Indigenous Saccharomyces cerevisiae Could Better Adapt to the Physicochemical Conditions and Natural Microbial Ecology of Prince Grape Must Compared with Commercial Saccharomyces cerevisiae FX10
Abstract
1. Introduction
2. Results
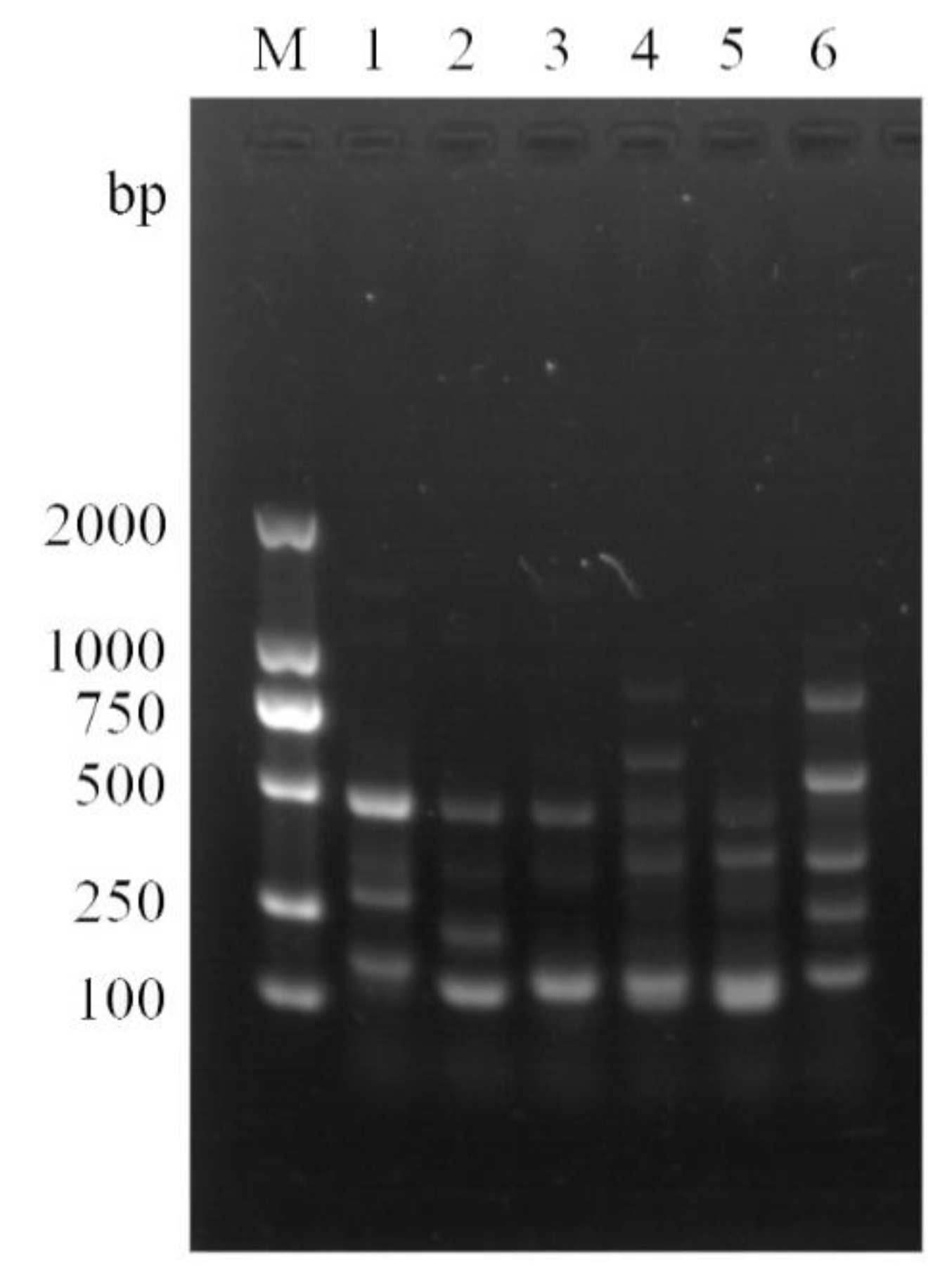
2.1. Interdelta Sequencing Typing of S. cerevisiae
2.2. Changes in Physicochemical Parameters during Fermentation
2.3. Changes in Volatile Compounds during Fermentation
2.4. Fungal Community Diversity and Richness Analysis
2.5. Dynamic Changes of the Fungal Community during Fermentation
2.6. Correlation Analysis between Microbiota and Volatile Compounds
3. Discussion
4. Materials and Methods
4.1. Grape Variety
4.2. Interdelta Sequence Typing
4.3. Winemaking and Sampling
4.4. DNA Extraction and Sequencing
4.5. Physicochemical Parameters Analysis
4.6. Volatile Compounds Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lema, C.; Garcia-Jares, C.; Orriols, I.; Angulo, L. Contribution of Saccharomyces and non-Saccharomyces populations to the production of some components of Albarino wine aroma. Am. J. Enol. Vitic. 1996, 47, 206–216. [Google Scholar]
- Belda, I.; Zarraonaindia, I.; Perisin, M.; Palacios, A.; Acedo, A. From vineyard soil to wine fermentation: Microbiome approximations to explain the “terroir” concept. Front. Microbiol. 2017, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Zarzoso, B.; Gostincar, A.; Bobet, R.; Uruburu, F.; Querol, A. Selection and molecular characterization of wine yeasts isolated from the ‘El Penedes’ area (Spain). Food Microbiol. 2000, 17, 553–562. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2017, 34, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Hansen, E.H.; Nissen, P.; Sommer, P.; Nielsen, J.C.; Arneborg, N. The effect of oxygen on the survival of non-Saccharomyces yeasts during mixed culture fermentations of grape juice with Saccharomyces cerevisiae. J. Appl. Microbiol. 2001, 91, 541–547. [Google Scholar] [CrossRef]
- Scacco, A.; Oliva, D.; Maio, D.S.; Polizzotto, G.; Genna, G.; Tripodi, G.; Lanza, C.M.; Verzera, A. Indigenous Saccharomyces cerevisiae strains and their influence on the quality of Cataratto, Inzolia and Grillo white wines. Food Res. Int. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Pietrafesa, R.; Massari, C.; Poeta, C.; Romano, P. Selection of indigenous Saccharomyces cerevisiae strains for Nero d’Avola wine and evaluation of selected starter implantation in pilot fermentation. Int. J. Food Microbiol. 2010, 144, 187–192. [Google Scholar] [CrossRef]
- Frezier, V.; Dubourdieu, D. Ecology of yeast-strain Saccharomyces cerevisiae during spontaneous fermentation in a Bordeaux winery. Am. J. Enol. Vitic. 1992, 43, 375–380. [Google Scholar]
- Nikolaou, E.; Soufleros, E.H.; Bouloumpasi, E.; Tzanetakis, N. Selection of indigenous Saccharomyces cerevisiae strains according to their oenological characteristics and vinification results. Food Microbiol. 2006, 23, 205–211. [Google Scholar] [CrossRef]
- Lopes, C.A.; Broock, V.M.; Querol, A.; Caballero, A.C. Saccharomyces cerevisiae wine yeast populations in a cold region in Argentinean Patagonia. A study at different fermentation scales. J. Appl. Microbiol. 2002, 93, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Rodriguez, M.E.; Sangorrin, M.; Querol, A.; Caballero, A.C. Patagonian wines: The selection of an indigenous yeast starter. J. Ind. Microbiol. Biotechnol. 2007, 34, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Suranska, H.; Vranova, D.; Omelkova, J. Isolation, identification and characterization of regional indigenous Saccharomyces cerevisiae strains. Braz. J. Microbiol. 2016, 47, 181–190. [Google Scholar] [CrossRef]
- Zhao, C.; Su, W.; Mu, Y.; Jiang, L.; Mu, Y. Correlations between microbiota with physicochemical properties and volatile flavor components in black glutinous rice wine fermentation. Food Res. Int. 2020, 138, 109800. [Google Scholar] [CrossRef] [PubMed]
- Zeravik, J.; Fohlerova, Z.; Milovanovic, M.; Kubesa, O.; Zeisbergerova, M.; Lacina, K.; Petrovic, A.; Glatz, Z.; Skladal, P. Various instrumental approaches for determination of organic acids in wines. Food Chem. 2016, 194, 432–440. [Google Scholar] [CrossRef]
- Peinado, R.A.; Mauricio, J.C.; Moreno, J. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 2006, 94, 232–239. [Google Scholar] [CrossRef]
- Lelova, Z.; Ivanova-Petropulos, V.; Masar, M.; Lisjak, K.; Bodor, R. Optimization and validation of a new capillary electrophoresis method with conductivity detection for determination of small anions in red wines. Food Anal. Methods 2018, 11, 1457–1466. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- de-la-Fuente-Blanco, A.; Saenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Thukral, A.K.; Bhardwaj, R.; Kumar, V.; Sharma, A. New indices regarding the dominance and diversity of communities, derived from sample variance and standard deviation. Heliyon 2019, 5, e02606. [Google Scholar] [CrossRef]
- Li, R.; Yang, S.; Lin, M.; Guo, S.; Han, X.; Ren, M.; Du, L.; Song, Y.; You, Y.; Zhan, J.; et al. The biogeography of fungal communities across different Chinese wine-producing regions associated with environmental factors and spontaneous fermentation performance. Front. Microbiol. 2022, 12, 636639. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, T.; Xu, X.; Ji, Y.; Jiang, X.; Shi, X.; Wang, B. Investigation of volatile compounds, microbial succession, and their relation during spontaneous fermentation of Petit Manseng. Front. Microbiol. 2021, 12, 717387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, W.; Yi, H.; Wang, B.; Xiao, J.; Zhou, X.; Xu, J.; Jiang, L.; Shi, X. Microbial community composition and its role in volatile compound formation during the spontaneous fermentation of ice wine made from Vidal grapes. Process Biochem. 2020, 92, 365–377. [Google Scholar] [CrossRef]
- Wang, H.L.; Hopfer, H.; Cockburn, D.W.; Wee, J. Characterization of microbial dynamics and volatile metabolome changes during fermentation of Chambourcy hybrid grapes from two Pennsylvania regions. Front. Microbiol. 2021, 11, 614278. [Google Scholar] [CrossRef]
- Antonelli, A.; Castellari, L.; Zambonelli, C.; Carnacini, A. Yeast influence on volatile composition of wines. J. Agric. Food Chem. 1999, 47, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Q.; Zhang, P.; Chen, D.; Howell, K.S. The fungal microbiome is an important component of vineyard ecosystems and correlates with regional distinctiveness of wine. Msphere 2020, 5, e00534-20. [Google Scholar] [CrossRef]
- Morrison-Whittle, P.; Goddard, M.R. From vineyard to winery: A source map of microbial diversity driving wine fermentation. Environ. Microbiol. 2018, 20, 75–84. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Sandionigi, A.; Bruni, I.; Bruno, A.; Lovicu, G.; Casiraghi, M.; Labra, M. Grape microbiome as a reliable and persistent signature of field origin and environmental conditions in Cannonau wine production. PLoS ONE 2017, 12, e0184615. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Gobbi, M.; Vero, L.D.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2014, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, S.; Barbato, D.; Guerrini, L.; Mari, E.; Buscioni, G.; Mangani, S.; Romboli, Y.; Galli, V.; Parenti, A.; Granchi, L. Selection of indigenous Saccharomyces cerevisiae strains and exploitation of a pilot-plant to produce fresh yeast starter cultures in a winery. Fermentation 2021, 7, 99. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. Mbio 2016, 7, e00631-16. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Pietrafesa, R.; Siesto, G.; Romaniello, R.; Condelli, N.; Romano, P. Selected indigenous Saccharomyces cerevisiae strains as profitable strategy to preserve typical traits of Primitivo wine. Fermentation 2019, 5, 87. [Google Scholar] [CrossRef]
- Hufnagel, J.C.; Hofmann, T. Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar] [CrossRef]
- Jones, P.R.; Gawel, R.; Francis, I.L.; Waters, E.J. The influence of interactions between major white wine components on the aroma, flavour and texture of model white wine. Food Qual. Prefer. 2008, 19, 596–607. [Google Scholar] [CrossRef]
- Fowles, G.W.A. Acids in grapes and wines: A review. J. Wine Res. 1992, 3, 25–41. [Google Scholar] [CrossRef]
- Ebeler, S.E. Analytical chemistry: Unlocking the secrets of wine flavor. Food Rev. Int. 2001, 17, 45–64. [Google Scholar] [CrossRef]
- Saenz-Navajas, M.-P.; Fernandez-Zurbano, P.; Ferreira, V. Contribution of nonvolatile composition to wine flavor. Food Rev. Int. 2012, 28, 389–411. [Google Scholar] [CrossRef]
- Volschenk, H.; van Vuuren, H.J.J.; Viljoen-Bloom, M. Malo-ethanolic fermentation in Saccharomyces and Schizosaccharomyces. Curr. Genet. 2003, 43, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Redzepovic, S.; Orlic, S.; Majdak, A.; Kozina, B.; Volschenk, H.; Viljoen-Bloom, M. Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int. J. Food Microbiol. 2003, 83, 49–61. [Google Scholar] [CrossRef]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine acidity: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 315–329. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, T.; Yang, H.; Li, E. Isolation and selection of non-Saccharomyces yeasts being capable of degrading citric acid and evaluation its effect on kiwifruit wine fermentation. Fermentation 2020, 6, 25. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; David, V.; Hemmler, D.; Schmitt-Kopplin, P.; Alexandre, H. Exploring yeast interactions through metabolic profiling. Sci. Rep. 2020, 10, 6073. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Sun, F.; Wang, W.; Liu, Y.; Wang, J.; Sun, J.; Mu, J.; Gao, Z. Effects of spontaneous fermentation on the microorganisms diversity and volatile compounds during ‘Marselan’ from grape to wine. LWT 2020, 134, 110193. [Google Scholar] [CrossRef]
- Liu, P.-T.; Lu, L.; Duan, C.-Q.; Yan, G.-L. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT 2016, 71, 356–363. [Google Scholar] [CrossRef]
- Moreira, N.; Pina, C.; Mendes, F.; Couto, J.A.; Hogg, T.; Vasconcelos, I. Volatile compounds contribution of Hanseniaspora guilliermondii and Hanseniaspora uvarum during red wine vinifications. Food Control 2011, 22, 662–667. [Google Scholar] [CrossRef]
- Capece, A.; Siesto, G.; Poeta, C.; Pietrafesa, R.; Romano, P. Indigenous yeast population from Georgian aged wines produced by traditional “Kakhetian” method. Food Microbiol. 2013, 36, 447–455. [Google Scholar] [CrossRef]
- Romano, P.; Caruso, M.; Capece, A.; Lipani, G.; Paraggio, M.; Fiore, C. Metabolic diversity of Saccharomyces cerevisiae strains from spontaneously fermented grape musts. World J. Microbiol. Biotechnol. 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Legras, J.-L.; Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterisation. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Zhao, Y.; Liu, L.-L.; Jia, B.; Zhao, F.; Huang, W.-D.; Zhan, J.-C. Copper Tolerance and biosorption of Saccharomyces cerevisiae during alcoholic fermentation. PLoS ONE 2015, 10, e0128611. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; He, X.; Huang, W.; You, Y.; Zhan, J. Enhancing ethanol tolerance via the mutational breeding of Pichia terricola H5 to improve the flavor profiles of wine. Fermentation 2022, 8, 149. [Google Scholar] [CrossRef]
- Chen, D.; Chia, J.; Liu, S. Impact of addition of aromatic amino acids on non-volatile and volatile compounds in lychee wine fermented with Saccharomyces cerevisiae MERIT.ferm. Int. J. Food Microbiol. 2014, 170, 12–20. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Li, H. Wine Tasting; China Science Press: Beijing, China, 2006. [Google Scholar]
- Zhang, L.; Tao, Y.; Wen, Y.; Wang, H. Aroma evaluation of young Chinese Merlot wines with denomination of origin. S. Afr. J. Enol. Vitic. 2013, 34, 307. [Google Scholar] [CrossRef]
- Sanchez-Palomo, E.; Garcia-Carpintero, E.G.; Alonso-Villegas, R.; Gonzalez-Vinas, M.A. Characterization of aroma compounds of Verdejo white wines from the La Mancha region by odour activity values. Flavour Fragr. J. 2010, 25, 456–462. [Google Scholar] [CrossRef]
- Vaquero, C.; Izquierdo-Canas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of Lachancea thermotolerans for biological vs. chemical acidification at pilot-scale in white wines from warm areas. Fermentation 2021, 7, 193. [Google Scholar] [CrossRef]
- Siebert, T.E.; Barker, A.; Pearson, W.; Barter, S.R.; de Barros Lopes, M.A.; Darriet, P.; Herderich, M.J.; Francis, I.L. Volatile compounds related to ‘stone fruit’ aroma attributes in Viognier and Chardonnay wines. J. Agric. Food Chem. 2018, 66, 2838–2850. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L. Intensity prediction of typical aroma characters of cabernet sauvignon wine in Changli County (China). LWT 2010, 43, 1550–1556. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.; Blagojevic, P.; Palic, R. Volatiles of the grape hybrid cultivar Othello (Vitis vinifera x (Vitis labrusca x Vitis riparia)) cultivated in Serbia. J. Essent. Oil Res. 2010, 22, 616–619. [Google Scholar] [CrossRef]
- Chaves, M.; Zea, L.; Moyano, L.; Medina, M. Changes in color and odorant compounds during oxidative aging of Pedro Ximenez sweet wines. J. Agric. Food Chem. 2007, 55, 3592–3598. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Lv, J.; Ma, Y.; Guan, X. Changes in the physicochemical components, polyphenol profile, and flavor of persimmon wine during spontaneous and inoculated fermentation. Food Sci. Nutr. 2020, 8, 2728–2738. [Google Scholar] [CrossRef] [PubMed]





| Grape Must (Day 0) | Wine | ||
|---|---|---|---|
| L59 | FX10 | ||
| Glucose (g/L) | 115.59 ± 0.05a | 0.64 ± 0.02b | 0.65 ± 0.00b |
| Fructose (g/L) | 133.19 ± 0.16a | 1.42 ± 0.03b | 1.44 ± 0.05b |
| Glycerol (g/L) | N.D. | 8.81 ± 0.06a | 8.31 ± 0.14b |
| Ethanol (% v/v) | N.D. | 13.10 ± 0.04 | 13.46 ± 0.21 |
| Citric acid (g/L) | 0.59 ± 0.01c | 1.14 ± 0.01a | 1.11 ± 0.01b |
| Tartaric acid (g/L) | 7.36 ± 0.09a | 4.73 ± 0.08c | 5.09 ± 0.10b |
| Malic acid (g/L) | 1.43 ± 0.01a | 1.02 ± 0.01c | 1.25 ± 0.02b |
| Succinic acid (g/L) | 0.55 ± 0.02b | 1.39 ± 0.04a | 1.37 ± 0.04a |
| Lactic acid (g/L) | N.D. | 0.73 ± 0.01 | 0.73 ± 0.02 |
| Acetic acid (g/L) | 0.19 ± 0.00c | 0.29 ± 0.01b | 0.34 ± 0.02a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, M.; Huang, W.; You, Y.; Zhan, J. Indigenous Saccharomyces cerevisiae Could Better Adapt to the Physicochemical Conditions and Natural Microbial Ecology of Prince Grape Must Compared with Commercial Saccharomyces cerevisiae FX10. Molecules 2022, 27, 6892. https://doi.org/10.3390/molecules27206892
Gao J, Wang M, Huang W, You Y, Zhan J. Indigenous Saccharomyces cerevisiae Could Better Adapt to the Physicochemical Conditions and Natural Microbial Ecology of Prince Grape Must Compared with Commercial Saccharomyces cerevisiae FX10. Molecules. 2022; 27(20):6892. https://doi.org/10.3390/molecules27206892
Chicago/Turabian StyleGao, Jie, Mingfei Wang, Weidong Huang, Yilin You, and Jicheng Zhan. 2022. "Indigenous Saccharomyces cerevisiae Could Better Adapt to the Physicochemical Conditions and Natural Microbial Ecology of Prince Grape Must Compared with Commercial Saccharomyces cerevisiae FX10" Molecules 27, no. 20: 6892. https://doi.org/10.3390/molecules27206892
APA StyleGao, J., Wang, M., Huang, W., You, Y., & Zhan, J. (2022). Indigenous Saccharomyces cerevisiae Could Better Adapt to the Physicochemical Conditions and Natural Microbial Ecology of Prince Grape Must Compared with Commercial Saccharomyces cerevisiae FX10. Molecules, 27(20), 6892. https://doi.org/10.3390/molecules27206892








