The Development of a Green Innovative Bioactive Film for Industrial Application as a New Emerging Technology to Protect the Quality of Fruits
Abstract
:1. Introduction
2. Material and Methods
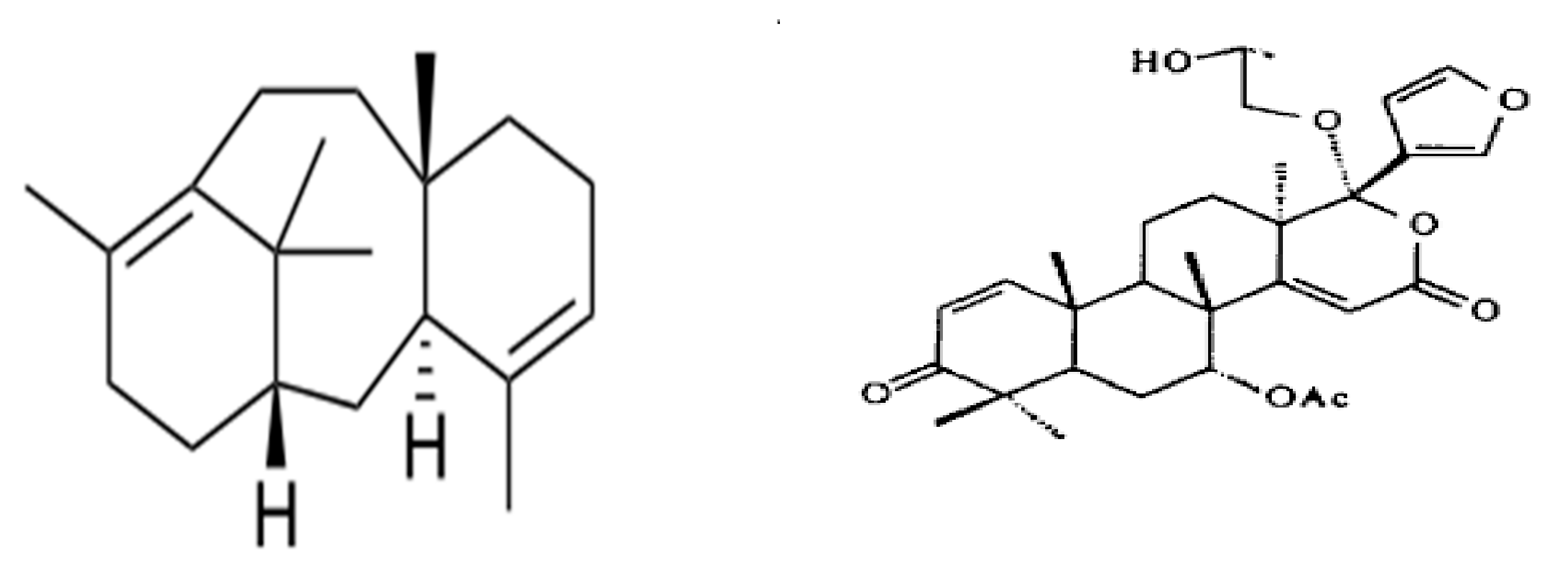
2.1. Isolation and Purification of Novel Bioactive Compounds from Leaves of Melia azedarach (Dharek) and Azadirachta indica for Films Preparation
2.2. Films Preparation
2.3. Standardization of Diterpenoids and Secomeliacins Compounds in Films Uses
2.4. Protocol of Novel Films Developmental Process
2.4.1. Degassing of Films
2.4.2. Bench Casting Process
2.4.3. Thickness of Films
2.5. Mechanical Properties of Novel Films
2.6. Film Microstructure
2.7. Stretch Modulus and Nanoindentation Process in Films
2.8. Moisture Contents and Water Solubility of Films
2.9. Collection of Fruits for Trials Process
2.10. Washing and Grading of Apple
2.11. Application of Films to an Apple Fruit
2.12. Stored Apples at Room Temperature
2.13. Films Effects on Physiological Rates and Oxidative Stress in Stored Apples
2.14. Quality Standards of (Golden) Apples during Storage Periods with Response to New Films
2.14.1. Total Soluble Solids (TSS)
2.14.2. Total Phenolic Contents (TPC)
2.14.3. Determination of DPPH Radicals Scavenging Activity
2.15. Quality Enzymes of (Golden) Apples after Films Application
2.15.1. Superoxide Dismutase (SOD)
2.15.2. Catalase Assay (CAT)
2.15.3. Peroxidase Assay (POX)
2.16. Screening of Essential Phytochemicals of Apples
2.17. Specification of HPLC-DAD Methods
2.17.1. Analysis of Chromatographic Conditions for Bioactive Compounds under Film Coating
2.17.2. Analysis of Major New Bioactive Compounds to Taste of Apple
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Data Available
Article Classification
Engineering Industrial Suitability
References
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Roy, S.K. Research achievement in the field of postharvest technology of fruits and vegetables. In Proc Workshop Appropriate Technology of Agro-Processing; CIPHET Ludhina: Ludhina, India, 1993; Volume 26, pp. 231–238. [Google Scholar]
- Alak, K.S.; Goswami, T.K. Controlled atmosphere storage of fruits and vegetables: A review. J. Food Sci. Technol. 2006, 43, 11–17. [Google Scholar]
- Balla, B.; Holb, I.J. Effect of three storage methods on fruit decay and brown rot of apple. Int. J. Hortic. Sci. Technol. 2007, 13, 55–57. [Google Scholar] [CrossRef]
- Ahmed, S.; Stoll, G. Biopesticides. In Biotechnology: Building on Farmers’ Knowledge; Haverkort, J.B., Hiemstra, W., Eds.; Macmillan Education Ltd.: London, UK, 1996. [Google Scholar]
- Harker, F.R.; Gunson, F.A.; Jaeger, S.R. The case for fruit quality. An interpretative review of consumer attitudes and preferences for apples. Postharvest Biol. Technol. 2003, 28, 333–347. [Google Scholar] [CrossRef]
- Jan, I.; Rab, A. Influence of storage duration on physico-chemical changes in fruit apple cultivars. J. Anim. Plant Sci. 2012, 22, 708–714. [Google Scholar]
- Khan, M.A.; Ahmad, I. Morphological studies on physical changes in apple fruit after storage at room temperature. J. Agric. Soc. Sci. 2005, 1, 102–104. [Google Scholar]
- Nilsson, T.; Gustavsson, K.H. Postharvest physiology of aroma apples in relation to position on the tree. Postharvest Bio. Technol. 2007, 43, 36–46. [Google Scholar] [CrossRef]
- Konopacka, D.; Plocharski, W.J. Effect of storage conditions on the relationship between apple firmness and texture acceptability. Postharvest Bio. Technol. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. The effect of calcium-infiltration on respiration, ethylene and aroma production of ’Golden Delicious’ apple fruits. Acta Hortic. 1993, 326, 131–140. [Google Scholar] [CrossRef]
- Sams, C.E.; Conway, C.S. Postharvest calcium infiltration improves fresh and processing quality of apples. Acta Hortic. 1993, 326, 123–130. [Google Scholar] [CrossRef]
- Hussain, P.R.; Meena, R.S.; Dar, M.A.; Wani, A.M. Effect of postharvest calcium chloride dip treatment and gamma irradiation on storage quality and shelf-life extension of Red Delicious apple. J. Food Sci. Technol. 2012, 49, 415–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafir, S.A.; Gadalla, S.O.; Murajei, B.N.; El-Nady, M.F. Physiological and anatomical comparison between four different apple cultivars under cold-storage conditions. Afr. J. Plant Sci. 2009, 3, 133–138. [Google Scholar]
- Poovaiah, B.W. Role of calcium in prolonging storage life of fruits and vegetables. Food Technol. 1986, 40, 86–89. [Google Scholar]
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of Neem (Azadirachta indica). Curr. Sci. 2002, 82, 1336–1345. [Google Scholar]
- Salib, J.Y.; Michael, H.N.; El-Nogoumy, S.I. New lactoyl glycoside Quercetin from Melia azedarach leaves. Chem. Nat. Compounds 2008, 44, 13–15. [Google Scholar] [CrossRef]
- Sharma, P.C.; Yelne, M.B.; Dennis, T.J. Data Base on Medicinal Plants Used in Ayurveda. In Central Council for Research in Ayurveda and Siddha; Department of Indian Medicine and Homeopathy, Ministry of Health and Family Welfare, Government of India: Chinnai, India, 2005; pp. 389–406. [Google Scholar]
- Bertuzzi, M.A.; Vidaurre, E.C.; Armada, M.; Gottifredi, J.C. Water vapor permeability of edible starch based films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Lima, Á.M.; Teixeira, J.A.; Moreira, R.A.; Vicente, A.A. Suitability of novel galactomannans as edible coatings for tropical fruits. J. Food Eng. 2009, 94, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Sobral, P.D.A.; Menegalli, F.C.; Hubinger, M.D.; Roques, M.A. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Cuq, J.-L.; Guilbert, S. Rheological models for the mechanical properties of myofibrillar protein-based films. J. Agric. Food Chem. 1996, 44, 1116–1122. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U. Postharvest L-cysteine application delayed pericarp browning, suppressed lipid peroxidation and maintained antioxidative activities of litchi fruit. Postharvest Bio. Technol. 2016, 121, 135–142. [Google Scholar] [CrossRef]
- Campbell, R.J.; Mobley, K.N.; Marini, R.P.; Pfeiffer, D.G. Growing conditions alter the relationship between SPAD-502 values and apple leaf chlorophyll. HortScience 1990, 25, 330–331. [Google Scholar] [CrossRef] [Green Version]
- Saadalla, M.M.; Quick, J.S.; Shanahan, J.F. Heat tolerance in winter wheat: II. Membrane thermostability and field performance. Crop Sci. 1990, 30, 1248–1251. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agricul. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Das, K.; Samanta, L.; Chainy, G.B.N. A Modified Spectrophotometric Assay of Superoxide Dismutase Using Nitrite Formation by Superoxide Radicals; NISCAIR-CSIR: New Delhi, India, 2000. [Google Scholar]
- Wheeler, C.R.; Salzman, J.A.; Elsayed, N.M.; Omaye, S.T.; Korte, D.W., Jr. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal. Biochem. 1990, 184, 193–199. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis; Verlag Chemie: Weinheim, Germany, 1974. [Google Scholar]
- Bajpai, V.K.; Dung, N.T.; Kwon, O.J.; Kang, S.C. Analysis and the potential applications of essential oil and leaf extracts of Silene armeria L. to control food spoilage and food-borne pathogens. Eur. Food Res. Technol. 2008, 227, 1613–1620. [Google Scholar] [CrossRef]
- Bhat, R.; Alias, A.K.; Paliyath, G. Essential Oils and Other Plant Extracts as Food Preservatives. In Progress in Food Preservation; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 539–580. [Google Scholar]
- Bassolé, I.H.N.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Dicko, M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Van Griensven, L.J. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef] [Green Version]
- Asadujjaman, M.D.; Saed, A.; Hossain, M.D.A.; Kumar Karmakar, U. Assessment of bioactivities of ethanolic extract of Melia azedarach (Meliaceae) leaves. J. Coast. Life Med. 2013, 1, 118–122. [Google Scholar]
- Laciar, A.; Vaca Ruiz, M.L.; Carrizo Flores, R.; Saad, J.R. Antibacterial and antioxidant activities of the essential oil of Artemisia echegarayi Hieron (Asteraceae). Rev. Argent. Microbiol. 2009, 41, 226–231. [Google Scholar] [PubMed]
- Davarynejad, G.; Zarei, M.; Ardakani, E.; Nasrabadi, M.E. Influence of putrescine application on storability, postharvest quality and antioxidant activity of two Iranian apricots (Prunus armeniaca L.) cultivars. Not. Sci. Biol. 2013, 5, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Nasir, M.; Khan, A.S.; Basra, S.A.; Malik, A.U. Foliar application of moringa leaf extract, potassium and zinc Lnfluence yield and fruit quality of ‘Kinnow’ mandarin. Sci. Hortic. 2016, 210, 227–235. [Google Scholar] [CrossRef]
- Abd El–Hamied, S.A.; El-Amary, E.I. Improving growth and productivity of “pear” trees using some natural plants extracts under north sinai conditions. J. Agric. Vet. Sci. 2015, 8, 1–9. [Google Scholar]
- Tajkarimi, M.; Ibrahim, S.A. Phytochemicals as anti-microbial food preservatives. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 207–235. [Google Scholar]
- Zhu, X.; Cao, J.; Wang, Q.; Jiang, W. Postharvest infiltration of BTH reduces infection of mango fruits (Mangifera indica L. cv. Tainong) by Colletotrichum gloeosporioides and enhances resistance inducing compounds. J. Phytopathol. 2008, 156, 68–74. [Google Scholar] [CrossRef]

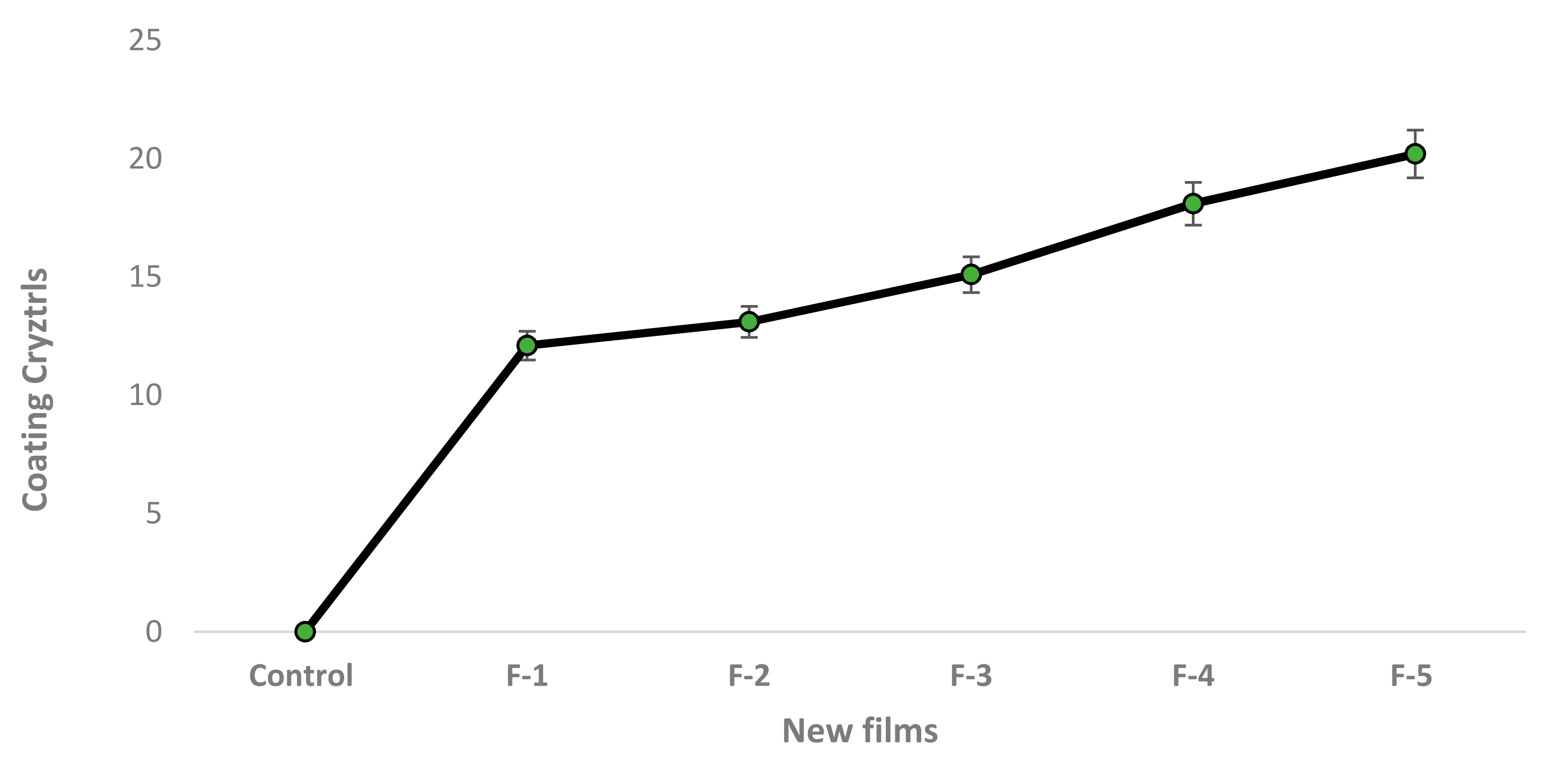
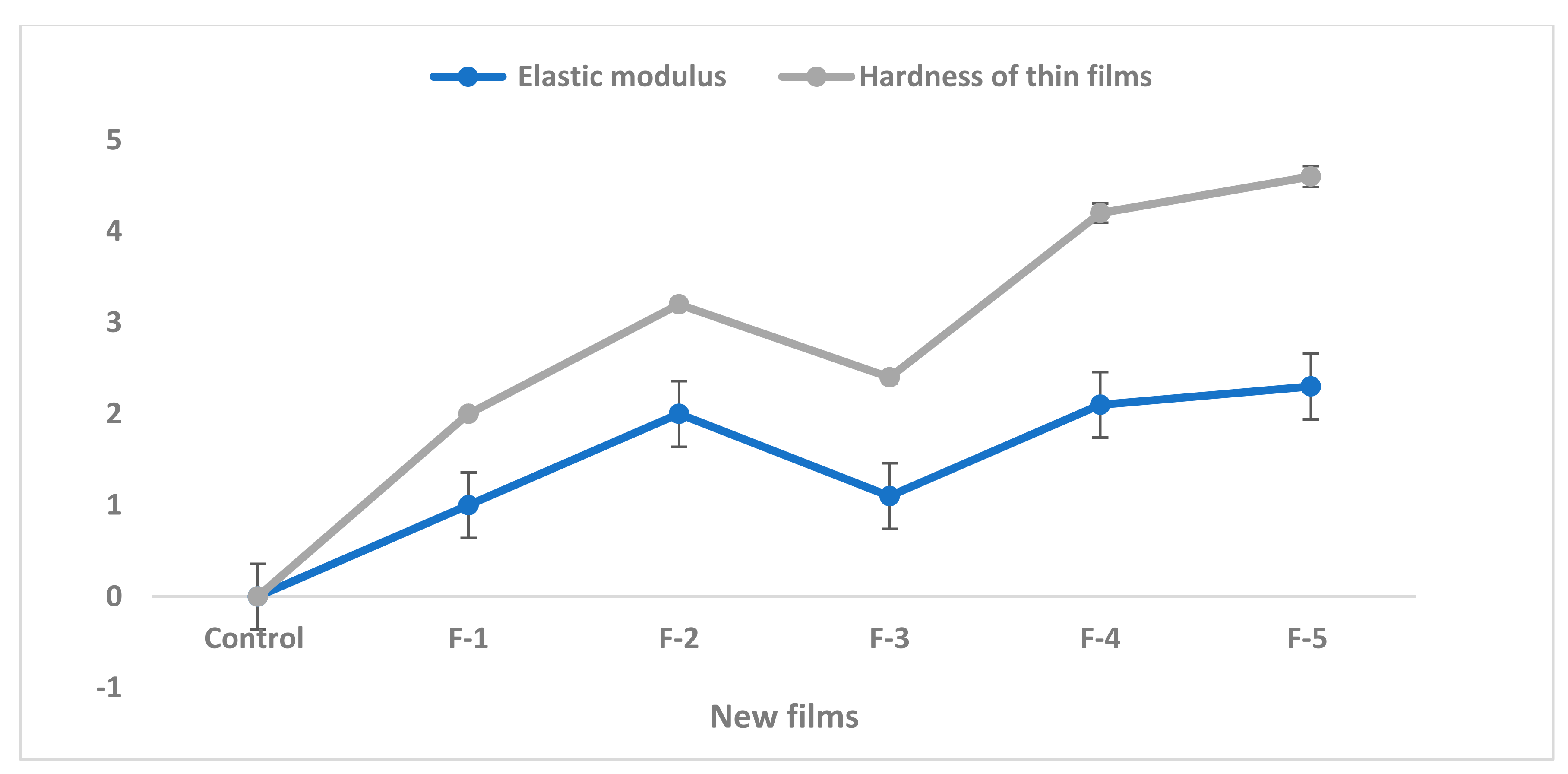

| Novel Films | |
|---|---|
| Film-1 | 10 mg of Diterpenoids + Secomeliacins film per fruit |
| Film-2 | 15 mg of Diterpenoids + Secomeliacins film per fruit |
| Film-3 | 20 mg of Diterpenoids + Secomeliacins film per fruit |
| Film-4 | 25 mg of Diterpenoids + Secomeliacins film per fruit |
| Film-5 | 30 mg of Diterpenoids + Secomeliacins film per fruite |
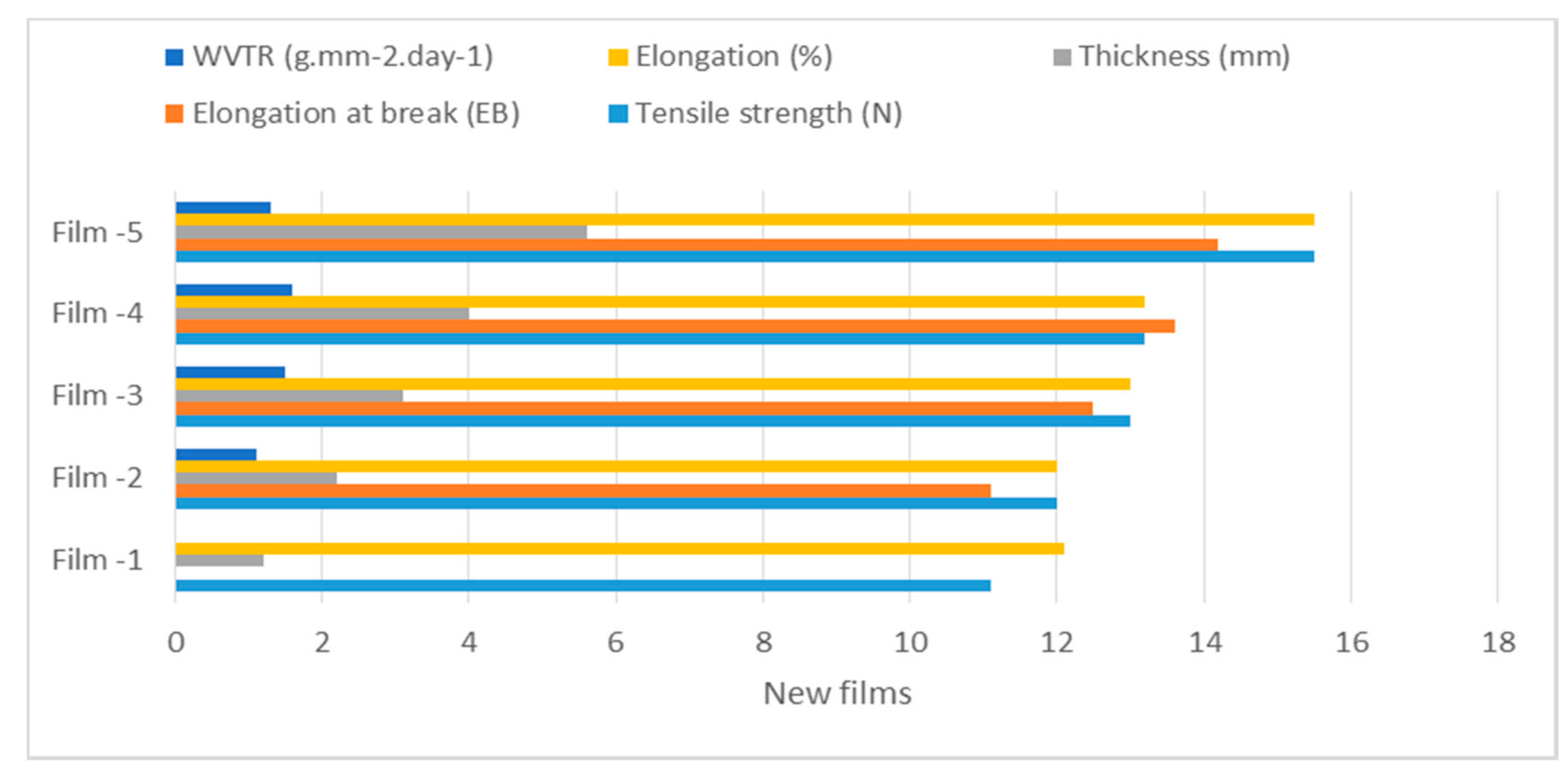
| New Film Specific Parameters | Film-1 | Film-2 | Film-3 | Film-4 | Film-5 |
|---|---|---|---|---|---|
| Tensile strength (N) | 11.1 ± 0.81 | 12.0 ± 0.80 | 13.0 ± 0.19 | 13.2 ±0.19 | 15.5± 0.19 |
| Elongation at break (EB) | 10.0 ± 0.89 | 11.1 ± 0.89 | 12.5 ± 0.89 | 13.6 ± 0.89 | 14.23 ± 0.29 |
| Thickness (mm) | 1.2 ± 0.89 | 2.2 ± 0.89 | 3.1 ± 0.89 | 4.0 ± 0.89 | 5.6 ± 0.83 |
| Elongation (%) | 12.1 ± 0.89 | 12.0 ± 0.89 | 13.0 ± 0.89 | 13.2 ± 0.89 | 15.5 ± 0.19 |
| WVTR (g·mm−2·day−1) | 2.0 ± 0.89 | 1.1 ± 0.89 | 1.5 ± 0.89 | 1.6 ± 0.89 | 1.3 ± 0.86 |
| Film Specific Parameters | Day after Storage (DAS) | Application of Films | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | Control | Film-1 | Film-2 | Film-3 | Film-4 | Film-5 | |
| Water vapor permeability (WVP) | 12 a | 12.0 a | 11.5 b | 10.0 c | 10.0 c | 12.1 b | 12.0 b | 13.0 e | 13.2 b | 15.5 a |
| Thermal stability | 13.2 a | 13.1 a | 12 b | 12.1 c | 9.00 f | 10.00 e | 10.5 d | 11.5 c | 11.6 b | 11.23 a |
| Films stability (mm) (df > 50) | 16.4 a | 16 b | 15.5 c | 15.2 d | 13.00 e | 14.2 b | 13.2 c | 13.1 d | 13.00 e | 14.6 a |
| New Film Specific Parameters | Days after Storage (DAS) | Film Application | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | Control | Film-1 | Film-2 | Film-3 | Film-4 | Film-5 | |
| Oxygen | 12 a | 11.0 b | 10.5 c | 9.0 d | 12.0 a | 10.1 b | 9.0 c | 8.01 d | 7.2 e | 6.5 f |
| Co2 | 13.2 a | 11.1 b | 10 c | 8.2 d | 13.1 a | 10.0 b | 8.1 b,c | 7.0 d | 6.01 e | 5.2 f |
| Ethylene | 13.4 a | 10.2 b | 9.5 c | 8.2 d | 13.0 a | 11.2 b | 10.2 c | 9.1 d | 8.00 e | 7.6 f |
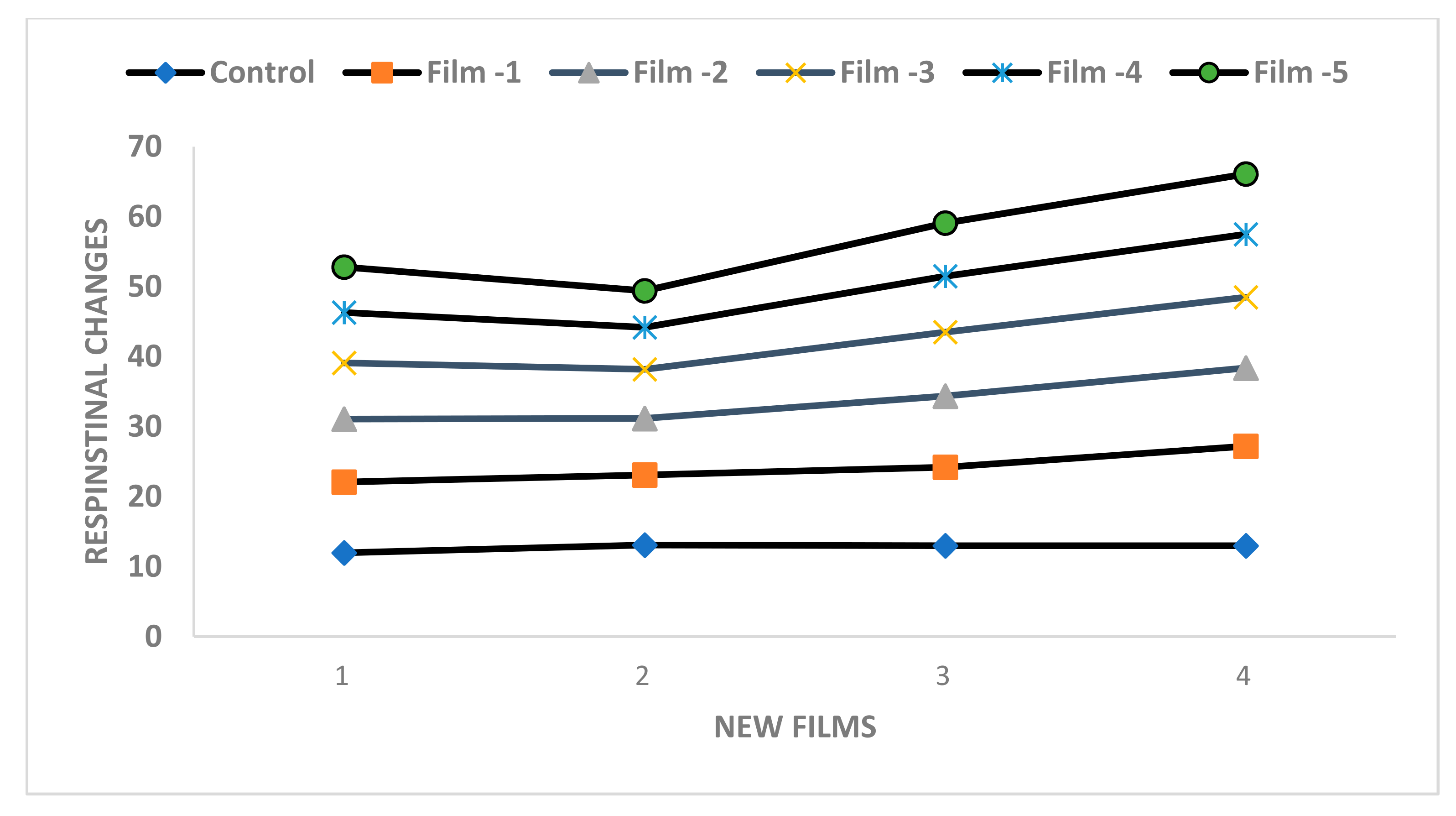
| Respirational rate | 16. 4 a | 12.2 b | 11.5 c | 10.2 d | 13.0 a | 14.2 b | 11.2 c | 10.1 d | 9.00 e | 8.6 f |
| Quality Enzymes Changes of Stored Fruits and Films | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| New Prepared Films | Catalase (CAT) (μ/g) | Per-Oxidase (POX) (μ/g) | Superoxide Dismutase (SOD) (μ/g) | ||||||
| DAS 3 | DAS 6 | DAS 9 | DAS 3 | DAS 6 | DAS 9 | DAS 3 | DAS 6 | DAS 9 | |
| Control | 4692.0 abcdef | 4997.3 abcde | 4398.1 abcdef | 6939 ns | 11,981 ns | 14,906 ns | 527.61 f | 638.74 cdef | 597.21 def |
| Film-1 | 3623.7 bcdef | 6394.7 a | 5547.3 abc | 6310 ns | 8454 ns | 9557 ns | 548.26 ef | 543.83 ef | 684.82 abcd |
| Film-2 | 3634.5 bcdef | 2658.6 f | 4381.3 abcdef | 6572 ns | 7665 ns | 6235 ns | 544.90 ef | 522.41 f | 659.77 bcde |
| Film-3 | 3272.0 def | 4454.2 abcdef | 5229.2 abcd | 5819 ns | 5546 ns | 10,191 ns | 544.56 ef | 638.64 cdef | 730.14 abc |
| Film-4 | 4297.9 abcdef | 3458.8 cdef | 3861.6 bcdef | 6386 ns | 4498 ns | 6804 ns | 543.57 ef | 517.67 f | 773.44 ab |
| Film-5 | 2902.5 ef | 4600.9 abcdef | 5691.6 ab | 8071 ns | 4928 ns | 7399 ns | 560.14 def | 780.94 ab | 790.70 a |
| New Prepared Films | Total Soluble Solid (TSS) | Total Antioxdants % Inhabitation of DPHH | Total Phenolic Compounds mg/100 g | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DAS 3 | DAS 6 | DAS 9 | DAS 3 | DAS 6 | DAS 9 | DAS 3 | DAS 6 | DAS 9 | |
| Control | 3.7000 a | 3.4000 ab | 2.3667 cd | 30.640 cde | 35.709 b | 46.769 a | 0.1720 abc | 0.1596 cd | 0.1637 bcd |
| Film-1 | 2.5333 bcd | 2.7333 abcd | 2.3667 cd | 34.480 bc | 44.260 a | 43.441 a | 0.1613 bcd | 0.1521 d | 0.1571 cd |
| Film-2 | 2.5000 bcd | 2.6667 bcd | 2.5000 bcd | 36.017 b | 26.493 e | 46.411 a | 0.1634 bcd | 0.1581 cd | 0.1605 cd |
| Film-3 | 2.9667 abc | 2.5333 bcd | 2.5667 bcd | 33.712 bc | 27.978 de | 45.387 a | 0.1595 cd | 0.1639 bcd | 0.1624 bcd |
| Film-4 | 2.1667 cd | 1.7667 d | 2.1667 cd | 34.736 bc | 32.381 bcd | 34.480 bc | 0.1687 bcd | 0.1783 ab | 0.1863 a |
| Film-5 | 2.4667 bcd | 2.0667 cd | 2.0667 cd | 35.044 bc | 30.691 cde | 33.354 bc | 0.1546 d | 0.1557 cd | 0.1659 bcd |
| DAS | New Films on Screening of Phytochemical in Apples (Golden Delicious) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Films | Saponins | Tanin | Sterols | Terperoids | Phlobatannins | Cardic Glycoside | Flvonoids | Quinines | |
| 3 | Control | + | + | + | + | + | − | + | + |
| 3 | Film-1 | + | + | + | + | + | − | + | + |
| 3 | Film-2 | + | + | + | + | + | − | + | + |
| 3 | Film-3 | + | + | + | + | + | − | + | + |
| 3 | Film-4 | + | + | + | + | + | − | + | + |
| 3 | Film-5 | + | + | + | + | + | − | + | + |
| 6 | Control | + | + | + | + | + | − | + | + |
| 6 | Film-1 | + | + | + | + | + | − | + | + |
| 6 | Film-2 | + | + | + | + | + | − | + | + |
| 6 | Film-3 | + | + | + | + | + | − | + | + |
| 6 | Film-4 | + | + | + | + | + | − | + | + |
| 6 | Film-5 | + | + | + | + | + | − | + | + |
| 9 | Control | + | + | + | + | + | − | + | + |
| 9 | Film-1 | + | + | + | + | + | − | + | + |
| 9 | Film-2 | + | + | + | + | + | − | + | + |
| 9 | Film-3 | + | + | + | + | + | + | + | + |
| 9 | Film-4 | + | + | + | + | + | + | + | + |
| 9 | Film-5 | + | + | + | + | + | + | + | + |
| Major Taste Compounds of Apples (u/g) 100 g | Day after Storage Changes | New Films Application of Apples | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | Control | Film-1 | Film-2 | Film-3 | Film-4 | Film-5 | |
| Phloridzin | 12.10 ± 0.80 | 12.00 ± 0.85 | 11.50 ± 0.81 | 10.00 ± 0.81 | 10.00 ± 0.84 | 12.10 ± 0.81 | 12.00 ± 0.81 | 11.00 ± 0.81 | 12.10 ± 0.81 | 12.50 ± 0.81 |
| Chlorogenic acid | 13.20 ± 0.81 | 13.10 ± 0.81 | 12.50 ± 0.81 | 12.10 ± 0.81 | 9.00 ± 0.21 | 10.00 ± 0.81 | 10.50 ± 0.81 | 11.50 ± 0.81 | 11.60 ± 0.81 | 11.23 ± 0.81 |
| Flavonoids | 16.40 ± 0.81 | 16.00 ± 0.80 | 15.50 ± 0.81 | 15.20 ± 0.81 | 13.00 ± 0.812 | 14.20 ± 0.81 | 13.20 ± 0.81 | 11.50 ± 0.81 | 13.00 ± 0.81 | 14.60 ± 0.81 |
| Feurlic acid | 12.50 ± 0.12 | 11.50 ± 0.80 | 11.10 ± 0.81 | 10.50 ± 0.81 | 11.00 ± 0.12 | 12.10 ± 0.81 | 12.30 ± 0.81 | 12.50 ± 0.81 | 13.10 ± 0.81 | 13.40 ± 0.81 |
| P-Coumaroylquinic | 18.10 ± 0.823 | 18.00 ± 0.14 | 17.00 ± 0.81 | 16.00 ± 0.81 | 16.00 ± 0.81 | 16.00 ± 0.81 | 16.50 ± 0.81 | 14.00 ± 0.81 | 15.00 ± 0.83 | 17.00 ± 0.81 |
| Physiological Parameters | Day after Storage (DAS) | Treated of New Films | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | Control | Film-1 | Film-2 | Film-3 | Film-4 | Film-5 | |
| Malondialdehyde (MDA) contents per gram FW | 12.1 a | 12.0 a | 11.5 b | 10.0 c | 10.0 d | 11.1 c | 12.2 b | 11.0 c | 12.3 b | 12.5 a |
| Chrophyll contents in leaves (%) | 13.2 a | 13.1 a | 12.5 b | 12.1 c | 9.00 f | 10.00 e | 10.5 d | 11.5 c | 11.6 b | 11.23 a |
| Lipid peroxidation assay per gram FW | 16.4 a | 16 b | 15.2 c | 15 d | 13.00 e | 14.2 b | 13.2 c | 13.1 d | 13.0 e | 14.6 a |
| Li and K | 12.0 a | 11.0 b | 10.5 c | 10 d | 11.00 f | 12.1 e | 12.3 d | 12.5 c | 13.1 b | 13.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, W.; Azmat, R.; Khojah, E.; Ahmed, R.; Qayyum, A.; Shah, A.N.; Abbas, A.; Moin, S.; Samra, B.N. The Development of a Green Innovative Bioactive Film for Industrial Application as a New Emerging Technology to Protect the Quality of Fruits. Molecules 2022, 27, 486. https://doi.org/10.3390/molecules27020486
Ahmed W, Azmat R, Khojah E, Ahmed R, Qayyum A, Shah AN, Abbas A, Moin S, Samra BN. The Development of a Green Innovative Bioactive Film for Industrial Application as a New Emerging Technology to Protect the Quality of Fruits. Molecules. 2022; 27(2):486. https://doi.org/10.3390/molecules27020486
Chicago/Turabian StyleAhmed, Waseem, Rafia Azmat, Ebtihal Khojah, Rasheed Ahmed, Abdul Qayyum, Adnan Noor Shah, Asad Abbas, Sumeira Moin, and Bassem N. Samra. 2022. "The Development of a Green Innovative Bioactive Film for Industrial Application as a New Emerging Technology to Protect the Quality of Fruits" Molecules 27, no. 2: 486. https://doi.org/10.3390/molecules27020486







