On the Nature of the Bonding in Coinage Metal Halides
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohanessian, G.; Goddard, W.A. Valence-bond concepts in transition metals: Metal hydride diatomic cations. Acc. Chem. Res. 1990, 23, 386–392. [Google Scholar] [CrossRef]
- Galbraith, J.M.; Shurki, A.; Shaik, S. A Valence Bond Study of the Bonding in First Row Transition Metal Hydride Cations: What Energetic Role Does Covalency Play? J. Phys. Chem. A 2000, 104, 1262–1270. [Google Scholar] [CrossRef]
- Mann, J.B.; Meek, T.L.; Knight, E.T.; Capitani, J.F.; Allen, L.C. Configuration Energies of the d-Block Elements. J. Am. Chem. Soc. 2000, 122, 5132–5137. [Google Scholar] [CrossRef]
- Kaupp, M. “Non-VSEPR” Structures and Bonding in d0 Systems. Angew. Chem. Int. Ed. 2001, 40, 3534–3565. [Google Scholar] [CrossRef]
- Kaupp, M. The role of radial nodes of atomic orbitals for chemical bonding and the periodic table. J. Comput. Chem. 2006, 28, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Radenković, S.; Danovich, D.; Shaik, S.; Hiberty, P.C.; Braïda, B. The nature of bonding in metal-metal singly bonded coinage metal dimers: Cu2, Ag2 and Au2. Comput. Theor. Chem. 2017, 1116, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Joy, J.; Danovich, D.; Kaupp, M.; Shaik, S. Covalent vs Charge-Shift Nature of the Metal–Metal Bond in Transition Metal Complexes: A Unified Understanding. J. Am. Chem. Soc. 2020, 142, 12277–12287. [Google Scholar] [CrossRef]
- Liakos, D.G.; Neese, F. Interplay of Correlation and Relativistic Effects in Correlated Calculations on Transition-Metal Complexes: The (Cu2O2)2+ Core Revisited. J. Chem. Theor. Comput. 2011, 7, 1511–1523. [Google Scholar] [CrossRef]
- Pyykko, P. Relativistic effects in structural chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Linares, M.; Braida, B.; Humbel, S. Valence Bond Approach of Metal−Ligand Bonding in the Dewar−Chatt−Duncanson Model. Inorg. Chem. 2007, 46, 11390–11396. [Google Scholar] [CrossRef]
- Shaik, S.; Hiberty, P.C. A Chemist’s Guide to Valence Bond Theory; Wiley-Interscience: New York, NY, USA, 2008. [Google Scholar]
- Shurki, A.; Braïda, B.; Wu, W. Valence Bond Theory with XMVB. In Complementary Bonding Analysis; Grabowsky, S., Ed.; De Gruyter STEM: Berlin, Germany, 2021; ISBN 978-3-11-066006-7. [Google Scholar]
- Wu, W.; Su, P.; Shaik, S.; Hiberty, P.C. Classical Valence Bond Approach by Modern Methods. Chem. Rev. 2011, 111, 7557–7593. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Hiberty, P. Valence Bond Theory—Its Birth, Struggles with Molecular Orbital Theory, Its Present State and Future Prospects. Molecules 2021, 26, 1624. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Polymeropoulos, E.E.A. Warshel: Computer Modeling of Chemical Reactions in Enzymes and Solutions, J. Wiley & Sons, Inc., New York, 1991, ISBN 0-47-1533955, 236 Seiten, Preis: £ 71,-. Ber. Der Bunsenges. Für Phys. Chem. 1992, 96, 1323–1324. [Google Scholar] [CrossRef]
- Bauer, P.; Barrozo, A.; Purg, M.; Amrein, B.A.; Esguerra, M.; Wilson, P.; Major, D.T.; Åqvist, J.; Kamerlin, S.C.L. Q6: A comprehensive toolkit for empirical valence bond and related free energy calculations. SoftwareX 2018, 7, 388–395. [Google Scholar] [CrossRef]
- Prah, A.; Purg, M.; Stare, J.; Vianello, R.; Mavri, J. How Monoamine Oxidase A Decomposes Serotonin: An Empirical Valence Bond Simulation of the Reactive Step. J. Phys. Chem. B 2020, 124, 8259–8265. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Wu, W.; Hiberty, P.C. Charge-shift bonding and its manifestations in chemistry. Nat. Chem. 2009, 1, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Braïda, B.; Hiberty, P.C. The essential role of charge-shift bonding in hypervalent prototype XeF2. Nat. Chem. 2013, 5, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Shaik, S.; Danovich, D.; Galbraith, J.M.; Braïda, B.; Wu, W.; Hiberty, P.C. Charge-Shift Bonding: A New and Unique Form of Bonding. Angew. Chem. Int. Ed. 2019, 59, 984–1001. [Google Scholar] [CrossRef]
- Shaik, S.; Danovich, D.; Braida, B.; Wu, W.; Hiberty, P.C. New Landscape of Electron-Pair Bonding: Covalent, Ionic, and Charge-Shift Bonds. In The Chemical Bond II. Structure and Bonding; Mingos, D.M.P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 170, pp. 169–212. ISBN 978-3-319-33522-3. [Google Scholar]
- Hiberty, P.C.; Danovich, D.; Shaik, S. A Conversation on New Types of Chemical Bonds. Israel J. Chem. 2021. [Google Scholar] [CrossRef]
- Wu, W.; Gu, J.; Song, J.; Shaik, S.; Hiberty, P.C. The Inverted Bond in [1.1.1] Propellane is a Charge-Shift Bond. Angew. Chem. Int. Ed. 2009, 48, 1407–1410. [Google Scholar] [CrossRef]
- Danovich, D.; Foroutan-Nejad, C.; Hiberty, P.C.; Shaik, S. Nature of the Three-Electron Bond. J. Phys. Chem. A 2018, 122, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Fayet, P.; Granzer, F.; Hegenbart, G.; Moisar, E.; Pischel, B. The role of small silver clusters in photography. Eur. Phys. J. D 1986, 3, 299–302. [Google Scholar] [CrossRef]
- Neipp, C.; Pascual, C.; Beléndez, A. Mixed phase-amplitude holographic gratings recorded in bleached silver halide materials. J. Phys. D Appl. Phys. 2002, 35, 957–967. [Google Scholar] [CrossRef]
- Kang, S.-K.; Yoon, A.S.-K.; Kim, Y.-M. Copper-Catalyzed Coupling Reaction of Terminal Alkynes with Aryl- and Alkenyliodonium Salts. Org. Lett. 2001, 3, 2697–2699. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Brust, M.; Schmidbaur, H. Gold—An introductory perspective. Chem. Soc. Rev. 2008, 37, 1759–1765. [Google Scholar] [CrossRef]
- Tolbatov, I.; Coletti, C.; Marrone, A.; Re, N. Reactivity of Gold(I) Monocarbene Complexes with Protein Targets: A Theoretical Study. Int. J. Mol. Sci. 2019, 20, 820. [Google Scholar] [CrossRef] [Green Version]
- Tolbatov, I.; Marzo, T.; Coletti, C.; La Mendola, D.; Storchi, L.; Re, N.; Marrone, A. Reactivity of antitumor coinage metal-based N-heterocyclic carbene complexes with cysteine and selenocysteine protein sites. J. Inorg. Biochem. 2021, 223, 111533. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, F. Structure and stability of coinage metal fluoride and chloride clusters (MnFn and MnCln, M = Cu, Ag, or Au; n = 1–6). J. Comput. Chem. 2012, 33, 2083–2091. [Google Scholar] [CrossRef]
- Rabilloud, F. Structure and Bonding in Coinage Metal Halide Clusters MnXn, M = Cu, Ag, Au; X = Br, I; n = 1–6. J. Phys. Chem. A 2012, 116, 3474–3480. [Google Scholar] [CrossRef]
- Li, X. Metalophilic interaction in gold halide: Quantum chemical study of AuX (X = F-at). J. Comput. Chem. 2014, 35, 923–931. [Google Scholar] [CrossRef]
- Li, X.; Geng, Z.-D. Investigation into the metallophilic interaction in coinage-metal halides: An ab initio study of CMX (CM = Cu and Ag, X = F − I). J. Mol. Model. 2015, 21, 205. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Peterson, K.A.; Puzzarini, C. Systematically convergent basis sets for transition metals. II. Pseudopotential-based correlation consistent basis sets for the group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) elements. Theor. Chim. Acta 2005, 114, 283–296. [Google Scholar] [CrossRef]
- Andrae, D.; Dolg, M.; Stoll, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hiberty, P.; Flament, J.; Noizet, E. Compact and accurate valence bond functions with different orbitals for different configurations: Application to the two-configuration description of F2. Chem. Phys. Lett. 1992, 189, 259–265. [Google Scholar] [CrossRef]
- Hiberty, P.C.; Humbel, S.; Byrman, C.P.; Van Lenthe, J.H. Compact valence bond functions with breathing orbitals: Application to the bond dissociation energies of F2 and FH. J. Chem. Phys. 1994, 101, 5969–5976. [Google Scholar] [CrossRef] [Green Version]
- Hiberty, P.C.; Shaik, S. Breathing-orbital valence bond method—A modern valence bond method that includes dynamic correlation. Theor. Chem. Acc. 2002, 108, 255–272. [Google Scholar] [CrossRef]
- Chirgwin, B.H.; Coulson, C.A. The electronic structure of conjugated systems. VI. Proc. R. Soc. London Ser. A Math. Phys. Sci. 1950, 201, 196–209. [Google Scholar] [CrossRef]
- Song, L.; Mo, Y.; Zhang, Q.; Wu, W. XMVB: A program for ab initio nonorthogonal valence bond computations. J. Comput. Chem. 2005, 26, 514–521. [Google Scholar] [CrossRef]
- Chen, Z.; Ying, F.; Chen, X.; Song, J.; Su, P.; Song, L.; Mo, Y.; Zhang, Q.; Wu, W. XMVB 2.0: A new version of Xiamen valence bond program. Int. J. Quantum Chem. 2014, 115, 731–737. [Google Scholar] [CrossRef]
- Guichemerre, M.; Chambaud, G.; Stoll, H. Electronic structure and spectroscopy of monohalides of metals of group I-B. Chem. Phys. 2002, 280, 71–102. [Google Scholar] [CrossRef]
- Dewar, M.J.S. A review of π Complex Theory. Bull. Soc. Chim. Fr. 1951, 18, C71. [Google Scholar]
- Chatt, J.; Duncanson, L.A. 586. Olefin co-ordination compounds. Part III. Infra-red spectra and structure: Attempted preparation of acetylene complexes. J. Chem. Soc. (Resumed) 1953, 2939–2947. [Google Scholar] [CrossRef]
- Braïda, B.; Chen, Z.; Wu, W.; Hiberty, P.C. Valence Bond Alternative Yielding Compact and Accurate Wave Functions for Challenging Excited States. Application to Ozone and Sulfur Dioxide. J. Chem. Theory Comput. 2020, 17, 330–343. [Google Scholar] [CrossRef]
- Bouabça, T.; Braïda, B.; Caffarel, M. Multi-Jastrow trial wavefunctions for electronic structure calculations with quantum Monte Carlo. J. Chem. Phys. 2010, 133, 44111. [Google Scholar] [CrossRef]
- Braïda, B.; Toulouse, J.; Caffarel, M.; Umrigar, C.J. Quantum Monte Carlo with Jastrow-valence-bond wave functions. J. Chem. Phys. 2011, 134, 084108. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Huber, K.P.; Herzberg, G. Molecular Spectra and Molecular Structure; Springer: Boston, MA, USA, 1979. [Google Scholar] [CrossRef]
- Evans, C.; Gerry, M.C. The Pure Rotational Spectra of AuCl and AuBr. J. Mol. Spectrosc. 2000, 203, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Danovich, D.; Wu, W.; Braïda, B.; Hiberty, P.C.; Shaik, S. Charge-Shift Bonding Emerges as a Distinct Electron-Pair Bonding Family from Both Valence Bond and Molecular Orbital Theories. J. Chem. Theory Comput. 2014, 10, 2410–2418. [Google Scholar] [CrossRef] [PubMed]

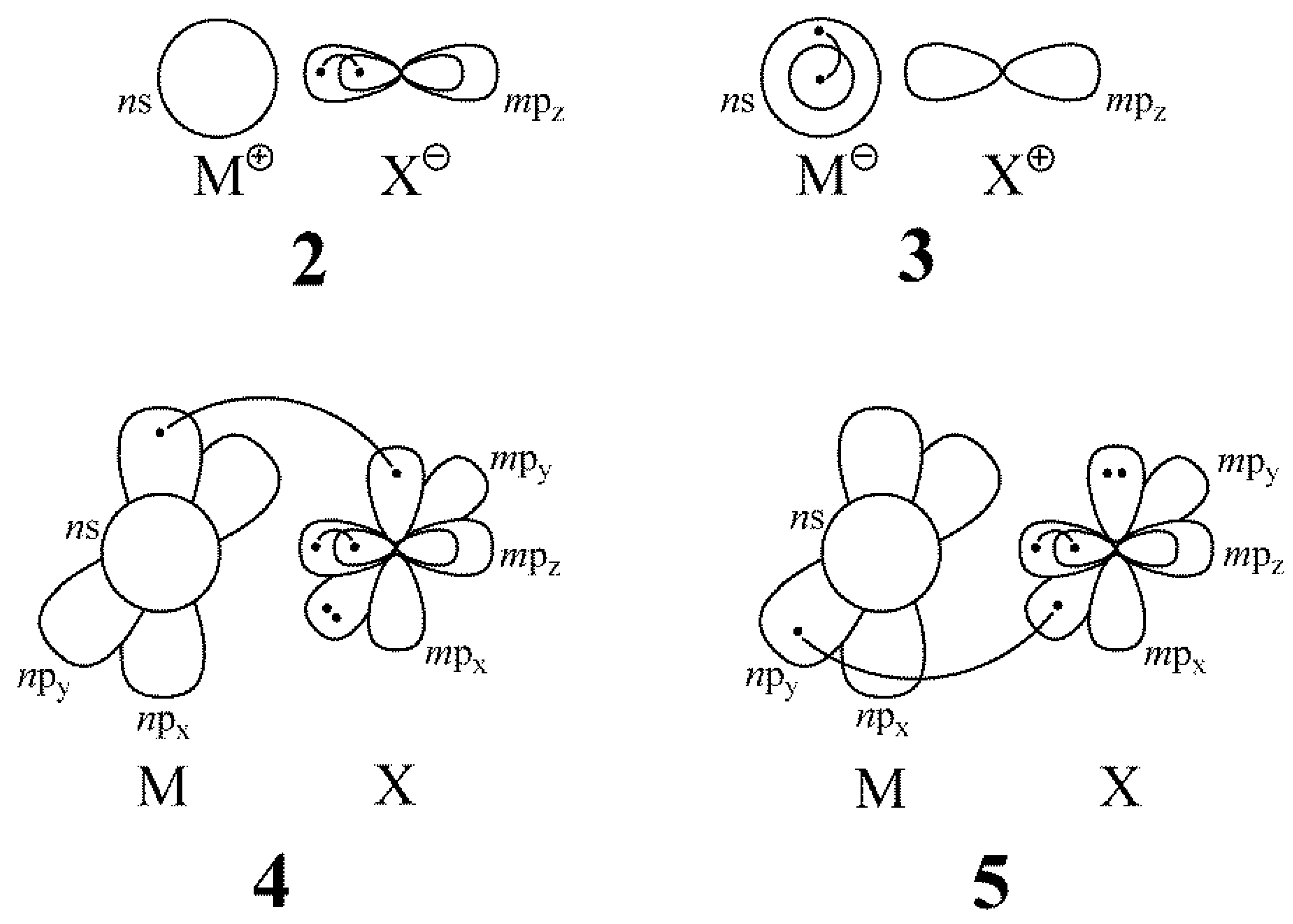
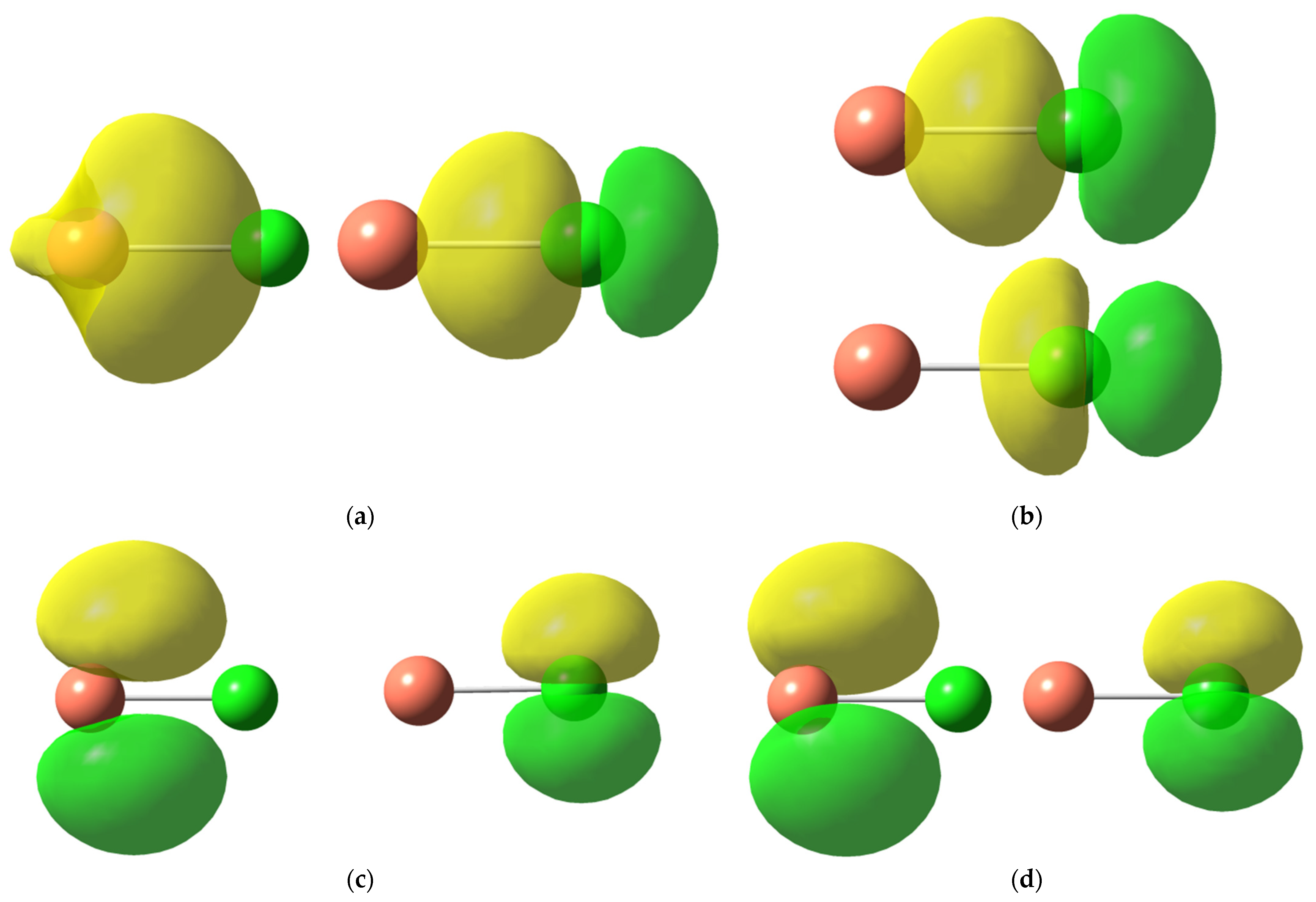
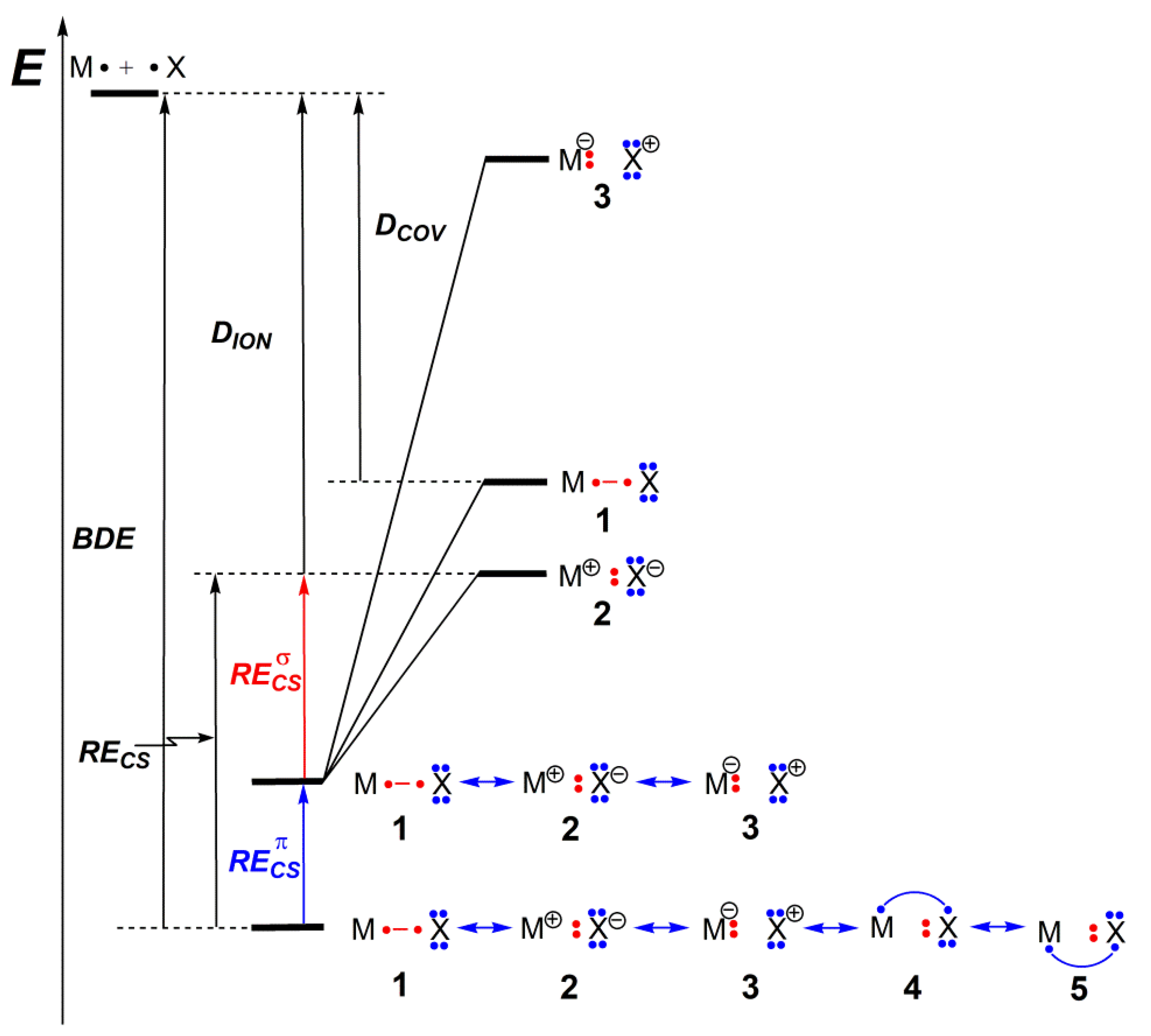
| MX | def2-TZVP | cc-pVQZ | aug-cc-pVQZ | Exp. [47] |
|---|---|---|---|---|
| CuF | 1.768 | 1.750 | 1.748 | 1.745 |
| CuCl | 2.103 | 2.068 | 2.062 | 2.051 |
| CuBr | 2.231 | 2.182 | 2.177 | 2.170 |
| AgF | 1.988 | 1.981 | 1.981 | 1.980 |
| AgCl | 2.295 | 2.294 | 2.288 | 2.281 |
| AgBr | 2.415 | 2.400 | 2.393 | 2.390 |
| AuF | 1.919 | 1.926 | 1.926 | 1.918 |
| AuCl | 2.214 | 2.220 | 2.216 | 2.199 |
| AuBr | 2.333 | 2.329 | 2.326 | 2.320 |
| MAE | 0.02 | 0.01 | 0.01 | |
| 0.06 | 0.02 | 0.02 |
| def2-TZVP | cc-pVQZ | aug-cc-pVQZ | Exp. | |||
|---|---|---|---|---|---|---|
| CCSD(T) | SD-BOVB | CCSD(T) | SD-BOVB | CCSD(T) | ||
| CuF | 91.8 | 90.4 a | 95.7 | 97.7 a | 99.0 | 98.9 [53]; 102.9 [54] |
| CuCl | 83.2 | 82.1 a | 85.8 | 85.0 a | 89.0 | 90.3 [53]; 90.6 [54] |
| CuBr | 77.3 | 74.9 a | 79.6 | 77.9 a | 83.3 | 79.1 [53] |
| AgF | 76.4 | 73.2 a | 77.8 | 81.6 a | 81.1 | 85.3 [53]; 83.9 [54] |
| AgCl | 71.8 | 70.8 a | 72.0 | 75.4 a | 74.7 | 66.7 [53]; 73.6 [54] |
| AgBr | 67.5 | 64.8 | 67.3 | 70.5 | 70.8 | 67.0 [53]; 71.5 [54] |
| AuF | 63.9 | 59.7 | 66.8 | 70.7 | 69.1 | 70.3 [53]; 73.8 [54] |
| AuCl | 64.0 | 61.0 | 65.5 | 63.1 a | 68.2 | 66.9 [53]; 72.2 [55] |
| AuBr | 60.9 | 56.5 | 62.0 | 64.2 | 66.3 | 50.9 [53]; 68.3 [55] |
| MAE | 5.0 | 7.6 (2.6) | 3.2 | 1.8 (1.5) | 0.00 | |
| MX | ||||||
|---|---|---|---|---|---|---|
| CuF | 21.8 | 24.1 | 16.9 | 18.7 | 38.7 | 42.8 |
| CuCl | 15.6 | 19.0 | 9.5 | 11.6 | 25.2 | 30.6 |
| CuBr | 15.0 | 20.0 | 7.9 | 10.5 | 22.9 | 30.5 |
| AgF | 28.2 | 38.5 | 12.5 | 17.0 | 40.7 | 55.5 |
| AgCl | 26.8 | 37.8 | 8.3 | 11.7 | 35.1 | 49.5 |
| AgBr | 27.3 | 42.1 | 6.4 | 9.8 | 33.7 | 51.9 |
| AuF | 55.4 | 92.8 | 10.1 | 17.0 | 65.5 | 109.8 |
| AuCl | 47.4 | 77.8 | 7.2 | 11.9 | 54.6 | 89.7 |
| AuBr | 44.3 | 78.5 | 6.1 | 10.9 | 50.4 | 89.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Đorđević, S.; Radenković, S.; Shaik, S.; Braïda, B. On the Nature of the Bonding in Coinage Metal Halides. Molecules 2022, 27, 490. https://doi.org/10.3390/molecules27020490
Đorđević S, Radenković S, Shaik S, Braïda B. On the Nature of the Bonding in Coinage Metal Halides. Molecules. 2022; 27(2):490. https://doi.org/10.3390/molecules27020490
Chicago/Turabian StyleĐorđević, Slađana, Slavko Radenković, Sason Shaik, and Benoît Braïda. 2022. "On the Nature of the Bonding in Coinage Metal Halides" Molecules 27, no. 2: 490. https://doi.org/10.3390/molecules27020490
APA StyleĐorđević, S., Radenković, S., Shaik, S., & Braïda, B. (2022). On the Nature of the Bonding in Coinage Metal Halides. Molecules, 27(2), 490. https://doi.org/10.3390/molecules27020490







