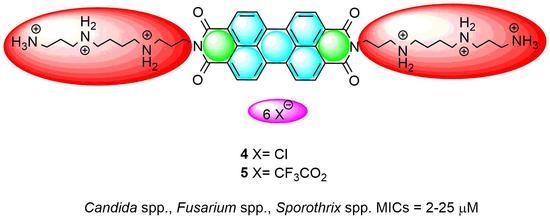
Antifungal Activity of Amphiphilic Perylene Bisimides
Abstract
:1. Introduction
2. Results
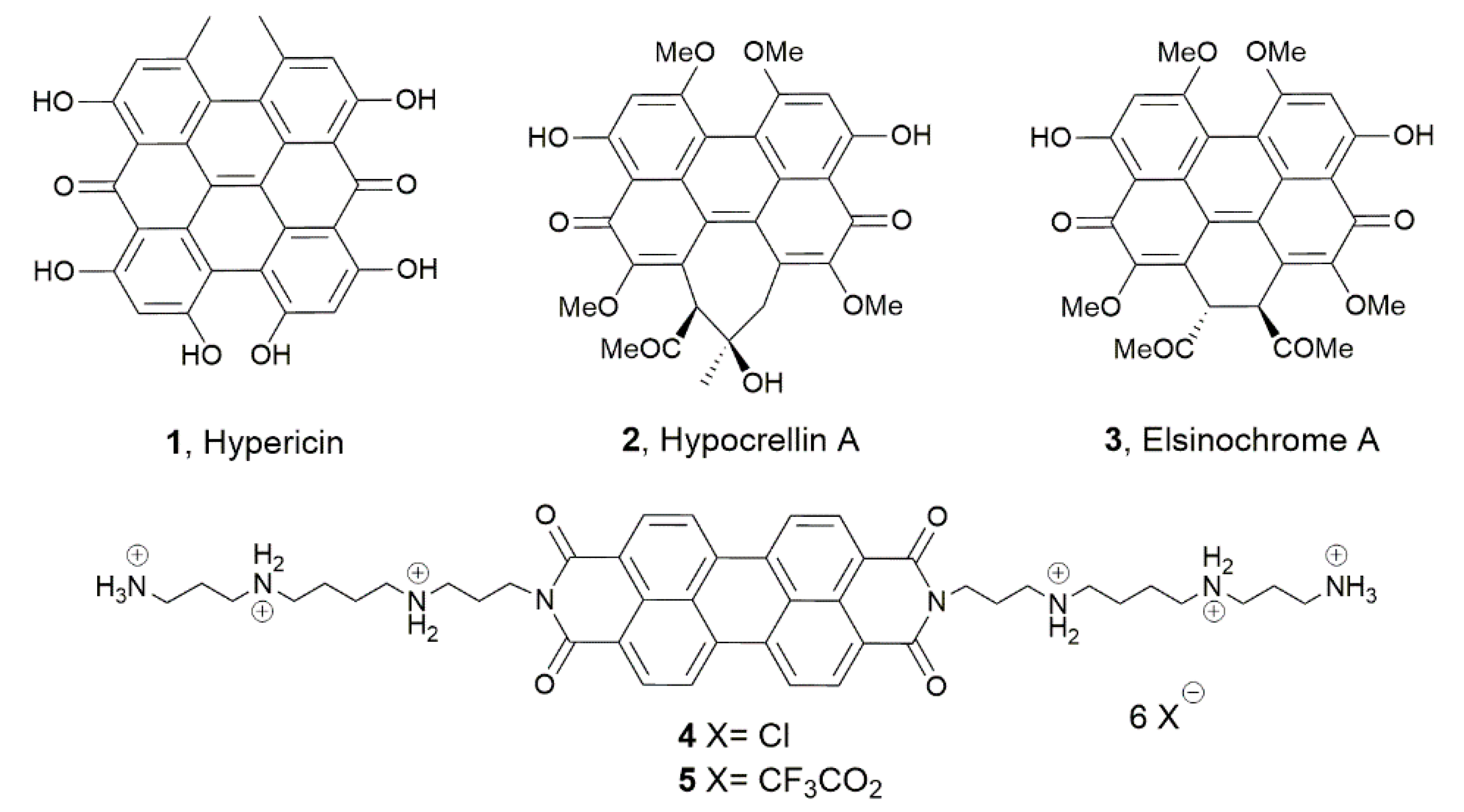
2.1. Chemistry
2.2. Antifungal Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
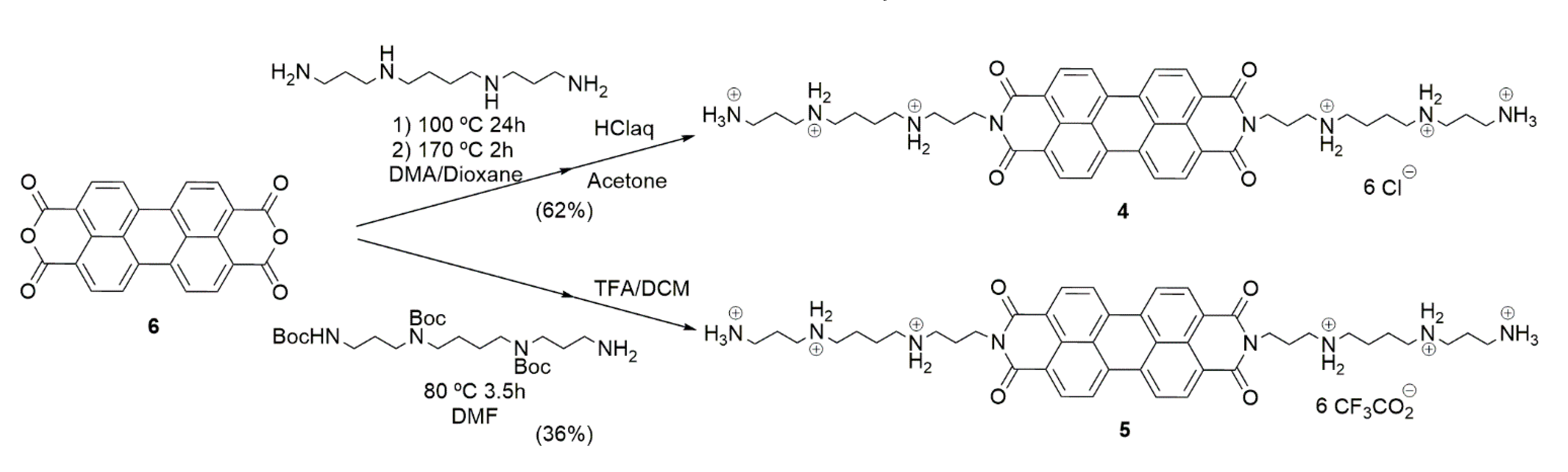
4.1.1. Synthesis of Compound 4
4.1.2. Synthesis of Compound 5
4.2. Bioassays
4.2.1. Antifungal Activity
Micro-Organisms
Antifungal Assay
4.2.2. Cytotoxic Activity
Cell Culture
Cytotoxicity Assay
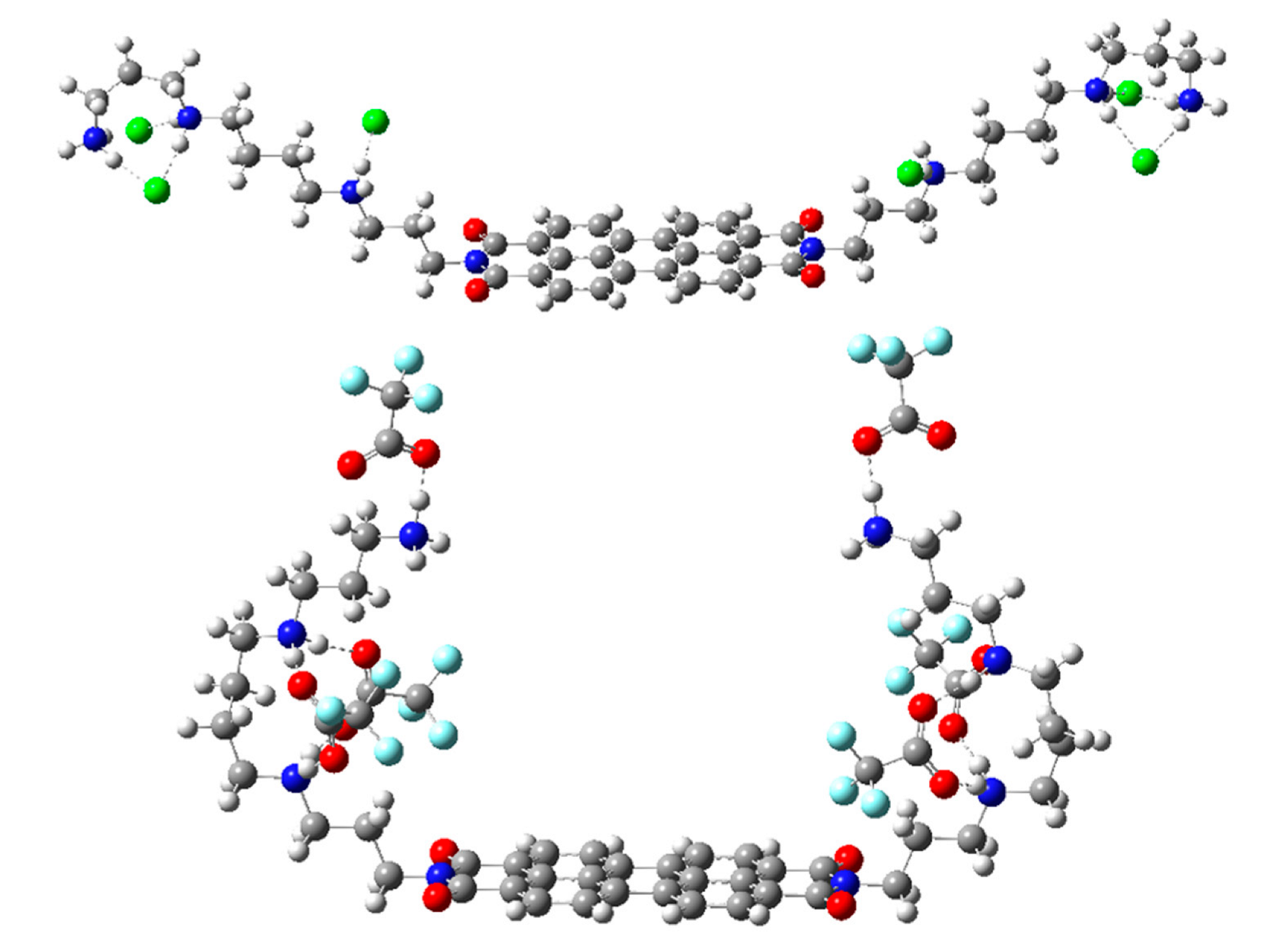
4.3. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Costa, P.S.; Mendes, V.; Veiga, F.F.; Negri, M.; Svidzinski, T.I.E. Relevant insights into onychomycosis’ pathogenesis related to the effectiveness topical treatment. Microb. Pathogen. 2022, 169, 105640. [Google Scholar] [CrossRef]
- Milobratović, D.; Janković, S.; Vukičević, J.; Marinković, J.; Janković, J.; Railić, Z. Quality of life in patients with toenail onychomycosis. Mycoses 2013, 56, 543–551. [Google Scholar] [CrossRef]
- Baran, R.; McLoone, N.; Hay, R.J. Could proximal white subungual onychomycosis be a complication of systemic spread? The lessons to be learned from Maladie Dermatophytique and other deep infections. Br. J. Dermatol. 2005, 153, 1023–1025. [Google Scholar] [CrossRef]
- Nenoff, P.; Krüger, C.; Ginter-Hanselmayer, G.; Tietz, H.-J. Mycology-an update. Part 1: Dermatomycoses: Causative agents, epidemiology and pathogenesis. J. Dtsch. Dermatol. Ges. 2014, 12, 188–209. [Google Scholar] [CrossRef]
- Stempel, J.M.; Hammond, S.P.; Sutton, D.A.; Weiser, L.M.; Marty, F.M. Invasive fusariosis in the voriconazole era: Single-center 13-year experience. Open Forum Infect. Dis. 2015, 2, ofv099. [Google Scholar] [CrossRef] [Green Version]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esnakula, A.K.; Summers, I.; Naab, T.J. Fatal disseminated fusarium infection in a human immunodeficiency virus positive patient. Case Rep. Infect. Dis. 2013, 2013, 379320. [Google Scholar] [CrossRef] [Green Version]
- Queiroz-Telles, F.; McGinnis, M.R.; Salkin, I.; Graybill, J.R. Subcutaneous mycoses. Infect. Dis. Clin. N. Am. 2003, 17, 59–85. [Google Scholar] [CrossRef]
- Bonifaz, A.; Vazquez-Gonzalez, D. Sporotrichosis: An update. G. Ital. Dermatol. Venereol. 2010, 145, 659–673. [Google Scholar]
- Ghannoum, M.; Isham, N.; Long, L. In vitro antifungal activity of ME1111, a new topical agent for onychomycosis, against clinical isolates of dermatophytes. Antimicrob. Agents Chemother. 2015, 59, 5154–5158. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Pinto, E.; Salgueiro, L. Natural Products: An Alternative to Conventional Therapy for Dermatophytosis? Mycopathologia 2017, 182, 143–167. [Google Scholar] [CrossRef]
- Yagan, S.; Yukruk, F.; Unlu, G.V. Antimicrobial activities of four perylenediimides. Afr. J. Microbiol. Res. 2015, 9, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Xing, M.-Z.; Zhang, X.-Z.; Sun, Z.-L.; Zhang, H.-Y. Perylenequinones act as broad-spectrum fungicides by generating reactive oxygen species both in the dark and in the light. J. Agric. Food Chem. 2003, 51, 7722–7724. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Švedienė, J.; Ložienė, K.; Paškevičius, A.; Kosyan, A.; Taran, N. Antifungal properties of hypericin, hypericin tetrasulphonic acid and fagopyrin on pathogenic fungi and spoilage yeasts. Pharm. Biol. 2016, 54, 3121–3125. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Sun, X.; Meng, L.; Wu, Q.; Wang, K.; Deng, Y. Antifungal activity of hypocrellin compounds and their synergistic effects with antimicrobial agents against Candida albicans. Microb. Biotechnol. 2021, 14, 430–443. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Roman, E.; Sanchez-Fresneda, R.; Casas, C.; Herrero, E.; Arguelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef] [Green Version]
- Roa-Linares, V.C.; Mesa-Arango, A.C.; González, M.A. Synthesis and antifungal activity of perylene bisimide derivatives. In Proceedings of the 18th International Electronic Conference on Synthetic Organic Chemistry, Online, 3 November 2014. [Google Scholar] [CrossRef]
- Spader, T.B.; Venturini, T.P.; Cavalheiro, A.S.; Mahl, C.D.; Mario, D.N.; Lara, V.M.; Santurio, J.; Alves, S.H. In vitro interactions between amphotericin B and other antifungal agents and rifampin against Fusarium spp. Mycoses 2011, 54, 131–136. [Google Scholar] [CrossRef]
- Vieira, D.B.; Carmona-Ribeiro, A.M. Cationic lipids and surfactants as antifungal agents: Mode of action. J. Antimicrob. Chemother. 2006, 58, 760–767. [Google Scholar] [CrossRef]
- Franceschin, M.; Lombardo, C.M.; Pascucci, E.; D’Ambrosio, D.; Micheli, E.; Bianco, A.; Ortaggi, G.; Savino, M. The number and distances of positive charges of polyamine side chains in a series of perylene diimides significantly influence their ability to induce G-quadruplex structures and inhibit human telomerase Bioorg. Med. Chem. 2008, 16, 2292–2304. [Google Scholar] [CrossRef]
- Rehm, S.; Stepanenko, V.; Zhang, X.; Rehm, T.H.; Würthner, F. Spermine-functionalized perylene bisimide dyes-highly dluorescent bola-amphiphiles in water. Chem. Eur. J. 2010, 16, 3372–3382. [Google Scholar] [CrossRef]
- Pajaziti, L.; Vasili, E. Treatment of onychomycosis-a clinical study. Med. Arch. 2015, 69, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.X.; Garneau-Tsodikova, S.; Green, K.D. A complex game of hide and seek: The search for new antifungals. Med. Chem. Commun. 2016, 7, 1285–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromolec. 2016, 92, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.R.; Sebai, S.C.; Shearman, G.C.; Ces, O.; Law, R.V.; Templer, R.H. Formulation affects the rate of membrane degradation catalyzed by cationic amphiphilic drugs. Ind. Eng. Chem. Res. 2008, 47, 650–655. [Google Scholar] [CrossRef]
- Xiao, M.; Fan, X.; Chen, S.C.-A.; Wang, H.; Sun, Z.-Y.; Liao, K.; Chen, S.-L.; Yan, Y.; Kang, M.; Hu, Z.-D.; et al. Antifungal susceptibilities of Candida glabrata species complex, Candida krusei, Candida parapsilosis species complex and Candida tropicalis causing invasive candidiasis in China: 3 year national surveillance. J. Antimicrob. Chemother. 2015, 70, 802–810. [Google Scholar] [CrossRef]
- Cursi, I.B.; Freitas, L.B.C.R.; Neves, M.L.P.F.; Silva, I.C. Onycomychosis due to Scytalidium spp.: A clinical and epidemiologic study at a University Hospital in Rio de Janeiro, Brazil. An. Bras. Dermatol. 2011, 86, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A. Fusarium onychomycosis: Prevalence, clinical presentations, response to itraconazole and terbinafine pulse therapy, and 1-year follow-up in nine cases. Int. J. Dermatol. 2015, 54, 1275–1282. [Google Scholar] [CrossRef]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida tropicalis antifungal cross-resistance is related to different azole target (Erg11p) modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef] [Green Version]
- Perlin, D.S. Mechanisms of echinocandin antifungal drug resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Guo, Y.; Yang, S.; Wang, C.; Fu, X.; Wang, J.; Mao, Y.; Zhang, J.; Li, Y. Cellular and molecular mechanisms of photodynamic hypericin therapy for nasopharyngeal carcinoma cells. J. Pharmacol. Exp. Ther. 2010, 334, 847–853. [Google Scholar] [CrossRef]
- Geall, A.; Blagbrough, I.S. Homologation of polyamines in the rapid synthesis of lipospermine conjugates and related lipoplexes. Tetrahedron 2000, 56, 2449–2460. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.A.; Morales, G.E.; Forero, J.E.; Roldan, J. Cytotoxic and antiviral activities of Colombian medicinal plant extracts of the Euphorbia genus. Mem. Inst. Oswaldo Cruz 2002, 97, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; Pople, J. Ab initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular interactions in solution: An overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.Y.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions: A Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comp. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]


| Fungal Strain (Number Strains) | MICs (µM) a,b | ||||
|---|---|---|---|---|---|
| 4 | 5 | Itraconazole | AmpB | Terbinafine | |
| Aspergillus flavus ATCC 204304 (1) | ≥25.6 (1) | ≥17.3 (1) | 0.18 | 0.54 | |
| Aspergillus fumigatus ATCC 204305 (1) | ≥25.6 (1) | ≥17.3 (1) | 0.35 | 1.08 | |
| Candida spp. (2), C. glabrata, C. tropicalis, C. albicans, C. krusei, C. lusitaniae | 6.4 (7) | 2.1 (7) | 0.06–1.42 (7) | 0.05–0.16 (7) | |
| Cryptococcus spp., C. neoformans, C.gattii (2) | 6.4 (2) | 2.1 (2) | |||
| Fusarium oxysporum (5) | 12.8 (1) ≥25.6 (4) | 4.3 (1) | ≥13.7 (1) | ||
| 8.6 (1) | ≥22.7 (5) | 6.9 (2) | |||
| 17.3 (3) | 3.4 (2) | ||||
| Fusarium oxysporum ATCC 48112 (1) | 9.6 (1) c | 4.3 (1) | ≥22.7 | 6.9 | |
| Fusarium proliferatum (1) | ≥25.6 (1) | ≥17.3 (1) | ≥22.7 | ≥13.7 | |
| Fusarium solani (2) | ≥25.6 (2) | 4.3 (1) | ≥22.7 (2) | 6.9 (1) | |
| ≥17.3 (1) | ≥13.7 (1) | ||||
| Fusarium spp. (9) | 6.4 (2) | 2.1 (2) 4.3 (1) ≥17.3 (6) | ≥22.7 (7) | ≥13.7 (7) | |
| ≥25.6 (7) | 2.8 (2) | 6.9 (2) | |||
| Scedosporium spp. (1) | 12.8 (1) | 4.3 (1) | |||
| Neoscytalidium dimidiatum (3) | 12.8 (3) | 4.3 (3) | ≥22.7 (3) | ≥13.7 (2) | |
| 0.9 (1) | |||||
| Neoscytalidium spp. (3) | 12.8 (3) | 2.1 (1) | ≥22.7 (3) | ≥13.7 (1) | |
| 4.3 (2) | 3.4 (2) | ||||
| Sporothrix spp. (10) | 12.8 (10) | 4.3 (10) | ≥22.7 (2) | ||
| ≤1.4 (8) | |||||
| Trichophyton mentagrophytes ATCC 24198 (1) | 6.4 (1) | 4.3 (1) | ≤ 0.03 |
| Compound | IC50 a | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| 4 | 37.6 ± 2.0 | 34.2 ± 0.8 | 32.6 |
| 5 | 10.9 ± 2.7 | 12.1 ± 2.0 | 19.4 |
| Fungal Strain | Time (h) | SI a | |
|---|---|---|---|
| 4 | 5 | ||
| Candida spp. | 24 | 5.9 | 5.1 |
| Aspergillus spp. | 48 | 1.3 | 0.7 |
| Cryptococcus spp. | 5.3 | 5.6 | |
| Fusarium spp. | 2.7–5.3 | 0.7–5.6 | |
| Scedosporium spp. | 2.7 | 2.8 | |
| Scytalidium spp. | 2.7 | 2.8–5.6 | |
| Sporothrix spp. | 72 | 2.5 | 4.5 |
| Trichophyton mentagrophytes | 5.1 | 4.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roa-Linares, V.C.; Mesa-Arango, A.C.; Zaragozá, R.J.; González-Cardenete, M.A. Antifungal Activity of Amphiphilic Perylene Bisimides. Molecules 2022, 27, 6890. https://doi.org/10.3390/molecules27206890
Roa-Linares VC, Mesa-Arango AC, Zaragozá RJ, González-Cardenete MA. Antifungal Activity of Amphiphilic Perylene Bisimides. Molecules. 2022; 27(20):6890. https://doi.org/10.3390/molecules27206890
Chicago/Turabian StyleRoa-Linares, Vicky C., Ana C. Mesa-Arango, Ramón J. Zaragozá, and Miguel A. González-Cardenete. 2022. "Antifungal Activity of Amphiphilic Perylene Bisimides" Molecules 27, no. 20: 6890. https://doi.org/10.3390/molecules27206890