The Optimization of Extraction Process, Antioxidant, Whitening and Antibacterial Effects of Fengdan Peony Flavonoids
Abstract
:1. Introduction
2. Results and Discussion
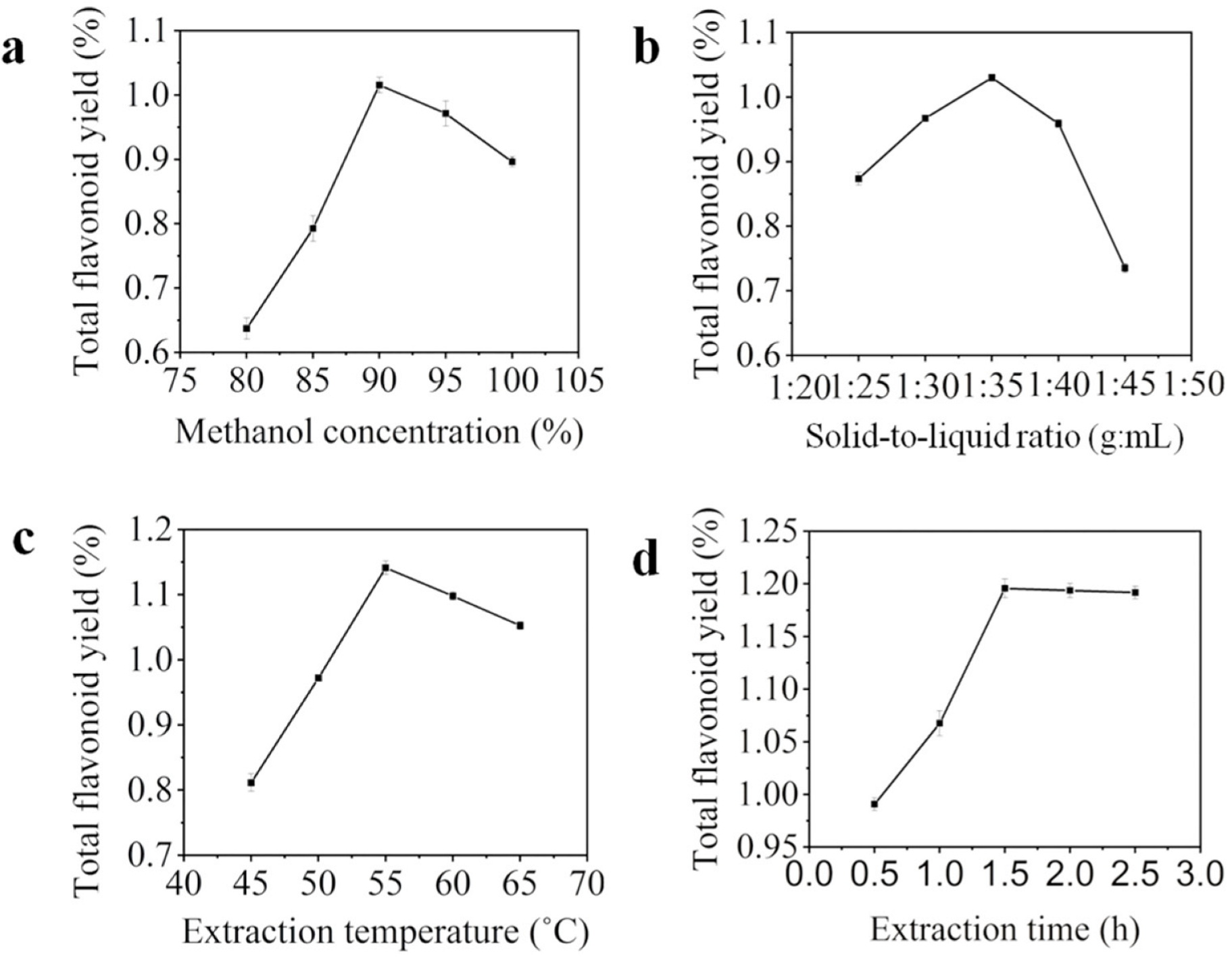
2.1. The Influence of Four Factors to Total Flavonoids Yield
2.2. Optimization for Total Flavonoids Yield
2.2.1. Model Fitting and Data Analysis Using RSM
2.2.2. Verification of Optimal Conditions
2.3. Antioxidant Activity of Fengdan Peony Flavonoid
2.4. Whitening Activity of Fengdan Peony Flavonoid
2.5. Antibacterial Activity
3. Materials and Methods
3.1. Material and Reagents
3.2. Extraction of Total Flavonoids
3.3. Determination of Fengdan Peony Total Flavonoids Yield
3.4. Single-Factor Experiment
3.5. RSM Experiment
3.6. Preparation of Fengdan Peony Total Flavonoids Powder
3.7. Antioxidant Activity Evaluation
3.7.1. DPPH Free Radical Assay
3.7.2. Hydroxyl Radical Assay
3.7.3. ABTS Free Radical Assay
3.8. Whitening Activity Evaluation
3.9. Antibacterial Activity Experiment
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yan, Z.; Xie, L.; Li, M.; Yuan, M.; Tian, Y.; Sun, D.; Zhang, Y.; Niu, L. Phytochemical components and bioactivities of novel medicinal food-peony roots. Food Res. Int. 2021, 140, 109902. [Google Scholar] [CrossRef]
- Deng, R.X.; Yang, X.; Wang, Y.X.; Du, M.Z.; Hao, X.T.; Liu, P. Optimization of ultrasound assisted extraction of monoterpene glycoside from oil peony seed cake. J. Food Sci. 2018, 83, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Ma, C.; Liu, C.; Gao, C.; Nie, R.; Wang, H. Hypolipidemic activity of peony seed oil rich in α-linolenic is mediated through inhibition of lipogenesis and upregulation of fatty acid β-oxidation. J. Food Sci. 2016, 81, H1001–H1009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ban, Q.; Wang, X.; Wang, Z. Green and efficient PEG-based ultrasonic-assisted extraction of polysaccharides from tree peony pods and the evaluation of their antioxidant activity in vitro. Biomed. Res. Int. 2018, 2121385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Shi, Q.Q.; Ji, D.; Niu, L.X.; Zhang, Y.L. Determination of the phenolic content, profile, and antioxidant activity of seeds from nine tree peony (Paeonia section Moutan DC.) species native to China. Food Res. Int. 2017, 97, 141–148. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Li, H.; Wu, Z.; Chen, B.; Lei, Y.; Shen, Y. In vitro and in silico antioxidant activity of novel peptides prepared from paeonia ostii ‘Feng Dan’ hydrolysate. Antioxidants 2019, 8, 433. [Google Scholar] [CrossRef] [Green Version]
- Tramontin, D.; Cadena-Carrera, S.E.; Assreuy, J.; Nunes, R.; Santin, J.R.; Bolzan, A.; Quadri, M. Response surface methodology (RSM) to evaluate both the extraction of triterpenes and sterols from jackfruit seed with supercritical CO2 and the biological activity of the extracts. J. Food Sci. Technol. 2021, 58, 3303–3313. [Google Scholar] [CrossRef]
- Tian, X.; Guo, S.; Zhang, S.; Li, P.; Wang, T.; Ho, C.T.; Pan, M.H.; Bai, N. Chemical characterization of main bioactive constituents in paeonia ostii seed meal and GC-MS analysis of seed oil. J. Food Biochem. 2020, 44, e13088. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, T.; Yin, D.X.; Song, P.; Hou, X.G.; Qi, Q.; Qi, Z.H. Major fatty acid profiles and bioactivity of seed oils from ten tree peony cultivars as a potential raw material source for the cosmetics and healthy Products. Chem. Biodivers. 2020, 17, e2000469. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiao, Y.; Zhang, B.; Chang, R.; Luo, D.; Yang, L.; Yang, Y.; Liu, D. Magnetically stabilized bed packed with synthesized magnetic silicone loaded with ionic liquid particles for efficient enrichment of flavonoids from tree peony petals. J. Chromatogr. A 2020, 1613, 460671. [Google Scholar] [CrossRef]
- De Queirós, L.D.; de Ávila, A.R.A.; Botaro, A.V.; Chirotto, D.B.L.; Macedo, J.A.; Macedo, G.A. Combined isoflavones biotransformation increases the bioactive and antioxidant capacity of soymilk. Appl. Microbiol. Biotechnol. 2020, 104, 10019–10031. [Google Scholar] [CrossRef]
- Ali, M.W.; Kim, I.D.; Bilal, S.; Shahzad, R.; Saeed, M.T.; Adhikari, B.; Nabi, R.B.S.; Kyo, J.R.; Shin, D.H. Effects of bacterial fermentation on the biochemical constituents and antioxidant potential of fermented and unfermented soybeans using probiotic bacillus subtilis (KCTC 13241). Molecules 2017, 22, 2200. [Google Scholar] [CrossRef] [Green Version]
- De Ávila, A.R.A.; de Queirós, L.D.; Ueta, T.M.; Macedo, G.A.; Macedo, J.A. Exploring in vitro effects of biotransformed isoflavones extracts: Antioxidant, antiinflammatory, and antilipogenic. J. Food Biochem. 2019, 43, e12850. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.; Schubert-Zsilavecz, M.; Wurglics, M. Ginkgo biloba extracts: A review of the pharmacokinetics of the active ingredients. Clin. Pharmacokinet. 2013, 52, 727–749. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Chen, Y.; Zheng, H.; Xie, B.; Sun, Z. Flavonoid compounds and antibacterial mechanisms of different parts of white guava (Psidium guajava L. cv. Pearl). Nat. Prod. Res. 2020, 34, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.S.; Liaw, E.T. Extraction optimization of flavonoids from hypericum formosanum and matrix metalloproteinase-1 inhibitory activity. Molecules 2017, 22, 2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process. Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Sun, X.L.; Li, W.G.; Li, J.; Zu, Y.G.; Hse, C.Y.; Xie, J.L.; Zhao, X.H. Process optimisation of microwave-assisted extraction of peony (Paeonia suffruticosa Andr.) seed oil using hexane-ethanol mixture and its characterisation. Int. J. Food Sci. Technol. 2016, 51, 2663–2673. [Google Scholar] [CrossRef]
- Suderman, N.; Sarbon, N.M. Optimization of chicken skin gelatin film production with different glycerol concentrations by response surface methodology (RSM) approach. J. Food Sci. Technol. 2020, 57, 463–472. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Zhang, J.; She, Z.; Jin, C.; Gao, M.; Zhao, Y. Optimization of operating conditions for the acidification metabolites production with waste sludge using response surface methodology (RSM). Environ. Sci. Pollut. Res. Int. 2019, 26, 30303–30312. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Y.; Shi, Y.; Zhao, K.; Tan, H.; Zeng, J.; Tang, Q.; Xie, H. Multiresponse optimization of ultrasonic-assisted extraction for Aurantii Fructus to obtain high yield of antioxidant flavonoids using a response surface methodology. Processes 2018, 6, 258. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 15, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Azahar, N.F.; Gani, S.S.A.; Mohd Mokhtar, N.F. Optimization of phenolics and flavonoids extraction conditions of Curcuma Zedoaria leaves using response surface methodology. Chem. Cent. J. 2017, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhou, X.P.; Liu, X.F. Reliability analysis of slopes using the improved stochastic response surface methods with multicollinearity. Eng. Geol. 2020, 271, 105617. [Google Scholar] [CrossRef]
- Apak, R. Current issues in antioxidant measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Oancea, S.; Perju, M.; Olosutean, H. Influence of enzyme-aided extraction and ultrasonication on the phenolics content and antioxidant activity of Paeonia officinalis L. petals. J. Serb. Chem. Soc. 2020, 85, 845–856. [Google Scholar] [CrossRef] [Green Version]
- Lan, S.L.; Eun, J.C.; Chang, H.K.; Jung, M.S.; Young, B.K.; Dong, H.S.; Hyun, W.C.; Yun, S.C.; Jun, S.K.; Jong, D.P. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Nicklisch, S.C.; Waite, J.H. Optimized DPPH assay in a detergent-based buffer system for measuring antioxidant activity of proteins. MethodsX 2014, 1, 233–238. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Messens, J. Bioplastic hydroxyl radical trapping. Nat. Chem. Biol. 2016, 12, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Y.; Lou, Z.; Song, L.; Wu, Y.; Gu, N.; Zhang, Y. In vitro cytotoxicity evaluation of graphene oxide from the peroxidase-like activity perspective. Colloids Surf. B 2017, 151, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, D.; Song, L.M.; Xu, Q.; Li, H.; Xu, H. Chemical profile and antioxidant activity of the oil from peony seeds (Paeonia suffruticosa Andr.). Oxid. Med. Cell. Longev. 2017, 2017, 9164905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Z.Z.; Ni, J.; Tang, J.M.; Sun, D.Y.; Yan, Z.G.; Zhang, J.; Niu, L.X.; Zhang, Y.L. Bioactive components, antioxidant and antimicrobial activities of Paeonia rockii fruit during development. Food Chem. 2021, 343, 128444. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Tu, X.F.; Thakur, K.; Zhang, Y.S.; Zhu, D.Y.; Zhang, J.G.; Wei, Z.J. Effects of different chemical modifications on the antioxidant activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2018, 112, 675–685. [Google Scholar] [CrossRef]
- Lin, D.; Wang, S.H.; Song, T.Y.; Hsieh, C.W.; Tsai, M.S. Safety and efficacy of tyrosinase inhibition of paeonia suffruticosa andrews extracts on human melanoma cells. J. Cosmet. Dermatol. 2019, 18, 1921–1929. [Google Scholar] [CrossRef]
- Xie, X.T.; Zhang, X.; Liu, Y.; Chen, X.Q.; Cheong, K.L. Quantification of 3,6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech 2020, 10, 189. [Google Scholar] [CrossRef]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohyd. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [Green Version]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health Care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Yan, Z.; Xie, L.; Tian, Y.; Li, M.; Ni, J.; Zhang, Y.; Niu, L. Insights into the phytochemical composition and bioactivities of seeds from wild peony species. Plants 2020, 9, 729. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Lopes, L.A.A.; Dos Santos Rodrigues, J.B.; Magnani, M.; de Souza, E.L.; de Siqueira-Júnior, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kaldhusdal, M.; Skjerve, E.; Hansen, M.K.; Hamnes, I.S.; David, B.; Hanssen, S.A.; Løvland, A. The incidence of necrotic enteritis in turkeys is associated with farm, season and faecal eimeria oocyst counts. BMC Vet. Res. 2021, 17, 292. [Google Scholar] [CrossRef]
- Yu, M.; Wang, B.; Qi, Z.; Xin, G.; Li, W. Response surface method was used to optimize the ultrasonic assisted extraction of flavonoids from Crinum asiaticum. Saudi J. Biol Sci. 2019, 26, 2079–2084. [Google Scholar] [CrossRef]
- Dai, C.Y.; Liao, P.R.; Zhao, M.Z.; Gong, C.; Dang, Y.; Qu, Y.; Qiu, L.S. Optimization of ultrasonic flavonoid extraction from Saussurea involucrate, and the ability of flavonoids to block melanin deposition in human melanocytes. Molecules 2020, 25, 313. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, Q.; Zhang, Y.; Liu, H. Total glucosides of peony protect cardiomyocytes against oxidative stress and inflammation by reversing mitochondrial dynamics and bioenergetics. Oxid. Med. Cell. Longev. 2020, 2020, 6632413. [Google Scholar] [CrossRef]
- Pham, D.C.; Nguyen, H.C.; Nguyen, T.L.; Ho, H.L.; Trinh, T.K.; Riyaphan, J.; Weng, C.F. Optimization of ultrasound-assisted extraction of flavonoids from Celastrus hindsii leaves using response surface methodology and evaluation of their antioxidant and antitumor activities. Biomed. Res. Int. 2020, 2020, 3497107. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhang, L.; Li, Q.; Jin, W.; Chen, W.; Han, J.; Zhang, Y. Simultaneous optimization for ultrasound-assisted extraction and antioxidant activity of flavonoids from Sophora flavescens using response surface methodology. Molecules 2018, 24, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Huang, J.; Xiao, G.; Chen, F.; Lee, B.; Ge, Q.; You, Y.; Liu, S.; Zhang, Y. Antioxidant capacities of fractions of bamboo shaving extract and their antioxidant components. Molecules 2016, 21, 996. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; Thakur, K.; Zhang, Y.Y.; Tu, X.F.; Zhang, Y.S.; Zhu, D.Y.; Zhang, J.G.; Wei, Z.J. Effects of different chemical modifications on the antibacterial activities of polysaccharides sequentially extracted from peony seed dreg. Int. J. Biol. Macromol. 2018, 116, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, G.S.; Kulkarni, R.D.; Naik, B.D. Evaluation of minimal inhibitory concentration of two new materials using tube dilution method: An in vitro study. J. Conserv. Dent. 2015, 18, 159–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Jiang, C.; Jing, H.; Jiang, C.; Wang, H.; Du, X.; Lou, Z. Separation of phenolics from peony flowers and their inhibitory activities and action mechanism on bacterial biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 4321–4332. [Google Scholar] [CrossRef] [PubMed]





| Source | Sum of Squares | df | Mean Square | F-Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 0.0249 | 9 | 0.0028 | 18.57 | 0.0004 | ** |
| A | 0.0015 | 1 | 0.0015 | 10.16 | 0.0153 | * |
| B | 0.0055 | 1 | 0.0055 | 37.03 | 0.0005 | ** |
| C | 0.0001 | 1 | 0.0001 | 0.4836 | 0.5092 | |
| AB | 0.0013 | 1 | 0.0013 | 8.70 | 0.0214 | * |
| AC | 0.0018 | 1 | 0.0018 | 11.85 | 0.0108 | * |
| BC | 0.0003 | 1 | 0.0003 | 2.18 | 0.1837 | |
| A 2 | 0.0040 | 1 | 0.0040 | 26.65 | 0.0013 | ** |
| B 2 | 0.0071 | 1 | 0.0071 | 48.00 | 0.0002 | ** |
| C 2 | 0.0019 | 1 | 0.0019 | 12.71 | 0.0092 | ** |
| Residual | 0.0010 | 7 | 0.0001 | |||
| Lack of fit | 0.0008 | 3 | 0.0003 | 3.68 | 0.1202 | |
| Pure Error | 0.0003 | 4 | 0.0001 | |||
| Cor Total | 0.0259 | 16 | ||||
| R2 = 0.9598 | ||||||
| Bacteria | Average Diameter of Inhibition Zone (cm) | |
|---|---|---|
| 30 mg/mL | 500 mg/mL | |
| B. subtilis | 1.35 ± 0.18 | 1.83 ± 0.06 |
| B. anthracis | 1.95 ± 0.05 | 2.20 ± 0.26 |
| S. aureus | 2.27 ± 0.26 | 2.65 ± 0.33 |
| C. perfringens | 2.63 ± 0.06 | 2.97 ± 0.06 |
| E. coli | - | - |
| S. typhimurium | - | - |
| P. aeruginosa | - | - |
| C. albicans | - | - |
| A. niger | - | - |
| 80% methanol | - | - |
| Factors | Variables |
|---|---|
| Methanol concentration (%) | 80; 85; 90; 95; 100 |
| Solid-to-liquid ratio (g/mL) | 1:25; 1:30; 1:35; 1:40; 1:45 |
| Extraction temperature (°C) | 45; 50; 55; 60; 65 |
| Extraction time (h) | 0.5; 1; 1.5; 2; 2.5 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A Solid-to-liquid ratio (g/mL) | 1:30 | 1:35 | 1:40 |
| B Extraction temperature (°C) | 50 | 55 | 60 |
| C extraction time (h) | 1 | 1.5 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Huang, Z.; Liu, Y.; Wang, H.; Qiu, M.; Qu, Y.; Yuan, W. The Optimization of Extraction Process, Antioxidant, Whitening and Antibacterial Effects of Fengdan Peony Flavonoids. Molecules 2022, 27, 506. https://doi.org/10.3390/molecules27020506
Lu J, Huang Z, Liu Y, Wang H, Qiu M, Qu Y, Yuan W. The Optimization of Extraction Process, Antioxidant, Whitening and Antibacterial Effects of Fengdan Peony Flavonoids. Molecules. 2022; 27(2):506. https://doi.org/10.3390/molecules27020506
Chicago/Turabian StyleLu, Jie, Zhiqiang Huang, Yusheng Liu, Huimin Wang, Min Qiu, Yinghui Qu, and Wenpeng Yuan. 2022. "The Optimization of Extraction Process, Antioxidant, Whitening and Antibacterial Effects of Fengdan Peony Flavonoids" Molecules 27, no. 2: 506. https://doi.org/10.3390/molecules27020506






