Integrated Metabolome and Lipidome Strategy to Reveal the Action Pattern of Paclobutrazol, a Plant Growth Retardant, in Varying the Chemical Constituents of Platycodon Root
Abstract
:1. Introduction
2. Results and Discussion
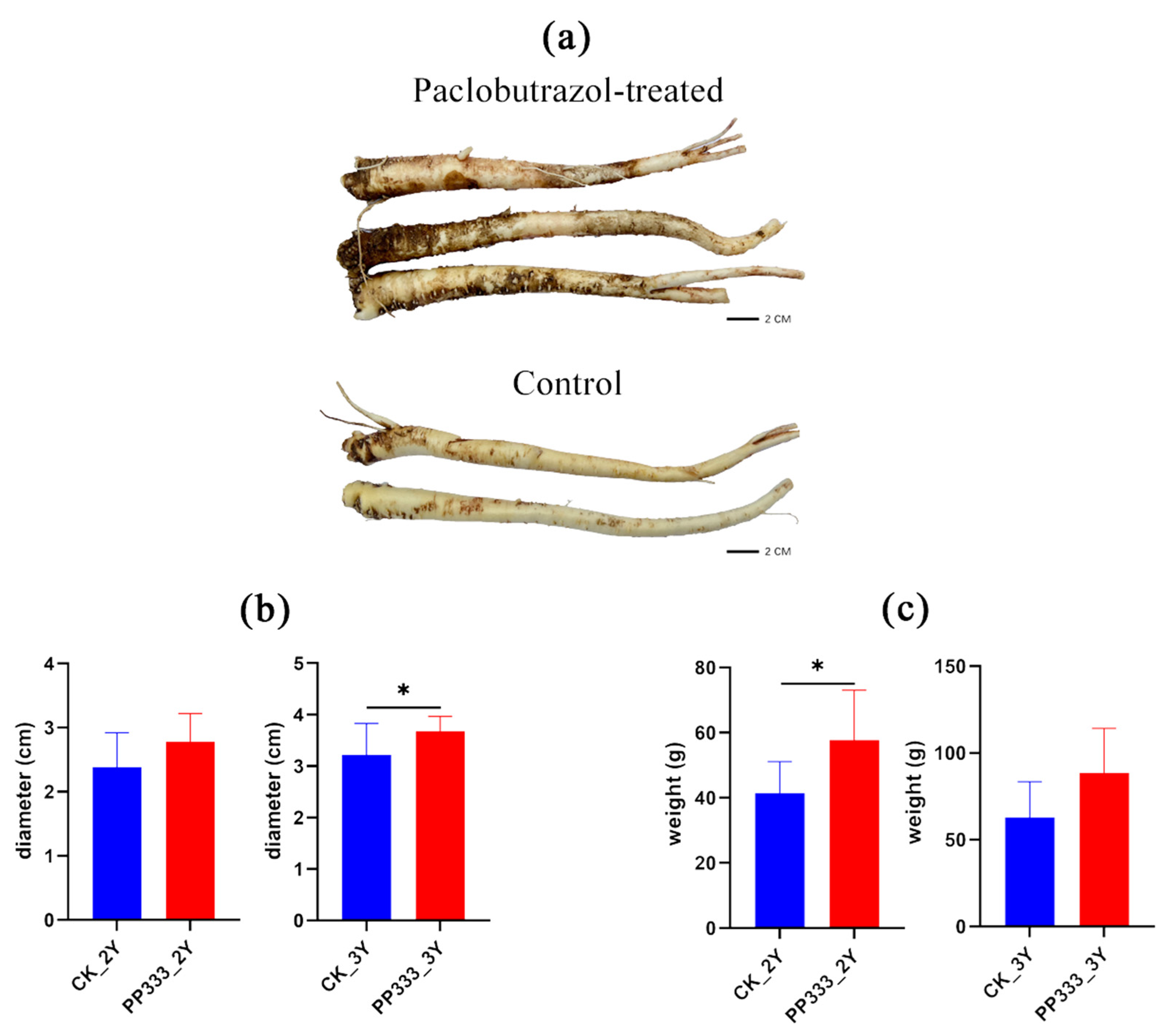
2.1. Effects of Paclobutrazol on the Growth of Platycodon Root
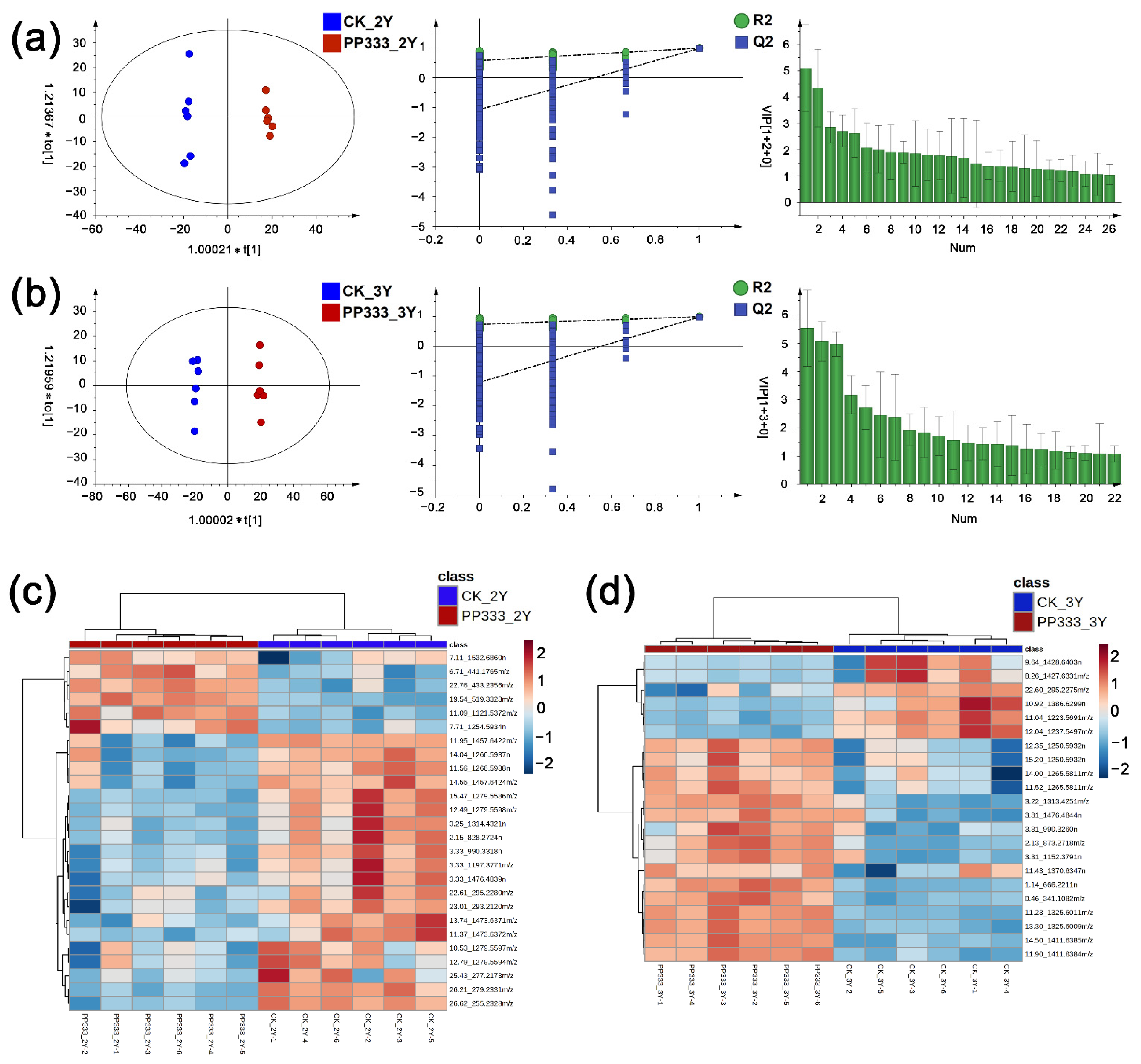
2.2. Effect of Paclobutrazol on Metabolic Profile of Platycodon Root
| NO. | Compound ID | Adducts | Formula | Mass Error/ppm | MS/MS Information | Identify | Treatments |
|---|---|---|---|---|---|---|---|
| 1 | 10.53_1279.5597m/z | [M−H]− | C59H92O30 | −0.25 | 1127.4875, 695.3644, 679.3696, 519.3330, 469.1564 | 3″-O-acetylplatyconic acid A | 2Y |
| 2 | 11.09_1121.5372m/z | [M+HCOO]− | C52H84O23 | −1.23 | 1075.5308, 753.4072, 681.3852, 665.3905, 541.1770, 469.1563 | platycoside J | 2Y |
| 3 | 11.37_1473.6372m/z | [M+HCOO]− | C65H104O34 | −1.31 | 1385.6228, 1367.6129, 843.4386, 825.4280, 781.4382, 663.3752 | 3″-O-acetyl platycodin D2 | 2Y |
| 4 | 11.56_1266.5938n | [M−H]− | C59H94O29 | −0.38 | 1223.5702, 723.3969, 681.3851, 663.3754, 541.1772, 469.1565 | platycodin C | 2Y, 3Y |
| 5 | 11.95_1457.6422m/z | [M+HCOO]− | C65H104O33 | −0.26 | 1369.6268, 827.4436, 809.4331, 765.4435, 647.3804, 541.1777 | 3″-O-acetylpolygalacin D2 | 2Y, 3Y |
| 6 | 12.49_1279.5598m/z | [M−H]− | C59H92O30 | −0.18 | 1249.5525, 1069.4838, 695.3647, 485.2907, 471.3113, 423.2904 | platycodin L | 2Y |
| 7 | 12.79_1279.5594m/z | [M−H]− | C59H92O30 | −0.52 | 995.4454, 849.3937, 717.3456, 695.3647, 469.2963, 409.3110 | 2″-O-acetylplatyconic acid A | 2Y |
| 8 | 13.74_1473.6371m/z | [M+HCOO]− | C65H104O34 | −1.43 | 1367.6112, 843.4381, 825.4280, 781.4389, 663.3743, 519.3329 | 2″-O-acetyl platycodin D2 | 2Y |
| 9 | 14.04_1266.5937n | [M−H]− | C59H94O29 | −0.05 | 1223.5674, 723.3954, 681.3850, 541.1770, 469.1561 | platycodin A | 2Y, 3Y |
| 10 | 14.55_1457.6424m/z | [M+HCOO]− | C65H104O33 | −1.30 | 1369.6276, 1147.5481, 827.4432, 809.4327, 647.3801, 491.1380 | 2″-O-acetylpolygalacin D2 | 2Y, 3Y |
| 11 | 15.47_1279.5586m/z | [M−H]− | C59H92O30 | −1.16 | 1249.5469, 1069.4835, 695.3639, 665.3540, 633.3631, 471.3111 | platycodin K | 2Y |
| 12 | 19.54_519.3323m/z | [M−H]− | C30H48O7 | −0.84 | 473.3248, 457.3316, 407.2954, 341.2212, 313.2396 | platycodigenin | 2Y |
| 13 | 7.11_1532.6860n | [M+HCOO]− | C69H112O37 | −1.47 | 1531.6792, 1239.5618, 989.4940, 665.3904, 541.1773, 469.1559 | platycoside D | 2Y |
| 14 | 7.71_1254.5934n | [M+HCOO]− | C58H94O29 | −1.08 | 1253.5785, 843.4378, 825.4278, 681.3845, 519.3325, 471.3116 | deapioplatycodin D3 | 2Y |
| 15 | 2.15_828.2724n | [M+HCOO]− | C30H52O26 | −2.72 | 827.2669, 647.2034, 485.1507, 341.1086, 323.0979, 179.0559 | 1-fructofuranosylnystose | 2Y, 3Y |
| 16 | 3.25_1314.4321n | [M+HCOO]− | C48H82O41 | −0.81 | 1313.4254, 1133.3627, 971.3085, 809.2560, 647.2036, 161.0457 | 1,1,1,1,1,1-kestooctaose | 2Y, 3Y |
| 17 | 3.33_1197.3771m/z | [M+HCOO]− | C42H72O36 | −1.20 | 1151.3725, 971.3081, 827.2674, 647.2037, 342.1127, 179.0562 | 1,1,1,1,1-kestoheptaose | 2Y, 3Y |
| 18 | 3.33_1476.4839n | [M+HCOO]− | C54H92O46 | −1.38 | 1475.4770, 1151.3725, 989.3190, 809.2569, 665.2148, 557.1721 | 1,1,1,1,1,1,1-kestononaose | 2Y, 3Y |
| 19 | 3.33_990.3318n | [M−H]− | C36H62O31 | −0.95 | 809.2565, 647.2037, 485.1510, 395.1200, 323.0982, 179.0562 | 1,1,1,1-kestohexaose | 2Y, 3Y |
| 20 | 6.71_441.1765m/z | [M+HCOO]− | C20H28O8 | −0.40 | 395.1709, 305.1243, 215.1082, 185.0976, 159.0817, 143.0708 | lobetyolin | 2Y |
| 21 | 22.76_433.2356m/z | [M−H]− | C21H39O7P | −1.00 | 433.2361, 279.2329, 152.9961 | LPA 18:2 | 2Y |
| 22 | 22.61_295.2280m/z | [M−H]− | C18H32O3 | 0.50 | 277.21745, 233.2285, 195.1394, 183.1024, 125.0952 | 9-hydroxy-10,12-octadecadienoic acid or isomer | 2Y, 3Y |
| 23 | 23.01_293.2120m/z | [M−H]− | C18H30O3 | −0.64 | 275.2026, 249.2222, 195.1391, 167.1086, 139.1137, 113.0974 | 9-oxo-10(E),12(E)-octadecadienoic acid or isomer | 2Y |
| 24 | 25.43_277.2173m/z | [M−H]− | C18H30O2 | −0.02 | 259.2069, 233.2278, 221.1551, 209.1552 | linolenic acid | 2Y |
| 25 | 26.21_279.2331m/z | [M−H]− | C18H32O2 | 0.47 | 279.2332, 261.2231, 233.1901 | linoleic acid | 2Y |
| 26 | 26.62_255.2328m/z | [M−H]− | C16H32O2 | −2.18 | 255.2336, 238.2253 | palmitic acid | 2Y |
| 27 | 11.04_1223.5691m/z | [M−H]− | C57H92O28 | −0.57 | 1091.5254, 681.3850, 663.3751, 635.3793, 519.3329, 469.1564 | platycodin D | 3Y |
| 28 | 10.92_1386.6299n | [M−H]− | C63H102O33 | −0.08 | 843.4389, 825.4283, 781.4385, 663.3751, 541.1780, 469.1566 | platycodin D2 | 3Y |
| 29 | 12.35_1250.5932n | [M+HCOO]− | C59H94O28 | −0.70 | 1249.5852, 1207.57340 665.3907, 469.1565, 409.1353 | 3″-O-acetylpolygalacin D | 3Y |
| 30 | 15.20_1250.5932n | [M−H]− | C59H94O28 | −1.25 | 1207.5743, 665.3903, 469.1563, 665.3903, 469.1563, 409.1351 | 2″-O-acetylpolygalacin D | 3Y |
| 31 | 12.04_1237.5497m/z | [M−H]− | C57H90O29 | −0.21 | 1207.5386, 1027.4747, 695.3643, 665.3541, 471.3116, 423.2906 | platycodin J | 3Y |
| 32 | 13.30_1325.6009m/z | [M+HCOO]− | C60H96O29 | −1.27 | 1279.5970, 827.4432, 809.4326, 765.4430, 485.3282 | deapi-2″-O-acetyl-polygalacin D2 | 3Y |
| 33 | 11.23_1325.6011m/z | [M+HCOO]− | C60H96O29 | −0.56 | 1325.6012, 1237.5801, 827.4433, 809.4328, 765.4435, | deapi-3″-O-acetyl-polygalacin D3 | 3Y |
| 34 | 11.43_1370.6347n | [M−H]− | C63H102O32 | 0.09 | 1045.5170, 941.4794, 827.4432, 737.4197, 665.3890, 469.1566 | polygalacin D2 | 3Y |
| 35 | 8.26_1427.6331m/z | [M+HCOO]− | C65H104O34 | −0.41 | 1385.6227, 1367.6132, 843.4382, 825.4271, 519.3330, 469.1565 | 3″-O-acetyl platycodin D3 | 3Y |
| 36 | 9.64_1428.6403n | [M+HCOO]− | C65H104O34 | −0.97 | 1385.6215, 1367.6215, 843.4380, 825.4275, 519.3324, 469.1565 | 2″-O-acetyl platycodin D3 | 3Y |
| 37 | 1.14_666.2211n | [M+HCOO]− | C24H42O21 | −0.51 | 711. 2197, 665.2143, 485.1514, 341.1095, 323.0981,179.0560 | nystose | 3Y |
| 38 | 0.46_341.1082m/z | [M−H]− | C12H22O11 | 0.09 | 272.0881, 221.0659, 179.0565, 161.0456 | sucrose | 3Y |

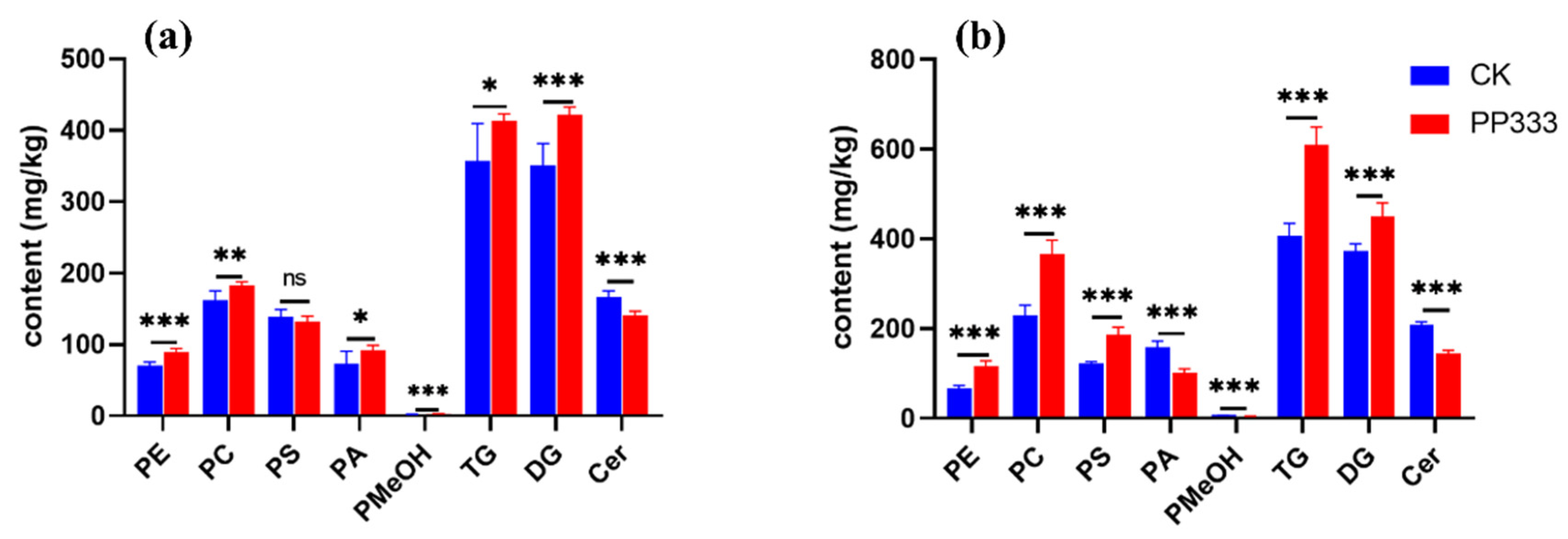
2.3. Effect of Paclobutrazol on Metabolic Profile of Platycodon Root
2.4. Multi-Quantitative Analysis of Key Components in Platycodon Root
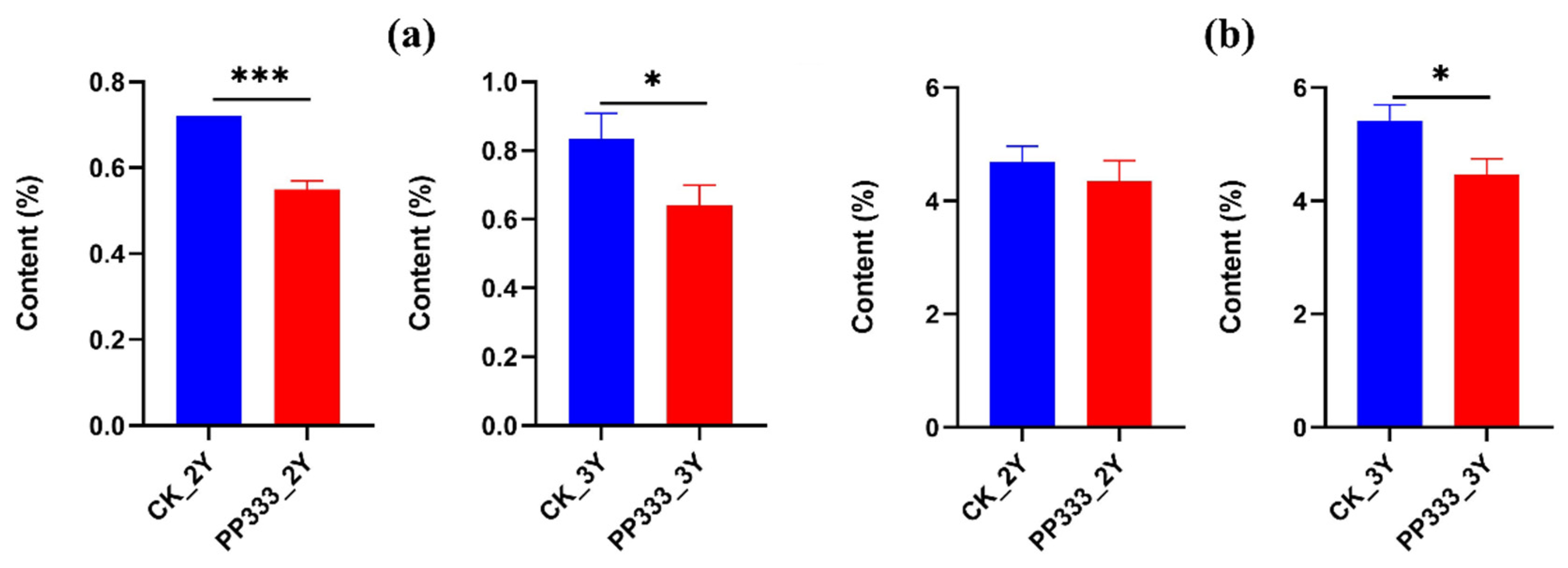
2.4.1. Effects of Paclobutrazol on the Content of Total Saponins
2.4.2. Effects of Paclobutrazol on the Content of Polysaccharides and Oligosaccharides
3. Materials and Methods
3.1. Multi-Quantitative Analysis of Key Components in Platycodon Root
3.2. Metabolome Analysis
3.3. Lipidome Analysis
3.4. Assay on Saponins
3.4.1. Total Saponins by UV
3.4.2. Total Saponins by UHPLC-ELSD
3.5. Assay on Saccharides
3.5.1. Water-Soluble Polysaccharides by UV
3.5.2. Oligosaccharides by UHPLC-ELSD
3.6. Data Analysis and Quality Assurance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandiflorus—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, J.J.; Hong, S.J.; Kim, D.S.; Boo, C.G.; Shin, E.C. Platycodon grandiflorum roots: A comprehensive study on odor/aroma and chemical properties during roasting. J. Food Biochem. 2020, 44, e13344. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Han, E.H.; Lim, M.K.; Lee, S.H.; Yu, H.J.; Lim, Y.H.; Kang, S. Fermented Platycodon grandiflorum Extracts Relieve Airway Inflammation and Cough Reflex Sensitivity In Vivo. J. Med. Food 2020, 23, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.W.; Li, Q.Y.; Liu, J.; Efferth, T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: Clinical experience and scientific basis. Phytomedicine 2021, 80, 153337. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Huang, Y.F.; Wang, W.Y.; Yang, L.; Zhou, H.; Sang, Z. Analysis on the current quality standards of Chinese materia Medica used in COVID-19 prevention and treatment. Pharm. Res. 2020, 160, 105074. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Bo, A.; Yang, M.; Xu, J.F.; Jiang, L.L.; Zhou, B.C.; Li, M.H. The Pharmacological Effects and Health Benefits of Platycodon grandiflorus—A Medicine Food Homology Species. Foods 2020, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.Y.; Sun, W.H.; Li, B.Z.; Shang, N.; Wang, Y.; Lv, W.Q.; Li, D.; Wang, L.J. Value-added application of Platycodon grandiflorus (Jacq.) A.DC. roots (PGR) by ultrasound-assisted extraction (UAE) process to improve physicochemical quality, structural characteristics and functional properties. Food Chem. 2021, 363, 130354. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Xie, H.; Li, R.; Wei, J.; Liu, Y. Environmental behavior of paclobutrazol in soil and its toxicity on potato and taro plants. Environ. Sci. Pollut. Res. Int. 2019, 26, 27385–27395. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Wang, Y.; Liu, C.; Chen, J.; Fan, S.; Xie, L.; Dong, Y.; Chen, S.; Zhou, W.; et al. Study on endocrine disruption effect of paclobutrazol and uniconazole on the thyroid of male and female rats based on lipidomics. Ecotoxicol. Environ. Saf. 2022, 234, 113386. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zeng, L.; Li, P.; Sun, T.; Wang, C.; Li, F.; Chen, Y.; Du, B.; Yang, Z. Influence of Plant Growth Retardants on Quality of Codonopsis Radix. Molecules 2017, 22, 1655. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.P.; Kim, J.; Lee, J.; Yoo, S.I.; Bae, W.; Geem, K.R.; Yu, J.; Jang, I.; Jo, I.H.; Cho, H.; et al. Gibberellin Signaling Promotes the Secondary Growth of Storage Roots in Panax ginseng. Int. J. Mol. Sci. 2021, 22, 8694. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Z.; Cui, S.; Xie, L.; Yu, J.; Tang, D.; Ma, X.; Mou, Y. Residue of Paclobutrazol and Its Regulatory Effects on the Secondary Metabolites of Ophiopogon japonicas. Molecules 2019, 24, 3504. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Tong, J.; Li, X. Evaluation of the Effects of Paclobutrazol and Cultivation Years on Saponins in Ophiopogon japonicus Using UPLC-ELSD. Int. J. Anal. Chem. 2020, 2020, 5974130. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ju, M.; Chu, S.; Xu, T.; Huang, Y.; Chan, Q.; Peng, H.; Gui, S. Quantitative and Chemical Fingerprint Analysis for the Quality Evaluation of Platycodi Radix Collected from Various Regions in China by HPLC Coupled with Chemometrics. Molecules 2018, 23, 1823. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kang, S.H.; Park, S.G.; Yang, T.J.; Lee, Y.; Kim, O.T.; Chung, O.; Lee, J.; Choi, J.P.; Kwon, S.J.; et al. Whole-genome, transcriptome, and methylome analyses provide insights into the evolution of platycoside biosynthesis in Platycodon grandiflorus, a medicinal plant. Hortic. Res. 2020, 7, 112. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, H.; Yuan, M.; Lan, L.; Hou, A.; Ji, S. Comprehensive characterization of the chemical constituents in Platycodon grandiflorum by an integrated liquid chromatography-mass spectrometry strategy. J. Chromatogr. A 2021, 1654, 462477. [Google Scholar] [CrossRef]
- Huang, W.; Lan, L.; Zhou, H.; Yuan, J.; Shui, M.; Mao, X.; Hu, Q.; Ji, S. Comprehensive profiling of Platycodonis radix in different growing regions using liquid chromatography coupled with mass spectrometry: From metabolome and lipidome aspects. RSC Adv. 2022, 12, 3897–3908. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.J.; Tan, G.F.; Zhou, W.Q.; Wang, G.L. Gibberellin and the plant growth retardant Paclobutrazol altered fruit shape and ripening in tomato. Protoplasma 2020, 257, 853–861. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, C.; Ren, W.; Li, B.; Lu, Y.; Pan, Z.; Cao, W. Flower and pod development, grain-setting characteristics and grain yield in Chinese milk vetch (Astragalus sinicus L.) in response to pre-anthesis foliar application of paclobutrazol. PLoS ONE 2021, 16, e0245554. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Papadopoulou, K.K.; Osbourn, A. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 439–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.J.; Kim, S.; Kim, J.J.; Jang, G.Y.; Moon, M.; Kim, H.D. Crude Saponin from Platycodon grandiflorum Attenuates Abeta-Induced Neurotoxicity via Antioxidant, Anti-Inflammatory and Anti-Apoptotic Signaling Pathways. Antioxidants 2021, 10, 1968. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, N.; Wang, Z.; Qi, Z.; Zheng, B.; Li, P.; Liu, J. Rapid characterization of chemical constituents of Platycodon grandiflorum and its adulterant Adenophora stricta by UPLC-QTOF-MS/MS. J. Mass. Spectrom. 2017, 52, 643–656. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.W.; Lee, S.; Kwon, R.H.; Na, H.; Kim, J.H.; Wee, C.D.; Yoo, S.M.; Lee, S.H. Characterization of Saponins from Various Parts of Platycodon grandiflorum Using UPLC-QToF/MS. Molecules 2021, 27, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, D.; Sun, C.; Li, Y.; Lu, H.; Wang, X. Comprehensive Lipidome and Metabolome Profiling Investigations of Panax quinquefolius and Application in Different Growing Regions Using Liquid Chromatography Coupled with Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 6710–6719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Duan, X.; Shang, B.; Hong, Y.; Sun, H. Analysis of lipidomics profile of rice and changes during storage by UPLC-Q-extractive orbitrap mass spectrometry. Food Res. Int. 2021, 142, 110214. [Google Scholar] [CrossRef]
- Xu, S.; Lv, X.; Wu, B.; Xie, Y.; Wu, Z.; Tu, X.; Chen, H.; Wei, F. Pseudotargeted Lipidomics Strategy Enabling Comprehensive Profiling and Precise Lipid Structural Elucidation of Polyunsaturated Lipid-Rich Echium Oil. J. Agric. Food Chem. 2021, 69, 9012–9024. [Google Scholar] [CrossRef]
- Hu, A.; Wei, F.; Huang, F.; Xie, Y.; Wu, B.; Lv, X.; Chen, H. Comprehensive and High-Coverage Lipidomic Analysis of Oilseeds Based on Ultrahigh-Performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 8964–8980. [Google Scholar] [CrossRef]
- Wang, X.Y.; Song, X.L.; Zhang, Y.; Luo, G.; Tai, H.C.; Lin, Z.H.; Zhu, P.L.; Sun, N.; Chu, Z.S.; Yu, Z.L.; et al. Evaluation of Beneficial and Adverse Effects of a Diet Supplemented with Schisandrae Fructus Seed Ethanol Extract on Lipid and Glucose Metabolism in Normal and Hypercholesterolemic/Hyperglycemic Mice. Evid.-Based Complement. Altern. Med. 2021, 2021, 8858962. [Google Scholar] [CrossRef]
- Hao, J.; Song, Y.; Tian, B.; Qi, C.; Li, L.; Wang, L.; Xing, Y.; Zhao, X.; Liu, J. Platycodon grandifloras polysaccharides inhibit mitophagy injury induced by Cr (VI) in DF-1 cells. Ecotoxicol. Environ. Saf. 2020, 202, 110901. [Google Scholar] [CrossRef]
- Zou, Y.F.; Chen, M.; Fu, Y.P.; Zhu, Z.K.; Zhang, Y.Y.; Paulsen, B.S.; Rise, F.; Chen, Y.L.; Yang, Y.Z.; Jia, R.Y.; et al. Characterization of an antioxidant pectic polysaccharide from Platycodon grandiflorus. Int. J. Biol. Macromol. 2021, 175, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Gao, J.N.; Shen, Y.; Kuang, H.X.; Xia, Y.G. A novel LC-MS/MS method for complete composition analysis of polysaccharides by aldononitrile acetate and multiple reaction monitoring. Carbohydr. Polym. 2021, 272, 118478. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, W.; Qiu, S.; Hou, J.; Wu, W.; Guo, D. Systematic profiling and comparison of the lipidomes from Panax ginseng, P. quinquefolius, and P. notoginseng by ultrahigh performance supercritical fluid chromatography/high-resolution mass spectrometry and ion mobility-derived collision cross section measurement. J. Chromatogr. A 2018, 1548, 64–75. [Google Scholar] [PubMed]
- The European Pharmacopoeia Commission. European Pharmacopoeia, 10th ed.; The European Pharmacopoeia Commission: Strasbourg, France, 2019. [Google Scholar]

| Content | Control | Paclobutrazol-Treated | ||
|---|---|---|---|---|
| 2-Year-Old | 3-Year-Old | 2-Year-Old | 3-Year-Old | |
| polysaccharides | 11.62% ± 2.03 | 9.40% ± 0.58 | 11.36% ± 1.33 | 11.52% ± 1.84 |
| oligosaccharides | 8.93% ± 0.85 | 9.41% ± 0.50 | 8.71% ± 0.48 | 8.91% ± 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, L.; Huang, W.; Zhou, H.; Yuan, J.; Miao, S.; Mao, X.; Hu, Q.; Ji, S. Integrated Metabolome and Lipidome Strategy to Reveal the Action Pattern of Paclobutrazol, a Plant Growth Retardant, in Varying the Chemical Constituents of Platycodon Root. Molecules 2022, 27, 6902. https://doi.org/10.3390/molecules27206902
Lan L, Huang W, Zhou H, Yuan J, Miao S, Mao X, Hu Q, Ji S. Integrated Metabolome and Lipidome Strategy to Reveal the Action Pattern of Paclobutrazol, a Plant Growth Retardant, in Varying the Chemical Constituents of Platycodon Root. Molecules. 2022; 27(20):6902. https://doi.org/10.3390/molecules27206902
Chicago/Turabian StyleLan, Lan, Weizhen Huang, Heng Zhou, Jiajia Yuan, Shui Miao, Xiuhong Mao, Qing Hu, and Shen Ji. 2022. "Integrated Metabolome and Lipidome Strategy to Reveal the Action Pattern of Paclobutrazol, a Plant Growth Retardant, in Varying the Chemical Constituents of Platycodon Root" Molecules 27, no. 20: 6902. https://doi.org/10.3390/molecules27206902






