Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization Analysis Results
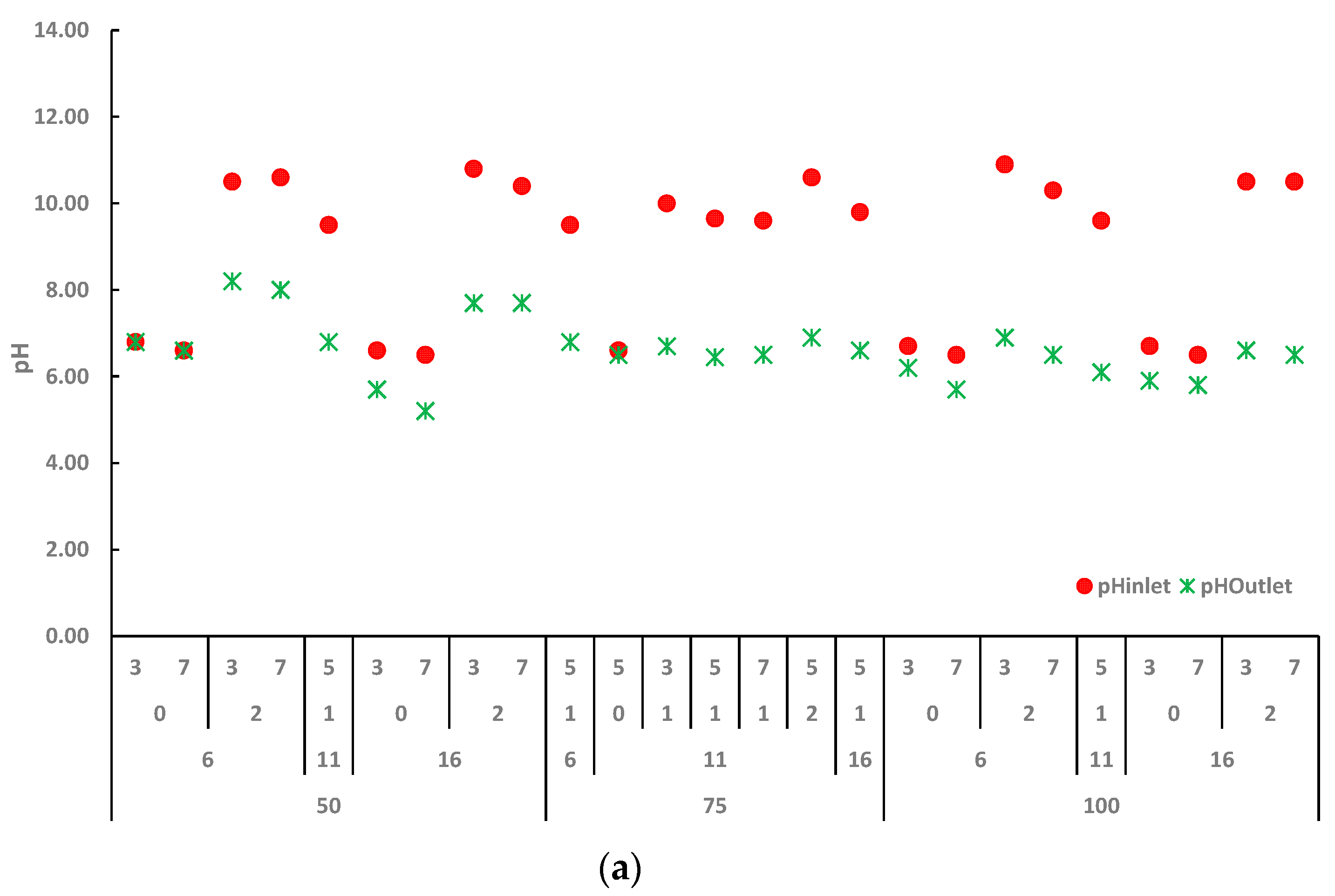
2.2. Effect of Ca(OH)2 Assisted Thermal Pretreatment Process on Switchgrass
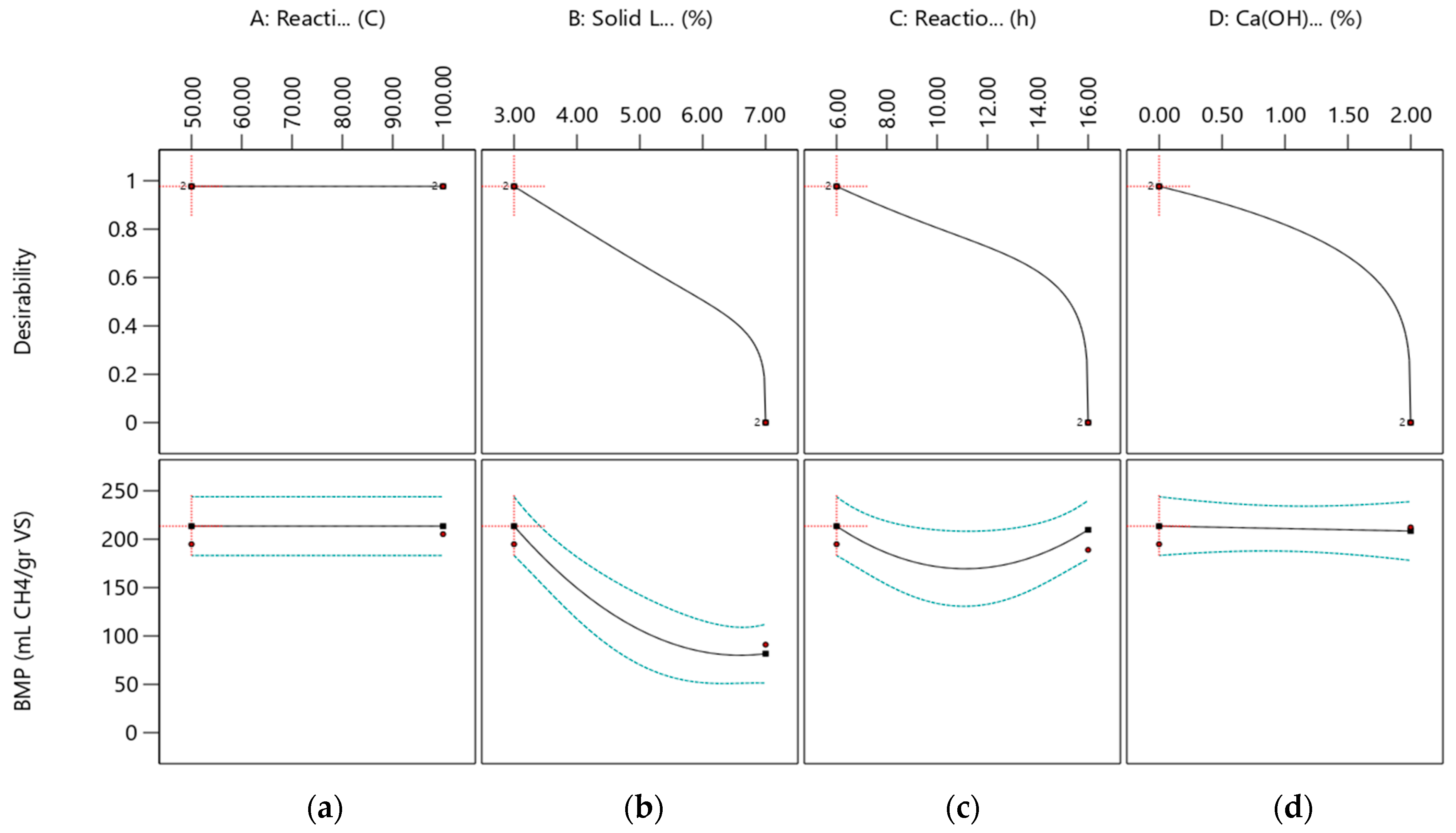
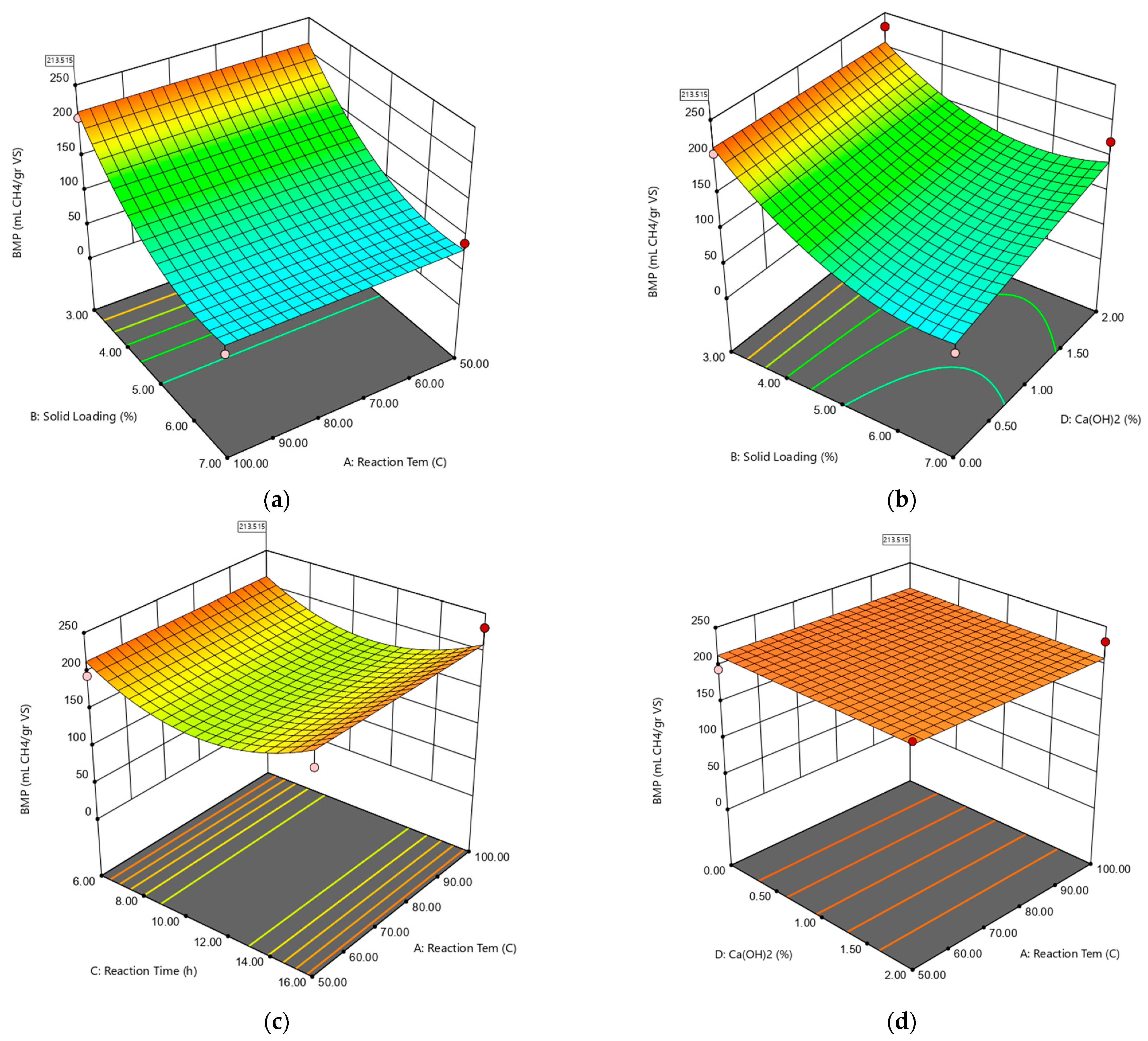
2.3. Modeling and Optimization of Ca(OH)2 Assisted Thermal Pretreatment Process
2.4. Effects of Ca(OH)2-Assisted Thermal Pretreatment Process on Switchgrass Surface Modification and Molecular Bond Changes
| Wavelength (cm−1) | Affected Chemical Bond and Impact | Reference |
|---|---|---|
| 470 | Resonance in bonds of Si-O-Si and (PO4)3− | [45,46] |
| 620 | Resonance in C-O, C=O bonds | [47] |
| 775 | Resonance in NH2 bonds | [48] |
| 895 | Resonance in β-glucosidic bonds, Indicator for crystalline and amorphous cellulose rate | [49,50,51] |
| 1050 | C-O, C=C, C-OH, and C-O-C tensions in cellulose and hemicellulose | [52,53] |
| 1180 | Asymmetrical C-O-C tension in cellulose and hemicellulose | [54] |
| 1245 | C-O adsorption of acetyl groups in hemicellulose | [52] |
| 1280 | C-H warping in crystallized cellulose | [55] |
| 1440 | O-H bond in linear warping of hemicellulose and lignin | [54] |
| 1465 | C-H deformation in lignin | [54] |
| 1580 | Resonance of aromatic rings in lignin | [51] |
| 1735 | Resonance of bonds in ketone and ester carbonyl groups | [55] |
| 2900–3400 | Resonance and tension of C-H and O-H bonds in cellulose | [51,53,56] |
2.5. Kinetic Modeling
3. Materials and Methods
3.1. Switchgrass
3.2. Switchgrass Characterization
3.3. Experimental Design and Analysis
3.4. Ca(OH)2-Assisted Thermal Pretreatment
3.5. Biochemical Methane Potential Test (BMP)
3.6. Scanning Electron Microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Kinetic Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Debnath, D.; Khanna, M.; Rajagopal, D.; Zilberman, D. The future of biofuels in an electrifying global transportation sector: Imperative, prospects and challenges. Appl. Econ. Perspect. Policy 2019, 41, 563–582. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Muszyński, P.; Haliniarz, M.; Brodowski, R.; Kowalczyk-Juśko, A.; Sekutowski, T.; Kurzyna-Szklarek, M. Agronomic aspects of switchgrass cultivation and use for energy purposes. Appl. Ecol. Environ. Res. 2018, 16, 5715–5743. [Google Scholar] [CrossRef]
- Başar, İ.A.; Perendeci, N.A. Optimization of zero-waste hydrogen peroxide-Acetic acid pretreatment for sequential ethanol and methane production. Energy 2021, 225, 120324. [Google Scholar] [CrossRef]
- Başar, İ.A.; Çoban, Ö.; Göksungur, M.Y.; Eskicioğlu, Ç.; Perendeci, N.A. Enhancement of lignocellulosic biomass anaerobic digestion by optimized mild alkaline hydrogen peroxide pretreatment for biorefinery applications. J. Environ. Manag. 2021, 298, 113539. [Google Scholar] [CrossRef] [PubMed]
- Larnaudie, V.; Ferrari, M.D.; Lareo, C. Switchgrass as an alternative biomass for ethanol production in a biorefinery: Perspectives on technology, economics and environmental sustainability. Renew. Sustain. Energy Rev. 2022, 158, 112115. [Google Scholar] [CrossRef]
- Alawad, I.; Ibrahim, H. Pretreatment of agricultural lignocellulosic biomass for fermentable sugar: Opportunities, challenges, and future trends. Biomass Convers. Biorefinery 2022, 1–29. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroualf, Y.; Barakat, A. Mild microwaves, ultrasonic and alkaline pretreatments for improving methane production: Impact on biochemical and structural properties of olive pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef]
- Tian, C.; Yan, M.; Huang, X.; Zhong, Y.; Lu, H.; Zhou, X. Highly acetylated lignocellulose prepared by alkaline extrusion pretreatment assisted acetylation reaction. Cellulose 2022, 29, 1487–1500. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Hu, M.; Yua, H.; Lia, Y.; Li, A.; Cai, Q.; Liu, P.; Tu, Y.; Wang, Y.; Hud, R.; Hao, B.; et al. Distinct polymer extraction and cellulose DP reduction for complete cellulose hydrolysis under mild chemical pretreatments in sugarcane. Carbohydr. Polym. 2018, 202, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, C.; Escudi, R.; Bernet, N.; Santa-Catalina, G.; Frederic, S.; Carrere, H. Conditions for efficient alkaline storage of cover crops for biomethane production. Bioresour. Technol. 2022, 348, 126722. [Google Scholar] [CrossRef]
- Madadi, M.; Wang, Y.; Xu, C.; Liu, P.; Wang, Y.; Xia, T.; Tu, Y.; Lin, X.; Song, B.; Yang, X.; et al. Using Amaranthus green proteins as universal biosurfactant and biosorbent for effective enzymatic degradation of diverse lignocellulose residues and efficient multiple trace metals remediation of farming lands. J. Hazard. Mater. 2021, 406, 124727. [Google Scholar] [CrossRef]
- Wu, L.; Feng, S.; Deng, J.; Yu, B.; Wang, Y.; He, B.; Peng, H.; Li, Q.; Hu, R.; Peng, L. Altered carbon assimilation and cellulose accessibility to maximize bioethanol yield under low-cost biomass processing in corn brittle stalk. Green Chem. 2019, 21, 4388. [Google Scholar] [CrossRef]
- Thomas, H.; Arnoult, S.; Brancourt-Hulmel, M.; Carrère, H. Methane production variability according to miscanthus genotype and alkaline pretreatments at high solid content. BioEnergy Res. 2019, 12, 325–337. [Google Scholar] [CrossRef]
- Kupry´s-Caruk, M.; Podlaski, S.; Kotyrba, D. Influence of double-cut harvest system on biomass yield, quality and biogas production from C4 perennial grasses. Biomass Bioenergy 2019, 130, 105376. [Google Scholar] [CrossRef]
- Frigon, J.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Masse, D.; Gilbert, Y.; Savoie, P.; Belanger, G.; Parent, G.; Babineau, D. Methane yield from switchgrass and reed canary grass grown in Eastern Canada. Bioresour. Technol. 2011, 102, 10286–10292. [Google Scholar] [CrossRef]
- Uma, S.; Thalla, A.K.; Devatha, C.P. Co-digestion of food waste and switchgrass for biogas potential: Effects of process parameters. Waste Biomass Valorization 2020, 11, 827–839. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, R.; Rojas-Sossa, J.P.; Isaguirre, C.; Mashburn, A.; Marsh, T.; Liu, Y.; Liao, W. Anaerobic co-digestion of energy crop and agricultural wastes to prepare uniform-format cellulosic feedstock for biorefining. Renew. Energy 2020, 147, 1358–1370. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, P.; Zhang, G.; Nabi, M.; Wang, S.; Ye, J.; Bao, S.; Zhang, Q.; Chen, N. Carbide slag pretreatment enhances volatile fatty acid production in anaerobic fermentation of four grass biomasses. Energy Convers. Manag. 2019, 199, 112009. [Google Scholar] [CrossRef]
- Ciggin, A.S. Anaerobic co-digestion of sewage sludge with switchgrass: Experimental and kinetic evaluation. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 15–21. [Google Scholar] [CrossRef]
- El-Mashad, H.M. Biomethane and ethanol production potential of Spirulina platensis algae and enzymatically saccharified switchgrass. Biochem. Eng. J. 2015, 93, 119–127. [Google Scholar] [CrossRef]
- Jackowiak, D.; Frigon, J.C.; Ribeiro, T.; Pauss, A.; Guiot, S. Enhancing solubilisation and methane production kinetic of switchgrass by microwave pretreatment. Bioresour. Technol. 2011, 102, 3535–3540. [Google Scholar] [CrossRef] [Green Version]
- Ünyay, H.; Yılmaz, F.; Başar, İ.A.; Perendeci, N.A.; Çoban, I.; Sahinkaya, E. Effects of organic loading rate on methane production from switchgrass in batch and semi-continuous stirred tank reactor system. Biomass Bioenergy 2022, 156, 106306. [Google Scholar] [CrossRef]
- Jin, G.; Bierma, T.; Walker, P.M. Low-heat, mild alkaline pretreatment of switchgrass for anaerobic digestion. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 565–574. [Google Scholar] [CrossRef]
- Jin, G.; Bierma, T. Low-heat alkaline pretreatment of biomass for dairy anaerobic codigestion. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Waste 2014, 49, 786–796. [Google Scholar] [CrossRef]
- Jin, G.; Bierma, T.; Walker, P.M. Biogas production from switchgrass under experimental conditions simulating U.S. digester operations. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 470–478. [Google Scholar] [CrossRef]
- Başar, İ.A.; Kokdemir Ünsar, E.; Ünyay, H.; Perendeci, N.A. Ethanol, methane, or both? Enzyme dose impact on ethanol and methane production from untreated energy crop switchgrass varieties. Renew. Energy 2020, 149, 287–297. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Wang, W.; Liu, G.; Chen, C. Assessment of pretreatment effects on anaerobic digestion of switchgrass: Economics-energy-environment (3E) analysis. Ind. Crops Prod. 2020, 145, 111957. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, H.; Yang, F.; Zhang, Y.; Gao, F. Microwave pretreatments of switchgrass leaf and stem fractions to increase methane production. Bioresources 2015, 10, 3922–3933. [Google Scholar] [CrossRef] [Green Version]
- Lemusa, E.R.; Brummer, C.; Moore, K.J.; Molstad, N.E.; Burras, C.E.; Barker, M.F. Biomass yield and quality of 20 switchgrass populations in southern Iowa, USA. Biomass Bioenergy 2002, 23, 433–442. [Google Scholar] [CrossRef]
- Niu, H.; Kong, X.; Li, L.; Sun, Y.; Yuan, Z.; Zhou, X. Analysis of Biogas from Switchgrass by Anaerobic Digestion. Bioresources 2015, 10, 7178–7187. [Google Scholar] [CrossRef] [Green Version]
- Imam, T.; Capareda, S. Characterization of Bio-Oil, Syn-Gas and Bio-Char From Switchgrass Pyrolysis at Various Temperatures. J. Anal. Appl. Pyrolysis 2011, 93, 170–177. [Google Scholar] [CrossRef]
- Masnadi, M.S.; Habibi, R.; Kopyscinski, J.; Hill, J.M.; Bi, X.; Lim, C.J.; Ellis, N.; Grace, J.R. Fuel characterization and co-pyrolysis kinetics of biomass and fossil fuels. Fuel 2014, 117, 1204–1214. [Google Scholar] [CrossRef]
- Sadaka, S.; Sharara, M.A.; Ashworth, A.; Keyser, P.; Allen, F.; Wright, A. Characterization of Biochar from Switchgrass Carbonization. Energies 2014, 7, 548–567. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Baxer, D. Trace element concentrations and associations in some biomass ashes. Fuel 2014, 129, 292–313. [Google Scholar] [CrossRef]
- Papa, G.; Rodriguez, S.; George, A.; Schievano, A.; Orzi, V.; Sale, K.L.; Singh, S.; Adani, F.; Simmons, B.A. Comparison of different pretreatments for the production of bioethanol and biomethane from corn stover and switchgrass. Bioresour. Technol. 2015, 183, 101–110. [Google Scholar] [CrossRef]
- Athmanathan, A.; Emery, I.R.; Kuczek, T.; Mosier, N.S. Impact of temperature, moisture, and storage duration on the chemical composition of switchgrass, corn stover, and sweet sorghum bagasse. Bioenergy Res. 2015, 8, 843–856. [Google Scholar] [CrossRef]
- Thomas, H.L.; Nolasco, H.F.P.; Carrère, H.; Lartaud, M.; Cao, T.V.; Baptiste, C.; Verdei, J.L. Alkaline Pretreatments for Sorghum and Miscanthus Anaerobic Digestion: Impacts at Cell Wall and Tissue Scales. BioEnergy Res. 2022, 15, 792–809. [Google Scholar] [CrossRef]
- Grigatti, M.; Barbanti, L.; Pritoni, G.; Venturi, G. Comparison of switchgrass (Panicum virgatum L.) genotypes as potential energy crop. In Proceedings of the 2nd World Conference and Technology Exhibition on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004; Available online: https://www.researchgate.net/publication/235781180_COMPARISON_OF_SWITCHGRASS_Panicum_virgatum_L_GENOTYPES_AS_POTENTIAL_ENERGY_CROP (accessed on 25 February 2022).
- Günerhan, Ü.; Us, E.; Dumlu, L.; Yılmaz, V.; Carrère, H.; Perendeci, A.N. Impacts of Chemical-Assisted Thermal Pretreatments on Methane Production from Fruit and Vegetable Harvesting Wastes: Process Optimization. Molecules 2020, 25, 500. [Google Scholar] [CrossRef] [Green Version]
- Dar, R.A.; Phutela, U.G. Enzymatic and hydrothermal pretreatment of newly isolated Spirulina subsalsa BGLR6 biomass for enhanced biogas production. Waste Biomass Valorization 2020, 11, 3639–3651. [Google Scholar] [CrossRef]
- Saikia, B.J.; Parthasarathy, G. Fourier Transform Infrared Spectroscopic Characterization of Kaolinite from Assam and Meghalaya, Northeastern India. J. Mod. Phys. 2010, 1, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Uysal, I.; Severcan, F.; Evis, Z. Characterization by Fourier transform infrared spectroscopy of hydroxyapatite co-doped with zinc and fluoride. Ceram. Int. 2013, 39, 7727–7733. [Google Scholar] [CrossRef]
- Litescu, S.C.; Teodor, E.D.; Truica, G.I.; Tach, A.; Radu, G.L. Fourier Transform Infrared Spectroscopy–Useful Analytical Tool for Non-Destructive Analysis. Infrared Spectrosc.-Mater. Sci. Eng. Technol. 2012, 353–368. [Google Scholar]
- Sumayya, A.; Panicker, C.Y.; Varghese, H.T.; Harikumar, B. Vibrational spectroscopic studies and AB inition calculations of l-glutamic acid 5-amide. RJC Rasayan J. Chem. 2008, 1, 548–555. [Google Scholar]
- Chandrasekaran, S.R.; Hopke, P.K.; Rector, L.; Allen, G.; Lin, L. Chemical composition of wood chips and wood pellets. Energy Fuels 2012, 26, 4932–4937. [Google Scholar] [CrossRef]
- Sun, C.; Liu, R.; Cao, W.; Yin, R.; Mei, Y.; Zhang, L. Impacts of Alkaline Hydrogen Peroxide Pretreatment on Chemical Composition and Biochemical Methane Potential of Agricultural Crop Stalks. Energy Fuels 2015, 29, 4966–4975. [Google Scholar] [CrossRef]
- Fu, S.F.; Wang, F.; Yuan, X.Z.; Yang, Z.M.; Luo, S.J.; Wang, C.S.; Guo, R.B. The thermophilic (55 °C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour. Technol. 2015, 175, 203–208. [Google Scholar] [CrossRef]
- Ang, T.N.; Ngoh, G.C.; Seak, A.; Chua, M.; Lee, M.G. Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses Elucidation of the effect of ionic liquid pretreatment on rice husk via structural analyses. Biotechnol. Biofuels 2012, 5, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Sun, R.; Fowler, P.; Baird, M.S. Comparative study of organosolv lignins from wheat straw. Ind. Crops Prod. 2006, 23, 180–193. [Google Scholar] [CrossRef]
- Gokulakumar, B.; Narayanaswamy, R. Fourier Transform–Infrared Spectra (FT-IR) Analysis of Root Rot Disease in Sesame (Sesamum Indicum). Rom. J. Biophys. 2008, 18, 217–223. [Google Scholar]
- Hsu, T.C.; Guo, G.L.; Chen, W.H.; Hwang, W.S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, I.; Alegria, I.; Zazpe, M.; Echeverria, M.; Echeverria, I. Diluted acid hydrolysis pretreatment of agri-food wastes for bioethanol production. Ind. Crops Prod. 2006, 24, 214–221. [Google Scholar] [CrossRef]
- Buffiere, P.; Loisel, D.; Bernet, N.; Delgenes, J.P. Towards new indicator for the prediction of solid waste anaerobic digestion properties. Water Sci. Technol. 2006, 53, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Wang, Q.; Jiang, Z.; Yang, X.; Ji, Y. Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenergy 2007, 31, 162–167. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Oliva, J.M.; Saez, F.; Castro, E. Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharification. Bioresour. Technol. 2008, 99, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- AWWA; WEF; APHA. Standard methods: 2540. In Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Water Environment Federation: Alexandria, VA, USA, 1997; pp. 55–61. [Google Scholar]
- Van Soest, P.J. Use of detergent in the analysis of fibrous feeds. A rapid method for the determination of fibre and lignin. J. Assoc. Anal. Chem. 1963, 46, 829e35. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.T.; Crocker, D. Determination of structural carbohydrates and lignin in biomass determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2012, 17. [Google Scholar]
- Perendeci, N.A.; Gokgol, S.; Orhon, D. Impact of alkaline H2O2 pretreatment on methane generation potential of greenhouse crop waste under anaerobic conditions. Molecules 2018, 23, 1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perendeci, N.A.; Ciggin, A.S.; Kokdemir Ünşar, E.; Orhon, D. Optimization of alkaline hydrothermal pretreatment of biological sludge for enhanced methane generation under anaerobic conditions. Waste Manag. 2020, 107, 9–19. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Yilmaz, V.; Taştan, B.E.; Gökgö, S.; Fardinpoor, M.; Namlı, A.; Steyer, J.P. Correlations between biochemical composition and biogas production during anaerobic digestion of microalgae and cyanobacteria isolated from different sources of Turkey. Bioresour. Technol. 2019, 281, 209–216. [Google Scholar] [CrossRef]
- Yilmaz, F.; Kökdemir Ünşar, E.; Perendeci, N.A.; Sahinkaya, E. Energy generation from multifarious wastes of alcohol distillery raki production process: Kinetic modeling of methane production. J. Environ. Chem. Eng. 2021, 9, 104838. [Google Scholar] [CrossRef]




| Component | Result | Std. Dev. |
|---|---|---|
| Solid | ||
| Total Solid, TS (g kg−1 Sample) | 938.12 | 0.54 |
| Volatile Solid, VS (g kg−1 Sample) | 824.31 | 3.36 |
| Van Soest Fractions | ||
| Neutral Detergent Solubles (%) | 26.54 | 0.48 |
| Hemicellulose (%) | 34.76 | 0.36 |
| Cellulose (%) | 33.13 | 0.44 |
| Acid Detergent Lignin (%) | 5.57 | 0.39 |
| Elemental Analysis | ||
| Carbon, C (%) | 40.13 | |
| Hydrogen, H (%) | 5.75 | |
| Nitrogen, N (%) | 0.87 | |
| Sulfur, S (%) | - | |
| Total Structural Carbohydrates (%) | 52.86 | 0.559 |
| Cellobiose (%) | 0 | |
| Glucose (%) | 29.41 | 0.347 |
| Xylose (%) | 17.36 | 0.167 |
| Galactose (%) | 3.23 | 0.018 |
| Arabinose (%) | 2.87 | 0.027 |
| Mannose (%) | 0 |
| Biochemical Methane Potential Model (Modified, Reduced Quadratic) | |||||
|---|---|---|---|---|---|
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
| Model | 83,292.64 | 6 | 13,882.11 | 19.01 | <0.0001 |
| B-Solid loading (SL, wt.%) as DM | 43,543.01 | 1 | 43,543.01 | 59.63 | <0.0001 |
| C-Reaction time (h) | 65.62 | 1 | 65.62 | 0.0899 | 0.7676 |
| D-Ca(OH)2 conc. (wt.%) | 3625.80 | 1 | 3625.80 | 4.97 | 0.0381 |
| BD | 4480.47 | 1 | 4480.47 | 6.14 | 0.0228 |
| B2 | 5584.90 | 1 | 5584.90 | 7.65 | 0.0123 |
| C2 | 5819.11 | 1 | 5819.11 | 7.97 | 0.0109 |
| Residual | 13,874.91 | 19 | 730.26 | ||
| Lack of Fit | 13,260.18 | 18 | 736.68 | 1.20 | 0.6269 |
| Pure Error | 614.73 | 1 | 614.73 | ||
| Cor Total | 97,167.54 | 25 | |||
| Fit Statistics | |||||
| Standard Deviation (Std. Dev.) | 27.02 | R2 | 0.8572 | ||
| Mean | 134.21 | Adjusted R2 | 0.8121 | ||
| Coefficient of Variation (CV) | 20.13 | Predicted R2 | 0.7646 | ||
| Adequate Precision | 10.7861 | ||||
| Model Equation in Terms of Coded Factors | |||||
| (mL CH4gVS−1) = +76.47 − 49.18 × B − 1.91 × C + 14.19 × D + 16.73 × BD + 41.28 × B² + 42.13 × C² | |||||
| Applied Pretreatment | Pretreatment Conditions | Results | Reference |
|---|---|---|---|
| Ca(OH)2 Assisted Thermal | Chemicals: Ca(OH)2: 0–2% Solid Loading: 3–7% Reaction Temperature: 50–100 °C Reaction Time: 6–16 h | BMP Raw Switchgrass (Shawnee): 217.1 mL CH4 gVS−1 CH4 Yield at Optimized Conditions: 248.7 mL CH4 gVS−1 at 3% solid loading as DM, 0% Ca(OH)2, 100 °C, 6 h. Enhancement Compared to Raw Switchgrass: 14.5% | This Work |
| H2O2 and Acetic Acid (Hac) Assisted Thermal | Chemicals: H2O2: 0–2% and Hac: 0–2% Reaction Temperature: 50–100 °C Reaction Time: 6–24 h | BMP Raw Switchgrass (Shawnee): 195.5 mL CH4 gVS−1 CH4 Yield at Optimized Conditions: 342.63 mL CH4 gVS−1 at 1.87% Hac, 0% H2O2, 50 °C, 6 h. Enhancement Compared to Raw Switchgrass: 75.2% | [3] |
| H2O2 Assisted Thermal | Chemicals: H2O2 1–3% Solid loading: 3–7% Reaction Temperature: 50–100 °C Reaction Time: 6–24 h | BMP Raw Switchgrass: 208.4 mL CH4 gVS−1 CH4 Yield at Optimized Conditions: 291.34 mL CH4 gVS−1 at 6.43% solid loading, 1.83% H2O2, 50 °C, 6.78 h. Enhancement Compared to Raw Switchgrass: 39.8% | [4] |
| Chemical Pretreatment (NaOH, KOH, Ca(OH)2, H2O2, HCl, H2SO4) Steam Explosion | Chemical Pretreatment Chemicals: NaOH, KOH, Ca(OH)2, H2O2, HCl, H2SO4 2, 3, 4, 5% w/v. Reaction Temperature: 25 °C Reaction Time: 12 h Solid loading: 8% (200 g Switchgrass/2.5 L) Steam Explosion Pressure: 1.2, 1.5, 1.8 Mpa Time: 20 Min. | BMP Raw Switchgrass: 46.3 mL CH4 gVS−1 CH4 Yield at Optimized Conditions: 197.2 mL CH4 gVS−1 at 4% NaOH, 25 °C, 12 h. Enhancement Compared to Raw Switchgrass: 325.9% CH4 Yield from Pretreated with 5% Ca(OH)2: 90.0 mL CH4 gVS−1 The effect of Ca(OH)2 pretreatment was not desirable compared to NaOH and KOH pretreatments. | [31] |
| Microwave Pretreatment | Final Reaction Temperature: 100, 150, 180 °C Reaction Time: 0–10–20 min. Temperature Increase: 5, 7.5, 10 °C/min | BMP Raw Switchgrass (Alamo): -mL CH4 gVS−1 CH4 Yield of Switchgrass Leaf at Optimized Conditions: 134.81 mL CH4 gVS−1 at 100 °C, 10 min, and 7.5 °C/min. Enhancement Compared to Raw Leaf Switchgrass: 9.1% CH4 Yield of Switchgrass Stem at Optimized Conditions: 99.35 mL CH4 gVS−1 at 150 °C, 10 min, and 10 °C/min. Enhancement Compared to Raw Stem Switchgrass: 5.2% | [32] |
| Chemical and Enzymatic Pretreatment | Chemical Pretreatment Chemicals: NaOH 1% (w/v) Liquid: Solid Ratio:10:1 Reaction Temperature: 50 °C Reaction Time: 12 h Enzymatic Pretreatment Reaction Time: 72 h Reaction Temperature: 50 °C Enzymes: Novozyme®188 (Cellobiase from Aspergillus niger) 35 FPU Celluclast®1.5 L (Cellulase from Tricho-derma reesei ATCC 26921) 61.5 CBU | BMP Raw Switchgrass (Kanlow): 197.39 mL CH4 gVS−1 CH4 Yield of Chemically Pretreated Switchgrass: 255.35 mL CH4 gVS−1 Enhancement Compared to Raw Switchgrass: 29.4% CH4 Yield of Chemically and Enzymatically Pretreated Switchgrass: 373.03 mL CH4 gVS−1 Enhancement Compared to Raw Switchgrass: 89% | [24] |
| Low Heat and Chemical Pretreatment | Chemicals: NaOH, Ca(OH)2, H2O2 0, 2.2, 5.5, 11, 22% NaOH, 6.6%H2O2 Reaction Temperature: Room Temp—100 °C Reaction Time: 3, 6, 24 h | BMP of Fine Grind Raw Switchgrass (Cave-in-Rock): 296 mL CH4 gVS−1 CH4 Yield of Switchgrass at Optimized Condition: 332 mL CH4 gVS−1 at 5.5% NaOH, 100 °C, 6 h. Enhancement Compared to Raw Switchgrass: 12.2% | [27] |
| Microwave Pretreatment | Reaction Temperature: 90–180 °C Reaction Time: 7.5–32.6 Min. | BMP of Fine Grind Raw Switchgrass (Kanlow): 296 mL CH4 gVS−1 CH4 Yield of Switchgrass at Optimized Condition: 320 mL CH4 gVS−1 at 150 °C Enhancement Compared to Raw Switchgrass: 8.1% Microwave pretreatment induced no significant effect on methane production. | [25] |
| Model | Parameter | Switchgrass | |||
| Pretreated at | Pretreated at | Pretreated at | Raw | ||
| 3% SL, 100 °C, 2% Ca(OH)2, 16 h | 3% SL, 50 °C, 0% Ca(OH)2, 6 h | 3% SL 100 °C, 0% Ca(OH)2, 6 h | |||
| M (mL CH4 gVS−1added) | 176.9 | 164.05 | 178.26 | 199.31 | |
| First Order | k (d−1) | 0.159 | 0.115 | 0.122 | 0.145 |
| Ppredicted (mL CH4 gVS−1added) | 173.92 | 161.68 | 170.88 | 188.71 | |
| R2 | 0.988 | 0.994 | 0.992 | 0.985 | |
| Adjusted R2 | 0.986 | 0.993 | 0.991 | 0.984 | |
| Difference (%) | 1.68 | 1.45 | 4.14 | 5.31 | |
| Cone | k (d−1) | 0.21 | 0.136 | 0.165 | 0.183 |
| n | 1.109 | 1.328 | 1.303 | 1.341 | |
| Ppredicted (mL CH4 gVS−1added) | 169.5 | 159.87 | 166.82 | 188.9 | |
| R2 | 0.993 | 0.997 | 0.992 | 0.983 | |
| Adjusted R2 | 0.992 | 0.996 | 0.991 | 0.982 | |
| Difference (%) | 4.18 | 2.55 | 6.41 | 5.22 | |
| Modified Gompertz | Rm (mL CH4 gVS−1added d−1) | 17.44 | 14.25 | 14.37 | 15.97 |
| λ (day) | 0.011 | 0.064 | 0.03 | 0.023 | |
| Ppredicted (mL CH4 gVS−1added) | 171.34 | 154.17 | 169.89 | 192.51 | |
| R2 | 0.954 | 0.984 | 0.967 | 0.976 | |
| Adjusted R2 | 0.949 | 0.982 | 0.963 | 0.973 | |
| Difference (%) | 3.14 | 6.02 | 4.69 | 3.41 | |
| Reaction Curve | Rm (mL CH4 gVS−1added d−1) | 26.33 | 20.19 | 20.33 | 22.7 |
| λ (day) | 0.018 | 0.059 | 0.04 | 0.038 | |
| Ppredicted (mL CH4 gVS−1added)) | 178.3 | 160.67 | 174.4 | 199.71 | |
| R2 | 0.99 | 0.994 | 0.992 | 0.986 | |
| Adjusted R2 | 0.989 | 0.994 | 0.992 | 0.984 | |
| Difference (%) | −0.79 | 2.06 | 2.16 | −0.2 | |
| Sample | Kinetic Parameters | Results | Reference | ||
|---|---|---|---|---|---|
| Switchgrass s-CSTR | First Order (FO) Cone (C) Modified Gompertz (MG) Reaction Curve (RC) | FO: k: −0.046–0.054 d−1 C: n: 1.7–1.9 MG: Rm: 7.1–9.3 mL CH4 gVS−1 d−1 RC: Rm: 9.45–12.6 mL CH4 gVS−1 d−1 | [26] | ||
| The Olive Pomace Alkaline Pretreatment (NaOH: 2%, 4% and 8% (w/w TS)) Microwave NaOH + Microwave | Modified Gompertz | Untreated Raw: Rm: 21.8 mL CH4 gVS−1 d−1 λ: 3.8 d NaOH Pretreatment: Rm: 22.4–50.9 mL CH4 gVS−1 d−1 λ: 0–5.6 d | [7] | ||
|
Switchgrass Chemical Pretreatment (NaOH, Ca(OH)2) | Modified Gompertz | Sample | μm (mL CH4 gVS−1 d−1) | λ (d) | [31] |
| Untreated SG | 1.9 | 3.1 | |||
| 4% NaOH | 17.9 | 2 | |||
| 5% Ca(OH)2 | 6.3 | 0.8 | |||
| Switchgrass Microwave Pretreatment (90–180 °C) | First Order | k: 0.080–0.134 The increase in the pretreatment temperature caused an increase in the k coefficient, except for 105 °C. The SG biodegradability accelerated. The 105 °C pretreatments showed similar results to the raw sample. | [25] | ||
| Microwave Pretreatment Leaf and Stem fraction | First Order | Leaf–k: 0.021–0.075 d−1 Stem–k: 0.021–0.070 d−1 The k values were increased by 44% and 68% at 150 °C and 180 °C, respectively, compared with the control. | [32] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akman, H.E.; Perendeci, N.A.; Ertekin, C.; Yaldiz, O. Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production. Molecules 2022, 27, 6891. https://doi.org/10.3390/molecules27206891
Akman HE, Perendeci NA, Ertekin C, Yaldiz O. Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production. Molecules. 2022; 27(20):6891. https://doi.org/10.3390/molecules27206891
Chicago/Turabian StyleAkman, Hasmet Emre, Nuriye Altınay Perendeci, Can Ertekin, and Osman Yaldiz. 2022. "Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production" Molecules 27, no. 20: 6891. https://doi.org/10.3390/molecules27206891
APA StyleAkman, H. E., Perendeci, N. A., Ertekin, C., & Yaldiz, O. (2022). Energy Crops and Methane: Process Optimization of Ca(OH)2 Assisted Thermal Pretreatment and Modeling of Methane Production. Molecules, 27(20), 6891. https://doi.org/10.3390/molecules27206891