Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3
Abstract
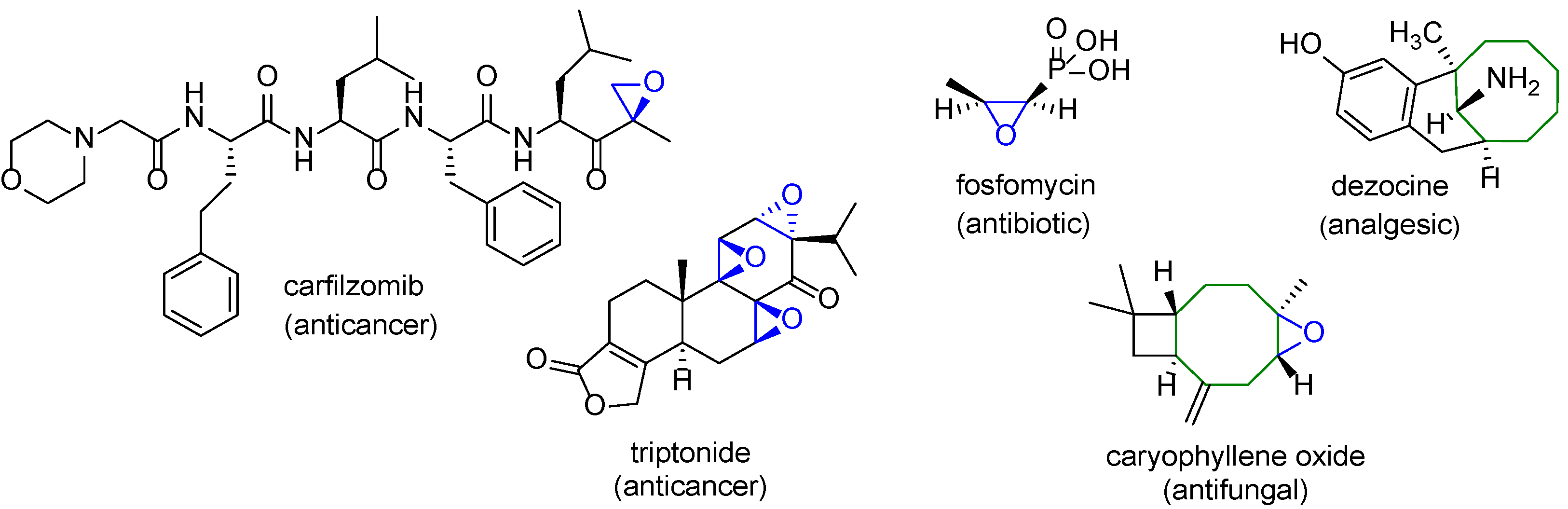
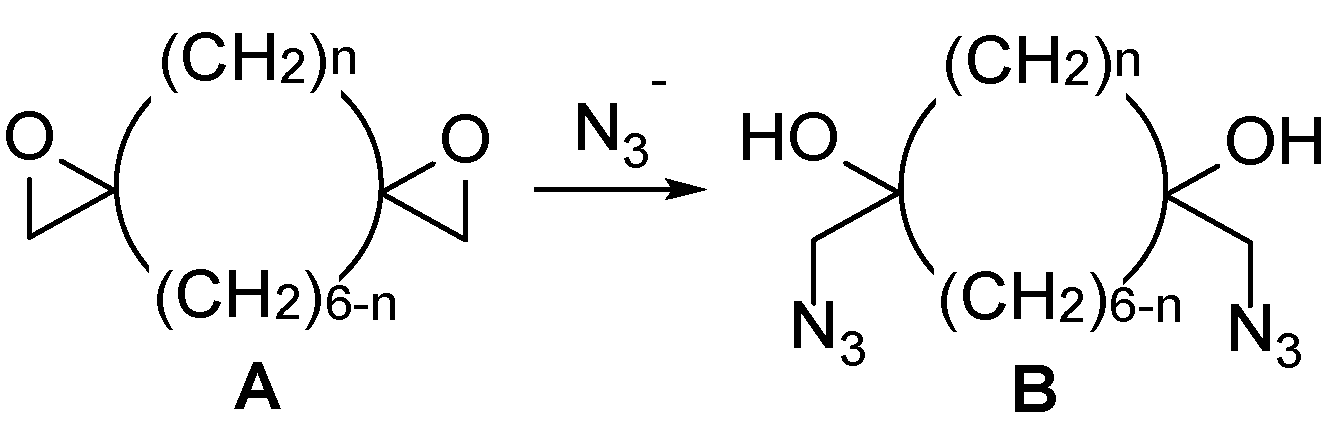
1. Introduction
2. Results and discussion
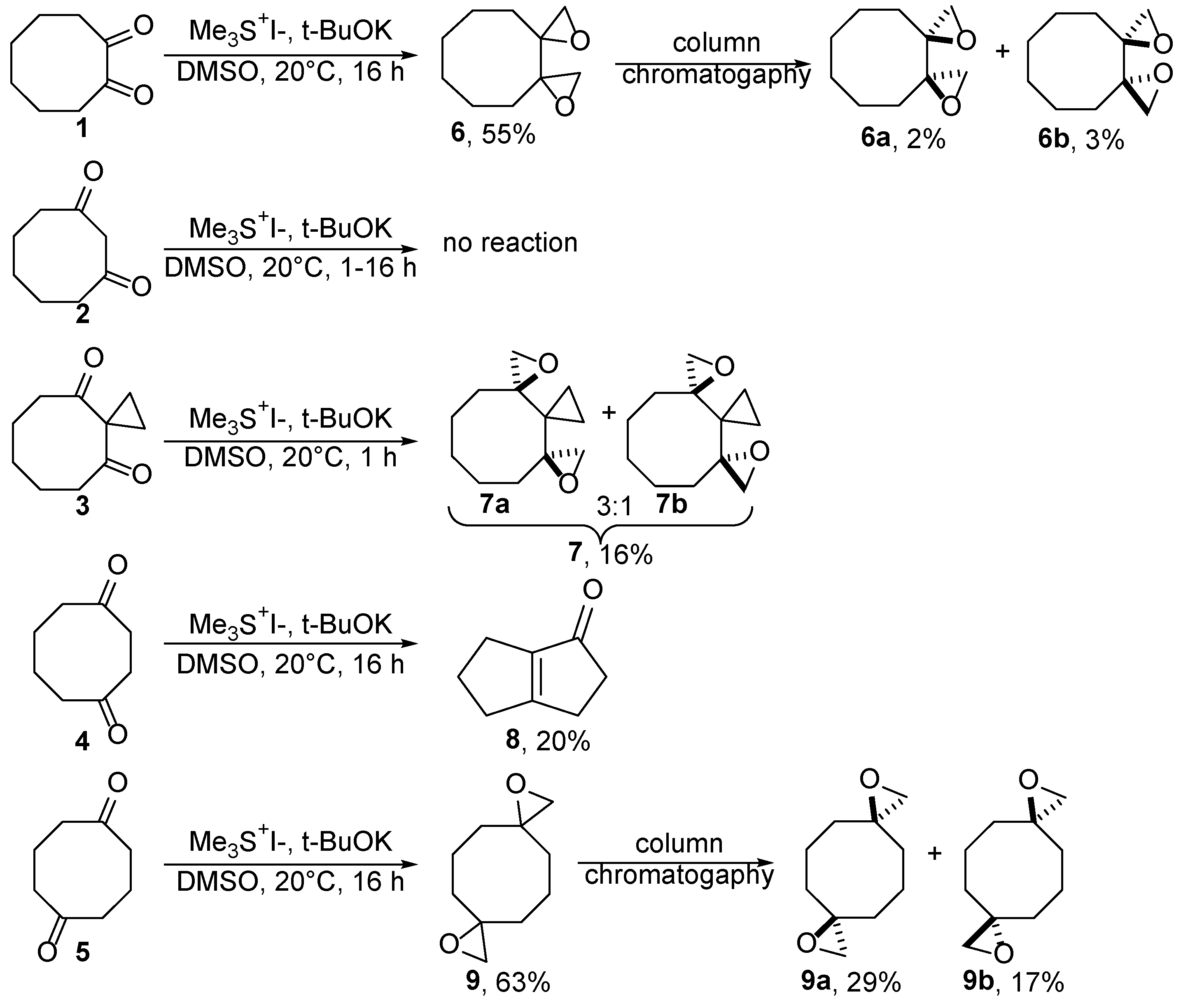
2.1. Synthesis of Bis(oxiranes) via Corey-Chaykovsky Reaction of Cyclooctanediones
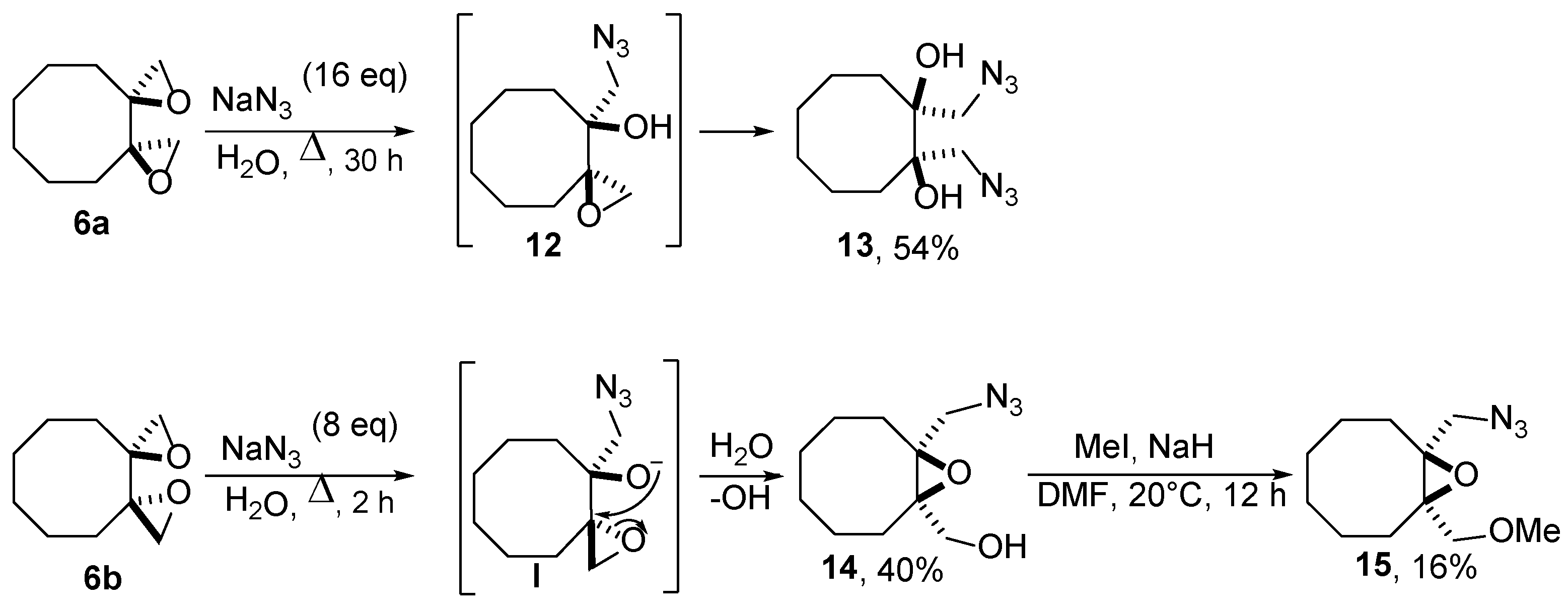
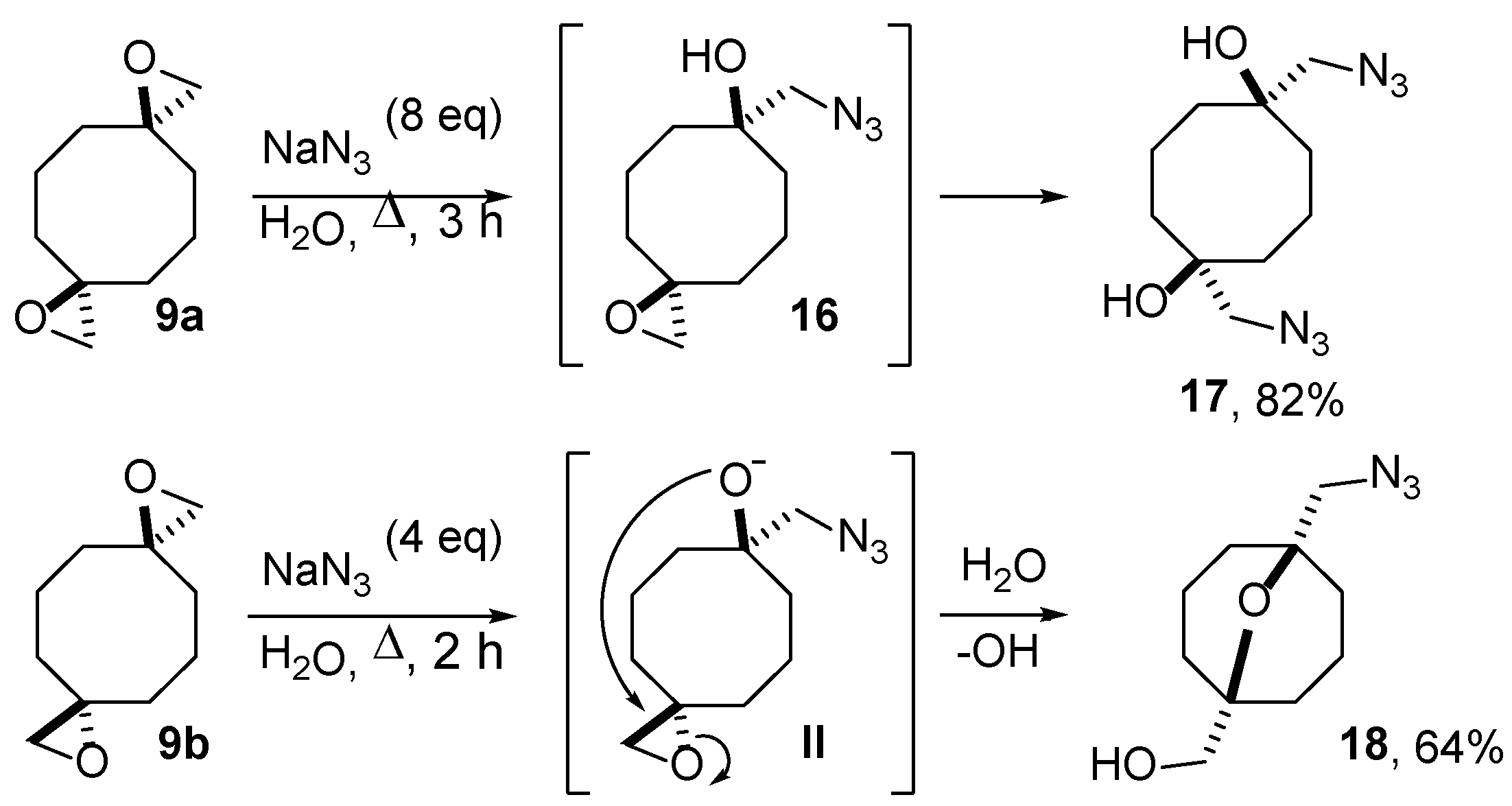
2.2. Ring Opening of Bis(oxiranes) with Sodium Azide
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of Bis(oxiranes) (General Method)
3.3. Ring Opening of Oxiranes upon Treatment with Sodium Azide (General Method)
3.4. Synthesis of (1R,8S)/(1S,8R)-1-(Azidomethyl)-8-(methoxymethyl)-9-oxabicyclo[6.1.0]nonane (15)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Delost, M.D.; Smith, B.J.; Anderson, D.T.; Njardarson, J.T. From oxiranes to oligomers: Architectures of U.S. FDA approved pharmaceuticals containing oxygen heterocycles. J. Med. Chem. 2018, 61, 10996–11020. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Molina, M.T.; Anjum, S. Naturally occurring cyclohexane epoxides: Sources, biological activities, and synthesis. Chem. Rev. 2004, 104, 2857–2900. [Google Scholar] [CrossRef] [PubMed]
- Gomesa, A.R.; Varelab, C.L.; Tavares-da-Silvab, E.J.; Roleira, F.M.F. Epoxide containing molecules: A good or a bad drug design approach. Eur. J. Med. Chem. 2020, 201, 112327. [Google Scholar] [CrossRef] [PubMed]
- Kalgutkar, A.S. Designing around structural alerts in drug discovery. J. Med. Chem. 2020, 53, 6276–6302. [Google Scholar] [CrossRef]
- Gehringer, M.; Laufer, S.A. Emerging and Re-Emerging Warheads for Targeted Covalent Inhibitors: Applications in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2019, 62, 5673–5724. [Google Scholar] [CrossRef]
- Moschona, F.; Savvopoulou, I.; Tsitopoulou, M.; Tataraki, D.; Rassias, G. Epoxide syntheses and ring-opening reactions in drug development. Catalysts 2020, 10, 1117. [Google Scholar] [CrossRef]
- Padwa, A.; Murphree, S.S. Epoxides and aziridines—A mini review. ARKIVOC 2006, 6–33. [Google Scholar] [CrossRef]
- Thirumalaikumar, M. Ring Opening Reactions of Epoxides. A Review. Org. Prep. Proced. Int. 2022, 54, 1–39. [Google Scholar] [CrossRef]
- Talukdar, R. Synthetically important ring opening reactions by alkoxybenzenes and alkoxynaphthalenes. RSC Adv. 2020, 10, 31363–31376. [Google Scholar] [CrossRef]
- Saddique, F.A.; Zahoor, A.F.; Faiz, S.; Ali, S.; Naqvi, R.; Usman, M.; Ahmad, M. Recent trends in ring opening of epoxides by amines as nucleophiles. Synth. Commun. 2016, 46, 831–868. [Google Scholar] [CrossRef]
- Meninno, S.; Lattanzi, A. Organocatalytic Asymmetric Reactions of Epoxides: Recent Progress. Chem. Eur. J. 2016, 22, 3632–3642. [Google Scholar] [CrossRef]
- Faiz, S.; Zahoor, A.F. Ring opening of epoxides with C-nucleophiles. Mol. Divers. 2016, 20, 969–987. [Google Scholar] [CrossRef]
- Guo, L.; Lamb, K.J.; North, M. Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Chen, Z.; Nasr, S.M.; Kazemi, M.; Mohammadi, M. A Mini-Review: Achievements in the Thiolysis of Epoxides. Mini. Rev. Org. Chem. 2020, 17, 352–362. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.; Koo, J.; Cho, W.; Lee, W.S.; Park, W.; Park, S.B. Diversity-oriented synthetic strategy for developing a chemical modulator of protein–protein interaction. Nat. Commun. 2016, 7, 13196. [Google Scholar] [CrossRef]
- Marcaurelle, L.A.; Comer, E.; Dandapani, S.; Duvall, J.R.; Gerard, B.; Kesavan, S.; Lee, M.D.; Liu, H.; Lowe, J.T.; Marie, J.-C.; et al. An aldol-based build/couple/pair strategy for the synthesis of medium- and large-sized rings: Discovery of macrocyclic histone deacetylase inhibitors. J. Am. Chem. Soc. 2010, 132, 16962–16976. [Google Scholar] [CrossRef]
- Clarke, A.K.; Unsworth, W.P. A happy medium: The synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 2020, 11, 2876–2881. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Andriasov, K.S.; Eremenko, M.G.; Grishin, Y.K.; Alferova, V.A.; Baranova, A.A.; Zefirov, N.A.; Zefirova, O.N.; Zarubaev, V.V.; Gracheva, Y.A.; et al. Bicyclic Isoxazoline Derivatives: Synthesis and Evaluation of Biological Activity. Molecules 2022, 27, 3546. [Google Scholar] [CrossRef]
- Hu, Y.-J.; Li, L.-X.; Han, J.-C.; Min, L.; Li, C.-C. Recent Advances in the Total Synthesis of Natural Products Containing Eight-Membered Carbocycles (2009−2019). Chem. Rev. 2020, 120, 5910–5953. [Google Scholar] [CrossRef]
- Averina, E.B.; Sedenkova, K.N.; Bakhtin, S.G.; Grishin, Y.K.; Kutateladze, A.G.; Roznyatovsky, V.A.; Rybakov, V.B.; Butov, G.M.; Kuznetsova, T.S.; Zefirov, N.S. symm-Tetramethylenecyclooctane: En Route to Polyspirocycles. J. Org. Chem. 2014, 79, 8163–8170. [Google Scholar] [CrossRef]
- Cope, A.C.; Martin, M.M.; McKervey, M.A. Transannular reactions in medium-sized rings. Q. Rev. Chem. Soc. 1966, 20, 119–152. [Google Scholar] [CrossRef]
- Reyes, E.; Uria, U.; Carrillo, L.; Vicario, J.L. Transannular reactions in asymmetric total synthesis. Tetrahedron 2014, 70, 9461–9484. [Google Scholar] [CrossRef]
- Cope, A.C.; Woo, G.L. Proximity Effects. XXXIV. Solvolysis of cis-Bicyclo[6.1.0]nonane. J. Am. Chem. Soc. 1963, 85, 3601–3608. [Google Scholar] [CrossRef]
- Talaz, O.; Saracoglu, N. A study on the synthesis of structural analogs of bis-indole alkaloid caulerpin: A step-by-step synthesis of a cyclic indole-tetramer. Tetrahedron 2010, 66, 1902–1910. [Google Scholar] [CrossRef]
- Sivaguru, P.; Ning, Y.; Bi, X. New Strategies for the Synthesis of Aliphatic Azides. Chem. Rev. 2021, 121, 4253–4307. [Google Scholar] [CrossRef]
- Mandler, M.D.; Degnan, A.P.; Zhang, S.; Aulakh, D.; Georges, K.; Sandhu, B.; Sarjeant, A.; Zhu, Y.; Traeger, S.C.; Cheng, P.T.; et al. Structural and Thermal Characterization of Halogenated Azidopyridines: Under-Reported Synthons for Medicinal Chemistry. Org. Lett. 2022, 24, 799–803. [Google Scholar] [CrossRef]
- Bednarek, C.; Wehl, I.; Jung, N.; Schepers, U.; Bräse, S. The Staudinger Ligation. Chem. Rev. 2020, 120, 4301–4354. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, X.; Jing, L.; Wu, G.; Kang, D.; Liu, X.; Zhan, P. Recent applications of click chemistry in drug discovery. Expert Opin. Drug Discov. 2019, 14, 779–789. [Google Scholar] [CrossRef]
- Farkas, F.; Wellauer, T.; Esser, T.; Sequin, U. The reaction of 1,2:3,4-diepoxy-2,3-dimethylbutane with nucleophiles. Helv. Chim. Acta 1991, 74, 1511–1519. [Google Scholar] [CrossRef]
- Kozlov, N.S.; Yakubovich, L.S.; Zhavnerko, K.A.; Prishchepenko, V.M. Reaction of cyclopentadiene trans-dioxide with amines. J. Org. Chem. USSR 1982, 18, 1842–1846. [Google Scholar]
- Nurieva, E.V.; Zefirov, N.A.; Mamaeva, A.V.; Grishin, Y.K.; Kuznetsov, S.A.; Zefirova, O.N. Synthesis of non-steroidal 2-methoxyestradiol mimetics based on the bicyclo[3.3.1]nonane structural motif. Mendeleev Commun. 2017, 27, 240–242. [Google Scholar] [CrossRef]
- Nurieva, E.V.; Semenova, I.S.; Nuriev, V.N.; Shishov, D.V.; Baskin, I.I.; Zefirova, O.N.; Zefirov, N.S. Diels-alder reaction as a synthetic approach to bicyclo[3.3.1]nonane colchicine analogs. Russ. J. Org. Chem. 2010, 46, 1892–1895. [Google Scholar] [CrossRef]
- Zefirova, O.N.; Plotnikova, E.D.; Nurieva, E.V.; Peregud, D.I.; Onufriev, M.V.; Gulyaeva, N.V. Synthesis of 2-thia-4-azabicyclo[3.3.1]non-3-en-3-amine–bridged nitric oxide synthase inhibitor with enhanced lipophilicity. Mendeleev Commun. 2013, 23, 76–77. [Google Scholar] [CrossRef]
- Wittig, G.; Krebs, A. Zur Existenz niedergliedriger Cycloalkine, I. Chem. Ber. 1961, 94, 3260–3275. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Webste, N.J.B. Mechanism of intramolecular photocycloadditions of cyclooctenones. J. Org. Chem. 1987, 52, 3603–3613. [Google Scholar] [CrossRef]
- Sedenkova, K.N.; Andriasov, K.S.; Stepanova, S.A.; Gloriozov, I.P.; Grishin, Y.K.; Kuznetsova, T.S.; Averina, E.B. Direct oxidation of cyclopropanated cyclooctanes as a synthetic approach to polycyclic cyclopropyl ketones. Eur. J. Org. Chem. 2018, 2018, 879–884. [Google Scholar] [CrossRef]
- Lyttle, M.H.; Streitwieser, A.; Miller, M.J. 1,5-Disubstituted cyclooctatetraenes. J. Org. Chem. 1989, 54, 2331–2335. [Google Scholar] [CrossRef]
- Fries, D.S.; Andrako, J.; Hudgins, P. Synthesis and preliminary pharmacological activity of aminoalkoxy isosteres of glycolate ester anticholinergics. J. Med. Chem. 1977, 20, 1250–1254. [Google Scholar] [CrossRef]
- Nagano, T.; Einaru, S.; Shitamichi, K.; Asano, K.; Matsubara, S. trans-Cyclooctenes as chiral ligands in rhodium-catalyzed asymmetric 1,4-additions. Eur. J. Org. Chem. 2020, 2020, 7131–7133. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Laikov, D.N. A new class of atomic basis functions for accurate electronic structure calculations of molecules. Chem. Phys. Lett. 2005, 416, 116–120. [Google Scholar] [CrossRef]
- Laikov, D.N. Optimization of atomic density-fitting basis functions for molecular two-electron integral approximations. J. Chem. Phys. 2020, 153, 114121. [Google Scholar] [CrossRef]
- Schreckenbach, G.; Ziegler, T. Calculation of NMR shielding tensors based on density functional theory and a scalar relativistic Pauli-type Hamiltonian. The application to transition metal complexes. Int. J. Quantum Chem. 1997, 61, 899–918. [Google Scholar] [CrossRef]
- Schreckenbach, G.; Ziegler, T. Calculation of NMR Shielding Tensors Using Gauge-Including Atomic Orbitals and Modern Density Functional Theory. J. Phys. Chem. 1995, 99, 606–611. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. Int. Ed. 2005, 54, 820–826. [Google Scholar] [CrossRef]
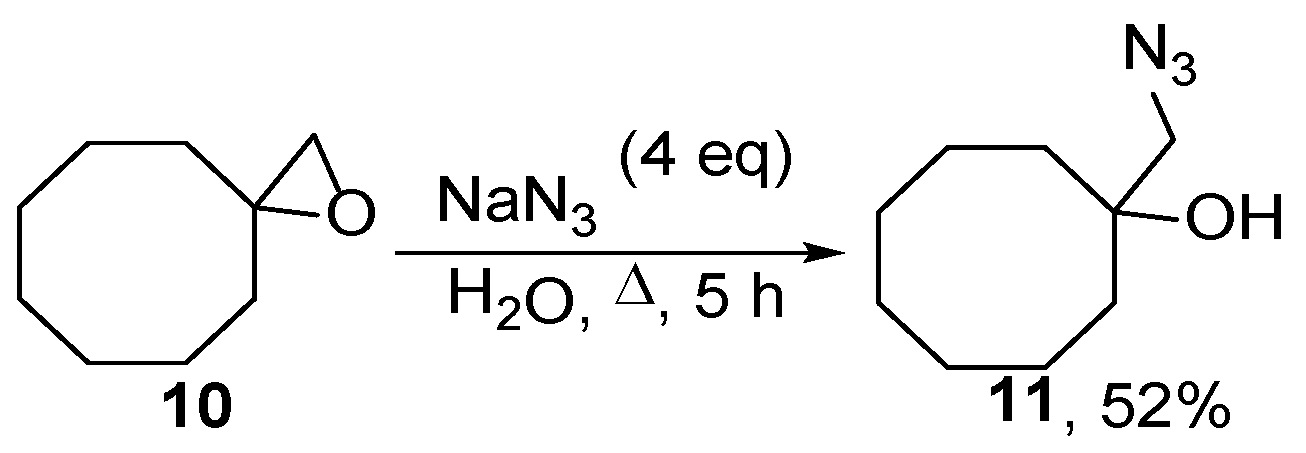
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedenkova, K.N.; Ryzhikova, O.V.; Stepanova, S.A.; Averin, A.D.; Kositov, S.V.; Grishin, Y.K.; Gloriozov, I.P.; Averina, E.B. Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3. Molecules 2022, 27, 6889. https://doi.org/10.3390/molecules27206889
Sedenkova KN, Ryzhikova OV, Stepanova SA, Averin AD, Kositov SV, Grishin YK, Gloriozov IP, Averina EB. Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3. Molecules. 2022; 27(20):6889. https://doi.org/10.3390/molecules27206889
Chicago/Turabian StyleSedenkova, Kseniya N., Olga V. Ryzhikova, Svetlana A. Stepanova, Alexei D. Averin, Sergei V. Kositov, Yuri K. Grishin, Igor P. Gloriozov, and Elena B. Averina. 2022. "Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3" Molecules 27, no. 20: 6889. https://doi.org/10.3390/molecules27206889
APA StyleSedenkova, K. N., Ryzhikova, O. V., Stepanova, S. A., Averin, A. D., Kositov, S. V., Grishin, Y. K., Gloriozov, I. P., & Averina, E. B. (2022). Bis(oxiranes) Containing Cyclooctane Core: Synthesis and Reactivity towards NaN3. Molecules, 27(20), 6889. https://doi.org/10.3390/molecules27206889





