The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature
Abstract
1. Introduction
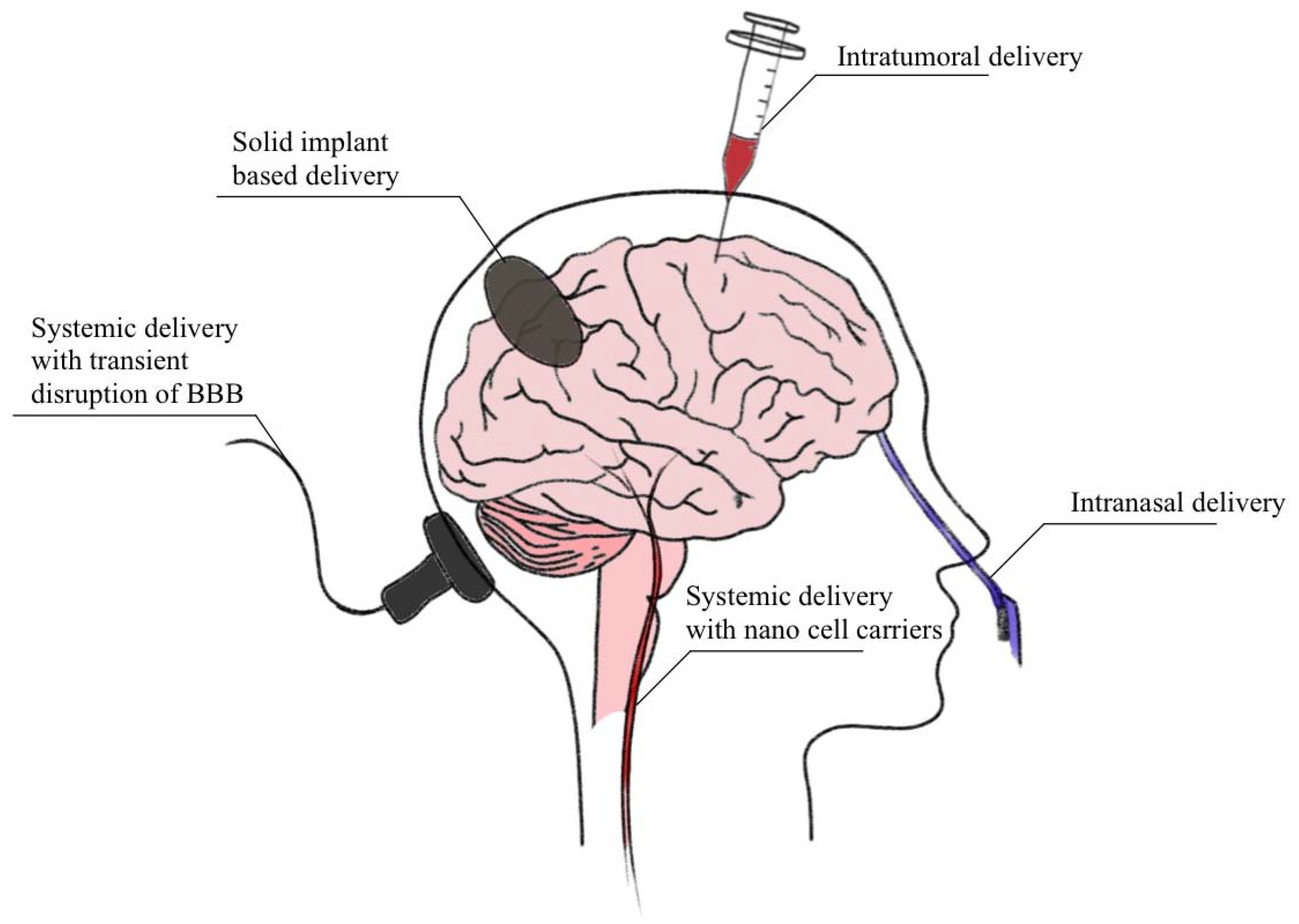
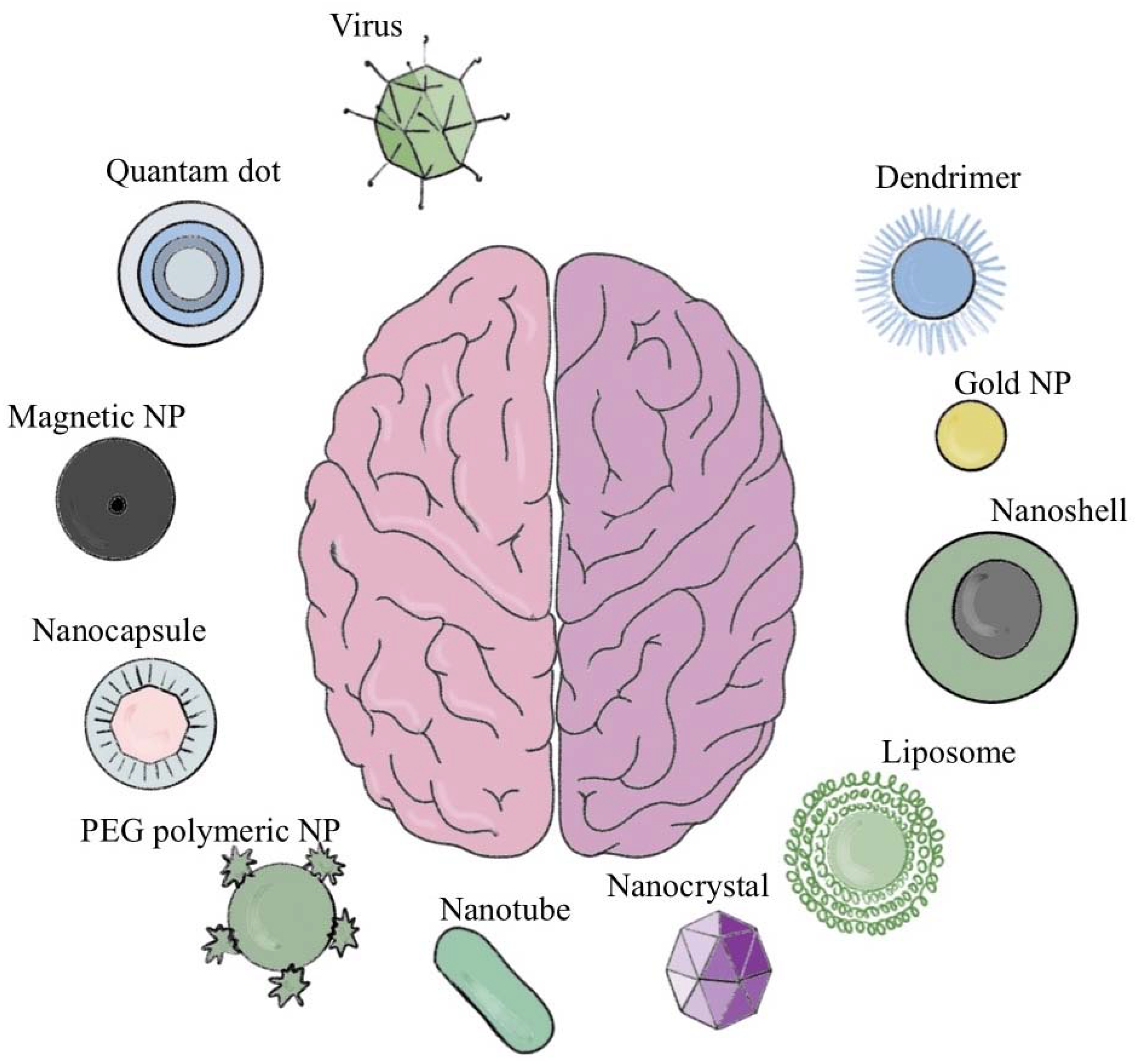
2. Current Brain Drug Delivery Techniques
3. Brain Tumors
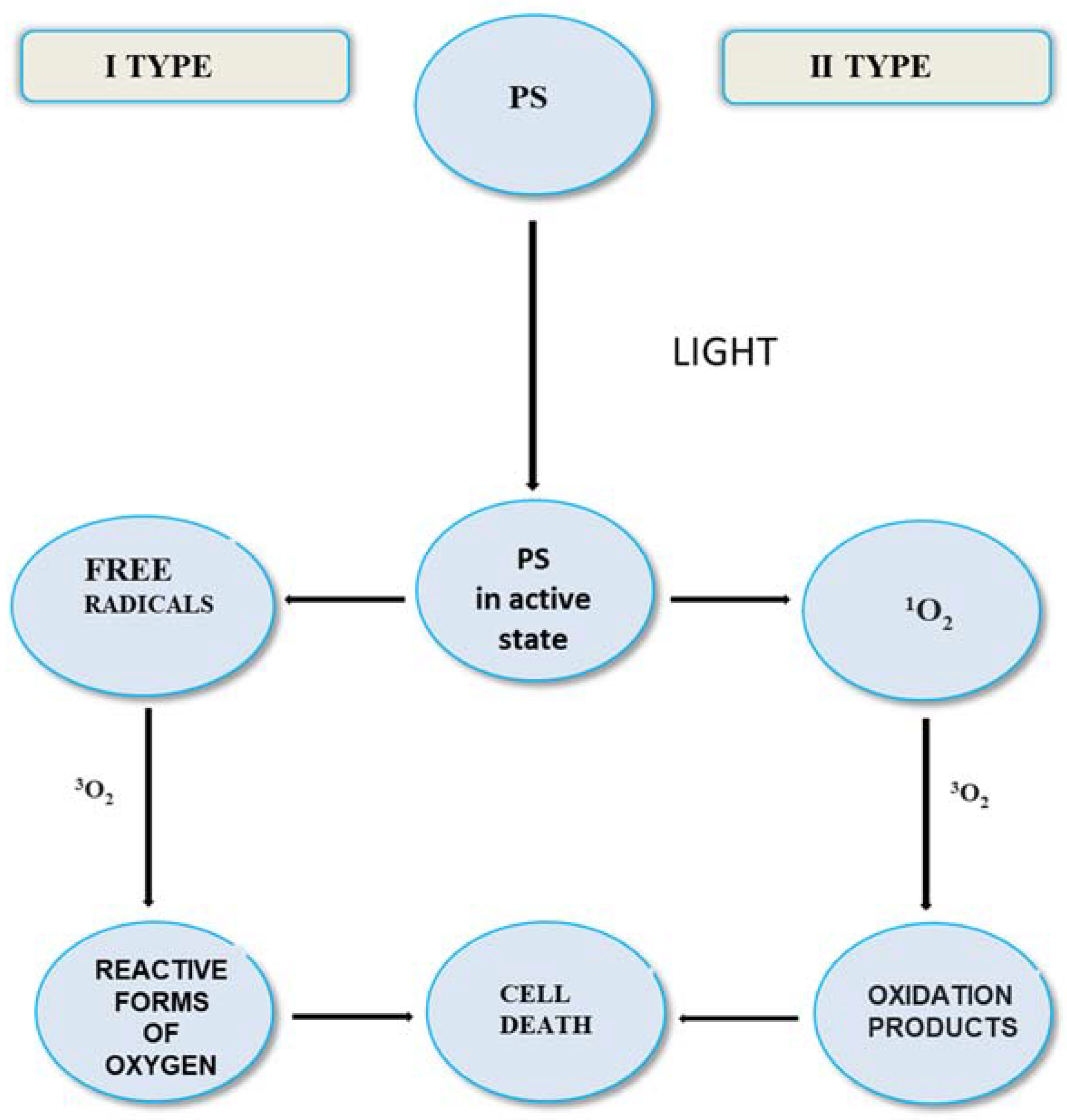
4. Advantages and Disadvantages of PDT Treatment
- “Free” photosensitizer from injection must be cleared from the body;
- Better selectivity needed of injected sensitizer for diseased cells and tissue;
- Problem of skin photosensitivity from injected sensitizer;
- Higher concentrations of 1O2 are needed at target sites;
- Hypoxic tumors are inherently difficult to treat with PDT, due to the oxygen requirement for their photodestruction.
- Far less “free” photosensitizer in body since it will be cleaved on-site by the fiber;
- Oxygen passage through fiber solves problem of hypoxia for tumor destruction;
- High precision eradication of tumors in diseased tissue adjacent to vital tissue;
- Existing endoscopic and micro-optic methods can be adapted to the new fiber device;
- Fiber method is less invasive, systemic administration of sensitizer not required;
- Newly acquired mechanistic understanding in our lab can be applied to increase singlet oxygen generation at water-fiber cap interfaces;
- Fiber system can better achieve sensitizer-O2 concentrations at a specific site concurrent with high excitation intensity to enhance local 1O2 concentrations.
5. Development of Photosensitizer and Fiber Optic Technology
6. Photosensitizers
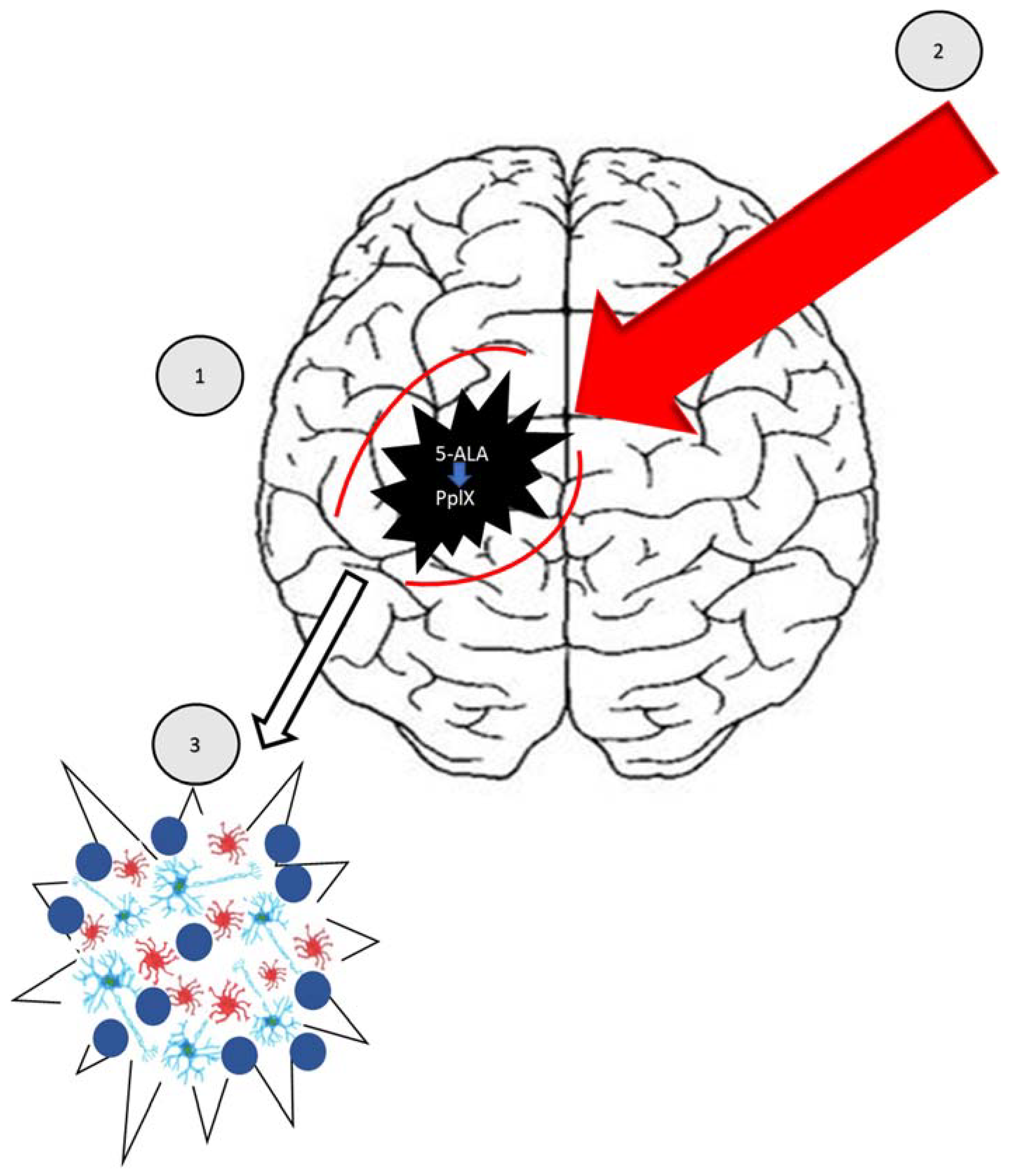
5-aminolevulinic Acid (Pro-Drug)
7. A Review of the Literature
Cellular View
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Didkowska, J.; Wojciechowska, U.; Michalek, I.M.; Caetano dos Santos, F.L. Cancer incidence and mortality in Poland in 2019. Sci. Rep. 2022, 12, 10875. [Google Scholar] [CrossRef]
- Patel, A.P.; Fisher, J.L.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Agius, D.; Alahdab, F.; Fitzmaurice, C. GBD 2016 Brain and Other CNS Cancer Collaborators. Global, regional, and national burden of brain and other CNScancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef]
- Pouchieu, C.; Baldi, I.; Gruber, A.; Berteaud, E.; Carles, C.; Loiseau, H. Descreptive epidemiology and risk factors of primary central nervous system tumors: Current knowledge. Rev. Neurol. 2016, 1, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Z.; Lv, H.; Li, F.; Sun, S.; Li, J.; Lee, C.S. Immune Checkpoint Blockade Mediated by a Small-Molecule Nanoinhibitor Targeting the PD-1/PD-L1 Pathway Synergizes with Photodynamic Therapy to Elicit Antitumor Immunity and Antimetastatic Effects on Breast Cancer. Small. 2019, 15, e1903881. [Google Scholar] [CrossRef]
- Sunil, V.; Teoh, J.H.; Mohan, B.C.; Mozhi, A.; Wang, C.H. Bioengineered immunomodulatory organelle targeted nanozymes for photodynamic immunometabolic therapy. J. Control Release 2022, 350, 215–227. [Google Scholar] [CrossRef]
- Choromańska, A.; Kulbacka, J.; Saczko, J. Terapia fotodynamiczna—Założenia, mechanizm, aplikacje kliniczne. Nowa Med. 2013, 1, 26–30. [Google Scholar]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X. Targeted drug delivery across the blood-brain barrier using ultrasound technique. Ther. Deliv. 2010, 1, 819–848. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Fecci, P.E.; Gromeier, M.; Sampson, J.H. Viruses in the treatment of brain tumors. Neuroimaging Clin. 2002, 12, 553–570. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood-Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Roet, M.; Hescham, S.A.; Jahanshahi, A.; Rutten, B.P.F.; Anikeeva, P.O.; Temel, Y. Progress in euromodulation of the brain: A role for magnetic nanoparticles? Prog. Neurobiol. 2019, 177, 1–14. [Google Scholar] [CrossRef]
- Baek, S.K.; Makkouk, A.R.; Krasieva, T.; Sun, C.H.; Madsen, S.J.; Hirschberg, H. Photothermal treatment of glioma; an in vitro study of macrophage-mediated delivery of gold nanoshells. J. Neurooncol. 2011, 104, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Male, D.; Gromnicova, R.; McQuaid, C. Gold Nanoparticles for Imaging and Drug Transport to the CNS. Int. Rev. Neurobiol. 2016, 130, 155–198. [Google Scholar] [PubMed]
- Zhu, Y.; Liu, C.; Pang, Z. Dendrimer-Based Drug Delivery Systems for Brain Targeting. Biomolecules 2019, 9, 790. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review. Int. J. Nanomed. 2020, 15, 2563–2582. [Google Scholar] [CrossRef]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies that Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Robles, P.; Fiest, K.M.; Frolkis, A.D.; Pringsheim, T.; Atta, C.; St. Germaine-Smith, C.; Day, L.; Lam, D.; Jette, N. The worldwide incidence and prevalence of primary brain tumors: A systematic review and meta-analysis. Neuro-Oncology 2015, 17, 776–783. [Google Scholar] [CrossRef]
- Hirschberg, H.; Berg, K.; Peng, Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 27. [Google Scholar] [CrossRef]
- Kulbacka, J.; Saczko, J.; Chwiłkowska, A.; Ługowski, M.; Banaś, T. Phototherapy—An alternative anticancer treatment. Borgis Med. Rodz. 2008, 4, 88–95. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nature 2003, 5, 380–387. [Google Scholar] [CrossRef]
- Allison, R.R. Photodynamic therapy: Oncologic horizons. Future Oncol. 2014, 10, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Peng, Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003, 23, 3591–3600. [Google Scholar] [PubMed]
- Kessel, D. Photodynamic therapy: From the beginning. Photodiag. Photodyn. Ther. 2004, 1, 3–7. [Google Scholar] [CrossRef]
- Pass, H.I. Photodynamic therapy in oncology: Mechanisms and clinical use. J. Natl. Cancer Inst. 1993, 85, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Bagnato, V.S.; Cuenca, R.; Downie, G.H.; Sibata, C.H. The future of photodynamic therapy in oncology. Future Oncol. 2006, 2, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Lukšienë, Ž. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar]
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar]
- Carruth, J.A. Clinical applications of photodynamic therapy. Int. J. Clin. Pract. 1998, 52, 39–42. [Google Scholar] [CrossRef]
- Scheffer, G.L.; Kool, M.; Heijn, M.; de Haas, M.; Pijnenborg, A.C.; Wijnholds, J.; van Helvoort, A.; de Jong, M.C.; Hooijberg, J.H.; Mol, C.A.; et al. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5 and MDR3 P-glycoprotein with panel of monoclonal antibodies. Cancer Res. 2000, 60, 5269–5277. [Google Scholar] [PubMed]
- Marcus, S.L.; McIntire, W.R. Photodynamic therapy systems and applications. Expert Opin. Emerg. Drugs 2002, 7, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef]
- Ricchelli, F. Photophysical properties of porphyrins in biological membranes. J. Photochem. Photobiol. B Biol. 1995, 29, 109–118. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis. Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Registry. Available online: http://onkologia.org.pl/nowotwory-mozgu/ (accessed on 7 June 2022).
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro Oncol. 2020, 22 (Suppl. S2), iv1–iv96. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Thambi, R.; Kandamuthan, S.; Sainulabdeen, S.; Vilasiniamma, L.; Abraham, T.R.; Balakrishnan, P.K. Histopathological Analysis of Brain Tumours- A Seven Year Study from a Tertiary Care Centre in South India. J. Clin. Diagn. Res. 2017, 11, 5–8. [Google Scholar] [CrossRef]
- Heesters, M.; Molenaar, W.; Go, G.K. Radiotherapy in supratentorial gliomas. A study of 821 cases. Strahlenther Onkol. 2003, 179, 606–614. [Google Scholar] [CrossRef]
- Woehrer, A.; Marosi, C.; Widhalm, G. Clinical neuropathology practice guide 1-2013: Molecular subtyping of glioblastoma: Ready for clinical use? Clin. Neuropathol. 2013, 32, 5–8. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013, 331, 139–146. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, K.; Sokotowska, J.; Szmidt, M.; Sysa, P. Glioblastoma multiforme—An overview. Contemp. Oncol. 2014, 18, 307–312. [Google Scholar]
- Schneider, T.; Mawrin, C.; Scherlach, C.; Skalej, M.; Firsching, R. Gliomas in adults. Dtsch. Ärzteblatt Int. 2010, 107, 799–807. [Google Scholar] [CrossRef]
- Gerrard, G.E.; Prestwich, R.J.; Franks, K.N.; Levy, D. Neuro-oncology practice in the U.K. Clin. Oncol. 2003, 15, 478–484. [Google Scholar] [CrossRef]
- Gupta, T.; Sarin, R. Poor-prognosis high-grade glioma: Evolving an evidence-based standard of care. Lancet Oncol. 2002, 3, 557–564. [Google Scholar] [CrossRef]
- Ejma, M.; Waliszewska-Prosół, M.; Hofman, A.; Bladowska, J.; Zub, L.W.; Podemski, R. Rare clinical form of glioblastoma multiforme. Postep. Hig. Med. Dosw. 2014, 27, 316–324. [Google Scholar] [CrossRef]
- Ortega, A.; Nuño, M.; Walia, S.; Mukherjee, D.; Black, K.L.; Patil, C.G. Treatment and survival of patients harboring histological variants of glioblastoma. J. Clin. Neurosci. 2014, 21, 1709–1713. [Google Scholar] [CrossRef]
- Paula, L.V.; Primo, F.L.; Tedesco, A.C. Nanomedicine associated with photodynamic therapy for glioblastoma treatment. Biophys. Rev. 2017, 9, 761–773. [Google Scholar] [CrossRef]
- Wei, Y.; Song, J.; Chen, Q. In vivo detection of chemiluminescence to monitor photodynamic threshold dose for tumor treatment. Photochem. Photobiol. Sci. 2011, 10, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Eng. J. Clin. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Pan, W.; Ferguson, S.; Lam, S. Patient and treatment factors associated with survival among adult glioblastomas patients: A USA population-based study from 2000–2010. J. Neurosci. 2015, 22, 1575–1581. [Google Scholar] [CrossRef]
- Stark, A.M.; van de Bergh, J.; Hedderich, J.; Mehdorn, H.M. Glioblastoma: Clinical characteristics, prognostic factors and survival in 492 patients. Clin. Neurol. Neurosurg. 2012, 114, 840–845. [Google Scholar] [CrossRef]
- Kessel, K.A.; Fischer, H.; Oechnser, M.; Zimmer, C.; Meyer, B.; Combs, S.E. Highprecision radiotherapy for meningiomas: Long-term results and patient-reported outcome (PRO). Strahlenther. Onkol. 2017, 193, 921–930. [Google Scholar] [CrossRef]
- Potemski, P. The molecular basis for treatment of gliomas. Onkol. W Prakt. Klin. 2010, 6, 73–78. [Google Scholar]
- Nowis, D.; Makowski, M.; Stokłosa, T.; Legat, M.; Issat, T.; Gołab, J. Direct tumor damage mechanisms of photodynamic therapy. Acta Biochim. Pol. 2005, 52, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Oleinick, N.L.; Evans, H.H. The photobiology of photodynamic therapy: Cellular targets and mechanisms. Radiat. Res. 1998, 150, S146–S156. [Google Scholar] [CrossRef]
- Soni, V.; Jain, A.; Khare, P.; Gulbake, A.; Jain, S.K. Potential approaches for drug delivery to the brain: Past, present, and future. Crit. Rev. Ther. Drug Carr. Syst. 2010, 27, 187–236. [Google Scholar] [CrossRef]
- Dima, V.F.; Vasiliu, V.; Dima, S.V. Photodynamic therapy: An update. Roum. Arch. Microbiol. Immunol. 1998, 57, 207–230. [Google Scholar] [PubMed]
- Furnari, F.B.; Fenton, T.; Bachoo, R.M.; Mukasa, A.; Stommel, J.M.; Stegh, A.; Hahn, W.C.; Ligon, K.L.; Louis, D.N.; Brennan, C.; et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007, 21, 2683–2710. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Aghi, M.K. Atypical meningiomas. Handb Clin Neurol. 2020, 170, 233–244. [Google Scholar] [PubMed]
- Kiesel, B.; Freund, J.; Reichert, D.; Wadiura, L.; Erkkilae, M.T.; Woehrer, A.; Hervey-Jumper, S.; Berger, M.S.; Widhalm, G. 5-ALA in suspected low-grade gliomas: Current Role, limitations, and new approaches. Front. Oncol. 2021, 11, 699301. [Google Scholar] [CrossRef]
- Moan, J.; Berg, K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991, 53, 549–553. [Google Scholar] [CrossRef]
- Niedre, M.; Patterson, M.S.; Wilson, B.C. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002, 75, 382–392. [Google Scholar] [CrossRef]
- Chen, J.; Stefflova, K.; Niedre, M.J.; Wilson, B.C.; Chance, B.; Glickson, J.D.; Zheng, G. Protease-triggered photosensitizing beacon based on singlet oxygen quenching and activation. J. Am. Chem. Soc. 2004, 126, 11450–11451. [Google Scholar] [CrossRef]
- Kanofsky, J.R. Quenching of singlet oxygen by human red cell ghosts. Photochem. Photobiol. 1991, 53, 93–99. [Google Scholar] [CrossRef]
- Kanofsky, J.R. Quenching of singlet oxygen by human plasma. Photochem. Photobiol. 1990, 51, 299–303. [Google Scholar] [CrossRef]
- Skovsen, E.; Snyder, J.W.; Lambert, J.D.C.; Ogilby, P.R. Lifetime and diffusion of singlet oxygen in a cell. J. Phys. Chem. B 2005, 109, 8570–8573. [Google Scholar] [CrossRef]
- Zebger, I.; Snyder, J.W.; Andersen, L.K.; Poulsen, L.; Gao, Z.; Lambert, J.D.; Kristiansen, U.; Ogilby, P.R. Direct optical detection of singlet oxygen from a single cell. Photochem. Photobiol. 2004, 79, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Wechsler-Reya, R.; Scott, M.P. The developmental biology of brain tumors. Annu. Rev. Neurosci. 2001, 24, 385–428. [Google Scholar] [CrossRef]
- Sanai, N.; Tramontin, A.D.; Quiñones-Hinojosa, A.; Barbaro, N.M.; Gupta, N.; Kunwar, S.; Lawton, M.T.; McDermott, M.W.; Parsa, A.T.; Manuel-García Verdugo, J.; et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427, 740–744. [Google Scholar] [CrossRef]
- Shirahata, Y.; Ohkohchi, N.; Itayak, H.; Satomi, S. New technique for gene transfection using laser irradiation. J. Investig. Med. 2001, 49, 184–190. [Google Scholar] [CrossRef]
- Zamadar, M.; Ghosh, G.; Mahendran, A.; Minnis, M.; Kruft, B.I.; Ghogare, A.; Aebisher, D.; Greer, A. Bioconjugated photosensitizer used for drug delivery via an optical Fiber. J. Am. Chem. Soc. 2011, 133, 7882–7891. [Google Scholar] [CrossRef]
- Mahmoudi, K.; Garvey, K.L.; Bouras, A.; Cramer, G.; Stepp, H.; Jesu Raj, J.G.; Bozec, D.; Busch, T.M.; Hadjipanayis, C.G. 5-Aminolevulinic Acid Photodynamic Therapy for the Treatment of High-Grade Gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and Applications of Photo-Triggered Theranostic Agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Yang, X.; Zheng, X.; Wen, S.; Wang, F.; Vidal, X.; Zhao, J.; Liu, D.; Zhou, Z.; et al. Amplified stimulated emission in upconversion nanoparticles for super-resolution nanoscopy. Nature 2017, 543, 229–233. [Google Scholar] [CrossRef]
- Yang, N.; Gong, F.; Cheng, L. Recent advances in upconversion nanoparticle-based nanocomposites for gas therapy. Chem. Sci. 2021, 13, 1883–1898. [Google Scholar] [CrossRef]
- Pohl, E.; Osterholtz, F. Silane Surfaces and Interfaces; Leyden, D., Ed.; Gordon & Breach: New York, NY, USA, 1986. [Google Scholar]
- Henningsen, J.; Hald, J. Dynamics of gas flow in hollow core photonic bandgap fibers. Appl. Opt. 2008, 47, 2790–2797. [Google Scholar] [CrossRef]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.B.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) in malignant brain tumors—Where do we stand? Photodiagnosis Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: Novel third-generation photosensitizers one step closer? Br. J. Pharmacol. 2008, 154, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Sibata, C.H. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagnosis Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef]
- Bechet, D.; Mordon, S.R.; Guillemin, F.; Barberi-Heyob, M.A. Photodynamic therapy of malignant brain tumours: A complementary approach to conventional therapies. Cancer Treat. Rev. 2014, 40, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, R.; Kawabata, S.; Miyatake, S.; Kuroiwa, T.; Easson, M.W.; Vicente, M.G. Application of a novel boronated porphyrin (H₂OCP) as a dual sensitizer for both PDT and BNCT. Lasers Surg. Med. 2011, 43, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Mordon, S.; Deleporte, P.; Reyns, N.; Vermandel, M. A novel device for intraoperative photodynamic therapy dedicated to glioblastoma treatment. Future Oncol. 2017, 13, 2441–2454. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Widhalm, G.; Stummer, W. What is the surgical benefit of using 5-ALA in fluorescence-guided malignant glioma cancer surgery? Neurosurgery 2015, 77, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Valdes, P.A.; Bekelis, K.; Harris, B.T.; Wilson, B.C.; Leblond, F.; Kim, A.; Simmons, N.E.; Erkmen, K.; Paulsen, K.D.; Roberts, D.W. 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in meningioma: Qualitative and quantitative measurements in vivo. Neurosurgery 2014, 10, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Stummer, W.; Reulen, H.J.; Novotny, A.; Stepp, H.; Tonn, J.C. Fluorescent resections of malignant gliomas—A review. Acta Neurochir. Suppl. 2003, 88, 9–12. [Google Scholar]
- Teng, L.; Nakada, M.; Zhao, S.G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.I. Ferrochelatase silencing increases the efficacy of fluorescence and 5-aminolevulinic acid photodynamic therapy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Palasuberniam, P.; Myers, K.A.; Wang, C.; Chen, B. Effects of Silencing Heme Biosynthesis Enzymes on 5-Aminolevulinic Acid-mediated Protoporphyrin IX Fluorescence and Photodynamic Therapy. Photochem. Photobiol. 2015, 91, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Kaneko, S. Fluorescence-Guided Resection of Malignant Glioma with 5-ALA. Int. J. Biomed. Imaging 2016, 2016, 6135293. [Google Scholar] [CrossRef]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J. ALA-Glioma Study Group. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Tetard, M.C.; Vermandel, M.; Mordon, S.; Lejeune, J.P.; Reyns, N. Experimental use of photodynamic therapy in high grade gliomas: A review focused on 5-aminolevulinic acid. Photodiagnosis Photodyn. Ther. 2014, 11, 319–330. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-guided surgery for high-grade gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef]
- Ishida, N.; Watanabe, D.; Akita, Y.; Nakano, A.; Yamashita, N.; Kuhara, T.; Yanagishita, T.; Takeo, T.; Tamada, Y.; Matsumoto, Y. Etretinate enhances the susceptibility of human skin squamous cell carcinoma cells to 5-aminolaevulic acid-based photodynamic therapy. Clin. Exp. Dermatol. 2009, 34, 385–389. [Google Scholar] [CrossRef]
- Piskorz, J.; Nowak, M.; Gośliński, T. Therapeutic and diagnostic applications of 5-aminolevulinic acid. Farm Pol. 2009, 65, 476–482. [Google Scholar]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Stummer, W.; Stepp, H.; Möller, G.; Ehrhardt, A.; Leonhard, M.; Reulen, H.J. Technical principles of microsurgical resection of malignant glioma tissue controlled by protoporphyrin-IX-fluorescence. Acta Neurochir. 1998, 140, 995–1000. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neurooncol. 2008, 87, 103–109. [Google Scholar] [CrossRef]
- Schwartz, C.; Ruhm, A.; Tonn, J.C.; Kreth, S.; Kreth, F.W. Interstitial photodynamic therapy for de-novo multiforme glioblastoma WHO IV. Neurooncology 2015, 17, 214–220. [Google Scholar]
- Eljamel, S. Photodynamic applications in brain tumors: A comprehensive review of the literature. Photodiagnosis Photodyn. Ther. 2010, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Pontén, J.; Macintyre, E.H. Long term culture of normal and neoplastic human glia. Acta Pathol. Microbiol. Scand. 1968, 74, 465–486. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martín, B.; Medina, M. Advances in the knowledge of the molecular biology of glioblastoma and its impact in patient diagnosis, stratification, and treatment. Adv. Sci. 2020, 7, 1902971. [Google Scholar] [CrossRef]
- Wolff, J.E.; Trilling, T.; Mölenkamp, G.; Egeler, R.M.; Jürgens, H. Chemosensitivity of glioma cells in vitro: A meta analysis. J. Cancer Res. Clin. Oncol. 1999, 125, 481–486. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Epidermal growth factor receptor cell proliferation signalling pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Ching, J.; Amiridis, S.; Stylli, S.S.; Bjorksten, A.R.; Kountouri, N.; Zheng, T.; Paradiso, L.; Luwor, R.B.; Morokoff, A.P.; O’Brien, T.J.; et al. The peroxisome proliferator activated receptor gamma agonist pioglitazone increases functional expression of the glutamate transporter excitatory amino acid transporter 2 (EAAT2) in human glioblastoma cells. Oncotarget 2015, 6, 21301–21314. [Google Scholar] [CrossRef] [PubMed]
- Strakova, N.; Ehrmann, J.; Bartos, J.; Malikova, J.; Dolezel, J.; Kolar, Z. Peroxisome proliferator-activated receptors (PPAR) agonists affect cell viability, apoptosis and expression of cell cycle related proteins in cell lines of glial brain tumors. Neoplasma 2005, 52, 126–136. [Google Scholar]
- Galeffi, F.; Turner, D.A. Exploiting Metabolic Differences in Glioma Therapy. Curr. Drug Discov. Technol. 2012, 9, 280–293. [Google Scholar] [CrossRef]
- Breuskin, D.; Szczygielski, J.; Urbschat, S.; Kim, Y.J.; Oertel, J. Confocal laser endomicroscopy in neurosurgery- an alternative to instantaneous sections? World Neurosurg. 2017, 100, 180–185. [Google Scholar] [CrossRef]
- Pellerino, A.; Franchino, F.; Soffietti, R.; Rudà, R. Overview on current treatment standards in high-grade gliomas. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 225–238. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature. Molecules 2022, 27, 6847. https://doi.org/10.3390/molecules27206847
Bartusik-Aebisher D, Żołyniak A, Barnaś E, Machorowska-Pieniążek A, Oleś P, Kawczyk-Krupka A, Aebisher D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature. Molecules. 2022; 27(20):6847. https://doi.org/10.3390/molecules27206847
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Aleksandra Żołyniak, Edyta Barnaś, Agnieszka Machorowska-Pieniążek, Piotr Oleś, Aleksandra Kawczyk-Krupka, and David Aebisher. 2022. "The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature" Molecules 27, no. 20: 6847. https://doi.org/10.3390/molecules27206847
APA StyleBartusik-Aebisher, D., Żołyniak, A., Barnaś, E., Machorowska-Pieniążek, A., Oleś, P., Kawczyk-Krupka, A., & Aebisher, D. (2022). The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature. Molecules, 27(20), 6847. https://doi.org/10.3390/molecules27206847








