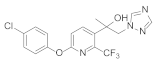
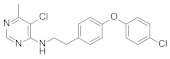
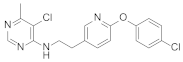
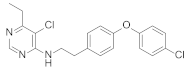
Abstract
Phenoxypyridine, the bioisostere of diaryl ethers, has been widely introduced into bioactive molecules as an active scaffold, which has different properties from diaryl ethers. In this paper, the bioactivities, structure-activity relationships, and mechanism of compounds containing phenoxypyridine were summarized, which may help to explore the lead compounds and discover novel pesticides with potential bioactivities.
1. Introduction
Diaryl ether [1] is an important active fragment in pesticide molecules, which has good lipid solubility, metabolic stability, cell membrane penetration, sufficient molecular flexibility [2], and can improve biological activity and photostability. So far, the structure of diaryl ether has been widely studied and applied, such as aryloxyphenoxypropionate herbicides, pyrethroid insecticides [3], and triazole fungicides. Pyridine [4], as a nitrogen-containing heterocyclic ring, plays an important role in agrochemicals, and its derivatives have a wide range of biological activities. The hydrophobicity (one of key properties affecting biological activity) of pyridine is significantly higher than that of the benzene ring [5]. Meanwhile, pyridine is an ionizable polar aromatic compound, which can optimize solubility and bioavailability of the lead compound [6]. Replacing the benzene ring with a pyridine ring [7] can usually increase the π-π stacking probability of the target molecule [8] and improve the biological activity (Table 1). Therefore, phenoxypyridine may have properties that are different from or even superior to those of diphenyl ether. The phenoxypyridine structure has been widely used in the molecular structure of pesticides. At present, there are many commercial pesticides containing the phenoxypyridine structure, as shown in Figure 1. The active skeleton is of great significance for the creation of new pesticides and there is no report on the summary of phenoxypyridine compounds. In this paper, we will summarize the research about the relevant phenoxypyridine derivatives in the pesticide field in the last ten years.

Table 1.
Comparison of the activity of diphenyl ethers and phenoxypyridine pesticides.
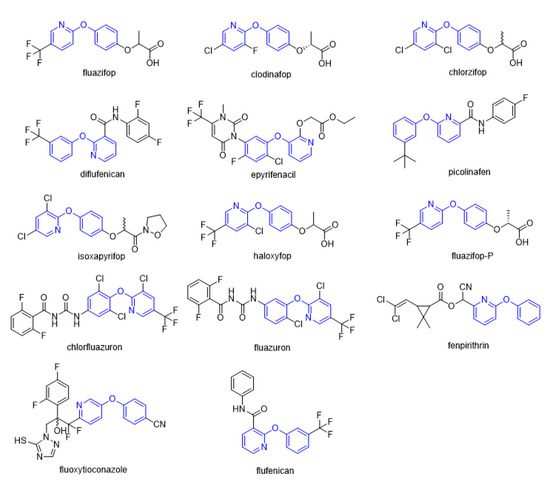
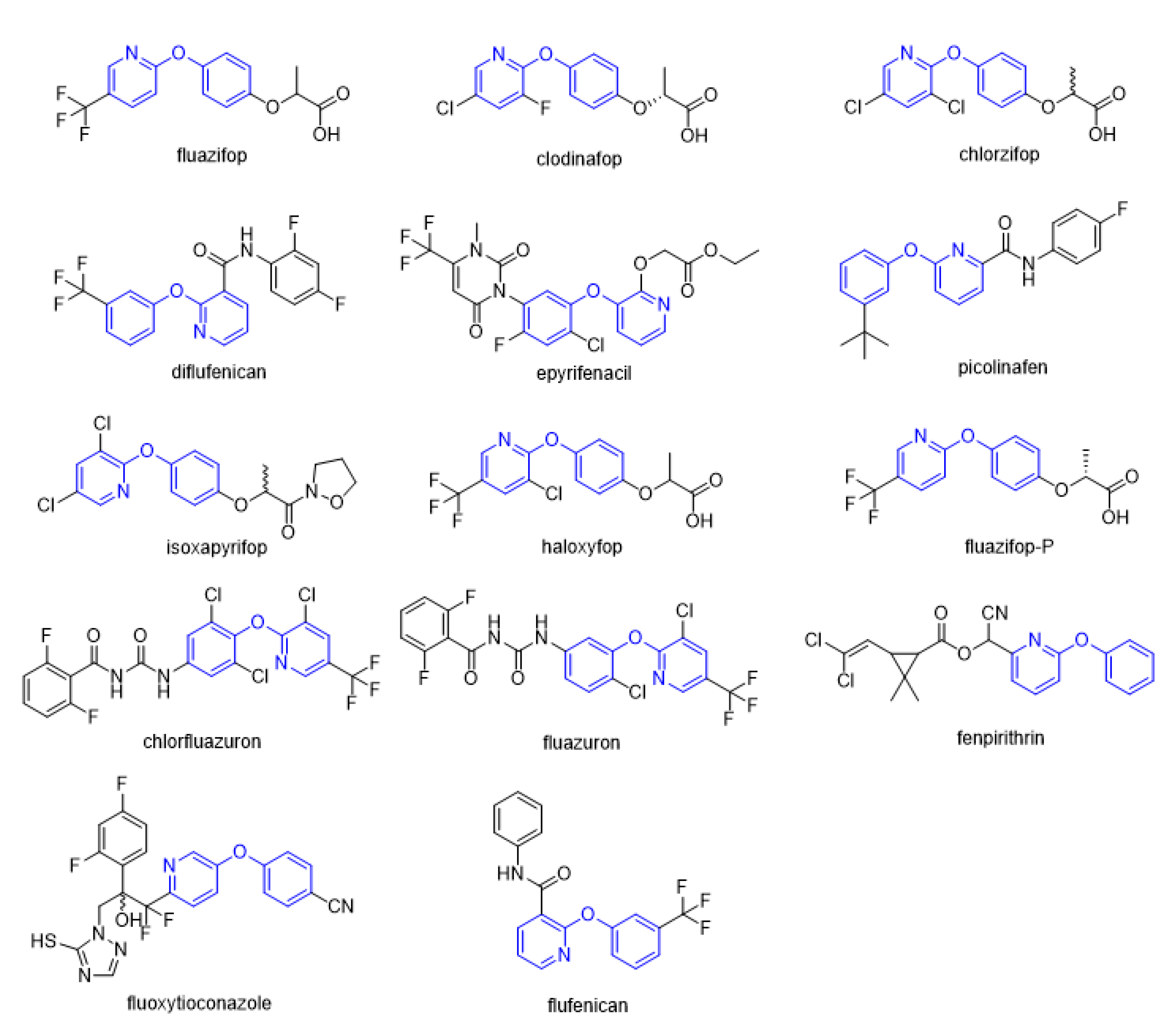
Figure 1.
Selected commercial pesticides containing phenoxypyridine.
2. Herbicides Containing Phenoxypyridine Scaffold
2.1. Acetyl CoA Carboxylase Inhibitors
Acetyl CoA carboxylase (ACCase) inhibitors [16,17] target ACCase [18] to inhibit fatty acid synthesis in gramineae plants. There are two classes of ACCase inhibitors: aryloxyphenoxypropionate [17] (AOPP or fop) and cyclohexanediones (CHD or dim). Aryloxyphenoxypropionate herbicides [19] occupy an important position in the world herbicide market which have characteristics of high efficiency, low toxicity, crop safety, and so on. In 1976, Ishihara discovered that the compound that was obtained by substituting the benzene ring on one side with a pyridine ring had higher herbicidal activity and launched the first aryloxyphenoxypropionate herbicide containing phenoxypyridine–pyrifenop [20]. Since then, extensive research on herbicides containing phenoxypyridine had been initiated.
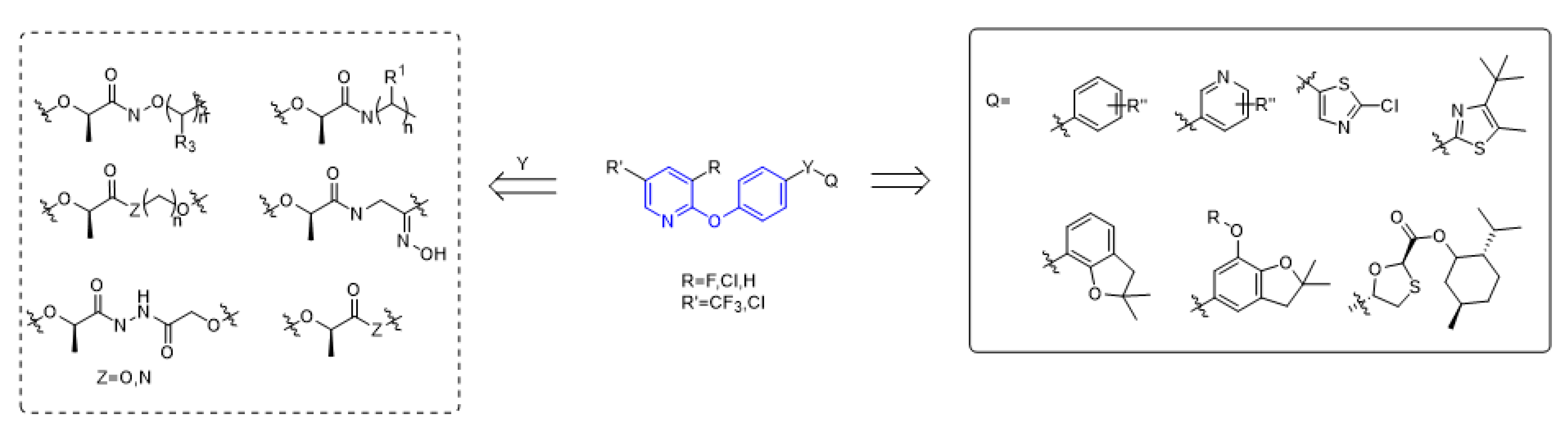
The structure of aryloxyphenoxypropionate herbicides containing phenoxypyridine is shown in the Figure 2, in which part A is phenoxypyridine with different substitutions, most of which were electron withdrawing groups, such as F, Cl, Br, NO2, CN, and CF3; part Y is the linking arm, where conformation R [19] was the active ingredient of herbicide; and part Q are various heterocycles, both aromatic and non-aromatic (pyridine, thiazole, benzofuran, etc.).
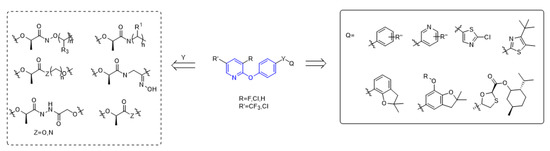
Figure 2.
The general structural formula of ACCase inhibitors.
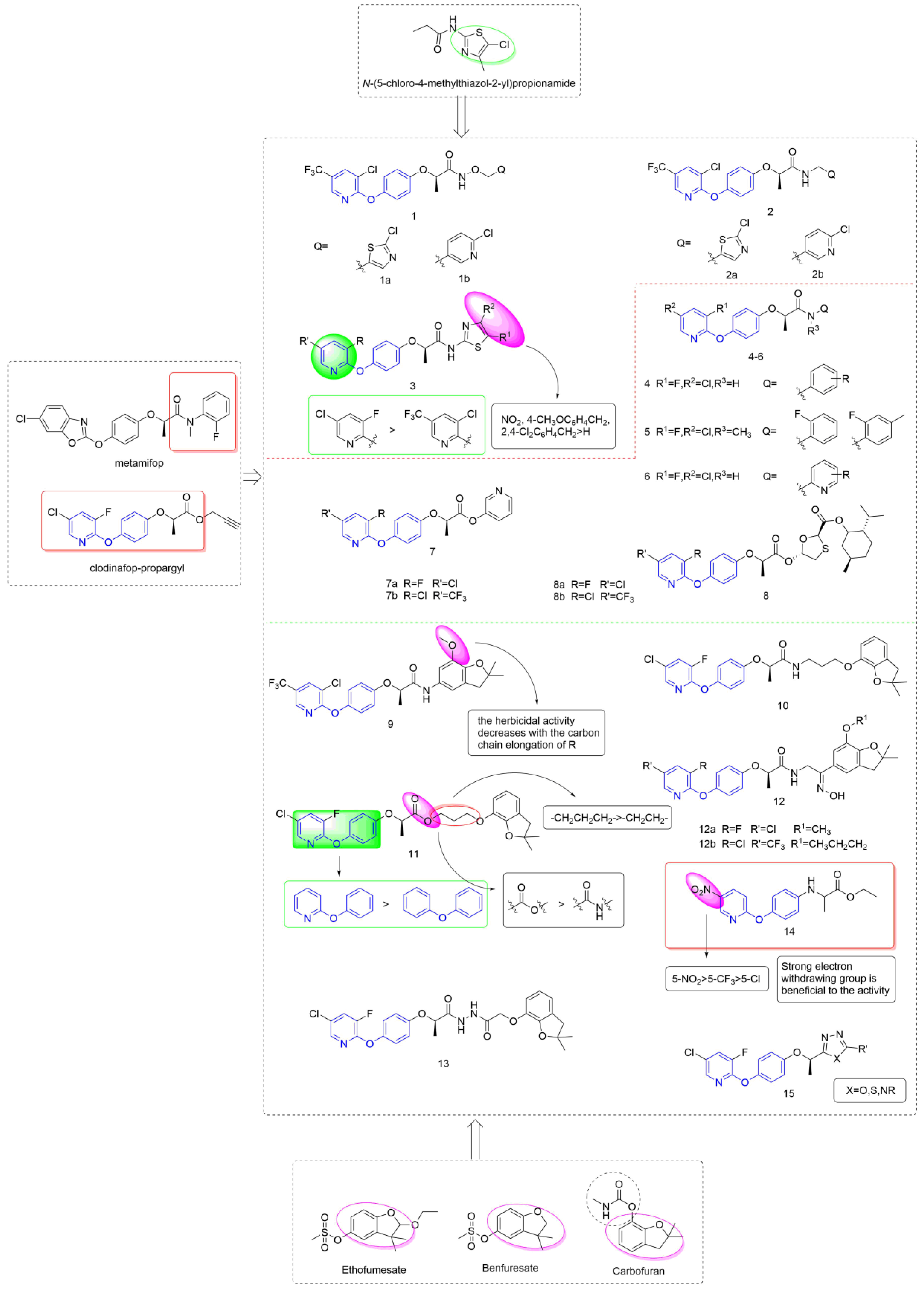
Taking metamifop and clodinafop as the leader, phenoxypyridine was linked to various aromatic rings through different linking arms to obtain active molecules with different structures, as shown in Figure 3. The synthesis of newer aryloxy phenoxycarboxylic acid amide derivative Compound 1 that was equipped with arylalkoxy was reported by Huang et al. [21] and assessed for herbicidal activities. Compounds 1a and 1b all showed 100% control efficiency against Digitaria sanguinalis in post-emergence applications, even at doses as low as 37.5 g a.i/ha and 18.75 g a.i/ha. Likewise, Wang et al. [22] designed and synthesized compound 2 by introducing an arylalkyl group into the structure of aryloxy phenoxycarboxylic acid amide. The inhibitory activity of Compounds 2a and 2b against Digitaria sanguinalis, Echinochloa crus-galli, and Setaria viridis under post-emergence was 100% at a dose of 60 g ai/ha. At the same time, Compound 2b was very safe for rice, and 2a was slightly less than 2b. The thiazole groups with different substituents were directly connected to an amido bond to yield Compound 3 [23], most of which showed a 100% inhibition rate against Digitaria sanguinalis, Echinochloa crus-galli and was comparable to metamifop. The effect of these compounds under post-emergence was slightly better than that under soil treatment, which could be used as post-seedling herbicide. The structure-activity relationship showed that 3-fluoro-5-clopyridine > 3-chloro-5-trifluoromethylpyridine; the order of influence of R1 groups was: NO2, 4-CH3OC6H4CH2, 2, 4-Cl2C6H4CH2>H.
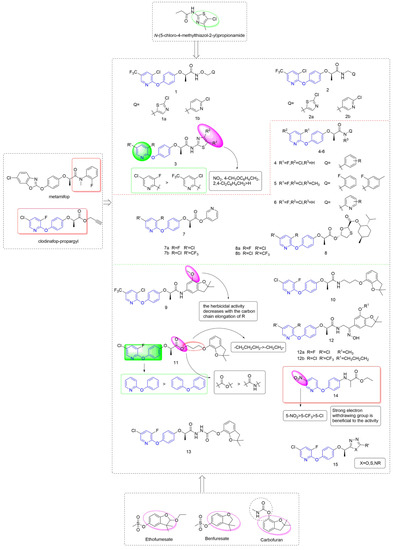
Figure 3.
ACCase inhibitors containing phenoxypyridine.
Using clodinafop-propargyl and metamifop as a lead structure, Compound 4 was synthesized by Yang et al. [24] through active group splicing and exhibited high selective herbicidal activity against monocotyledonous grass weeds (Beckmannia syzigachne (Steud.) Fern., Polypogon fugax Nees ex Steud., and Poa annua L.) at 150 g/ha. The chlorine-substituted target compound showed higher inhibitory activity against Polypogon fugax Nees ex Steud. than the compound with fluorine substituted. The control effect of Compound 5 that was synthesized by Xiao et al. [25] against Digitaria sanguinalis, Echinochloa crus-galli, and Setaria viridis was 100% at the dose of 85 g ai/ha. Compound 6 [26] showed 100% inhibitory activity against Echinochloa crus-galli and Setaria viridis at the concentration of 15 g ai/ha, and was safe for rice. Liu et al. proposed that aryloxyphenoxyalkanoic acid ester analogues 7 [27] and 8 [28] showed selective herbicidal activity against monocotyledonous weeds (Digitaria sanguinalis, Echinochloa crus-galli, and Setaria viridis) with more than 90% efficacy in both post-emergence and soil treatment at 5 g/mu.
Lin et al. [29,30] integrated a benzofuran unit into the scaffold of aryloxy phenoxycarboxylic acid amide to yield Compound 9. Compound 9 exhibited 100% control efficiency at the concentration of 2250 g/hm2 at both pre- and post-emergence applications. According to the SARs, substituents on pyridine had little effect on the herbicidal activity. The group of R plays a crucial role in herbicidal activities, and the herbicidal activity decreases with the increase of the carbon chain length of R. The aryloxyphenoxy propionamide could be linked with benzofuran via an alkoxy chain to give Compound 10 [31]. Compound 10 displayed a 98.7% inhibition rate against Echinochloa crus-galli whether with treatment by post-emergence or soil treatment at 25 g/mu. The linker between phenoxypyridine and benzofuran in Compound 10 was changed to an amido bond by Yan et al. [32], and the resulting compounds presented significantly better herbicidal activity against monocotyledonous weeds than dicotyledonous weeds. For monocotyledonous weeds, the herbicidal effect of post-emergence treatment was equivalent to that of pre-emergence treatment. Further analysis revealed that Compound 11 (100% inhibitory activity) exhibited better herbicidal activity than clodinafop-propargyl (89.9% and 84.7% inhibitory activity) against Echinochloa crus-galli either pre- or post-emergence application at 375 g.ai/ha.
The structure-activity relationship showed that the herbicidal activity of propionate derivatives somewhat exceeded that of propionamide derivatives. The results showed that increased lipid solubility was beneficial to the herbicidal activity of these compounds to a certain extent; the activity was significantly increased after the introduction of pyridine; in addition, the substitution in the pyridine ring had an important effect on the activity. For propionate derivatives, the activities of the compounds with n = 3 of alkyl chain were better than n = 2. However, for propionamide derivatives, increasing the length of the alkyl chain was less effective. In addition, it was confirmed by an enzyme activity test that 11 was a pro-herbicide [33], which acts in plants by hydrolyzing the ester into acid.
The structure of oxime was introduced to the skeleton by Hu et al. [34] to give Compound 12. Further analysis revealed that Compound 12a exhibited the highest herbicidal activity (100% inhibition rate) against Digitaria sanguinalis and Echinochloa crus-galli under soil treatment at a dose of 100 g/mu, and the control effect of Compound 12b under post-emergence was 100%. The phenoxypyridine could be linked with benzofuran via acylhydrazine to give aryloxyphenoxypropionic hydrazide derivatives and the herbicidal activity was tested by Yang et al. [35]. At the dose of 75 g/hm2, Compound 13 showed greater than 90% inhibition against Beckmannia syzigachne (Steud.) Fern. Under soil treatment, close to 100% inhibition against Eleusine indica (L.) Gaertn. when used post-emergence, and a certain inhibition effect on dicotyledonous weeds.
Xu et al. [36] took haloxyfop-methyl as the lead compound, introduced the structure of aryloxyanilino group based on bioisosterism, and designed a series of compounds containing pyridoxyanilino propionic acid/ethyl acetate. The new compounds showed a certain herbicidal activity against Echinochloa crus-galli, and the IC50 of 14 was 27.692 mg/L, which was at the similar level to that of the control haloxyfop-methyl (26.959 mg/L). Preliminary structure-activity relationships revealed that the new compounds exhibited enhanced herbicidal activity with the introduction of the strong electron-withdrawing substituent nitro on the pyridine ring. Moreover, the bioactivity of the compound with an electron-withdrawing substituent at position 3 of the pyridine ring was higher than at position 5. This provides a novel structural skeleton for the study of this class of compounds. Compound 15, which was reported by Kalhor et al. [37], showed fair to good activity, in which the 1,2,4 triazole structure contributed to the improvement of herbicidal activity and crop selectivity.
2.2. Protoporphyrinogen IX Oxidase Inhibitors
Protoporphyrinogen oxidase (PPO) [38] is a key enzyme in the biosynthesis of chlorophyll and heme in plants and is one of the important targets for the creation of novel herbicides. At present, PPO-inhibiting herbicides mainly include diphenyl ethers, phenylpyrazoles, triazolinones, N-phenyl phthalimides, and diazoles [39]. Among these herbicides, diphenyl ethers (DPEs) [40] had been widely studied by researchers in the creation of novel pesticides due to their high efficiency, low toxicity, high selectivity, and simple synthesis process; Ye Fei’s team committed to the research and development of PPO inhibitors for a long time. Several series of compounds (Figure 4) containing phenoxypyridine had been designed, studied for greenhouse herbicidal activity, PPO inhibitory activity, crop selectivity, and structure-activity relationships (SARs). These studies fully confirmed that phenoxypyridine provided good herbicidal activity.
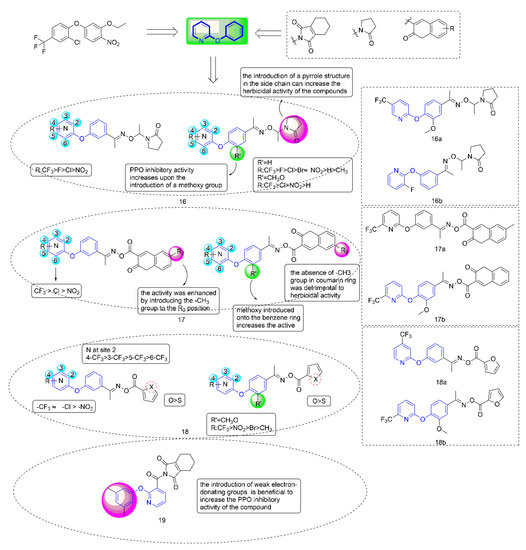
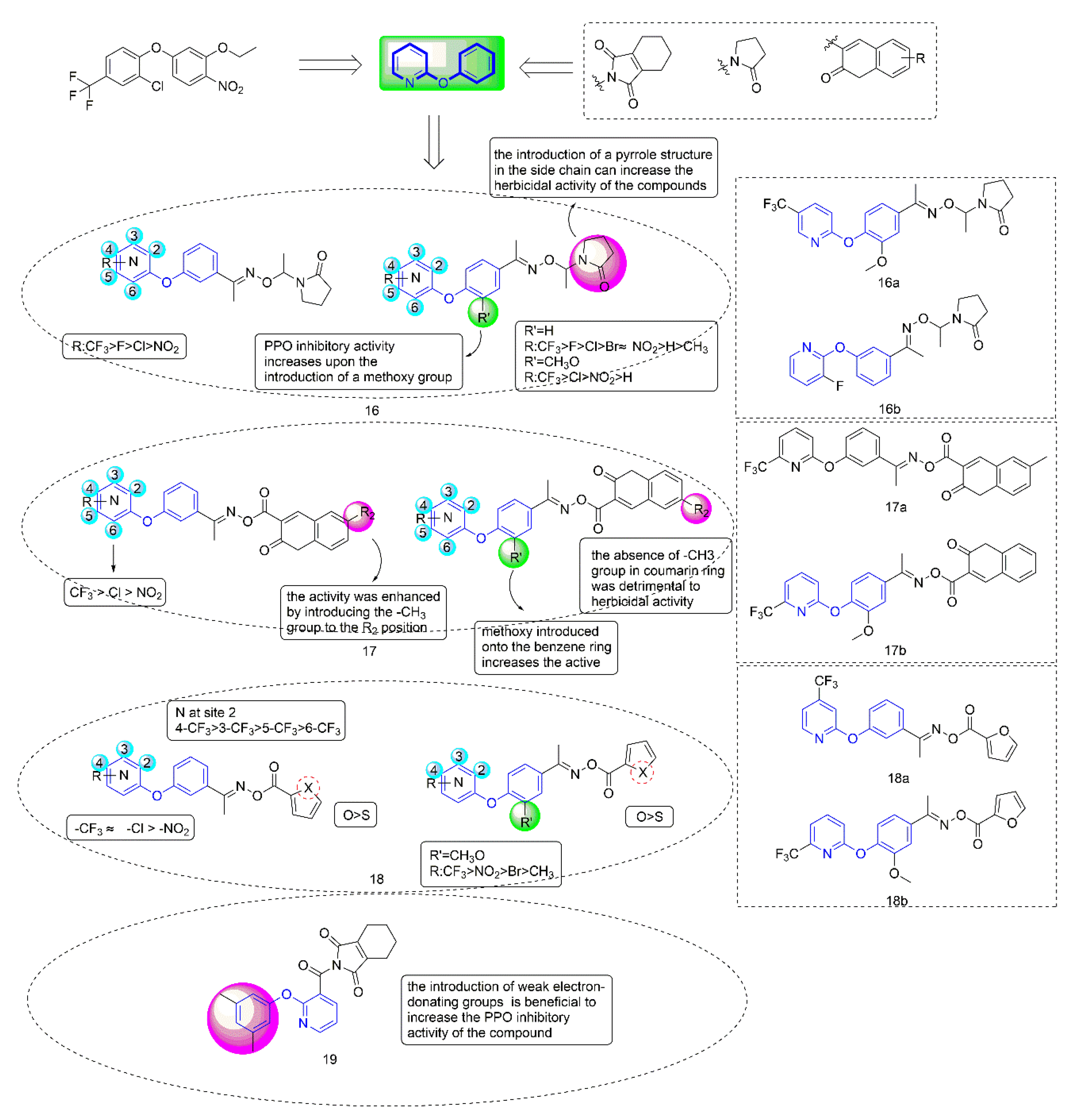
Figure 4.
Protoporphyrinogen IX oxidase inhibitors containing phenoxypyridine.
PPO inhibitors were known to compete with protogen IX by mimicking part of its structure, so they introduced pyrrolidone into the second ring side chain of diphenyl ether structure to simulate the three rings of protoporphyrinogen IX. Diphenyl ether derivatives with oxime substituents could significantly improve the herbicide activity and crop selectivity. Therefore, the Compound 16 series were designed and synthesized with the introduction of both oxime and pyrrolidone. Compounds 17 and 18 were synthesized by introducing coumarin and five-membered heterocycle, respectively.
When diphenyl ether was replaced by phenoxypyridine, the herbicidal activity and PPO inhibitory activity of the compounds were significantly increased, and herbicidal spectrum was significantly expanded. Most of the compounds showed strong PPO inhibitory activity in vitro, which was consistent with their herbicidal activity. Most compounds distinctly presented better inhibitory effects on dicotyledonous weeds than monocotyledonous weeds. Among them, the IC50 of Compound 16a [41] (IC50 = 0.041 mg/L), 16b [42] (IC50 = 0.0262 mg/L), 17a [43] (IC50 = 0.01937 mg/L), 17b [44] (IC50 = 0.01252 mg/L), 18a [45] (IC50 = 0.032 mg/L), and 18b [8] (IC50 = 0.0468 μmol/L) against PPO was consistent with or better than that of oxyfluorfen. At 150 g a.i./ha, Compound 16a achieved 100% inhibition against A. theophrasti for post-emergence treatment. The herbicidal activity of Compounds 17a and 17b reached level A at 300 g a.i./ha−1.
The structure-activity relationship indicated that the herbicidal activity of the compounds with electron-withdrawing substituents on the pyridine ring was significantly higher than that of the compounds with electron-donating substituents. The introduction of the pyrrole structure in the side chain could significantly improve the herbicidal activity of the compound. The introduction of a coumarin ring at the para position of phenoxypyridine was shown to enhance the inhibitory activity of target compounds. Meanwhile, the type of substituents that were introduced on the coumarin ring had a significant effect on the herbicidal activity of the compound. Compounds containing furan rings showed better herbicidal activity than compounds containing thiophene rings. The most critical finding for Compound 18 was that the introduction of a trifluoromethyl group on the pyridine ring increased the inhibitory activity against PPO and varied when changing the substitution position.
The typical characteristic of PPO inhibitors, which were previously known as albino herbicides, is that the weeds are bleached and curled to death by inhibiting chlorophyll synthesis. Most weeds exhibited unique bleaching that was consistent with the symptoms following PPO herbicides application. Compared with the corresponding diphenyl ether compounds, the compounds containing phenoxypyridine could significantly reduce Ca and Cb contents of A. retroflexus, indicating that the compounds containing phenoxypyridine had a better bleaching effect. Multiple crops showed strong tolerance to 16a (rice, peanut, and cotton), 16b (rice, peanut, and cotton), 17a (maize, cotton, and soybean), 18a (rice, wheat, maize, and soybean), and 18b (rice, wheat, maize, and soybean) at 300 g ai/ha. Field tests showed that the compound had a good inhibitory effect on weed growth. The amino acid residues PHE-392 and ARG-98 were important groups that were involved in the catalysis of porphyrins in organisms. Molecular docking results showed that compounds 16a, 16b, 17a, 18a, and 18b acted more tightly on the active site than oxyfluorfen. Most of them form two hydrogen bonds with surrounding amino acid residue AGR-98.
Considering that N-phenyl-phthalimide herbicides [46] had the advantages of fast degradation rate, short residual time, and no pollution to the environment [47], Zhao et al. [48] introduced tetrahydrophthalimide to improve the selectivity and degradability of herbicides. Compound 19 had an IC50 value of 0.00667 mg/L against PPO, and exhibited similar herbicidal activity to oxyfluorfen. The structure-activity relationships indicated that the introduction of weak electron-donating groups on the benzene ring of the compounds was beneficial to increase the PPO inhibitory activity of the compounds. When the phenoxypyridine structure was replaced with phenylthiopyridine, the PPO inhibitory activity of the compound was significantly reduced. Similarly, most of the tested weeds bleached and died. In general, Compound 19 had a bleaching effect on weeds, acted more tightly on the active site, and showed higher safety and selectivity, making 19 a potential new herbicide candidate in the field.
2.3. Other Herbicides
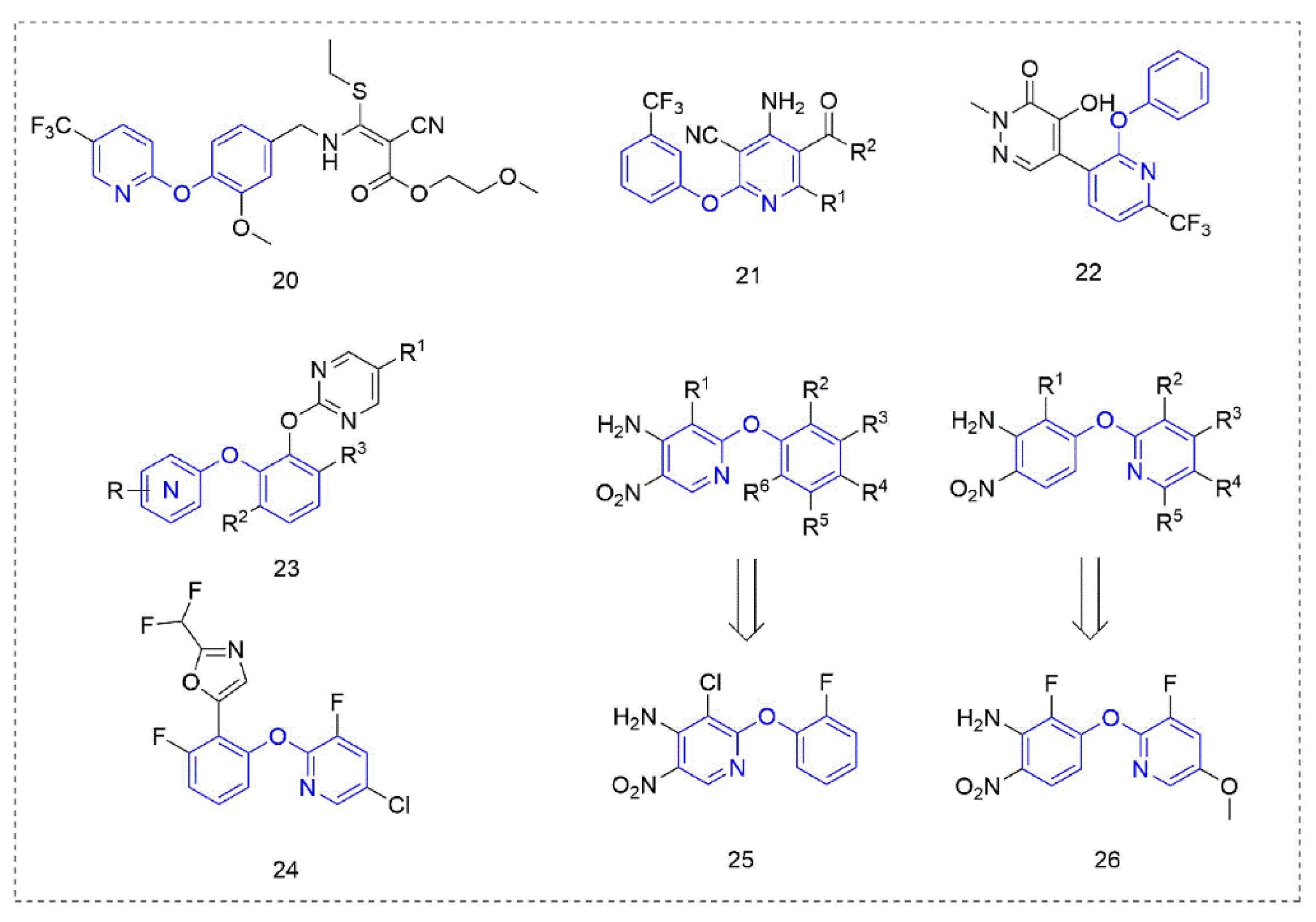
Cyanoacrylate derivatives [49] are photosystem II (PS II) inhibitors [50], which can control weeds by interfering with electron transfer in the photosynthetic system of the plant, preventing photosynthesis. This special mechanism makes cyanoacrylate extremely safe for animals, in line with the requirements of the current social market for new herbicides. The compounds (Figure 5) that were obtained by linking the trifluoromethyl-substituted phenoxypyridine unit with cyanoacrylate skeleton showed good herbicidal activity. The herbicidal activity of the target Compound 20 [51] against Digitaria sanguinalis, Echinochloa crus-galli, Abutilon theophrasti Medicus, Amaranthus retroflexus L., and Eclipta prostrata (L.) L. was 100%.
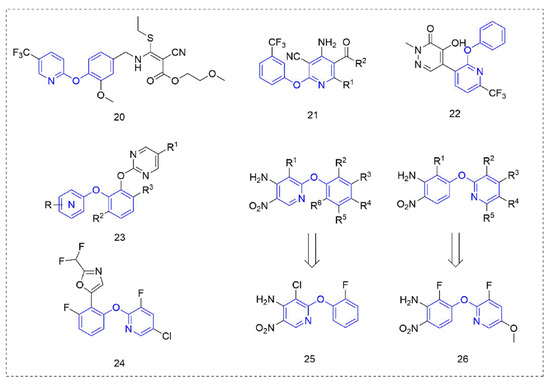
Figure 5.
Other compounds with herbicidal activity containing phenoxypyridine.
The mechanism of phytoene desaturase (PDS) inhibitors [52] is to inhibit the catalytic action of phytoene desaturase in the biosynthesis pathway of carotenoids, and then inhibit plant photosynthesis and cause the plant to stop growing until it dies. Therefore, PDS inhibitors belong to carotenoid biosynthesis inhibitors, and the most obvious manifestation of plants that are treated are albino symptoms [53]. Compound 21 was designed by Zhai et al. [54] based on picolinafen and diflufenican and showed moderate herbicide activity against Brassica campestris L at a concentration of 100 mg/L. Compounds, where R1 was an electron-donating substituent, showed better activity than those with an electron-withdrawing substituent, and when R2 was a methoxy group, the activity was better than that of an ethoxy group.
The pyridazinone Compound 22, reported by Syngenta [55], showed 80–100% activity against Solanum nigrum L. and Amaranthus retroflexus L. at 25 g a.i./ha. The bis(aryl)catechol derivatives 23 designed and synthesized by DuPont [56] had excellent inhibitory activity against a variety of weeds. The novel herbicidal phenoxypyridine compounds that were reported by Syngenta [57] showed improved properties compared to the known pyrimidine compounds—especially improving crop (soybean) selectivity. Compound 24 had significant effects on various weeds (Lolium perenne, Solanum nigrum, Amaranthus retoflexus, Setaria faberi, Echinochloa crus-galli, and Ipomoea hederacea) at a concentration of 500 g/ha. In 2020, two kinds of phenoxypyridine-containing compounds with herbicidal activity were discovered and reported by Bayer [58,59]. At 1280 g/ha, Compounds 25 and 26 showed more than 90% activity against a variety of weeds whether with treatment by preemergence (Amaranthus retroflexus, Stellaria media, and Veronica persica) or post-emergence (Poa annua, Amaranthus retroflexus, Stellaria media, and Bassia scoparia).
3. Fungicides and Bactericides Containing Phenoxypyridine Scaffold
3.1. Complex I Inhibitors
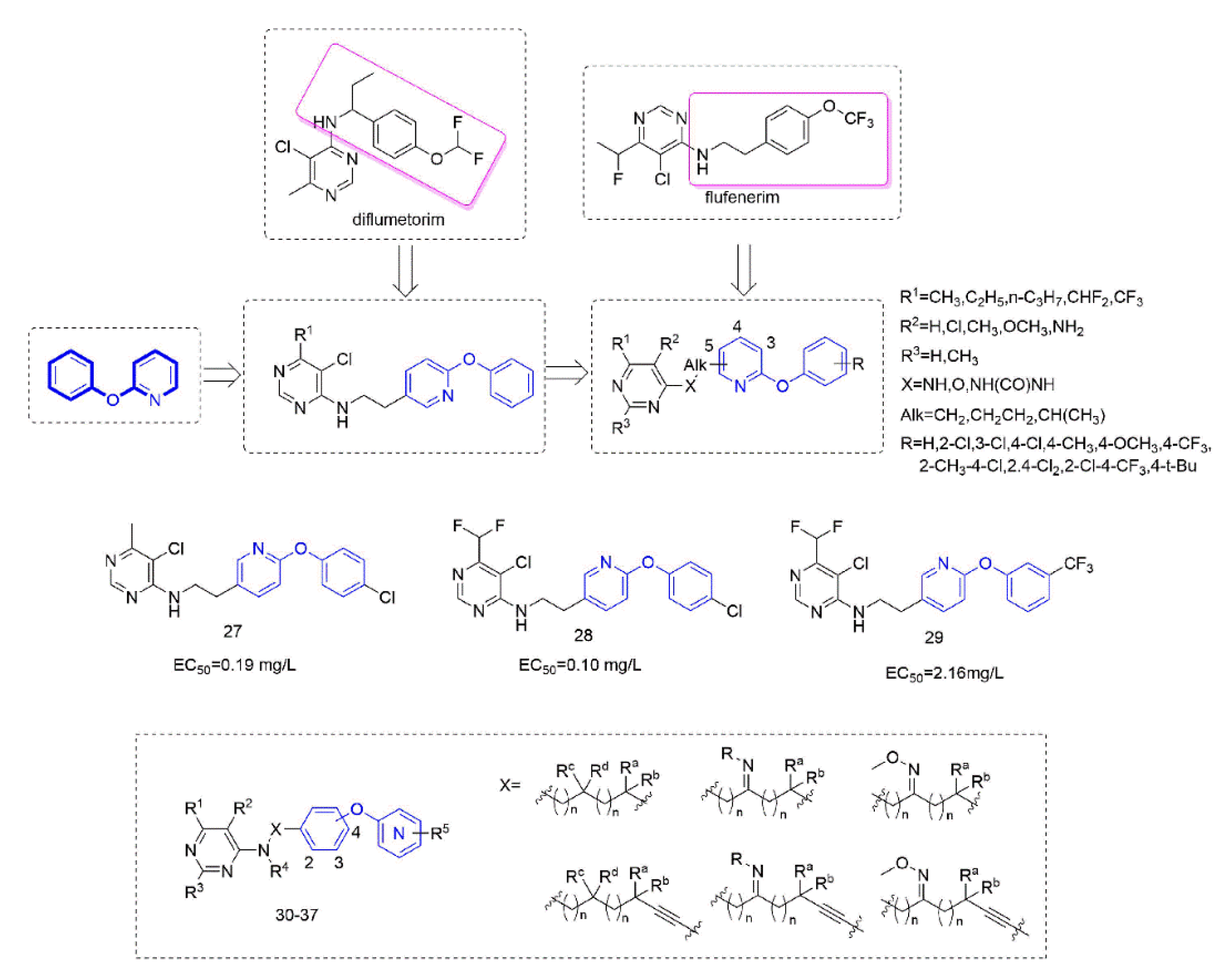
Diflumetorim is a member of aminoalkylpyrimidines [60] targeting mitochondrial complex I (MET I) [61] which has a unique mode of action that is different from the MET I inhibitor acting as insecticide [62]. Therefore, it has no cross-resistance with existing traditional fungicides and is safe for non-target organisms. Liu and co-workers devoted to the research of pyrimidine amine compounds (Figure 6), and the fungicidal activity of the compounds that were synthesized by introducing a phenoxypyridine structure was significantly improved.
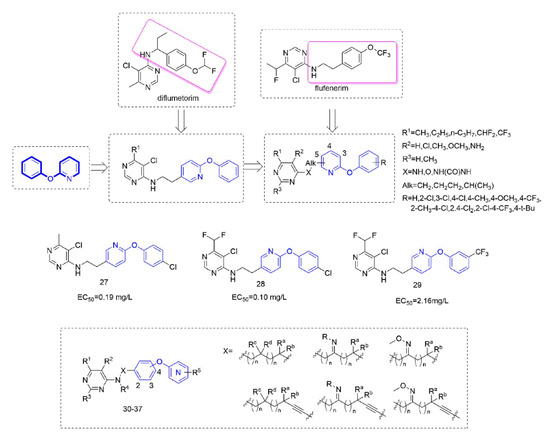
Figure 6.
Complex I inhibitors containing phenoxypyridine.
Several series of aminoalkylpyrimidine analogs containing phenoxypyridine fragments were designed and synthesized to study the control effect of cucumber downy mildew and the structure-activity relationships. The structure-activity relationships indicated that the compounds with Alk = CH2CH2 exhibited higher fungicidal activity than the corresponding analogues with Alk = CH2. When the pyrimidine group was attached to the pyridine ring at position 3 or 4, the fungicidal activity of these compounds decreases sharply. The substitutions of R1 and R2 on the pyrimidine ring were critical to exert fungicidal activity, while R3 does not contribute significantly to enhance fungicidal activity. Compounds containing phenoxypyridine had better activity than those containing diphenyl ether. Among them, the activity of Compounds 27 [63] (EC50 = 0.19 mg/L) and 28 [15] (EC50 = 0.10 mg/L) against cucumber downy mildew was significantly higher than that of diflumetorim (EC50 = 23.06 mg/L). In addition, the researchers found that the introduction of phenoxypyridine led to a significant increase in the activity against southern corn rust (SCR). The newly designed Compound 29 [64] displayed an EC50 value of 2.16 mg/L, which was superior to the commercial control diflumetorim. (EC50 = 53.26 mg/L). In the past few years, BASF had reported several aminoalkylpyrimidine derivates 30–37 [65,66,67,68,69,70,71,72]. As shown in the Figure 6, phenoxypyridine was linked to aminoalkylpyrimidine in various link arms resulting in some molecules with good protective fungicidal activity.
3.2. Complex III Inhibitors
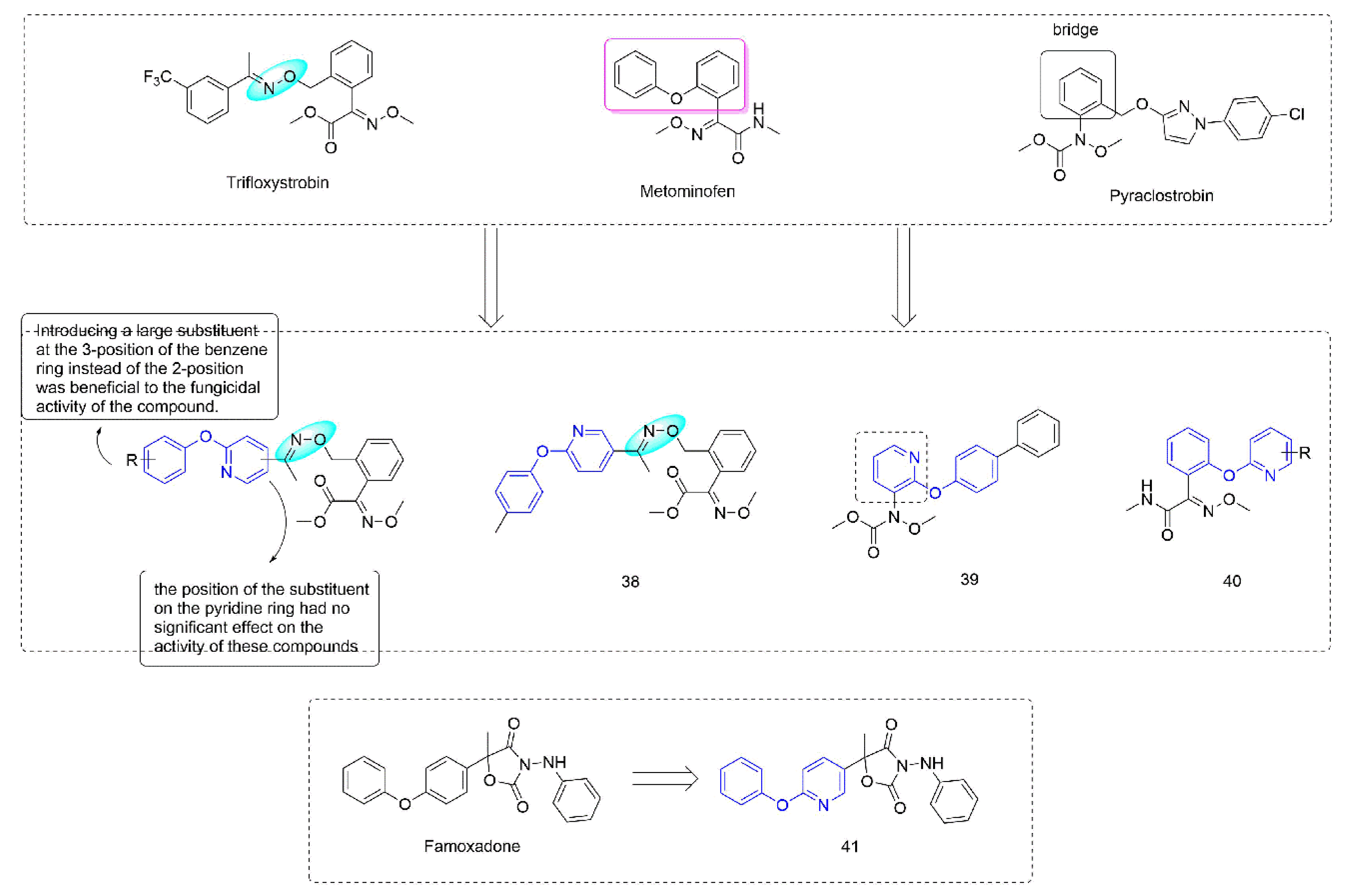
Strobilurin [73] were derived from strobilurin A [74], a natural antibiotic with bactericidal activity, and were a kind of agricultural fungicide with great development potential and market vitality [75,76]. Strobilurins act on the Qo site of mitochondrial electron transport chain complex III and are also known as Qo site inhibitors. Some strobilurin derivatives containing phenoxypyridine are shown in Figure 7. A series of strobilurin analogues containing oxime ether structures were synthesized through introducing a phenoxypyridine group by Liu et al. [77]. Most of the compounds showed good fungicidal activity, with a significantly broadened antifungal spectrum compared to the compounds containing diaryl ether previously that were reported by BASF [78], among which the EC50 of 38 [79] against Sclerotinia sclerotiorum could reach 0.47 μg/mL. The trans-configuration was a dominant configuration. The disubstituted compounds on the benzene ring were less active than the monosubstituted compounds. Wang et al. [80] constructed a phenoxypyridine structure by modifying the bridge structure in strobilurins. The newly synthesized compounds showed certain fungicidal activity, among which the IC50 values of 39 against Botrytis cinerea and Sclerotinia sclerotiorum could reach 0.98 μg/mL and 0.64 μg/mL, respectively. The alkoxyiminoacetamide derivatives 40, reported by Hayase et al. [81], had good activity against a variety of pathogenic fungi (Botrytis cinerea, Pseudoperonospora cubensis, Sphaerotheca fuliginea, and Pyricularia oryzae). The pyramoxadone 41, developed by Qin et al. [9], had strong inhibitory activity against a variety of plant pathogens (Rhizoctonia solani, Pythium aphanidermatum, Pyricularia grisea, Phytophthora capsica, and Phomopsis asparagi (Sacc.) Bubak). Meanwhile, the IC50 value of the inhibitory activity of pyramoxadone against sporangium release of Phytophthora capsica was 13.85 μg/mL.
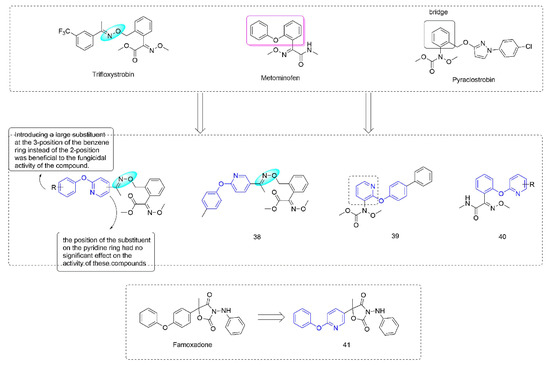
Figure 7.
Complex III inhibitors containing phenoxypyridine.
3.3. Sterol Biosynthesis Inhibitors
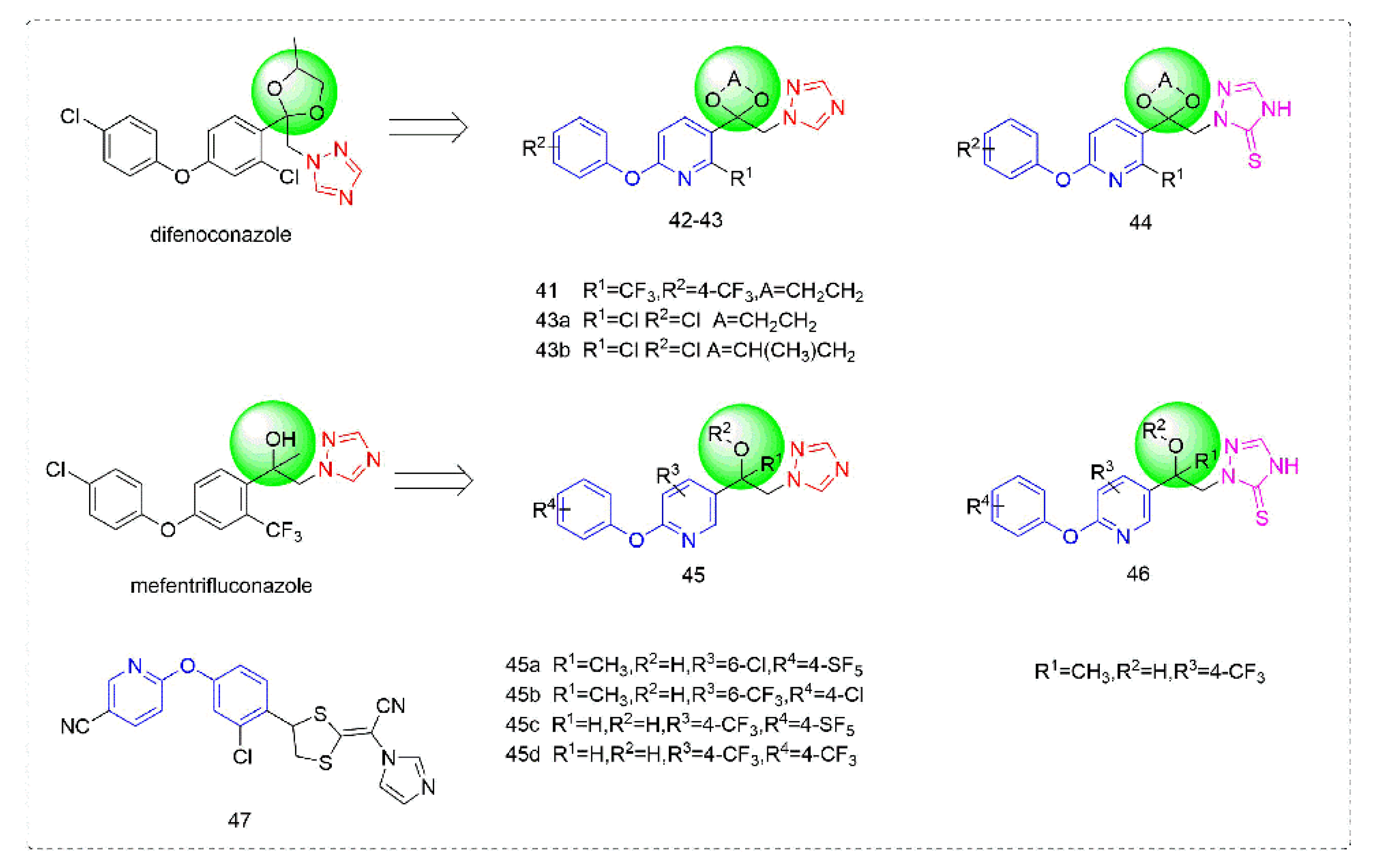
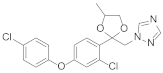
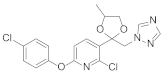
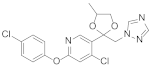
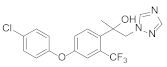
Triazole fungicides are a new type of fungicide with broad spectrum, high efficiency, low residue, long effect, good systemic translocation, and both protective and curative effects. Triazole fungicides belong to ergosterol biosynthesis inhibitors, which mainly inhibit the activity of sterol 14α-demethylase in sterol biosynthesis to achieve fungicidal effects [82,83]. The triazole derivatives (Figure 8) that were synthesized by Bayer exhibited good protective activity against a variety of pathogenic fungi (Puccinia recondite, Sphaerotheca fuliginea, Uromyces appendiculatus, and Blumeria). The protective activity of compound 42 [84] against Septoria tritici reached 100% at 100 mg/L. The ED50 of Compound 43a [11] against Alternaria and Pyricularia oryzae Cav. reached 0.12 mg/L and 0.56 mg/L, respectively, while 43b [11] was 2.7 mg/L, 0.008 mg/L and 1.2 mg/L against Botrytis cinerea, Sphaerotheca fuliginea and Pyricularia oryzae Cav. 44a, 45b, 42c, and 45d [14] showed 100% protective activity against Sphaerotheca at 10 mg/L. Compound 46 [85] had 90–100% control effect against various pathogens at 500 mg/L. Some imidazole derivatives (such as clotrimazole, ketoconazole, imidazole, and oxazole) also inhibited 14 α-demethylase (CYP51). Jeanmart et al. [86] reported a series of novel compounds that were based on the modification of imidazole-based ketene dithioacetals lanoconazole and luliconazole. Compound 47 with the ketene dithioacetal [87,88] scaffold showed certain fungicidal activity, with 79% inhibitory activity against Alternaria solani.
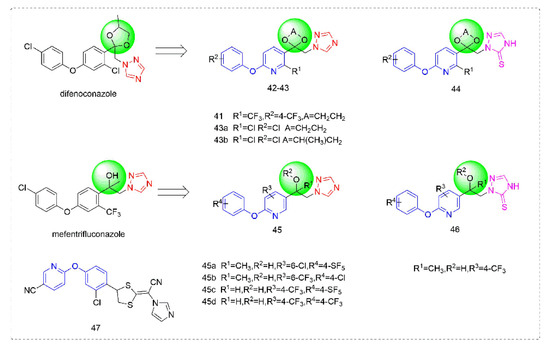
Figure 8.
Sterol biosynthesis inhibitors containing phenoxypyridine.
3.4. Succinate Dehydrogenase Inhibitors
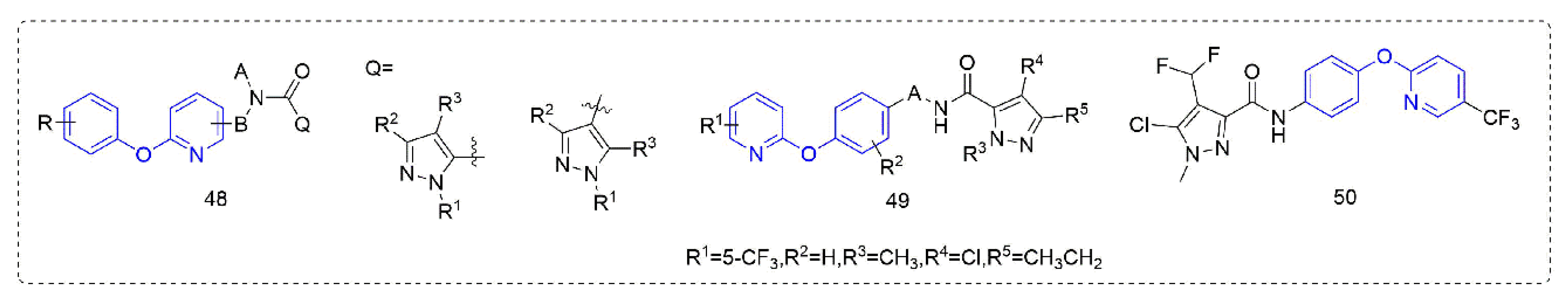
Succinate dehydrogenase inhibitors are a class of fungicides with a long history of development, accounting for a considerable proportion of fungicides. Succinate dehydrogenase inhibitors mainly bind to the ubiquinone pocket of SDH and mainly affect the electron transfer of the respiratory chain, to inhibit the growth of pathogenic fungi and eventually lead to death. Most of the pyrazole amide, such as Compounds 48 (Figure 9), that were designed and synthesized by Guan et al. [89] showed good protective activity against Pseudoperonospora cubensis (Berk.et Curt.) Rostov., Blumeria graminis, and Puccinia sorghi in addition to certain insecticidal activity. The control effect of Compound 49 [90] against Pseudoperonospora cubensis (Berk.et Curt.) Rostov. was 100% at 12.5 ppm, and the control effect in the field was also better than that of dimethomorph. The activity of Compound 50 which was synthesized by Sun et al. [91] against Pyricularia grisea was significantly better than that of diphenyl ether and other skeleton compounds, with an EC50 value of 2.286 μg/mL, similar to fluxapyroxad (2.101 μg/mL), more than eight-fold higher than isopyrazam and more than 15-fold higher than the aminopyralid boscalid. Preliminary mechanistic studies suggested that these compounds may not be SDH inhibitors, but inhibited fungal growth by inducing plant defense responses.
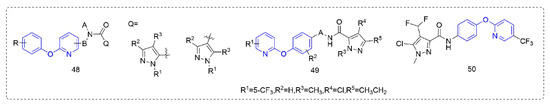
Figure 9.
Succinate dehydrogenase inhibitors containing phenoxypyridine.
3.5. Other Fungicides and Bactericides
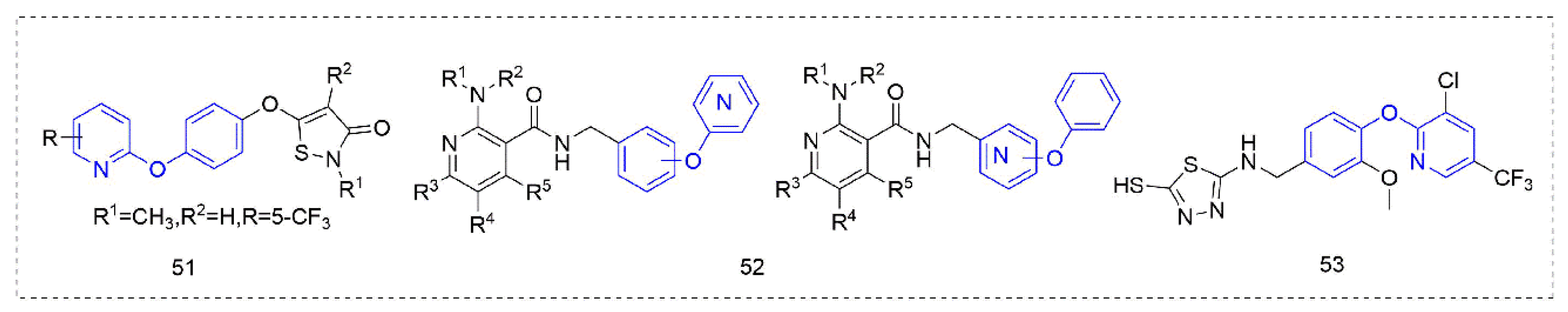
Some other types of compounds containing phenoxypyridine structures with fungicidal or bactericidal activity are summarized in Figure 10. Phenoxypyridine was linked to isothiazolinone, resulting in Compound 51 [92] with good control effects on Blumeria graminis, Botrytis cinereal, and Pyricularia grisea at low doses. The introduction of chlorine at 4-position of isothiazolinone made the compound lose its inhibitory effect on Botrytis cinereal [93]. The Compounds 52 that were synthesized by Nippon Soda Co., Ltd. (Tokyo, Japan) [94] showed more than a 75% control effect against cucumber gray mold at 500 mg/L and did not cause any damage to the plant.
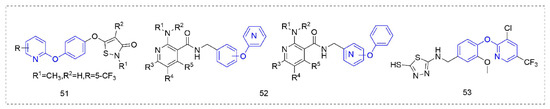
Figure 10.
Other compounds with fungicidal or bactericidal activity containing phenoxypyridine.
A series of vanillin derivatives [95] containing 1,3,4-thiadiazole moiety were synthesized and their antibacterial activities were evaluated against Xanthomonas oryzae pv. oryzae (Xoo) and Xanthomonas oryzae pv. oryzicola (Xoc). Among them, Compound 53 [96] showed good antibacterial activity against Xoo and Xoc with EC50 values of 38.74 μg/mL and 46.97 μg/mL, respectively. The preliminary mechanism of action of these compounds were explored, and it was found that these compounds could inhibit the production of exopolysaccharides of Xoo and increase the permeability of the cell membrane.
4. Insecticides Containing Phenoxypyridine Scaffold
4.1. Transient Receptor Potential Vanilloid Channel Blockers
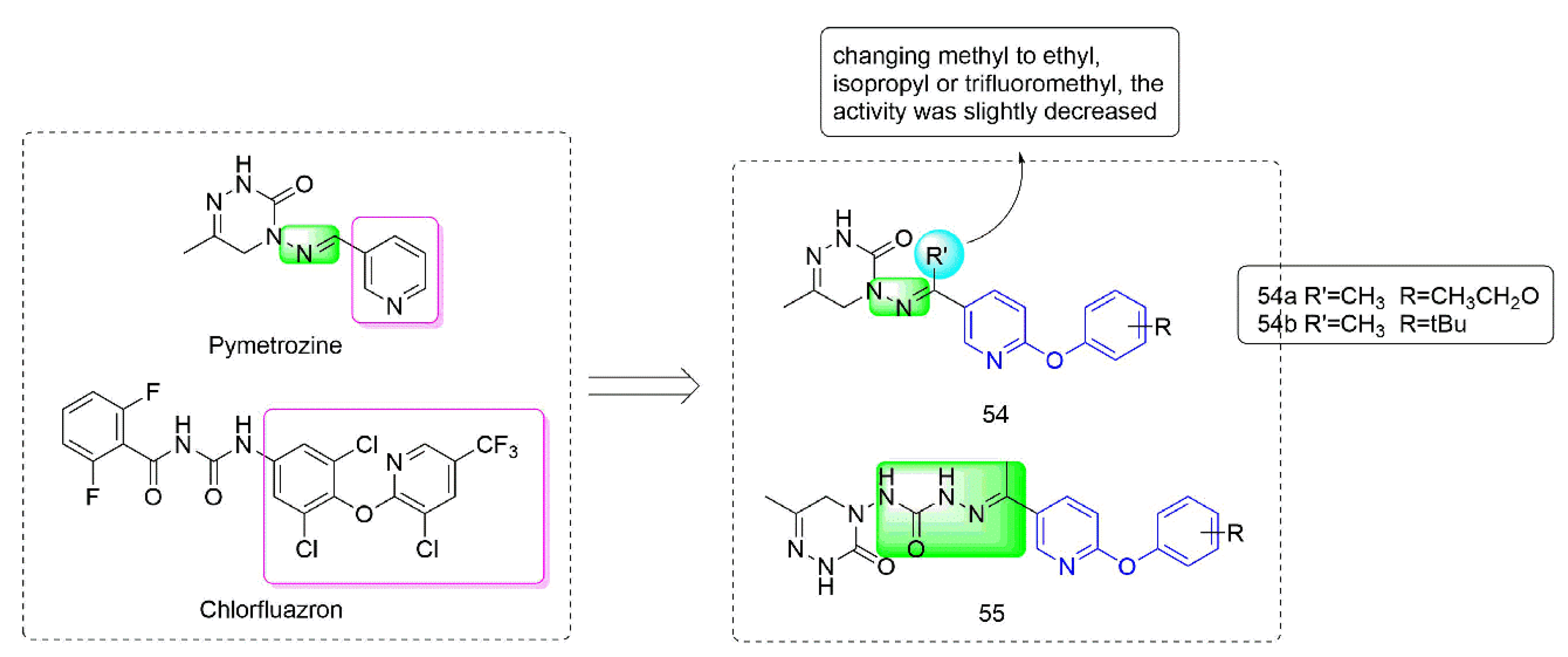
Pymetrozine [97] is a triazinone insecticide [98] that acts on the specific insecticide target protein transient receptor potential vanilloid (TRPV) ion channel, and showed no cross-resistance with other insecticides [99]. Compounds 54–55 (Figure 11) were developed by Nankai University with both phenoxypyridine groups and triazinone groups. The activity against aphids of Compound 54, which was synthesized by Yang et al. [100] by constructing phenoxypyridine structure and introducing a methyl group to the imino group, was significantly improved. At the concentration of 5 mg/kg, the activities against aphids of 54a (80%) and 54b (80%) were both higher than those of pymetrozine (30%). Meanwhile, 54 also exhibited significant insecticidal activity against mosquitoes and lepidopteran pests (cotton bollworm, corn borer, and oriental stick insect). By modifying the linker arm, Wang et al. [101,102] designed and synthesized a series of triazinone derivatives 55 containing an acylhydrazone structure. These compounds had certain activities against aphids, cotton bollworm, corn borer, and armyworm.
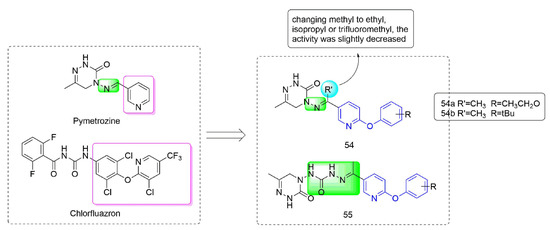
Figure 11.
Transient receptor potential vanilloid channel blockers containing phenoxypyridine.
4.2. Complex I Inhibitors
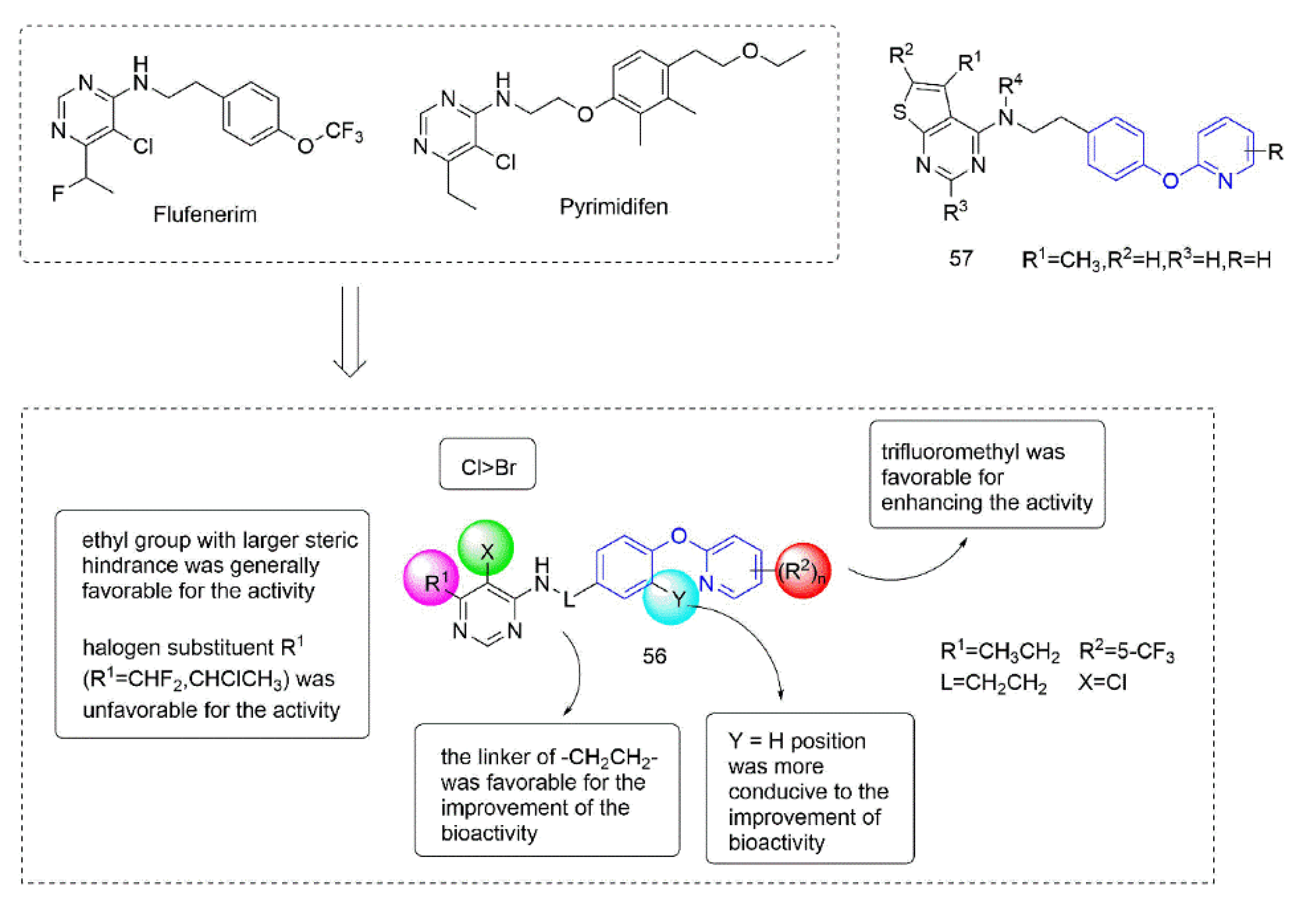
Some insecticides and acaricides (flufenerim, purimidifen, tebufenpyrad, and tolfenpyrad [103]) worked by inhibiting the mitochondrial electron transport (MET) at complex I to disrupt respiration, known as complex I inhibitors [104]. Most of the 4-aminopyrimidine [105] derivatives that were synthesized by Wang et al. [106] through intermediate derivatization methods showed good activity against Myzus persicae, among which 56 (Figure 12) had the highest activity and the lowest LC50 value of 0.34 mg/L. The structure-activity relationships suggested that the linker of -CH2CH2- was favorable for bioactivity; the halogen substituent at the X position (X = Cl, Br) was more beneficial to the activity; for R1, the ethyl group with large steric resistance was generally conducive to improve the activity. The substituted thienopyrimidine amines 57 (Figure 12) that were synthesized by Chai et al. [107] had broad-spectrum insecticidal and acaricidal activity, which were very effective against lepidoptera pests, homoptera, and mites even at a very low dose, especially against aphids, Tetranychus cinarcini, Plutella xylodes, and armyworm.
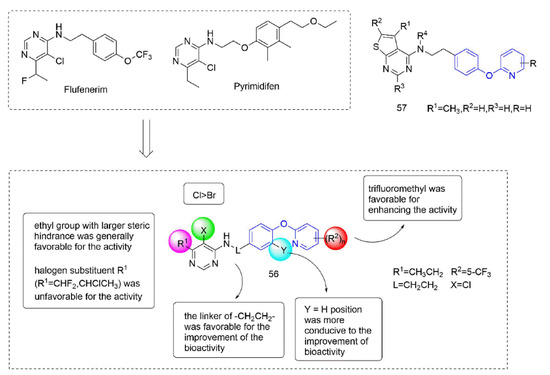
Figure 12.
Complex I inhibitors (4-aminopyrimidine) containing phenoxypyridine.
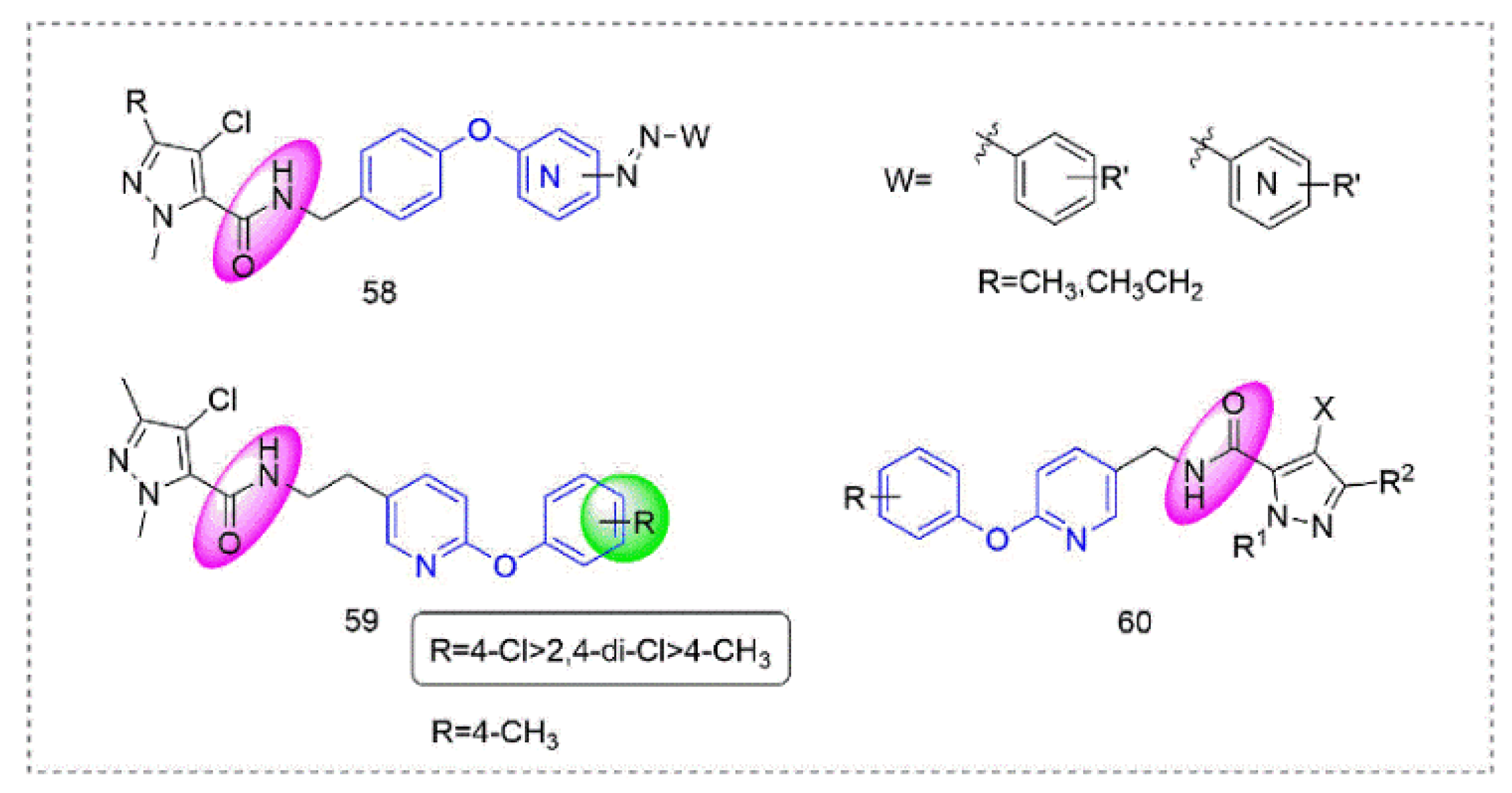
Pyrazole-5-carboxamide insecticides 58 (Figure 13) containing an azo structure were synthesized by Shao et al. [108], many of which had 100% activity against Aphis craccivora Koch and Tetranychus cinnabarinus. Compound 59 [109] showed broad-spectrum insecticidal activity and a 100% mortality rate against Plutella xylostella and Myzus persicae at 600 mg/L. At the same time, several compounds had good activity against Blumeria graminis and southern corn rust. Pyrazole derivatives 60 that were designed and synthesized by Okada et al. [110] had good insecticidal activity against various insect pests (Plutella xylostella, Nilaparvata lugens, and the eggs and adults of Tetranychus urticae).
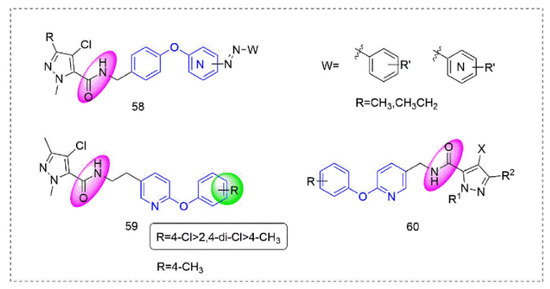
Figure 13.
Complex I inhibitors (Pyrazole-5-carboxamide) containing phenoxypyridine.
4.3. Other Insecticides
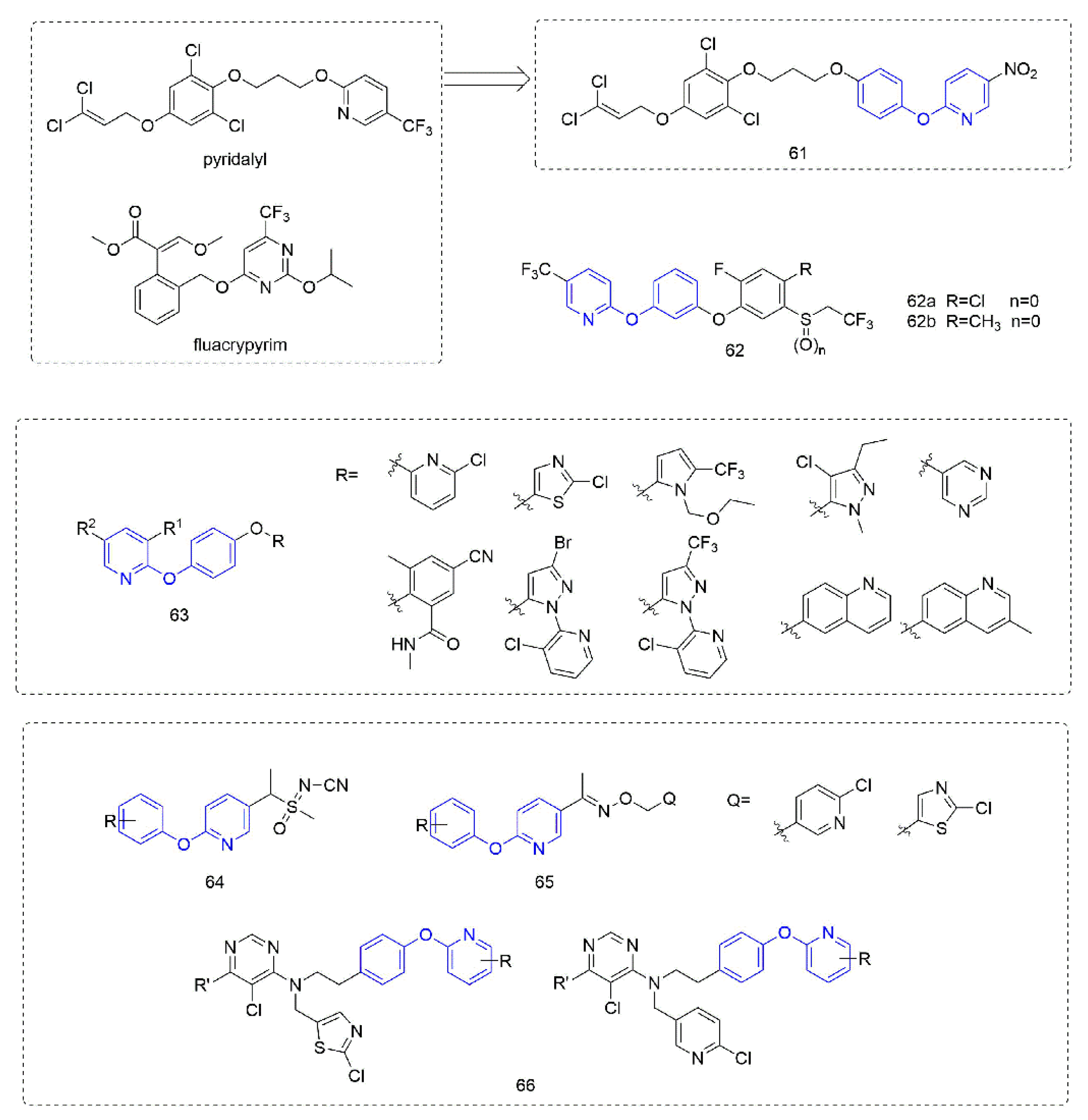
Phenoxypyridine-containing compounds with insecticidal activity are summarized in Figure 14. Pyridalyl [111] inhibited cellular protein synthesis in insect cell lines but not mammalian cell lines. The novel dihalopropene ether insecticides that were synthesized by Liu et al. [112] exhibited good insecticidal activity. The LC50 of Compound 61, which introduced phenoxypyridine, was 4.05 mg/L and 9.82 mg/L against M. separate and P. litura, respectively, was better than the control pyridalyl (LC50 = 4.81 mg/L and 10.07 mg/L) and better than the compounds with other aromatic ring substitutions. Alkylphenyl sulfide derivatives 62 that was reported by Kumiai Chemical Industry Co., Ltd. [113] had more than 90% control of Tetranychus urticae (Koch) at a concentration of 4 mg/L. Inspired by juvenile hormone, the analogues 63 that were prepared by Li et al. [114] with the introduction of phenoxypyridine were more than 85% effective against Nilaparvata lugens at a concentration of 200 mg/L. Using phenoxypyridine molecular plug-ins, the sulfoximine and oxime ether, Compounds 64 and 65 with insecticidal activity were synthesized by Liu et al. [115] and Du et al. [116]. The neonicotinoids 66 that was designed and synthesized by Tang et al. [117] had certain activities against lepidoptera, homoptera, coleoptera, and the larvae and adults of orthoptera.
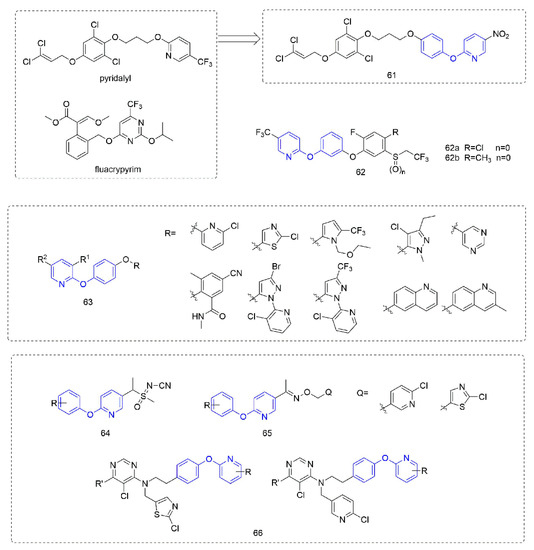
Figure 14.
Other compounds with insecticidal activity containing phenoxypyridine.
5. Conclusions
In pesticide applications, phenoxypyridine played an important role in the development of lead compounds. Compounds that were derived by linking phenoxypyridine to different active fragments or changing the substituents of phenoxypyridine exhibited a wide range of biological activity, such as herbicidal, fungicidal, bactericidal, and insecticidal activities. In this paper, the derivatives with different activities were classified. The summary of the structure-activity relationship of the derivatives indicated that structural modifications at different positions of phenoxypyridine could improve its activity. Previous studies had focused on compounds that were linked to the phenoxy group at position 2 of pyridine, possibly due to the difficulty of synthesis, so the relationship between the position of the N atom on pyridine and biological activity was unclear. The inhibitory effects of these compounds may be performed by different mechanisms and, therefore, further studies on the mechanism (or targets) are necessary for better evaluations. Still, a lot of activity of phenoxypyridine needs to be prospected in bactericides. In conclusion, phenoxypyridine could be considered as the promising active scaffold for pesticides.
Author Contributions
Conceptualization, Y.L. and Z.Q.; literature search, Y.L., B.F., Y.X. and B.R.; writing original draft preparation, Y.L.; writing review and editing, Y.L. and Z.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21877125).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bedos-Belval, F.; Rouch, A.; Vanucci-Bacque, C.; Baltas, M. Diaryl ether derivatives as anticancer agents—A review. Medchemcomm 2012, 3, 1356–1372. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, H.; Yang, J.F.; Zhu, X.L.; Qu, R.Y.; Yang, G.F. Diaryl Ether: A Privileged Scaffold for Drug and Agrochemical Discovery. J. Agric. Food Chem. 2020, 68, 9839–9877. [Google Scholar] [CrossRef] [PubMed]
- Ujihara, K. The history of extensive structural modifications of pyrethroids. J. Pestic. Sci. 2019, 44, 215–224. [Google Scholar] [CrossRef]
- Shimizu, S.; Watanabe, N.; Kataoka, T.; Shoji, T.; Abe, N.; Morishita, S.; Ichimura, H. Pyridine and Pyridine Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; Volume 30, p. a22-399. [Google Scholar]
- Guan, A.Y.; Liu, C.L.; Sun, X.F.; Xie, Y.; Wang, M.A. Discovery of pyridine-based agrochemicals by using Intermediate Derivatization Methods. Bioorganic Med. Chem. 2016, 24, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Antiviral Activities of Pyridine Fused and Pyridine Containing Heterocy-cles, A Review (from 2000 to 2020). Mini-Rev. Med. Chem. 2021, 21, 2584–2611. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Li, Q.; Wen, K.; Ismail, I.; Liu, D.D.; Niu, C.W.; Wen, X.; Yang, G.F.; Xi, Z. Synthesis and Herbicidal Activity of Pyrido[2,3-d]pyrimidine-2,4-dione-Benzoxazinone Hybrids as Protoporphyrinogen Oxidase Inhibitors. J. Agric. Food Chem. 2017, 65, 5278–5286. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Peng, J.F.; Liu, F.Y.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Design, Synthesis, and Herbicidal Activity of Diphenyl Ether Derivatives Containing a Five-Membered Heterocycle. J. Agric. Food Chem. 2022, 70, 1003–1018. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.K.; Ovejero, R.F.L.; Belchior, G.G.; Maymone, G.P.L.; Dayan, F.E. ACCase-inhibiting herbicides: Mechanism of action, resistance evolution and stewardship. Sci. Agric. 2021, 78, e20190102. [Google Scholar] [CrossRef]
- Kukorelli, G.; Reisinger, P.; Pinke, G. ACCase inhibitor herbicides—Selectivity, weed resistance and fitness cost: A review. Int. J. Pest Manag. 2013, 59, 165–173. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, G.; Zhang, A.; Zheng, L.; Cao, S. Study on the enantioselectivity inhibition mechanism of acetyl-coenzyme A carboxylase toward haloxyfop by homology modeling and MM-PBSA analysis. J. Mol. Model. 2012, 18, 3783–3792. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.A.; Pernich, D.J. Origin of enantiomeric selectivity in the aryloxyphenoxypropionic acid class of herbicidal acetyl coenzyme A carboxylase (ACCase) inhibitors. J. Agric. Food Chem. 2002, 50, 4554–4566. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Langelueddeke, P.; Leditschke, H.; Nahm, H.; Schwerdtle, F. Herbicidal α-(4-phenoxyphenoxy)alkanecarboxylic Acid Derivatives. DE Patent US3954442A, 4 May 1976. [Google Scholar]
- Huang, M.; Liu, A.; Nie, S.; Liu, Q.; Lei, M.; Gao, D.; Ren, Y.; Zhang, P.; Han, K.; He, L. N-(arylalkoxy)aryloxyphenoxy Carboxylic Acid Amide Compounds, and Preparation Method and Application Thereof. Chinese Patent CN104277034A, 14 January 2015. [Google Scholar]
- Wang, X.; Liu, A.; Liu, Q.; Lei, M.; Ren, Y.; Ou, X.; Huang, L.; Han, K.; Gao, G.; Wu, M. [N-(Aralkyl)aryloxy]phenoxycarboxylic Acid Amide Compounds as Agrochemicals and Their Preparation, Pharmaceutical Compositions and Use in the Treatment of Plant Diseases. Chinese Patent WO2015000392A1, 8 January 2015. [Google Scholar]
- Ying, Z.H.; Chen, A.Y.; Hu, A.X.; Ye, J. Synthesis, Crystal Structure and Herbicidal Activity of N-(Thiazol-2-yl)-2-(4-aryloxyphenoxy)propionamides. Chin. J. Org. Chem. 2017, 37, 149–156. [Google Scholar] [CrossRef]
- Yang, S.; Ding, C.R.; Liu, X.H.; Weng, J.Q.; Yuan, J.; Tan, C.X. Synthesis and Herbicidal Activity of Chiral Aryloxyphenoxypropionic Amides Compounds. Chin. J. Org. Chem. 2019, 39, 3588–3593. [Google Scholar] [CrossRef]
- Xiao, T.; Li, S.; Feng, Y.; Wang, X.; Tian, X.; Chen, Z. Preparation of Aryloxyphenoxypropionamide Compounds as Weedicides. Chinese Patent CN104876922A, 2 September 2015. [Google Scholar]
- Liu, A.; Ren, Y.; Lei, M.; Pang, H.; Liu, Q.; Huang, L.; He, L.; Han, K.; Gao, G.; He, L. N-pyridine Aryloxy Phenoxy Carboxylic Acid Derivative, Preparation Method and Application. Chinese Patent CN105315199A, 10 February 2016. [Google Scholar]
- Liu, Q.; Zhou, H.; Zhu, R.; Chen, L. Pyridine-3-yl Aryloxy Phenoxy Alkyl Acid Ester and Its Application as Agricultural Herbicide. Chinese Patent CN106632007A, 10 May 2017. [Google Scholar]
- Liu, Q.; Zhou, H.; Lu, G.; Peng, Y. Preparation of Aryloxyphenoxy Alkyl Acid Derivatives as Herbicides. Chinese Patent CN106632293A, 10 May 2017. [Google Scholar]
- Lin, D.; Xiao, M.W.; Yang, Z.H.; Li, B.B.; Hu, A.X.; Ye, J. Synthesis and Herbicidal Activity of N-(2,2-Dimethyl-7-alkoxy-2,3-dihydrobenzofuran-5-yl)-2-(4-arylxoyphenoxy)propionamides. Chem. Res. Chin. Univ. 2017, 33, 74–79. [Google Scholar] [CrossRef]
- Hu, A.; Lin, D.; Li, B.; Ye, J.; Ou, X. Process for Preparation and Application of 2-[4-(pyridyl-2-yloxy)phenoxy]amide Derivative. Chinese Patent CN106478613A, 8 March 2017. [Google Scholar]
- Hu, A.; Yang, Z.; Ye, J.; Li, Y. Preparation of N-[(dihydrobenzofuran-7-oxo)alkyl]-2-aryloxy Amide Derivatives as Herbicides. Chinese Patent CN107382980A, 24 November 2017. [Google Scholar]
- Yan, Z.Z.; Yang, Z.H.; Deng, X.L.; Lin, D.; Wu, M.F.; Li, J.M.; Chen, A.Y.; Ye, J.; Hu, A.X.; Liao, H.D. Novel aryloxyphenoxypropionate derivates containing benzofuran moiety: Design, synthesis, herbicidal activity, docking study and theoretical calculation. Pestic. Biochem. Physiol. 2019, 154, 78–87. [Google Scholar] [CrossRef]
- Krahmer, H.; Walter, H.; Jeschke, P.; Haaf, K.; Baur, P.; Evans, R. What makes a molecule a pre- or a post-herbicide—How valuable are physicochemical parameters for their design? Pest Manag. Sci. 2021, 77, 4863–4873. [Google Scholar] [CrossRef]
- Hu, A.X.; Chen, A.Y.; Li, B.B.; Yang, Z.H.; Li, Y.H. Preparation of N-(2-hydroxyiminoethyl)amide Derivatives Useful as Herbicides. Chinese Patent CN105859669A, 17 August 2016. [Google Scholar]
- Yang, Z.; Gu, L.; Wang, M.; Li, G.; Chang, X.; Wang, X.; Ma, M.; Yu, J.; Wang, X.; Wang, C. Preparation of Pyridine Derivative Compound as Herbicide. Chinese Patent CN108003142A, 8 May 2018. [Google Scholar]
- Xu, Z.H.; Zhang, T.; Wang, S.K.; Li, J.K. Synthesis and Herbicidal Activities of Novel Ethyl 2-(4-(Pyridin-2-yl-oxy)phenyl-amino)propanoates/acetates. Chin. J. Org. Chem. 2017, 37, 526–532. [Google Scholar] [CrossRef]
- Kalhor, M.; Dadras, A. Synthesis, Characterization, and Herbicidal Activities of New 1,3,4-Oxadiazoles, 1,3,4-Thiadiazoles, and 1,2,4-Triazoles Derivatives Bearing (R)-5-Chloro-3-fluoro-2-phenoxypyridine. J. Heterocycl. Chem. 2013, 50, 220–224. [Google Scholar] [CrossRef]
- Dayan, F.E.; Barker, A.; Tranel, P.J. Origins and structure of chloroplastic and mitochondrial plant protoporphyrinogen oxidases: Implications for the evolution of herbicide resistance. Pest Manag Sci. 2018, 74, 2226–2234. [Google Scholar] [CrossRef]
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef]
- Rouhollahi, A.; Ghasemi, J.B.; Babaee, E. Quantitative Structure Activity Relationship Modeling of Environmentally Important Diphenyl Ether Herbicides Using MLR and PLS. Curr. Anal. Chem. 2010, 6, 3–10. [Google Scholar] [CrossRef][Green Version]
- Zhao, L.X.; Hu, J.J.; Wang, Z.X.; Yin, M.L.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Novel phenoxy-(trifluoromethyl)pyridine-2-pyrrolidinone-based inhibitors of protoporphyrinogen oxidase: Design, synthesis, and herbicidal activity. Pestic Biochem. Physiol. 2020, 170, 104684. [Google Scholar] [CrossRef]
- Zhao, L.X.; Peng, J.F.; Hu, J.J.; Zou, Y.L.; Yin, M.L.; Wang, Z.X.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, herbicidal activity, and the molecular docking study of novel diphenyl ether derivatives as protoporphyrinogen IX oxidase inhibitors. J. Mol. Struct. 2022, 1258, 132670. [Google Scholar] [CrossRef]
- Zhao, L.X.; Wang, Z.X.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Phenoxypyridine derivatives containing natural product coumarins with allelopathy as novel and promising proporphyrin IX oxidase-inhibiting herbicides: Design, synthesis and biological activity study. Pestic Biochem. Physiol. 2021, 177, 104897. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Wang, Z.X.; Peng, J.F.; Zou, Y.L.; Hui, Y.Z.; Chen, Y.Z.; Gao, S.; Fu, Y.; Ye, F. Design, synthesis, and herbicidal activity of novel phenoxypyridine derivatives containing natural product coumarin. Pest Manag. Sci. 2021, 77, 4785–4798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.X.; Peng, J.F.; Liu, F.Y.; Zou, Y.L.; Gao, S.; Fu, Y.; Ye, F. Discovery of novel phenoxypyridine as promising protoporphyrinogen IX oxidase inhibitors. Pestic Biochem. Physiol. 2022, 184, 105102. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sun, Y.A.; Kuang, A.; Jin, D. Advances on development of tetrahydrophthalimide herbicides. Nongyao 2001, 40, 7–9, 11. [Google Scholar]
- Uclés, S.; Hakme, E.; Ferrer, C.; Fernández-Alba, A.R. Analysis of thermally labile pesticides by on-column injection gas chromatography in fruit and vegetables. Anal. Bioanal Chem. 2018, 410, 6861–6871. [Google Scholar] [CrossRef]
- Zhao, L.X.; Jiang, M.J.; Hu, J.J.; Zou, Y.L.; Cheng, Y.; Ren, T.; Gao, S.; Fu, Y.; Ye, F. Design, Synthesis, and Herbicidal Activity of Novel Diphenyl Ether Derivatives Containing Fast Degrading Tetrahydrophthalimide. J. Agric. Food Chem. 2020, 68, 3729–3741. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, H.P.; Wang, H.; Yang, S.; Jin, L.H.; Hu, D.Y.; Pang, L.L.; Xue, W. Synthesis and antiviral activity of novel chiral cyanoacrylate derivatives. J. Agric. Food Chem. 2005, 53, 7886–7891. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Q.M. Recent Advances in the Pesticide Activities of 2-Cyanoacrylate Derivatives. J. Agric. Food Chem. 2021, 69, 12933–12946. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Ni, Y.D.; Miao, H.Y.; Chen, J.; Zhang, Y.; Shi, L.; Zhang, H.J.; Zhou, H.Y.; Li, J.F.; Qian, H.W. Preparation of Trifluoromethylpyridyloxy Aryl Group-Containing Cyanoacrylate Derivative as Herbicide. Chinese Patent CN111410630A, 14 July 2020. [Google Scholar]
- Zhang, D.; Zhou, N.; Yang, L.J.; Yu, Z.L.; Ma, D.J.; Wang, D.W.; Li, Y.H.; Liu, B.; Wang, B.F.; Xu, H.; et al. Discovery of (5-(Benzylthio)-4-(3-(trifluoromethyl)phenyl)-4H-1,2,4-triazol-3-yl) Methanols as Potent Phytoene Desaturase Inhibitors through Virtual Screening and Structure Optimization. J. Agric. Food Chem. 2022, 70, 10144–10157. [Google Scholar] [CrossRef]
- Koschmieder, J.; Fehling-Kaschek, M.; Schaub, P.; Ghisla, S.; Brausemann, A.; Timmer, J.; Beyer, P. Plant-type phytoene desaturase: Functional evaluation of structural implications. PLoS ONE 2017, 12, e0187628. [Google Scholar] [CrossRef]
- Zhai, Y.E.; Shi, D.Q. Synthesis and Herbicidal Activity of 2-Alkyl(aryl)-4-amino-3-[alkyl(alkoxy)carbonyl]-5-cyano-6-[(3-trifluoromethyl)phenoxy]-pyridines. J. Heterocycl. Chem. 2013, 50, 1039–1042. [Google Scholar] [CrossRef]
- Bhonoah, Y.; Elliott, A.C.; Gaulier, S.; Ling, K.; Mitchell, G.; Morris, J.A.; Rzepa, P.R.; Viner, R.C. Preparation of Herbicidal Pyridazinone Derivatives. GB Patent WO2013050421A1, 11 April 2013. [Google Scholar]
- Reddy, R.P.; Balagopal, L. Bis(aryl)catechol Derivatives as Herbicides and Their Preparation. US Patent WO2016010731A1, 21 January 2016. [Google Scholar]
- Wailes, J.S.; Black, J.; Morris, J.A.; Briggs, E.; Tate, J.A.; Aspinall, M.B.; Ng, S. Aryloxypyridines as Herbicides and Their Preparation. GB Patent WO2020094524A1, 14 May 2020. [Google Scholar]
- McLeod, M.C.; Barber, D.M.; Braun, R.; Jakobi, H.; Asmus, E.; Schmutzler, D.; Machettira, A.B. Preparation of Substituted Pyridinyloxyanilines, Their Salts and Use of Said Compounds as Herbicidal Agents. DE Patent EP3747867A1, 9 December 2020. [Google Scholar]
- McLeod, M.C.; Barber, D.M.; Braun, R.; Jakobi, H.; Asmus, E.; Schmutzler, D.; Machettira, A.B. Substituted Phenoxypyridines, Their Salts and Use of Said Compounds as Herbicidal Agents. DE Patent EP3747868A1, 9 December 2020. [Google Scholar]
- Wang, W.; Liu, Y.; Xue, Z.; Li, J.; Wang, Z.; Liu, X. Activity of the Novel Fungicide SYP-34773 against Plant Pathogens and Its Mode of Action on Phytophthora infestans. J. Agric. Food Chem. 2021, 69, 11794–11803. [Google Scholar] [CrossRef]
- Walter, H. NADH Inhibitors (Complex I). Mod. Crop Prot. Compd. 2012, 2, 670–691. [Google Scholar]
- Sparks, T.C.; DeAmicis, C.V.; Dekeyser, M.A.; Furuya, T.; Nakano, M.; Fujioka, S. Inhibitors of Mitochondrial Electron Transport: Acaricides and Insecticides. Mod. Crop Prot. Compd. 2012, 3, 1156–1201. [Google Scholar]
- Guan, A.Y.; Liu, C.L.; Chen, W.; Yang, F.; Xie, Y.; Zhang, J.B.; Li, Z.N.; Wang, M.A. Design, Synthesis, and Structure-Activity Relationship of New Pyrimidinamine Derivatives Containing an Aryloxy Pyridine Moiety. J. Agric. Food Chem. 2017, 65, 1272–1280. [Google Scholar] [CrossRef]
- Guan, A.Y.; Wang, M.A.; Yang, J.L.; Wang, L.Z.; Xie, Y.; Lan, J.; Liu, C.L. Discovery of a New Fungicide Candidate through Lead Optimization of Pyrimidinamine Derivatives and Its Activity against Cucumber Downy Mildew. J. Agric. Food Chem. 2017, 65, 10829–10835. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Wang, M.A.; Chen, W.; Yang, F.; Yang, J.L.; Zhao, Y.; Li, Z.N.; Liu, C.L. Design, synthesis and antifungal activity of new substituted difluoromethylpyrimidinamine derivatives. J. Fluor. Chem. 2017, 201, 49–54. [Google Scholar] [CrossRef]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113716A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113720A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113773A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.M.W.; Lohmann, J.K.; Montag, J.; Vrettou-Schultes, M. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113778A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. DE Patent WO2013113787A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. Patent WO2013135671A1, 19 September 2013. [Google Scholar]
- Grammenos, W.; Craig, I.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.; Lohmann, J.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. Patent WO2013113863A1, 8 August 2013. [Google Scholar]
- Grammenos, W.; Craig, I.R.; Boudet, N.; Mueller, B.; Dietz, J.; Lauterwasser, E.M.W.; Lohmann, J.K.; Montag, J. Preparation of Pyrimidine Compounds as Fungicides. Patent WO2013113715A1, 8 August 2013. [Google Scholar]
- Zhang, X.; Liu, H.; Gao, Y.; Wang, H.; Guo, B.; Li, J. Synthesis and Antifungal Activities of New Type β-Methoxyacrylate-Based Strobilurin Analogues. Chin. J. Chem. 2012, 30, 1517–1524. [Google Scholar] [CrossRef]
- Chai, B.S.; Liu, C.L.; Li, H.C.; He, X.M.; Luo, Y.M.; Huang, G.; Zhang, H.; Chang, J.B. Design, synthesis and acaricidal activity of novel strobilurin derivatives containing pyrimidine moieties. Pest Manag. Sci. 2010, 66, 1208–1214. [Google Scholar] [CrossRef]
- Chen, J.; Shi, J.; Yu, L.; Liu, D.; Gan, X.; Song, B.; Hu, D. Design, Synthesis, Antiviral Bioactivity, and Defense Mechanisms of Novel Dithioacetal Derivatives Bearing a Strobilurin Moiety. J. Agric. Food Chem. 2018, 66, 5335–5345. [Google Scholar] [CrossRef]
- Hao, Z.S.; Wang, W.B.; Yu, B.; Qi, X.; Lv, Y.; Liu, X.Y.; Chen, H.Y.; Kalinina, T.A.; Glukhareva, T.V.; Fan, Z.J. Design, Synthesis, and Evaluation of Fungicidal Activity of Novel Pyrazole-Containing Strobilurin Derivatives(dagger). Chin. J. Chem. 2021, 39, 1531–1537. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, D.Y.; Yin, F.H.; Li, J.Q.; Xiao, Y.M.; Fu, B.; Qin, Z.H. The application of “plug-in molecules” method in novel strobilurin fungicides screening. RSC Adv. 2020, 10, 42804–42809. [Google Scholar] [CrossRef]
- Mueller, B.; Sauter, H.; Roehl, F.; Doetzer, R.; Lorenz, G.; Ammermann, E. Preparation of Carbamates and Plant-Protecting Agents Containing Them. DE Patent WO9315046A1, 5 August 1993. [Google Scholar]
- Qin, Z.; Yang, D. High-Activity Imino Phenylacetate Compounds and Preparation Method and Application Thereof. Chinese Patent CN106946770A, 14 July 2017. [Google Scholar]
- Wang, L.L.; Zhao, S.S.; Kong, X.T.; Cao, L.L.; Tian, S.; Ye, Y.H.; Qiao, C.H. Design, synthesis and fungicidal evaluation of novel pyraclostrobin analogues. Bioorganic Med. Chem. 2018, 26, 875–883. [Google Scholar] [CrossRef]
- Hayase, Y.; Kataoka, T.; Takenaka, H.; Ichinari, M.; Masuko, M.; Takahashi, T.; Tanimoto, N. Preparation of Substituted phenyl(alkoxyimino)acetamides and Their Use as Fungicides. JP Patent EP398692A2, 1990. [Google Scholar]
- Qin, Z. Preparation of Pyra-Famoxadone as Agricultural Fungicide. Chinese Patent CN103396410A, 20 November 2013. [Google Scholar]
- Cools, H.J.; Fraaije, B.A. Update on mechanisms of azole resistance in Mycosphaerella graminicola and implications for future control. Pest Manag. Sci. 2013, 69, 150–155. [Google Scholar] [CrossRef]
- Snelders, E.; Camps, S.M.T.; Karawajczyk, A.; Schaftenaar, G.; Kema, G.H.J.; van der Lee, H.A.; Klaassen, C.H.; Melchers, W.J.G.; Verweij, P.E. Triazole Fungicides Can Induce Cross-Resistance to Medical Triazoles in Aspergillus fumigatus. PLoS ONE 2012, 7, e31801. [Google Scholar] [CrossRef]
- Coqueron, P.; Miller, R.; Bernier, D.; Naud, S.; Genix, P.; Wittrock, S.; Kennel, P.; Brunet, S.; Hoffmann, S.; Meissner, R. Preparation of Triazole Derivatives for Use as Fungicides. DE Patent WO2018054832A1, 29 March 2018. [Google Scholar]
- Coqueron, P.; Miller, R.; Bernier, D.; Naud, S.; Genix, P.; Kennel, P.; Brunet, S.; Wittrock, S.; Hoffmann, S. Preparation of Triazole Derivatives for Use as Fungicides. DE Patent WO2018054829A1, 29 March 2018. [Google Scholar]
- Hoffmann, S.; Sudau, A.; Dahmen, P.; Wachendorff-Neumann, U.; Meissner, R.; Geist, J.; Bernier, D.; Vors, J.; Coqueron, P.; Wittrock, S.; et al. Preparation of Triazole Derivatives, Intermediates Thereof and Their Use as Fungicides. Patent WO2017029179A1, 23 February 2017. [Google Scholar]
- Goertz, A.; Meissner, R.; Miller, R.; Naud, S.; Bernier, D.; Genix, P.; Brunet, S.; Kennel, P.; Coqueron, P. Preparation of Triazolethione Derivatives as Fungicides for Crop Protection and Plant Growth Regulators. DE Patent WO2018145933A1, 16 August 2018. [Google Scholar]
- Jeanmart, S.; Gagnepain, J.; Maity, P.; Lamberth, C.; Cederbaum, F.; Rajan, R.; Jacob, O.; Blum, M.; Bieri, S. Synthesis and fungicidal activity of novel imidazole-based ketene dithioacetals. Bioorg. Med. Chem. 2018, 26, 2009–2016. [Google Scholar] [CrossRef]
- Aydinli, S.G.; Bulut, E.; Deniz, N.G.; Sayil, C.; Komarovska-Porokhnyavets, O.; Lubenets, V.; Zvarych, V.; Stasevych, M.; Nesterkina, M.; Kravchenko, I. New ketene dithioacetals generated from 2-nitroperchlorobutadiene and investigation of their antibacterial, antifungal, anticonvulsant and antidepressant activities. Chem. Biodivers. 2022, 19, e202100931. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Bi, X.H.; Liu, Q. Recent developments of ketene dithioacetal chemistry. Chem. Soc. Rev. 2013, 42, 1251–1286. [Google Scholar] [CrossRef] [PubMed]
- Guan, A.Y.; Yang, F.; Wang, J.F.; Chen, W.; Li, K.K.; Sun, X.F.; Chen, X.M.; Xie, Y.; Song, Y.Q.; Liu, C.L. Pyrazole Amide Compound as Agrochemical Insecticides and Bactericide and Its Preparation. Chinese Patent CN104974136A, 14 October 2015. [Google Scholar]
- Liu, C.; Wang, L.; Lan, J.; Sun, X.; Sun, Q.; Zhang, J. Use of Compound of Pyrazole Amides as Agricultural Fungicide. CN Patent WO2013064079A1, 10 May 2013. [Google Scholar]
- Sun, S.S.; Chen, L.; Huo, J.Q.; Wang, Y.; Kou, S.; Yuan, S.T.; Fu, Y.N.; Zhang, J.L. Discovery of Novel Pyrazole Amides as Potent Fungicide Candidates and Evaluation of Their Mode of Action. J. Agric. Food Chem. 2022, 70, 3447–3457. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, J.; Zhou, J.; Guan, A.; Wang, J.; Xu, Y.; Li, M.; Liu, C. Isothiazolinone Compound as Agrochemical Fungicide and Its Preparation. Chinese Patent CN103570642A, 12 February 2014. [Google Scholar]
- Xu, Y.; Zhang, J.; Li, M.; Zhou, J.-Z.; Wang, J.-F.; Liu, C.-L. Synthesis and biological activity of isothiazolone derivatives. Nongyao 2014, 53, 712–714. [Google Scholar]
- Inagaki, J.; Yamanaka, H. Preparation of Pyridines and Agricultural/Horticultural Microbicides Containing Them. JP Patent WO2014013951A1, 23 January 2014. [Google Scholar]
- Liu, D.; Zhang, J.; Zhao, L.; He, W.; Liu, Z.; Gan, X.; Song, B. First Discovery of Novel Pyrido[1,2-a]pyrimidinone Mesoionic Compounds as Antibacterial Agents. J. Agric. Food Chem. 2019, 67, 11860–11866. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Xu, X.; Guo, J.; Wang, Z.; Li, P. Aromatic (hetero)cyclic Ether Compound Having Insecticidal Activity, Preparation Method and Application. CN Patent WO2020156512A1, 6 August 2020. [Google Scholar]
- Wang, L.X.; Niu, C.D.; Salgado, V.L.; Lelito, K.; Stam, L.; Jia, Y.L.; Zhang, Y.; Gao, C.F.; Wu, S.F. Pymetrozine activates TRPV channels of brown planthopper Nilaparvata lugens. Pestic Biochem. Physiol. 2019, 153, 77–86. [Google Scholar] [CrossRef]
- Ghareeb, E.A.; Mahmoud, N.F.H.; El-Bordany, E.A.; El-Helw, E.A.E. Synthesis, DFT, and eco-friendly insecticidal activity of some N-heterocycles derived from 4-((2-oxo-1,2-dihydroquinolin-3-yl)methylene)-2-phenyloxazol-5(4H)-one. Bioorganic Chem. 2021, 112, 104945. [Google Scholar] [CrossRef]
- Wu, Q.; Cai, H.; Yuan, T.; Li, S.; Gan, X.; Song, B. Novel vanillin derivatives containing a 1,3,4-thiadiazole moiety as potential antibacterial agents. Bioorg. Med. Chem. Lett. 2020, 30, 127113. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.X.; Song, H.J.; Li, Y.Q.; Wang, Q.M. Additive effects on the improvement of insecticidal activity: Design, synthesis, and insecticidal activity of novel pymetrozine derivatives. Bioorg. Med. Chem. 2016, 24, 391–402. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Yang, Y.; Liu, Y.; Wang, Z. Triazinone Derivative Containing Acylhydrazone Structure and Preparation Method Therefor, and Insecticidal and Bactericidal Uses Thereof. CN Patent WO2019056247A1, 28 March 2019. [Google Scholar]
- Wang, Q.; Yang, Y.; Wang, Z.; Liu, Y. Triazone Derivatives Containing Acylhydrazone Structure, Preparation Method and Application Thereof in Insecticide and Antimicrobial Agent. Chinese Patent CN107266381A, 20 October 2017. [Google Scholar]
- Song, H.J.; Liu, Y.X.; Xiong, L.X.; Li, Y.Q.; Yang, N.; Wang, Q.M. Design, synthesis, and insecticidal activity of novel pyrazole derivatives containing alpha-hydroxymethyl-N-benzyl carboxamide, alpha-chloromethyl-N-benzyl carboxamide, and 4,5-dihydrooxazole moieties. J. Agric. Food Chem. 2012, 60, 1470–1479. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Xiong, L.; Li, Y.; Yang, N.; Wang, Q. Design, synthesis, and insecticidal evaluation of new pyrazole derivatives containing imine, oxime ether, oxime ester, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I. J. Agric. Food Chem. 2013, 61, 8730–8736. [Google Scholar] [CrossRef] [PubMed]
- Gadhachanda, V.R.; Wu, B.; Wang, Z.; Kuhen, K.L.; Caldwell, J.; Zondler, H.; Walter, H.; Havenhand, M.; He, Y. 4-Aminopyrimidines as novel HIV-1 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Z.; Pan, S.; Zhu, M.; Guan, A.; Sun, X.; Zhang, J.; Song, Y.; Liu, C.; Yang, X. A new potential aphicide against Myzus persicae: Design, synthesis and 3D-QSAR of novel phenoxypyridine derivatives containing 4-aminopyrimidine. J. Mol. Struct. 2022, 1262, 132949. [Google Scholar] [CrossRef]
- Chai, B.S.; Zhang, J.B.; Song, Y.Q.; Yang, J.C.; Li, K.K.; Wang, L.Z.; Sun, X.F.; Liu, C.L. Preparation of Substituted Thienopyrimidinamine Compounds and Their Application as Insecticidal and Acaricidal Agents. Chinese Patent CN105218557A, 6 January 2016. [Google Scholar]
- Shao, X.; Li, Z.; Zhang, Y.; Zhou, C.; Cheng, J.; Xu, X.; Xu, Z. Preparation and Application of Pyrazolamide Compounds Containing Azo Structure. Chinese Patent CN113512002A, 19 October 2021. [Google Scholar]
- Chen, W.; Guan, A.Y.; Yang, L.; Cheng, Y.L.; Xu, Y.Q.; Ding, T.; Fang, X.M. The Synthesis and Biological Activity of 4-Chloro-1,3-dimethyl-N-(2-(6-phenoxypyridin-3-yl)ethyl)-1H-pyrazole-5-carboxamide. Agrochemicals 2014, 53, 316–318. [Google Scholar]
- Okada, I.; Suzuki, S.; Okui, S.; Takahashi, Y.; Fukuchi, T.; Nakajima, T. Preparation of [(5-pyrazolylcarboxamido)alkyl]pyridine Derivatives and Insecticidal, Miticidal, and Fungicidal Compositions Containing Them as Active Ingredients. JP Patent EP329020A1, 1989. [Google Scholar]
- Powell, G.F.; Ward, D.A.; Prescott, M.C.; Spiller, D.G.; White, M.R.H.; Turner, P.C.; Earley, F.G.P.; Phillips, J.; Rees, H.H. The molecular action of the novel insecticide, Pyridalyl. Insect Biochem. Mol. Biol. 2011, 41, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.; Liu, M.H.; Yu, H.; Ou, X.M.; Liu, X.P.; Ren, Y.G.; Long, C.Y.; Huang, L.; Yu, W.Q.; Shi, G.R.; et al. Discovery of nitropyridyl-based dichloropropene ethers as insecticides. Bioorg. Med. Chem. Lett. 2019, 29, 1430–1433. [Google Scholar] [CrossRef] [PubMed]
- Domon, K.; Toriyabe, K.; Ogawa, Y.; Bessho, J.; Kawamoto, K.; Watanabe, A.; Komatsu, M.; Matsuda, T.; Ito, S. Preparation of Alkylphenylsulphide Derivatives as Pest Control Agents. JP Patent WO2013157229A1, 24 October 2013. [Google Scholar]
- Liu, Y.; Wang, M.; Xu, Y.; Wu, Y.; Fu, B.; Li, J.; Xiao, Y.; Qin, Z. Design, synthesis, and biological activity of sulfoximine derivatives. J. Heterocycl. Chem. 2022, 59, 729–738. [Google Scholar] [CrossRef]
- Du, S.; Yang, D.; Wan, C.; Zhao, F.; Qin, Z. Design, synthesis and insecticidal activities of aryloxypyridinyl ethanone oxime ethers. Nongyaoxue Xuebao 2021, 23, 62–68. [Google Scholar] [CrossRef]
- Tang, J.; Wang, R.; Liu, J.; Wu, X.; Niu, J. 4-(2-Phenylethylamino)pyrimidine-nicotine Derivatives as Pesticides and Their Preparation, Pharmaceutical Compositions and Use in the Pest Control. Chinese Patent CN103232434A, 7 August 2013. [Google Scholar]
- Nesterov, A.; Spalthoff, C.; Kandasamy, R.; Katana, R.; Rankl, N.B.; Andres, M.; Jahde, P.; Dorsch, J.A.; Stam, L.F.; Braun, F.J.; et al. TRP Channels in Insect Stretch Receptors as Insecticide Targets. Neuron 2015, 86, 665–671. [Google Scholar] [CrossRef]
- Min, L.J.; Wang, H.; Bajsa-Hirschel, J.; Yu, C.S.; Wang, B.; Yao, M.M.; Han, L.; Cantrell, C.L.; Duke, S.O.; Sun, N.B.; et al. Novel Dioxolane Ring Compounds for the Management of Phytopathogen Diseases as Ergosterol Biosynthesis Inhibitors: Synthesis, Biological Activities, and Molecular Docking. J. Agric. Food Chem. 2022, 70, 4303–4315. [Google Scholar] [CrossRef] [PubMed]
- Ulmschneider, S.; Dietz, J.; Renner, J.; Grote, T.; Grammenos, W.; Mueller, B.; Lohmann, J.K.; Vrettou-Schultes, M. Triazole Compounds Carrying a Sulfur Substituent and Their Use as Fungicides. DE Patent WO2010146116A1, 23 December 2010. [Google Scholar]
- Dietz, J.; Riggs, R.; Boudet, N.; Lohmann, J.; Craig, I.R.; Haden, E.; Lauterwasser, E.; Mueller, B.; Grammenos, W.; Grote, T. Preparation of halogenalkylphenoxyphenyltriazolylethanol Derivatives for Use as Fungicides. Patent WO2013007767A1, 17 January 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).