2.1. Model Fitting of the Extraction Process
A three level and two factor central composite design was applied to make the response surface optimization for the PHWE of the hydrophilic bioactive compounds from black rosehip (
Table 1). The extraction temperature (X1) and solvent-to-solid ratio (X2) were chosen as the two main independent variables, and the measured independent variables in 13 experiments are given in
Table 2. Other factors were kept constant throughout all experiments: the pressure of 100 bar and the extraction time of 60 min. Dried whole fruit consisting of flesh and seed parts (5% of residual moisture) was used as solid raw material for the extraction. In the static extraction mode, the solvent-to-solid ratio and moisture content of the feed should be taken into account to obtain better extraction yields [
18]. Previous optimization studies for the pressurized liquid extraction or pressurized hot water extraction of plant phenolics demonstrated that both the temperature and the solvent-to-solid ratio were important factors affecting yield [
19,
20,
21].
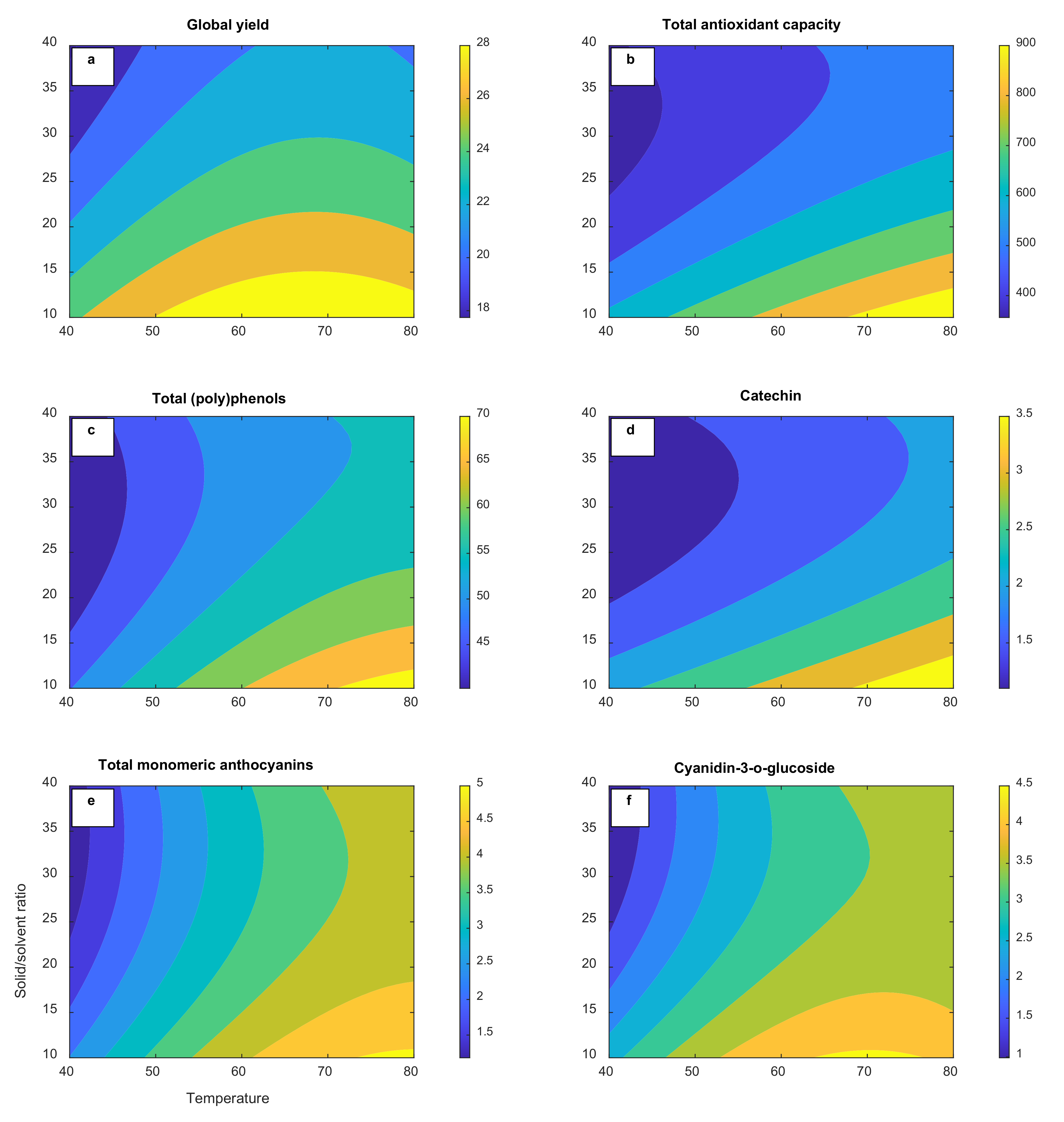
The RSM model was used to generate 3D and contour response surface plots in order to graphically represent the relationship between independent (temperature and solvent-to-solid ratio) and dependent variables (global yield, total antioxidant capacity, total (poly)phenols, catechin, total monomeric anthocyanins, and cyanidin-3-
O-glucoside). As illustrated in
Figure 1a–f, temperature and the solvent-to-solid ratio significantly contribute to all the responses analyzed. The efficiency of the extraction process is quantitatively related to the global yield. Global yields in the range of 17.6–29.6% were achieved under the described operation conditions suggesting that a good amount of the soluble components can be acquired using PHWE (
Table 2). Solvent choice affects the extraction efficiency as well as the extract properties. Similar yields (29.7%) have been reported in water extracts in previous studies [
22]. The 3D response surface plots can be found in the
Supplementary Figure S1.
In the case of the phenolic and anthocyanin compounds, optimization of the temperature is necessary in PHWE in order to take full advantage of the enhanced solubility and mass transfer but also to minimize the thermal degradation [
4]. All responses were substantial when the extraction temperature was raised from 40 °C to 80 °C, probably due to the greater extraction efficiency with better mass transfer related to the increase in solubility (
p < 0.05).
Figure 1d–f depicts a slight plateau at temperatures above 75 °C in the response surfaces of the total antioxidant capacity, total monomeric anthocyanins, and cyanidin-3-
O-glucoside. One explanation for this might be a partial thermal degradation of the anthocyanin major antioxidant compounds in agreement with the previous report of Liazid et al. (2014) [
23]. Our preliminary experiments confirmed a 2.2-fold anthocyanin yield decrease when the PHWE process was performed at 100 °C (data not shown). A similar trend has been reported by Koyu et al. (2017) when the temperature augmented from 60 °C to 80 °C during the optimization of the subcritical water extraction of
Morus nigra L. fruits at 150 bar for 60 min [
24]. Hence, an extraction temperature of 75 °C was set as the optimum condition that maximizes the desirability function of the RSM tool for the production of the extract rich in (poly)phenols to be encapsulated as higher temperatures did not confer any rise in yield. As can be seen from
Table 3, the model exhibited a significant (
p < 0.01) relationship between the total (poly)phenols and independent variables, with predicted R
2 (0.8227) in reasonable agreement with the adjusted R
2 (0.9454). Considerable interaction effects (
p < 0.05) among the extraction temperature and solvent-to-solid ratio were also observed on all of the dependent variables analyzed, except global yield, according to the ANOVA analysis.
Another important parameter for the recovery of (poly)phenols is the solvent-to-solid ratio. Minimal consumption of the solvent is the second crucial aspect after maximization of the yield in an industrial scale extraction [
25]. The higher solvent-to-solid ratio (
v/
w), the lower the amounts of extracted total (poly)phenols and total monomeric anthocyanins were, as can be inferred from the negative significant linear coefficients (
p < 0.05) in
Table 3. The extraction yield is expected to gradually rise with the increasing solid–liquid ratio according to mass transfer principles [
26]. However, the results imply that the solute diffusion did not enhance with increasing the solvent-to-solid ratio in our static extraction setting. Previous authors have suggested that a large solvent volume may result in insufficient energy to facilitate cell wall breakage for an efficient extraction of plant secondary metabolites [
27]. The extraction yield is also dependent on how the ratio between the solvent and the solid is regulated; that is, keeping the solvent volume or the solid mass constant [
25]. In our experimental setting, the solvent-to-solid ratio was adjusted by changing the solid mass while the solvent volume was kept constant. A similar pattern in yield was observed by Clodoveo, Crupi, Muraglia, and Corbo (2022) when the solid–solvent ratio increased in the ultrasound-assisted extraction of polyphenols from carob pods using aqueous ethanol as the solvent [
28].
The phenolic and anthocyanin constituents of the black rosehip extract obtained via different conditions of PHWE were in the same range as those previously reported in the literature [
15] with slight quantitative differences. Water-soluble total (poly)phenol values in different rosehip species ranged from 150.8 mg to 299.2 mg GAE/100 g in dry weight, of which
R. spinosissima had the highest (poly)phenolic content as well as antioxidant capacity [
10] which is a lower yield than our study. Total monomeric anthocyanins were lower than those found by Odabas and Koca (2021) [
9], using microwave-assisted aqueous two-phase extraction with ethanol/ammonium sulfate. The PHW extracts obtained optimized conditions and yielded remarkable total antioxidant capacity values via the CUPRAC assay (
Table 4). Pashazadeh, Zannou, Galanakis, Aldawoud, Ibrahim, and Koca (2021) reported DPPH radical scavenging and ferric-reducing antioxidant power results of 110 ± 2 mmol TE/g and 698 ± 15 mmol ISE/g, respectively, for black rosehip using aqueous methanol after the convective drying process. A number of antioxidant capacity assays are present in the literature as they do not correlate well due to differences in the test procedures as well as the surrounding chemistry of the antioxidant compounds [
29]. Greater antioxidant potential in our extracts may also be attributed to a possible higher presence of other hydrophilic antioxidant constituents as PHWE is also an efficient method for vitamin C extraction [
30]. The spectrophotometric analysis of the PHW extracts revealed total monomeric anthocyanin contents in the range of 1.19–4.98 mg per g of dry fruit (
Table 2).
The regression analysis of the experimental data was subjected to a regression coefficient to obtain second-order polynomial equations in terms of coded variables and are given as follows:
The fitted polynomial equations are presented as contour plots in order to visualize the relationship between the experimental levels of each factor and the response. The results of the ANOVA, the goodness-of-fit, and the adequacy of the models are summarized in
Table 3. Considering all response surface models, the “lack of fit” was nonsignificant (
p > 0.05). The adjusted determination coefficients (R
2 adj) were also close to R
2. These findings confirm the adequacy of the model terms to represent the experimental data and to predict the six analyzed parameters. The extraction temperature and solvent-to-solid ratio had significant effects (
p < 0.05) on the analyzed responses.
Current trends strongly favor the scaling-up of nonconventional green extraction techniques for recovering phenolic compounds that consume less organic solvent(s), involve minimal operational steps, provide high throughput capability, and assure the highest yield at lower costs. Santos and coworkers (2012) reported a 40-fold lower cost of manufacturing, and 2.15- and 1.66-fold more anthocyanins and total phenolic compounds obtained from jabuticaba skins by pressurized liquid extraction using ethanol as a solvent compared to conventional low-pressure solvent extraction [
31]. Using pressurized hot water as the solvent of extraction, the PHWE technique is an even greener version of pressurized liquid extraction as water is perhaps the most available naturally occurring liquid on Earth [
4,
32]. We did not perform an economic evaluation for the feasibility of a large-scale PHWE operation within the scope of this study. However, future studies should focus on the economic feasibility of the global process.
2.2. Validation of Optimal Conditions
The main advantage of RSM is the simultaneous maximization of multiple responses in the investigated experimental domain. Based on the above findings, the PHWE conditions giving the best levels of several factors were attained, and they showed that a temperature of 75 °C and a solvent-to-solid ratio of 10:1 mL g
−1 were optimal, working under 100 bar. The adequacy of the model for predicting optimum yields was experimentally tested using the determined optimized conditions. The optimal extract of BRE revealed mean values of 68.90 ± 3.94, 3.90 ± 0.21, 5.28 ± 0.41, 4.08 ± 0.51 mg, and 930.48 ± 24.37 mmol per g of dry fruit for total (poly)phenols, catechin, total monomeric anthocyanins, cyanidin-3-
O-glucoside, and total antioxidant capacity, respectively, in the verification experiments. Being similar (
p > 0.05) to the respective predicted ones, these findings allow validation of the experimental design and demonstrate the effectiveness and good adequacy of the RSM (
Table 4). The optimum conditions for extraction were compared to the predicted values of RSM using an independent sample
t-test. The PHWE process provided a good polyphenol yield with a high antioxidant capacity for a notably shorter extraction time of 60 min, compared to the conventional extraction process. The HPLC analysis showed that cyanidin-3-O-glucoside was the most abundant anthocyanin in all of the PHWE extracts, followed by peonidin-3-O-glucoside, cyanidin-3-O-galactoside, and delphinidin-3-O-glucoside (data not shown) in agreement with the literature data [
9].
2.3. Characterization of Encapsulated Extract Formulations
Table 5 shows the particle size measurements using the dynamic light scattering technique to characterize the uncoated and coated liposomes in liquid dispersion form before and after spray drying. Nano scale vesicle sizes of the liposomes achieved by the use of microfluidization. The biopolymer coating of the liposomes slightly increased the particle size, and loading BRE into the liposomes resulted in bigger particles (
p < 0.05). According to the polydispersity index (PDI) as a measure of the width of the particle size distribution, values of between 0.1 and 0.25 prove a narrow size distribution, while values above 0.5 illustrate a very broad size distribution [
33]. The relatively high PDI measurements for chitosan-coated samples could be the result of the intrinsic factors of chitosan including PDI, the degree of acetylation, and molecular weight [
34].
The addition of maltodextrin prior to spray drying resulted in no alteration in particle size as maltodextrin is an osmotically inactive substance (
p > 0.05) [
35], but it did cause an appreciable increment in the diameter after reconstitution (
Table 5). Being a neutral polysaccharide, the effect of the maltodextrin addition on the ζ potential of the liposomal dispersions was also insignificant as expected (
p > 0.05). By contrast, the ζ potential of the anionic liposomal dispersions (−25.77 ± 1.12 mV) drastically increased to 52.97 ± 2.45 mV and 21.97 ± 0.85 mV by coating oppositely charged chitosan and whey protein, respectively, (
p < 0.05). On the other hand, the spray-drying process caused a marginal reduction in the ζ potential as is depicted by the values after reconstitution (
p < 0.05). A high ζ potential and low PDI values are taken as indicators of the physical stability of the nanoparticles [
36].
The encapsulation efficiency was satisfactory in all samples in terms of the total (poly)phenols (
Table 6). The uncoated BRE liposome, chitosan-coated BRE liposome, and whey protein-coated BRE liposome were found to be 67.2 ± 0.7, 76.7 ± 0.8, and 87.2 ± 4.0, respectively, (
p < 0.05). Better encapsulation efficiencies were favored in the biopolymer- coated liposomes as reported in other studies [
37]. Unlike the freeze-drying process which is disadvantageous in terms of cost and time, spray drying is the most often used process in drug delivery systems for converting aqueous materials into a dried and powder form [
38]. However, the retention of the (poly)phenolic compounds during processing is required to preserve the functional properties. High temperatures during spray drying may have a detrimental effect on the thermo-labile (poly)phenols [
39]. The total (poly)phenols of the fabricated powders ranged from 55.62 to 61.79 mg per L after reconstitution (
Table 6). The amount of (poly)phenols in the samples decreased owing to oxygen and heat exposure during spray drying. The retention efficiency of the total (poly)phenols in the biopolymer-coated liposomal powders was found to be significantly higher than the spray-dried BRE (
p < 0.05). These results are in agreement with our previous research in which chitosan-coated mulberry extract nanoliposomes spray dried with 20% MD, using the same process conditions, yielded total phenolic and anthocyanin retention efficiencies of 69% and 56%, respectively [
40]. In a similar pattern as observed with the retention of phenolics, the retention of the antioxidant capacity of the powders was confirmed with the CUPRAC assay. The chitosan-coated nanoliposomes loaded with flaxseed–peptide fractions retained about 90% and 86% of DPPH and ABTS free radical scavenging activity after spray drying [
41].
The physicochemical properties of the fabricated powders are given in
Table 7. Moisture content is a critical property of powder products as it indicates the residual water in powders. A range of 1–6% is pursued in the industry for the storage stability of powder [
42]. In the current study, the moisture content of all spray-dried samples was below 6% with no significant difference among the samples (
p > 0.05). Meanwhile, the yield of the powders changed between 60.5% and 72.3%, with a high solubility above 90% (
p > 0.05) for all of them. Odabaş and Koca (2020) reported similar values for powder yield and solubility that were 52.39 ± 0.65% and 91.14 ± 1.79%, respectively, for spray-dried black rosehip extract microcapsules in maltodextrin–gum arabic (3:1) [
43].
The color measurements indicated the entrapment of anthocyanins within the biopolymer-coated liposomes, resulting in lower a * values than the spray-dried BRE (
p < 0.05) in
Table 7. The a * coordinate is related to the anthocyanin content in black rosehip fruit that is the pigment responsible for the redness of the fabricated powders. Compared to blank formulations, the chitosan coating also attenuated the whiteness index in the liposomal samples (
p > 0.05), whereas the whey protein coating yielded a significant decrease (
p < 0.05). The resultant spray-dried powders had a light pink color, while the blank powders were white, which is also demonstrated by the luminosity (L *) values. The visual appearance of the prepared powders is given in
Supplementary Figure S2.
The SEM micrographs of the spray-dried particles of the different formulations are shown in
Figure 2. The dried particles exhibited spherical structures with some dents and wrinkles on their surfaces. Either rapid evaporation of water within the particles due to the high inlet temperature or the slow water diffusion rate due to the low inlet temperature cause particle shrinkage during spray drying [
44]. The lack of apparent cracks or fissures on the particles in all formulations demonstrate good protection of the internal material encapsulated in the liposomes. The small particles attached to the surface of the capsules might indicate the incomplete encapsulation of the internal material.
2.4. Effect of Simulated Digestion on the Total (Poly)phenol and Total Antioxidant Capacity of the Bioaccessibility of Free and Encapsulated Extracts
To determine the total (poly)phenol and total antioxidant capacity in the bioaccessible fraction of the encapsulated black rosehip extract, they were incubated under simulated gastrointestinal conditions. (Poly)phenols are considered bioaccessible when they are released from the food matrix during digestion and solubilized in a form that the enterocytes can absorb [
45]. The percentage total of (poly)phenols in the bioaccessible fraction of the chitosan-coated (69.3 ± 2.2%) and whey protein-coated (65.3 ± 9.7%) liposomal extracts were approximately 2.3-fold higher compared to the spray-dried extract (30.3 ± 1.3%) and 5.6-fold higher compared to the nonencapsulated extract (12.4 ± 1.0%) (
Figure 3). The total antioxidant capacity in the bioaccessible fraction followed the same trend as the total (poly)phenols for spray-dried BRE, demonstrating an approximate 2.9- and 4.1-fold increase in the radical scavenging activity and cupric ion reducing antioxidant capacity, respectively. Similarly, chitosan- and whey protein-coated liposomal powders had 3.8- and 8.6-fold higher radical scavenging activity and 5.0- and 8.1-fold higher cupric ion reducing antioxidant capacity compared to the nonencapsulated extract after in vitro digestion. These results suggest that the encapsulation process and biopolymer coating greatly enhanced the amount of phenolic and antioxidant activity in the bioaccessible fraction (
p < 0.05). A similar trend has been previously reported for the liposomal-encapsulated mulberry waste extract by our research group [
40]. The application of such delivery systems in dry form yielded better results than in dispersion form [
46]. When the liposomal- encapsulated cocoa phenolics in a spray-dried form were incorporated into an Ayran beverage, the bioaccessibility of the cocoa phenolics increased approximately 2.5 fold in comparison with their aqueous dispersion form.
A chitosan coating has been well established to increase the in vitro bioaccessibility of polyphenols in liposomal delivery systems [
40,
47,
48,
49]. Recent studies have also reported significant improvements in astaxanthin [
50] and curcumin [
51] bioaccessibility after the whey protein-coating of liposomes. In our study, while both coatings increased the total (poly)phenol and total antioxidant capacity in the bioaccessible fraction, there was no significant difference between the liposomes coated with chitosan and whey protein (
p > 0.05). It is noteworthy that this is the first study in which the effect of chitosan and whey protein coating on the bioaccessibility of (poly)phenols was compared. Whey proteins, as a byproduct from cheese or the casein manufacturing process, are well-utilized in the food industry as they are nutritious, widely available, and cheap [
52]. In comparison, chitosan, which is obtained through the deacetylation of chitin from crustacean exoskeletons, is a more costly biopolymer (more than one thousand euros per kilogram at Aldrich) despite being a source from abundant marine wastes [
53]. However, in our study, the amount of chitosan used for the liposome coating was 10 times less than the needed amount of whey protein indicating no substantial economical difference between both the biopolymers in the coating process.