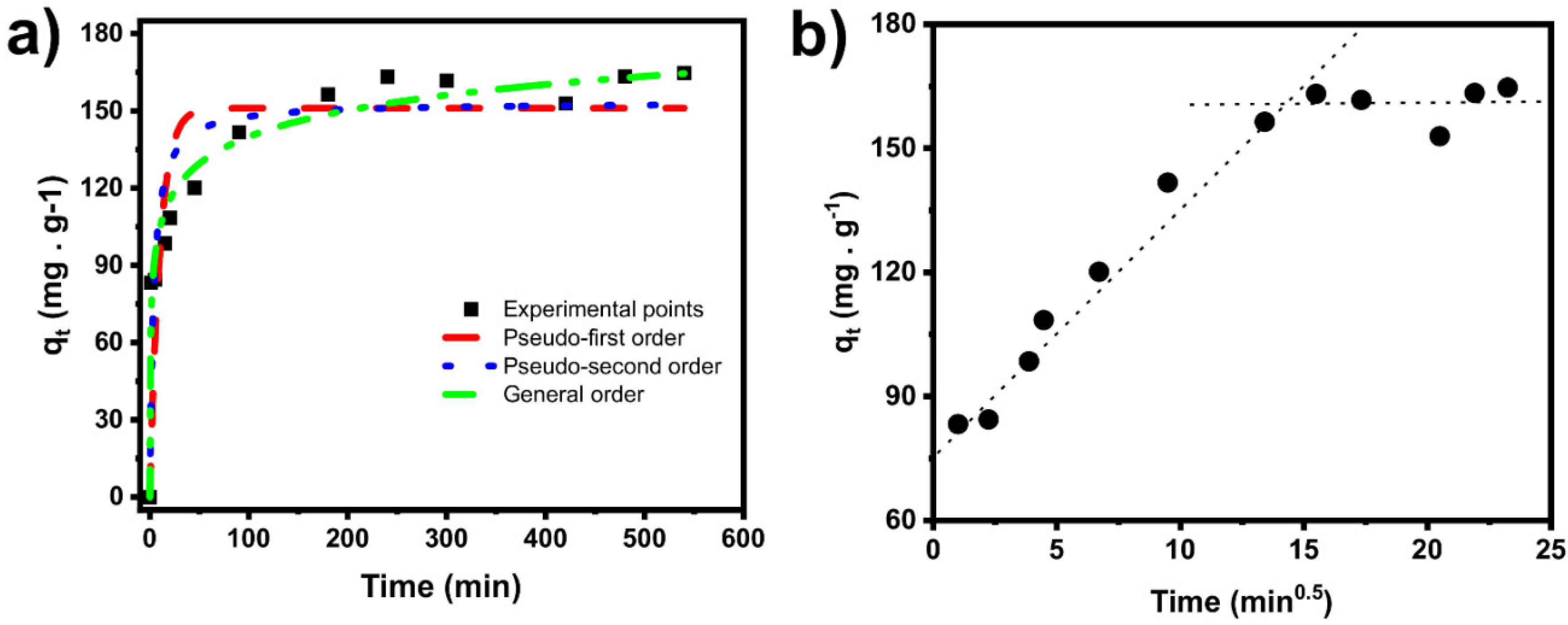
2.3.2. Kinetic Study
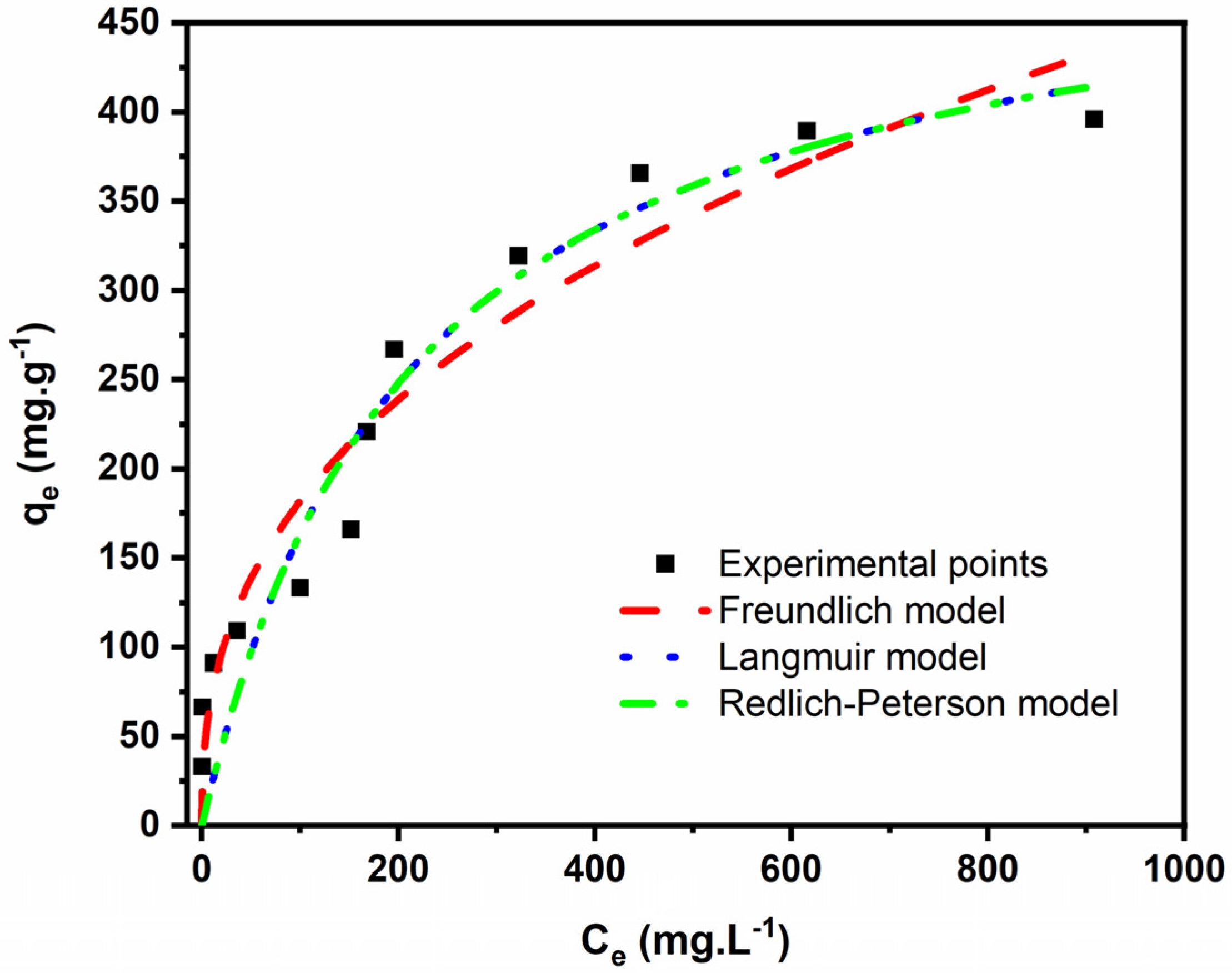
2.3.3. Isotherm Study
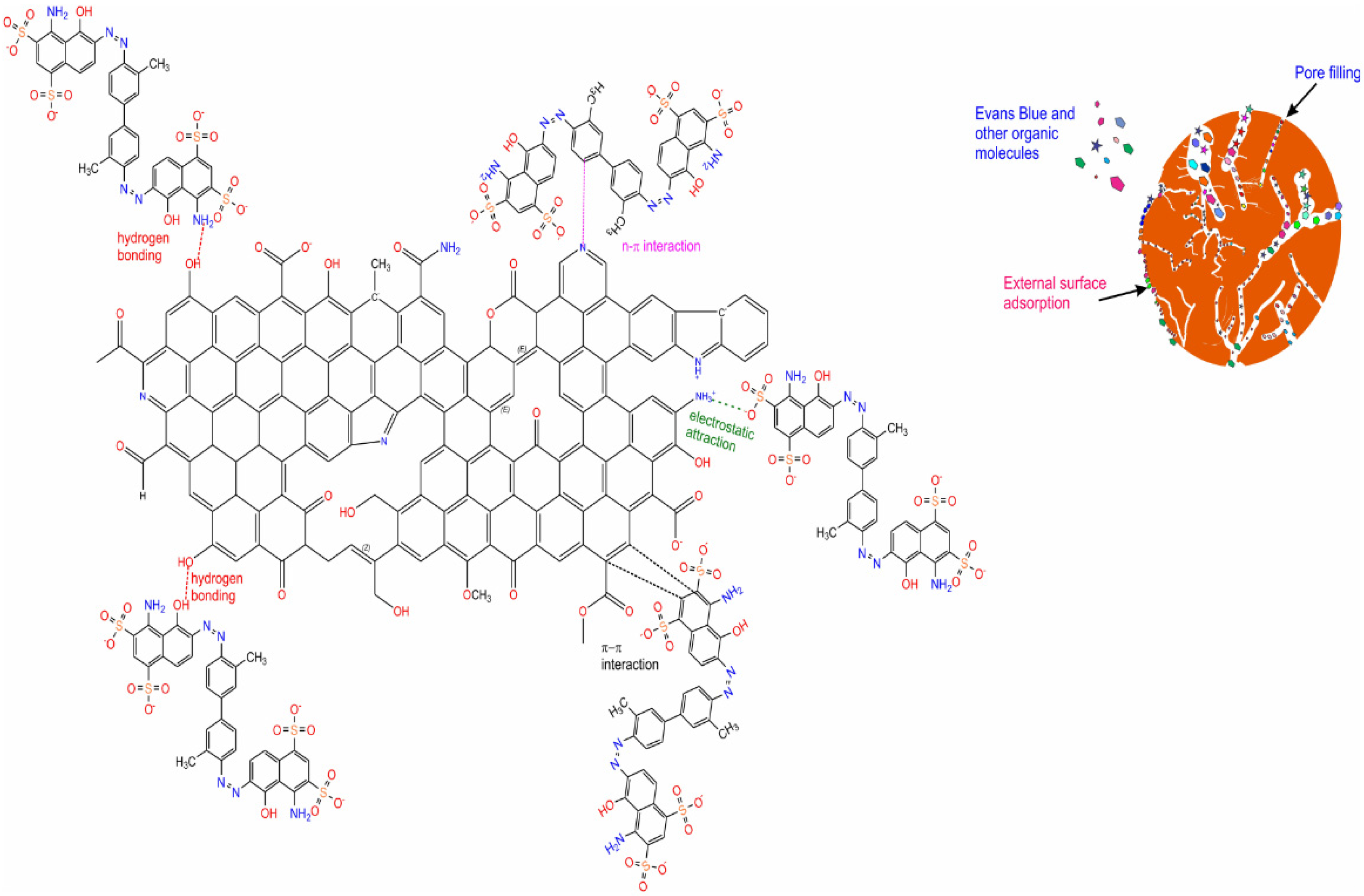
2.3.4. EB Dye Mechanism of Adsorption on Biochar
2.3.5. EB Adsorption Performance over Norway Spruce BC and Other Adsorbents: Comparison with the Literature
2.3.6. Synthetized Wastewater Treatment Tests
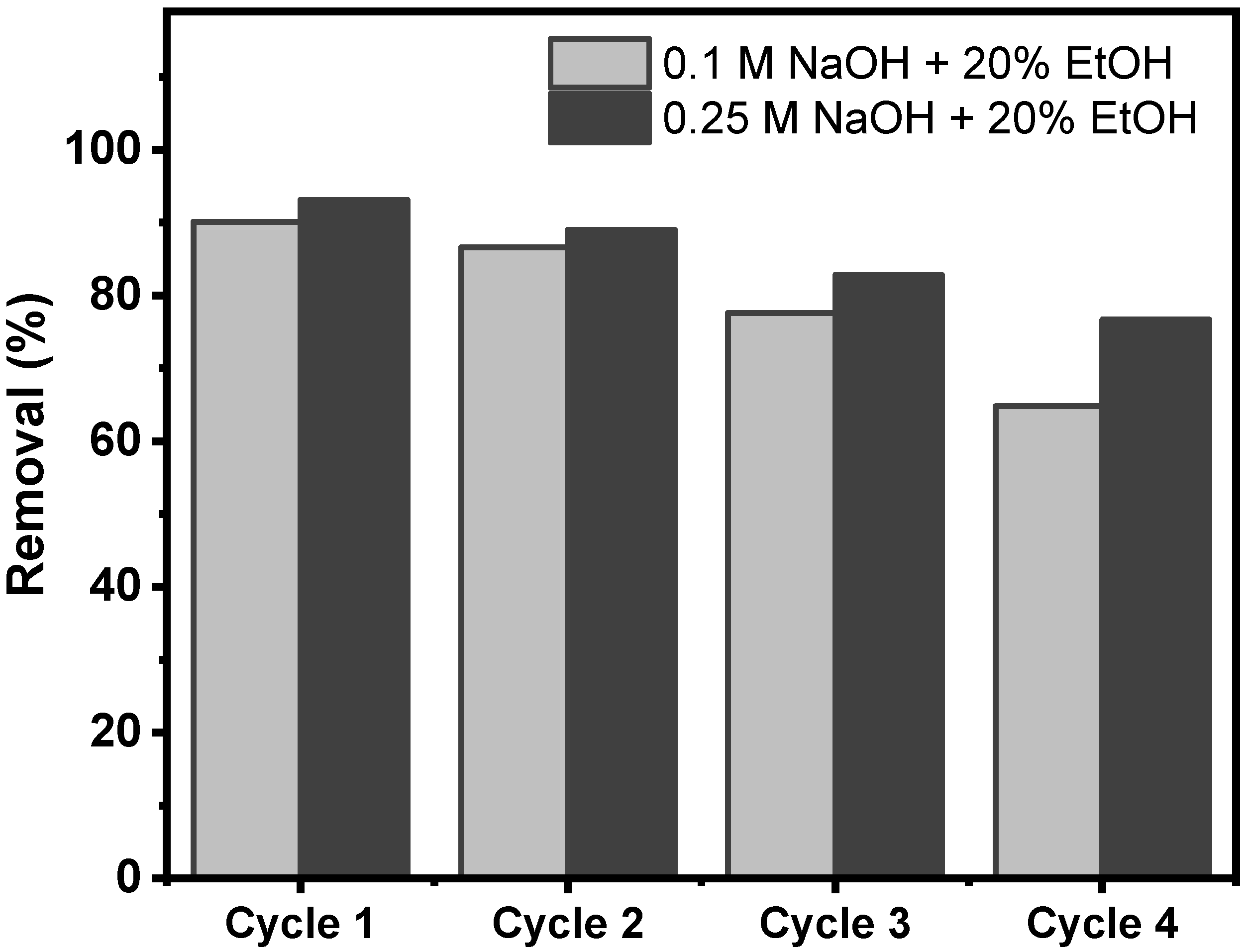
2.3.7. Regeneration Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation Process
3.3. Response Surface Methodology (RSM)
BC Characterization
3.4. Evans Blue (EB) Removal Process
3.4.1. Batch Adsorption Tests
3.4.2. Kinetic and Isotherm Models of Adsorption
3.4.3. Application to Synthetized Effluents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Enaime, G.; Baçaoui, A.; Yaacoubi, A.; Lübken, M. Biochar for Wastewater Treatment—Conversion Technologies and Appli-cations. Appl. Sci. 2020, 10, 3492. [Google Scholar] [CrossRef]
- Li, S.; Chan, C.Y.; Sharbatmaleki, M.; Trejo, H.; Delagah, S. Engineered Biochar Production and Its Potential Benefits in a Closed-Loop Water-Reuse Agriculture System. Water 2020, 12, 2847. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable Biomass Activated Carbons as Elec-trodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Larsson, S.H.; Mathieu, M.; Thyrel, M.; Pham, T.N. Application of design of experiments (DoE) for optimised production of micro- and mesoporous Norway spruce bark activated carbons. Biomass Convers. Biorefinery 2021, 1–19. [Google Scholar] [CrossRef]
- dos Reis, G.; de Oliveira, H.; Larsson, S.; Thyrel, M.; Lima, E.C. A Short Review on the Electrochemical Performance of Hierarchical and Nitrogen-Doped Activated Biocarbon-Based Electrodes for Supercapacitors. Nanomaterials 2021, 11, 424. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, Z.; Si, M.; Zhu, F.; Yang, W.; Zhao, F.; Shi, Y. Application of biochars in the remediation of chromium con-tamination: Fabrication, mechanisms, and interfering species. J. Hazard. Mater. 2021, 407, 124376. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review of biochar production techniques, characterization, stability, and applications for circular bio-economy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Molina-Sabio, M.; Rodríguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. Colloids Surfaces A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Leite, A.B.; Saucier, C.; Lima, E.C.; Dos Reis, G.S.; Umpierres, C.S.; Mello, B.L.; Shirmardi, M.; Dias, S.; Sampaio, C.H. Activated carbons from avocado seed: Optimisation and application for removal of several emerging organic compounds. Environ. Sci. Pollut. Res. 2017, 25, 7647–7661. [Google Scholar] [CrossRef] [Green Version]
- Dos Reis, G.S.; Wilhelm, M.; Silva, T.C.D.A.; Rezwan, K.; Sampaio, C.H.; Lima, E.C.; De Souza, S.M.G.U. The use of the design of experiments for the evaluation of the production of surface rich activated carbon from sewage sludge via micro-wave and conventional pyrolysis. Appl. Therm. Eng. 2016, 93, 590–597. [Google Scholar] [CrossRef]
- Duan, X.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Yang, K.; Zhang, Z. Process Optimization for the Preparation of Activated Carbon from Jatropha Hull Using Response Surface Methodology. Energy Sources Part A Recover. Util. Environ. Eff. 2011, 33, 2005–2017. [Google Scholar] [CrossRef]
- Moralı, U.; Demiral, H.; Şensöz, S. Optimization of activated carbon production from sunflower seed extracted meal: Taguchi design of experiment approach and analysis of variance. J. Clean. Prod. 2018, 189, 602–611. [Google Scholar] [CrossRef]
- Abioye, A.M.; Abdulkadir, L.N.; Sintali, I.S.; Bawa, M.A.; Ani, F.N. Temperature Controlled Microwave-Induced CO2 Ac-tivated Carbon: Optimization Using Box-Behnken Design. Adv. Eng. Res. 2020, 198, 129–135. [Google Scholar]
- Viotti, P.V.; Moreira, W.M.; dos Santos, O.A.A.; Bergamasco, R.; Vieira, A.M.S.; Vieira, M. Diclofenac removal from water by adsorption on Moringa oleifera pods and activated carbon: Mechanism, kinetic and equilibrium study. J. Clean. Prod. 2019, 219, 809–817. [Google Scholar] [CrossRef]
- Salari, M.; Rakhshandehroo, G.R.; Nikoo, M.R. Degradation of ciprofloxacin antibiotic by Homogeneous Fenton oxidation: Hybrid AHP-PROMETHEE method, optimization, biodegradability improvement and identification of oxidized by-products. Chemosphere 2018, 206, 157–167. [Google Scholar] [CrossRef]
- Banerjee, P.; Sau, S.; Das, P.; Mukhopadhayay, A. Optimization and modelling of synthetic azo dye wastewater treatment using Graphene oxide nanoplatelets: Characterization toxicity evaluation and optimization using Artificial Neural Network. Ecotoxicol. Environ. Saf. 2015, 119, 47–57. [Google Scholar] [CrossRef]
- Satuf, M.L.; Pierrestegui, M.J.; Rossini, L.; Brandi, R.J.; Alfano, O.M. Kinetic modeling of azo dyes photocatalytic degradation in aqueous TiO2 suspensions. Toxicity and biodegradability evaluation. Catal. Today 2011, 161, 121–126. [Google Scholar] [CrossRef]
- Yao, L.; Xue, X.; Yu, P.; Ni, Y.; Chen, F. Evans Blue Dye: A Revisit of Its Applications in Biomedicine. Contrast Media Mol. Imaging 2018, 2018, 7628037. [Google Scholar] [CrossRef] [Green Version]
- Vergis, B.R.; Kottam, N.; Krishna, R.H.; Nagabhushana, B. Removal of Evans Blue dye from aqueous solution using magnetic spinel ZnFe2O4 nanomaterial: Adsorption isotherms and kinetics. Nano-Struct. Nano-Objects 2019, 18, 100290. [Google Scholar] [CrossRef]
- Jinendra, U.; Bilehal, D.; Nagabhushana, B.M.; Jithendra Kumara, K.S.; Kollur, S.P. Nano-catalytic behavior of highly effi-cient and regenerable mussel-inspired Fe3O4@CFR@GO and Fe3O4@CFR@TiO2 magnetic nanospheres in the reduction of Evans blue dye. Heliyon 2021, 7, e06070. [Google Scholar] [CrossRef]
- Yagmur, E.; Gokce, Y.; Tekin, S.; Semerci, N.I.; Aktas, Z. Characteristics and comparison of activated carbons prepared from oleaster (Elaeagnus angustifolia L.) fruit using KOH and ZnCl2. Fuel 2020, 267, 117232. [Google Scholar] [CrossRef]
- Guclu, C.; Alper, K.; Erdem, M.; Tekin, K.; Karagoz, S. Activated carbons from co-carbonization of waste truck tires and spent tea leaves. Sustain. Chem. Pharm. 2021, 21, 100410. [Google Scholar] [CrossRef]
- Bag, O.; Tekin, K.; Karagoz, S. Microporous activated carbons from lignocellulosic biomass by KOH activation. Full. Nanotub. Carbon Nanostructures 2020, 28, 1030–1037. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Junior, O.F.C.; Sreńscek-Nazzal, J. Preparation of low-cost activated carbons from amazonian nutshells for CO2 storage. Biomass Bioenergy 2021, 144, 105925. [Google Scholar] [CrossRef]
- Efeovbokhan, V.E.; Alagbe, E.; Odika, B.; Babalola, R.; Oladimeji, T.E.; Abatan, O.G.; Yusuf, E.O. Preparation and character-ization of activated carbon from plantain peel and coconut shell using biological activators. J. Phys. Conf. Ser. 2019, 1378, 032035. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.-P.; Huang, L.; Gao, X.; Cheng, Y.; Yao, B.; Hu, Z.; Wan, J.; Xiao, X.; Zhou, J. Activated carbon derived from melaleuca barks for outstanding high-rate supercapacitors. Nanotechnology 2015, 26, 304004. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Mehmood, S.; Qaswar, M.; Ali, S.; Khan, Z.H.; Ying, H.; Chen, D.Y.; Núñez-Delgado, A. Oxidized biochar ob-tained from rice straw as adsorbent to remove uranium (VI) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 105104. [Google Scholar] [CrossRef]
- Chen, R.; Li, L.; Liu, Z.; Lu, M.; Wang, C.; Li, H.; Ma, W.; Wang, S. Preparation and characterization of activated carbons from tobacco stem by chemical activation. J. Air Waste Manag. Assoc. 2017, 67, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Tong, X.; Zhuo, H.; Zhong, L.; Peng, X.; Wang, S.; Sun, R. 3D hierarchical porous N-doped carbon aerogel from re-newable cellulose: An attractive carbon for high-performance supercapacitor electrodes and CO2 adsorption. RSC Adv. 2016, 6, 15788–15795. [Google Scholar] [CrossRef]
- Reyhani, A.; Golikand, A.N.; Mortazavi, S.Z.; Irannejad, L.; Moshfegh, A.Z. The effects of multi-walled carbon nanotubes graphitization treated with different atmospheres and electrolyte temperatures on electrochemical hydrogen storage. Electrochim. Acta 2010, 55, 4700–4705. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Smith, E.; Dent, G. Modern Raman Spectroscopy—A Practical Approach; Wiley: Chichester, UK, 2004. [Google Scholar]
- Ranguin, R.; Ncibi, M.C.; Cesaire, T.; Lavoie, S.; Jean-Marius, C.; Grutzmacher, H.; Gaspard, S. Development and character-isation of nanostructured hybrid material with vitamin B12 and bagasse-derived activated carbon for anaerobic chlordecone (Kepone) removal. Environ. Sci. Pollut. Res. Int. 2020, 27, 41122–41131. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, G.S.; Lima, E.C.; Sampaio, C.H.; Rodembusch, F.S.; Petter, C.O.; Cazacliu, B.G.; Dotto, G.L.; Hidalgo, G.E.N. Novel kaolin/polysiloxane based organic-inorganic hybrid materials: Sol−gel synthesis, characterization, and photocatalytic proper-ties. J. Solid State Chem. 2018, 260, 106–116. [Google Scholar] [CrossRef]
- Prola, L.; Machado, F.; Bergmann, C.P.; de Souza, F.E.; Gally, C.R.; Lima, E.C.; Adebayo, M.; Dias, S.; Calvete, T. Adsorption of Direct Blue 53 dye from aqueous solutions by multi-walled carbon nanotubes and activated carbon. J. Environ. Manag. 2013, 130, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Salomón, Y.L.D.O.; Georgin, J.; Reis, G.; Lima, E.C.; Oliveira, M.L.S.; Franco, D.S.P.; Netto, M.S.; Allasia, D.; Dotto, G.L. Utilization of Pacara Earpod tree (Enterolobium contortisilquum) and Ironwood (Caesalpinia leiostachya) seeds as low-cost biosorbents for removal of basic fuchsin. Environ. Sci. Pollut. Res. 2020, 27, 33307–33320. [Google Scholar] [CrossRef] [PubMed]
- Chandra, I.K.; Ju, Y.-H.; Ayucitra, A.; Ismadji, S. Evans blue removal from wastewater by rarasaponin–bentonite. Int. J. Environ. Sci. Technol. 2012, 10, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Kefif, F.; Ezziane, K.; Bahmani, A.; Bettahar, N.; Mayouf, S. Evans Blue dye removal from contaminated water on calcined and uncalcined Cu-Al-CO3Cu-Al-CO3 layered double hydroxide materials prepared by coprecipitation. Bull. Mater. Sci. 2019, 42, 14. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, C.; Nagabhushana, B.; Raghu, M.S.; Pratibha, S.; Dhananjaya, N.; Narayana, A. Perovskite lanthanum aluminate nanoparticles applications in antimicrobial activity, adsorptive removal of Direct Blue 53 dye and fluoride. Mater. Sci. Eng. C 2019, 101, 674–685. [Google Scholar] [CrossRef]
- Bouraada, M.; Bessaha, H.; De Ménorval, L.C. Removal of Evans Blue and Yellow thiazole dyes from aqueous solution by Mg-Al-CO3 Layered Double Hydroxides as anion-exchanger. Mediterr. J. Chem. 2014, 3, 894–906. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Bin Mahbub, M.K.; Wilhelm, M.; Lima, E.C.; Sampaio, C.H.; Saucier, C.; Dias, S. Activated carbon from sewage sludge for removal of sodium diclofenac and nimesulide from aqueous solutions. Korean J. Chem. Eng. 2016, 33, 3149–3161. [Google Scholar] [CrossRef]
- Kasperiski, F.M.; Lima, E.C.; dos Reis, G.S.; da Costa, J.B.; Dotto, G.L.; Dias, S.L.P.; Cunha, M.R.; Pavan, F.A.; Correa, C.S. Preparation of CTAB-functionalized aqai stalk and its efficient application as adsorbent for the removal of Direct Blue 15 and Direct Red 23 dyes from aqueous media. Chem. Eng. Commun. 2018, 205, 1520–1536. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and Equilibrium Models of Adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Springer: Cham, Switzerland, 2015; pp. 33–69. [Google Scholar]
- Thue, P.S.; Umpierres, C.S.; Lima, E.C.; Lima, D.R.; Machado, F.M.; dos Reis, G.S.; da Silva, R.S.; Pavan, F.A.; Tran, H.N. Single-step pyrolysis for producing magnetic activated carbon from tucumã (Astrocaryum aculeatum) seed and nickel(II) chloride and zinc(II) chloride Application for removal of nicotinamide and propranolol. J. Hazard. Mater. 2020, 398, 122903. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, D.F.; dos Reis, G.S.; Lima, E.C.; De Brum, I.A.; Thue, P.S.; Cazacliu, B.G.; Lima, D.; dos Santos, A.H.; Dotto, G.L. Efficient adsorbent based on construction and demolition wastes functionalized with 3-aminopropyltriethoxysilane (APTES) for the removal ciprofloxacin from hospital synthetic effluents. J. Environ. Chem. Eng. 2020, 8, 103875. [Google Scholar] [CrossRef]
- Lima, V.V.C.; Nora, F.B.D.; Peres, E.C.; Reis, G.S.; Lima Éder, C.; Oliveira, M.L.; Dotto, G.L. Synthesis and characterization of biopolymers functionalized with APTES (3-aminopropyltriethoxysilane) for the adsorption of sunset yellow dye. J. Environ. Chem. Eng. 2019, 7, 103410. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Larsson, S.H.; Thyrel, M.; Pham, T.N.; Claudio Lima, E.; de Oliveira, H.P.; Dotto, G.L. Preparation and Appli-cation of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents. Coatings 2021, 11, 772. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Lima, D.R.; da Silva, R.S.; Thue, P.S.; Seliem, M.K.; Sher, F.; dos Reis, G.S.; Larsson, S.H. Removal of captopril pharmaceutical from synthetic pharmaceutical-industry wastewaters: Use of activated carbon derived from Bu-tia catarinensis. J. Environ. Chem. Eng. 2020, 8, 104506. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Lima, E.C.; Benetti, A.D.; Thue, P.S.; Cunha, M.R.; Cimirro, N.F.G.M.; Sher, F.; Hadi Dehghani, M.; dos Reis, G.S.; Dotto, G.L. Preparation of hybrids of wood sawdust with 3-aminopropyltriethoxysilane. Application as an adsor-bent to remove Reactive Blue 4 dye from wastewater effluents. J. Taiwan Inst. Chem. Eng. 2021, 125, 141152. [Google Scholar] [CrossRef]

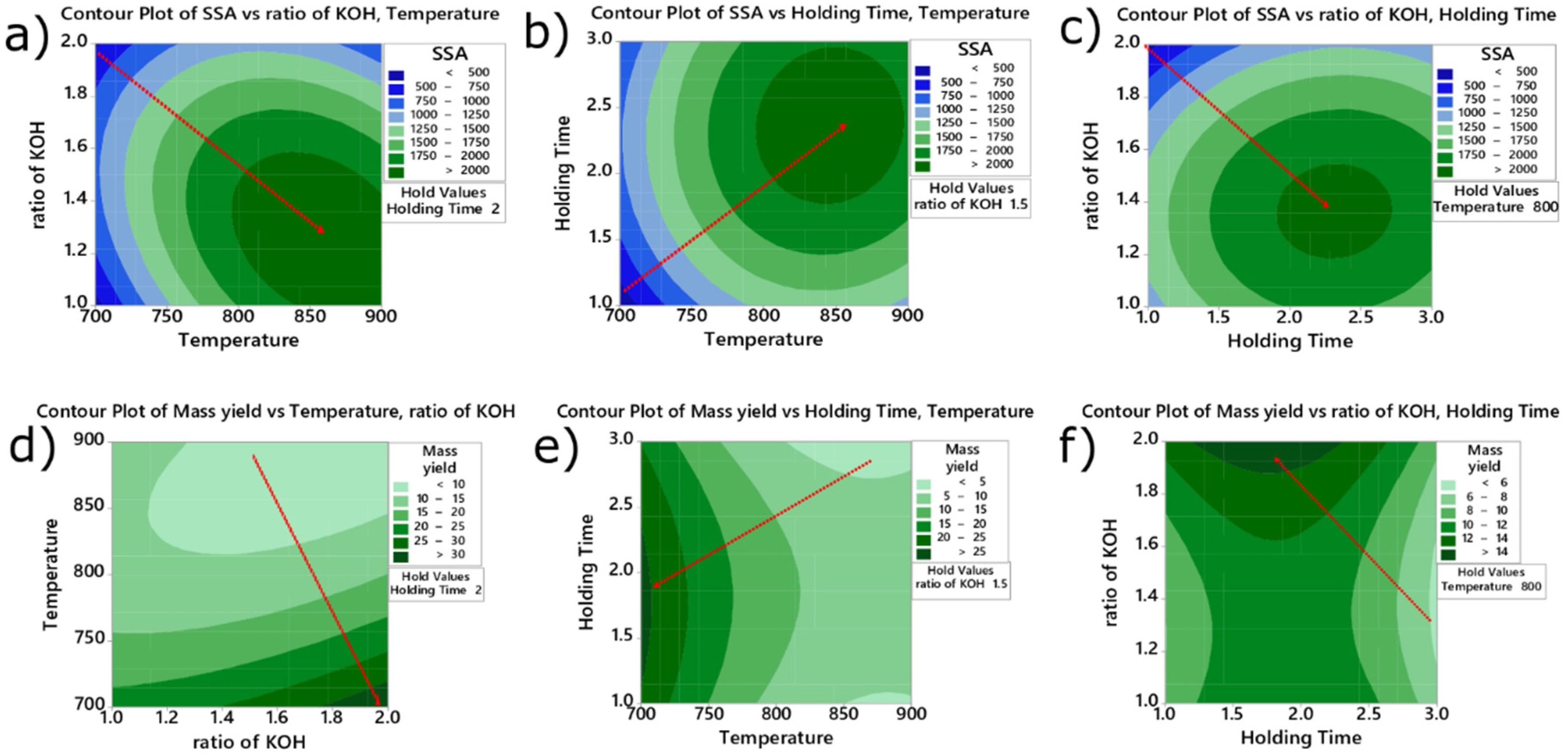

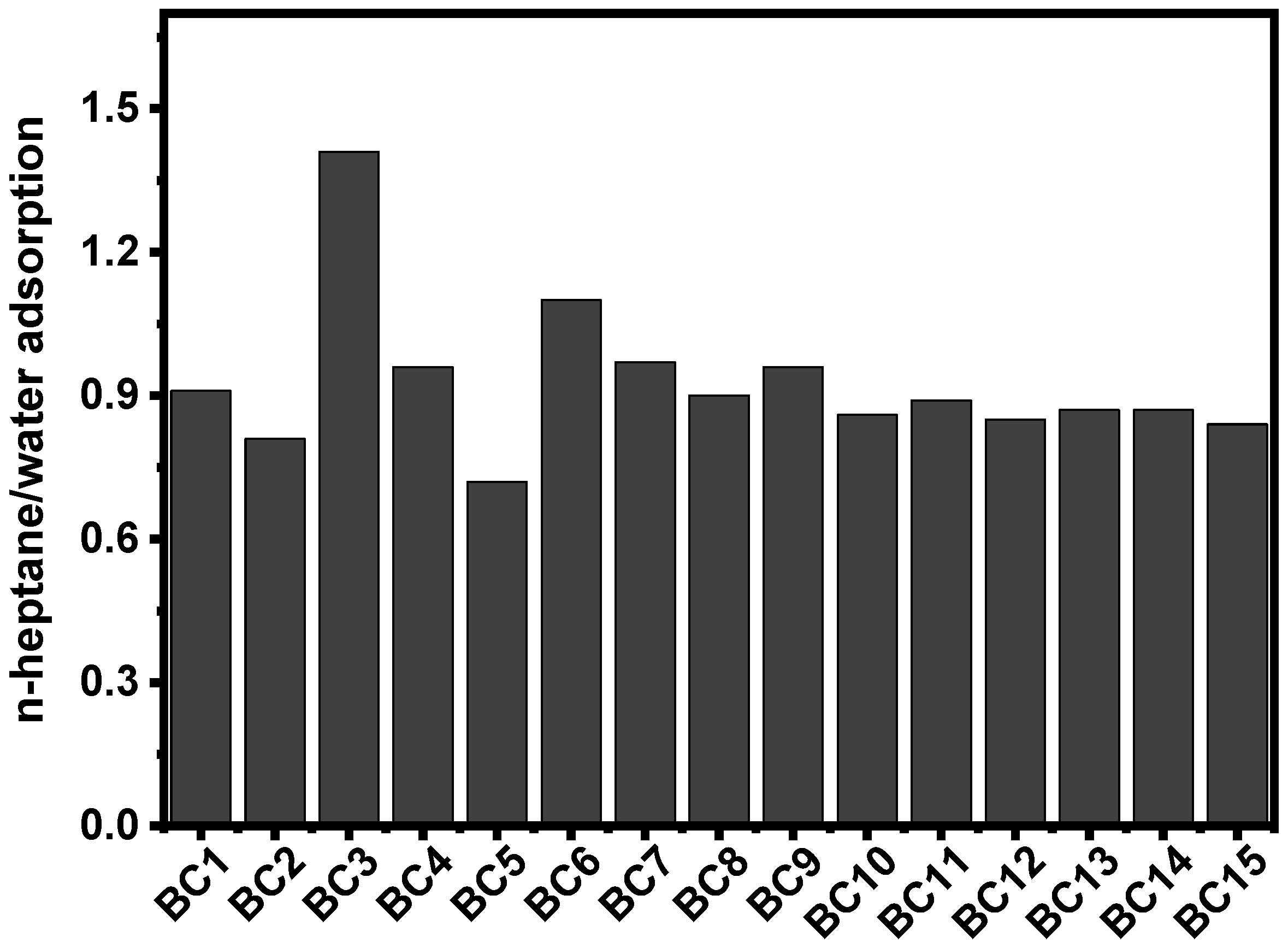


| Experiment Samples | Coded Samples | Temperature (°C) | Holding Time (h) | Ratio | SSA (m²·g−1) | Mass Yield (%) |
|---|---|---|---|---|---|---|
| 700:1:1.5 | BC1 | 700 | 1 | 1.5 | 346 | 23.6 |
| 900:1:1.5 | BC2 | 900 | 1 | 1.5 | 1355 | 3.0 |
| 700:3:1.5 | BC3 | 700 | 3 | 1.5 | 557 | 21.3 |
| 900:3:1.5 | BC4 | 900 | 3 | 1.5 | 1812 | 3.9 |
| 700:2:1 | BC5 | 700 | 2 | 1 | 726 | 19.3 |
| 900:2:1 | BC6 | 900 | 2 | 1 | 2209 | 11.3 |
| 700:2:2 | BC7 | 700 | 2 | 2 | 274 | 36.8 |
| 900:2:2 | BC8 | 900 | 2 | 2 | 418 | 10.2 |
| 800:1:1 | BC9 | 800 | 1 | 1 | 754 | 12.1 |
| 800:3:1 | BC10 | 800 | 3 | 1 | 1415 | 8.2 |
| 800:1:2 | BC11 | 800 | 1 | 2 | 572 | 11.5 |
| 800:3:2 | BC12 | 800 | 3 | 2 | 1434 | 5.0 |
| 800:2:1.5 | BC13 | 800 | 2 | 1.5 | 2011 | 10.6 |
| 800:2:1.5 | BC14 | 800 | 2 | 1.5 | 2002 | 12.7 |
| 800:2:1.5 | BC15 | 800 | 2 | 1.5 | 1943 | 10.7 |
| Biomass | Temperature (°C) | Holding Time (h) | Ratio | SSA (m²·g−1) | Ref. |
|---|---|---|---|---|---|
| Norway spruce bark | 900 | 2 | 1:1 | 2209 | This work |
| Oleaster fruits flesh | 800 | 1 | 1:3 | 1816 | [21] |
| Spent tea leaves | 800 | 1 | 1:1 | 820.7 | [22] |
| Oak wood sawdust | 800 | 1 | 1:0.5 | 1662 | [23] |
| Amazonian nut shells | 800 | 1 | 1:1 | 1624 | [24] |
| Sample’s Name | Smeso (m2·g−1) | Smicro (m².g−1) | Smeso% (%) | Smicro% (%) |
|---|---|---|---|---|
| BC6 | 499 | 1710 | 22.6 | 77.4 |
| BC4 | 825 | 1061 | 43.7 | 56.3 |
| BC11 | 99 | 627 | 13.6 | 86.4 |
| BC7 | 124 | 150 | 45.3 | 54.7 |
| Kinetic Models | |
|---|---|
| Pseudo first-order model | |
| q1 (mg g−1) | 148.7 |
| k1 (min−1) | 0.09438 |
| R2 | 0.5832 |
| R2adj | 0.5534 |
| SD (mg g−1) | 31.73 |
| Pseudo second-order model | |
| q2 (mg g−1) | 158.7 |
| k2 (g mg−1 min−1) | 9.602 × 10−4 |
| R2 | 0.7209 |
| R2adj | 0.7010 |
| SD (mg g−1) | 25.96 |
| General-order model | |
| qn (mg·g−1) | 630.0 |
| kn ((g·mg−1)n−1·min−1) | 4.420 × 10−28 |
| n (-) | 33.87 |
| R2 | 0.9681 |
| R2adj | 0.9617 |
| t0.5 | 1.5 |
| t0.95 | 139.4 |
| SD (mg g−1) | 432.0 |
| Isotherm Models | |
|---|---|
| Langmuir | |
| Qmax (mg g−1) | 511.5 |
| KL (L mg−1) | 0.004700 |
| R2 | 0.9198 |
| R2adj | 0.9117 |
| SD (mg g−1)2 | 39.08 |
| Freundlich | |
| KF ((mg g−1)(mg L−1)−1/nF) | 29.66 |
| nF | 2.540 |
| R2 | 0.9318 |
| R2adj | 0.9250 |
| SD (mg g−1)2 | 36.02 |
| Redlich–Peterson | |
| KRP((mg·g−1)·(mg·L−1)−1) | 2.403 |
| a ((mg·L−1)−b) | 0.004700 |
| B | 1.000 |
| R2 | 0.9198 |
| R2adj | 0.9019 |
| SD (mg g−1) | 41.19 |
| Biomass | Dosage (g·L−1) | pH | Isotherm Model | Kinetic Model | Qmax (mg·g−1) | Ref. |
|---|---|---|---|---|---|---|
| Magnetic spinel ZnFe2O4 nanomaterial | 0.2 | 7.0 | Freundlich | Pseudo Second Order | 45.45 | [19] |
| Commercial activated carbon | 1.5 | 2.0 | Liu | General-order | 135.2 | [36] |
| Multiwalled carbon nanotube | 1.5 | 2.0 | Liu | General-order | 409.4 | [36] |
| Rarasaponin–bentonite | 10.0 | - | Toth | Pseudo First Order | 495.8 | [38] |
| Calcined Cu-Al-CO3 layered double hydroxide materials | 4.0 | 6.0 | Langmuir | - | 333.3 | [39] |
| Perovskite lanthanum aluminate nanoparticles | 0.6 | 7.0 | Langmuir | pseudo-second-order | 40.82 | [40] |
| Mg-Al-CO3 | 0.5 | 6.0 | langmuir | 107.5 | [41] | |
| Aqai palm stalk (Euterpe oleracea) | 2.5 | 2.0 | Sips | Avrami fractional order | 45.1 | [43] |
| Norway spruce bark BC | 1.5 | 7.0 | Redlich–Peterson | General-order | 396.1 * | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guy, M.; Mathieu, M.; Anastopoulos, I.P.; Martínez, M.G.; Rousseau, F.; Dotto, G.L.; de Oliveira, H.P.; Lima, E.C.; Thyrel, M.; Larsson, S.H.; et al. Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption. Molecules 2022, 27, 456. https://doi.org/10.3390/molecules27020456
Guy M, Mathieu M, Anastopoulos IP, Martínez MG, Rousseau F, Dotto GL, de Oliveira HP, Lima EC, Thyrel M, Larsson SH, et al. Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption. Molecules. 2022; 27(2):456. https://doi.org/10.3390/molecules27020456
Chicago/Turabian StyleGuy, Marine, Manon Mathieu, Ioannis P. Anastopoulos, María G. Martínez, Frédéric Rousseau, Guilherme L. Dotto, Helinando P. de Oliveira, Eder C. Lima, Mikael Thyrel, Sylvia H. Larsson, and et al. 2022. "Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption" Molecules 27, no. 2: 456. https://doi.org/10.3390/molecules27020456
APA StyleGuy, M., Mathieu, M., Anastopoulos, I. P., Martínez, M. G., Rousseau, F., Dotto, G. L., de Oliveira, H. P., Lima, E. C., Thyrel, M., Larsson, S. H., & dos Reis, G. S. (2022). Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption. Molecules, 27(2), 456. https://doi.org/10.3390/molecules27020456










