Supramolecular Design Strategy of a Water-Soluble Diphenylguanidine-Cyclodextrin Polymer Inclusion Complex
Abstract
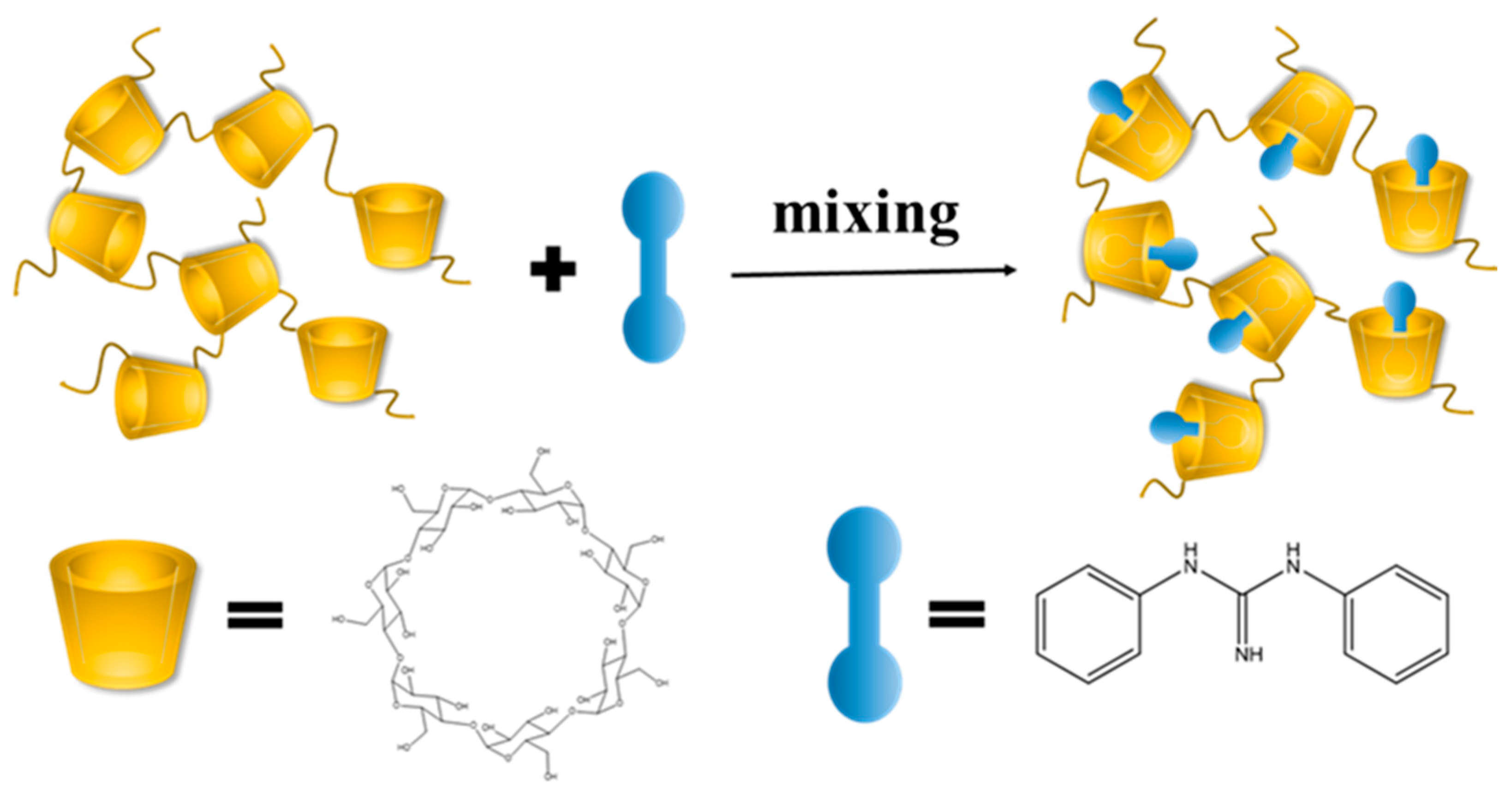
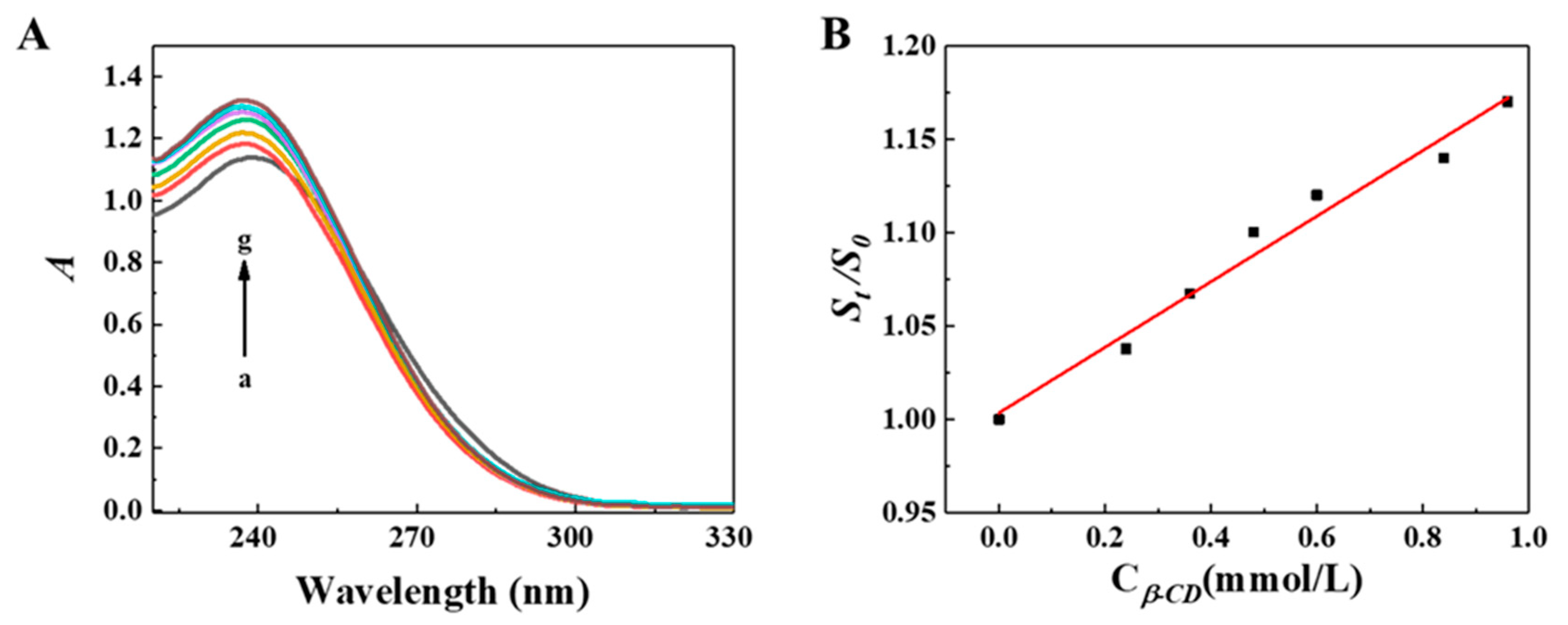
1. Introduction
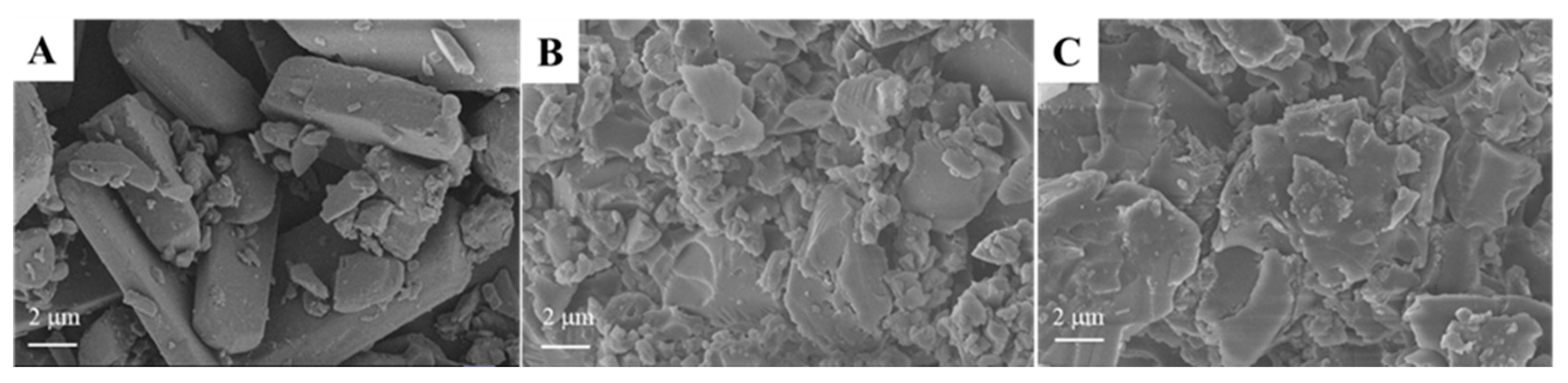
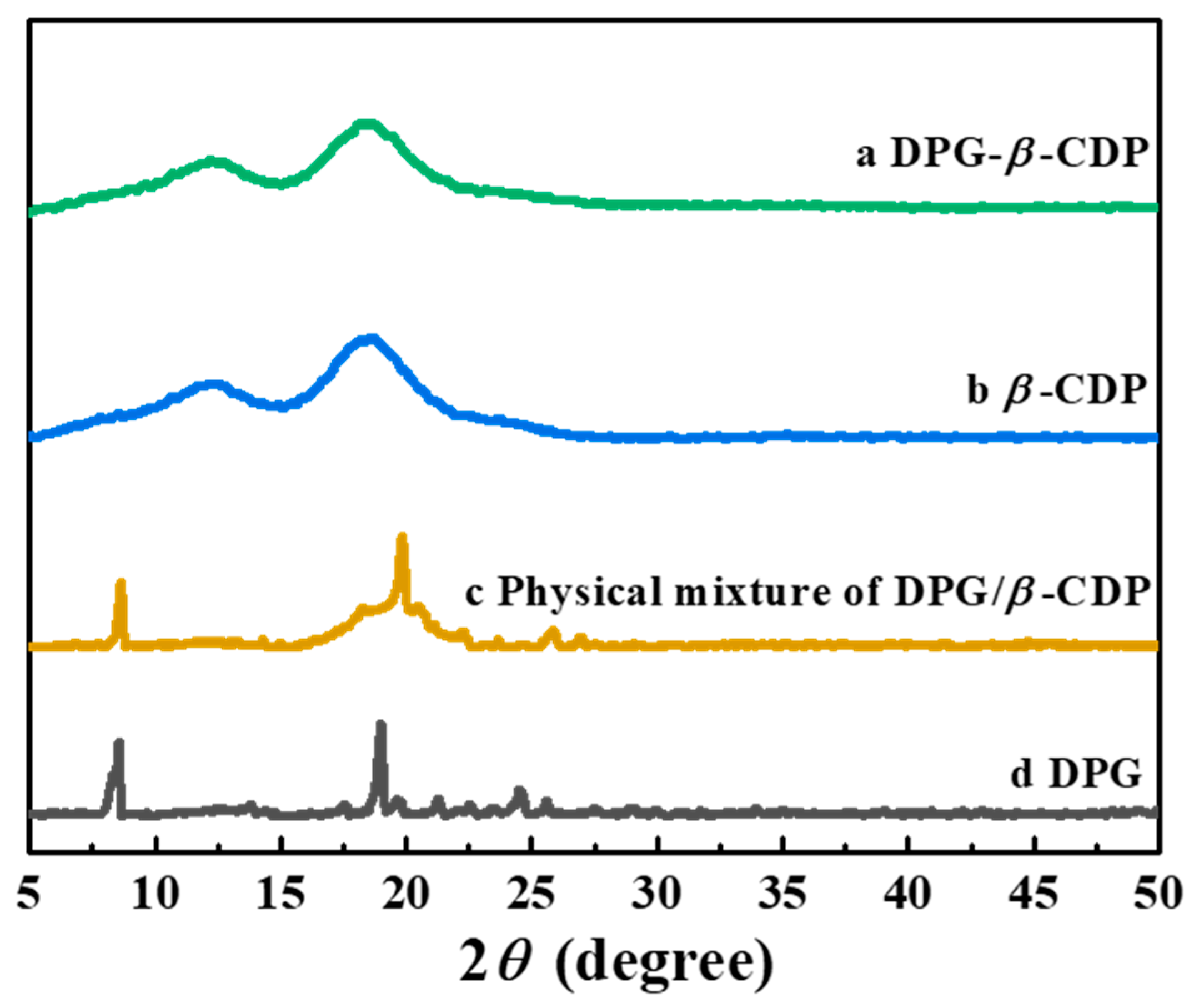
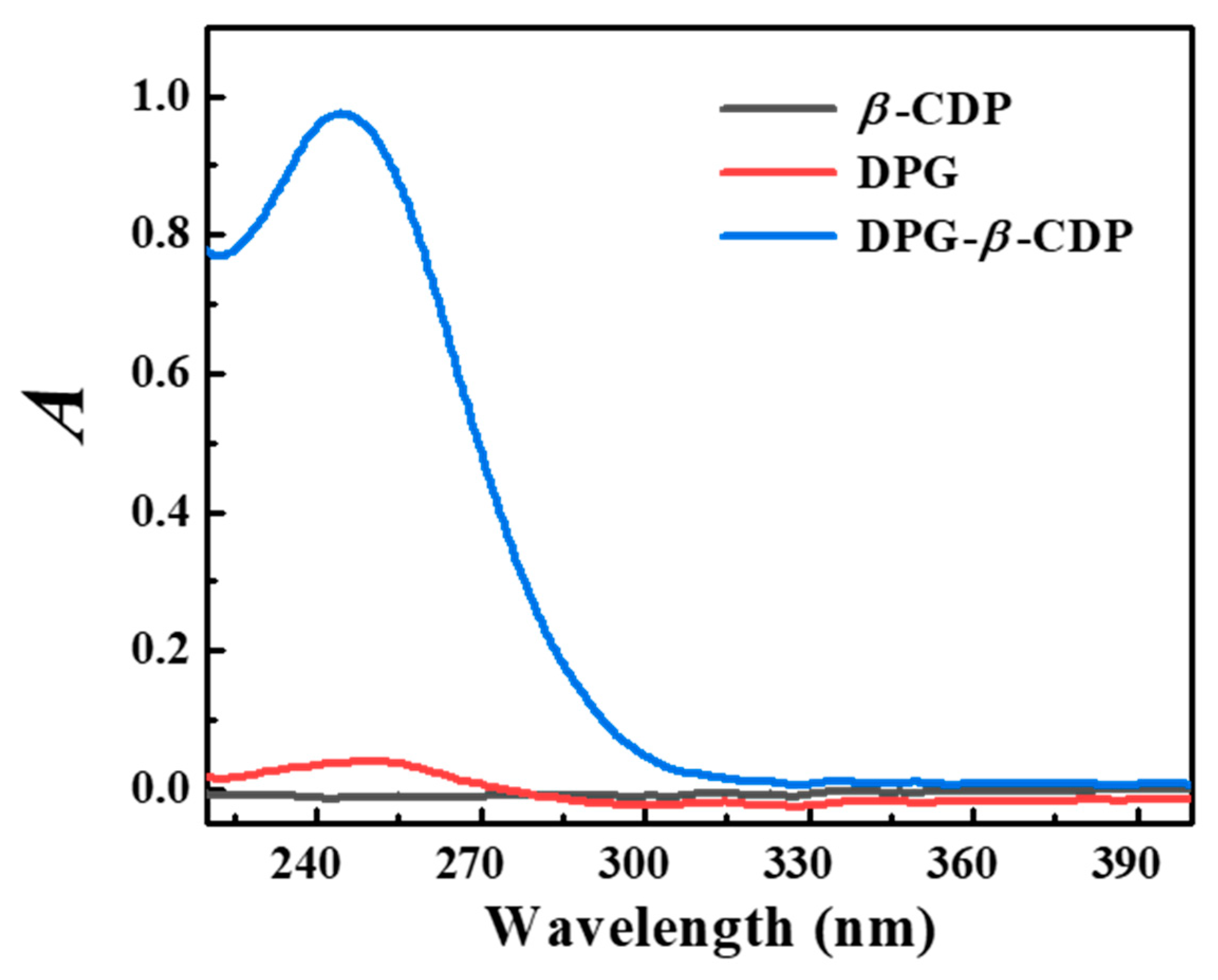
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hernández Santana, M.; den Brabander, M.; García, S.; van der Zwaag, S. Routes to Make Natural Rubber Heal: A Review. Polym. Rev. 2018, 58, 585–609. [Google Scholar] [CrossRef]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Heideman, G.; Datta, R.N.; Noordermeer, J.W.M.; van Baarle, B. Activators in Accelerated Sulfur Vulcanization. Rubber Chem. Technol. 2004, 77, 512–541. [Google Scholar] [CrossRef]
- Akiba, M.; Hashim, A.S. Vulcanization and crosslinking in elastomers. Prog. Polym. Sci. 1997, 22, 475–521. [Google Scholar] [CrossRef]
- Sieira, B.J.; Montes, R.; Touffet, A.; Rodil, R.; Cela, R.; Gallard, H.; Quintana, J.B. Chlorination and bromination of 1,3-diphenylguanidine and 1,3-di-o-tolylguanidine: Kinetics, transformation products and toxicity assessment. J. Hazard. Mater. 2020, 385, 121590. [Google Scholar] [CrossRef]
- Peter, K.T.; Tian, Z.; Wu, C.; Lin, P.; White, S.; Du, B.; McIntyre, J.K.; Scholz, N.L.; Kolodziej, E.P. Using High-Resolution Mass Spectrometry to Identify Organic Contaminants Linked to Urban Stormwater Mortality Syndrome in Coho Salmon. Environ. Sci. Technol. 2018, 52, 10317–10327. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, T.; Cheng, C.; Wang, Q.; Lin, S.; Liu, C.; Han, X. Research Progress on Synthesis and Application of Cyclodextrin Polymers. Molecules 2021, 26, 1090. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Yu, H.; Lin, H.; Xie, Y.; Qu, M.; Jiang, M.; Shi, J.; Hong, H.; Xu, H.; Li, L.; Liao, G.; et al. MUC1 vaccines using β-cyclodextrin grafted chitosan (CS-g-CD) as carrier via host-guest interaction elicit robust immune responses. Chin. Chem. Lett. 2022, 33, 4882–4885. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Celebioglu, A.; Saporito, A.F.; Uyar, T. Green Electrospinning of Chitosan/Pectin Nanofibrous Films by the Incorporation of Cyclodextrin/Curcumin Inclusion Complexes: pH-Responsive Release and Hydrogel Features. ACS Sustain. Chem. Eng. 2022, 10, 4758–4769. [Google Scholar] [CrossRef]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio. 2022, 16, 100429. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Advances in the development of cyclodextrin-based nanogels/microgels for biomedical applications: Drug delivery and beyond. Carbohydr. Polym. 2022, 297, 120033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, P.; Lin, L.; Guo, F.; Wang, Q.; Piao, Y.; Diao, G. Study of a water-soluble supramolecular complex of curcumin and β-cyclodextrin polymer with electrochemical property and potential anti-cancer activity. Chin. Chem. Lett. 2022, 33, 4043–4047. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.-G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, L.; Guo, J.; Wu, M.; Park, S.; Yao, H.; Paek, S.H.; Diao, G.; Piao, Y. Design Strategy for Vulcanization Accelerator of Diphenylguanidine/Cyclodextrin Inclusion Complex for Natural Rubber Latex Foam with Enhancing Performance. Research 2022, 2022, 9814638. [Google Scholar] [CrossRef]
- Zhao, B.; Jiang, L.; Jia, Q. Advances in cyclodextrin polymers adsorbents for separation and enrichment: Classification, mechanism and applications. Chin. Chem. Lett. 2022, 33, 11–21. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Yu, C.; Li, T.; Zhang, X.; Xiong, C.; Wang, T. Efficient and Spectrally Stable Blue Light-Emitting Diodes Based on Diphenylguanidine Bromide Passivated Mixed-Halide Perovskites. ACS Appl. Electron. Mater. 2021, 3, 4912–4918. [Google Scholar] [CrossRef]
- Guo, F.; Guo, J.; Zheng, Z.; Xia, T.; Chishti, A.N.; Lin, L.; Zhang, W.; Diao, G. Polymerization of pyrrole induced by pillar[5]arene functionalized graphene for supercapacitor electrode. Chin. Chem. Lett. 2022, 33, 4846–4849. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Wang, J.; Wang, C.; Wang, J.; Ding, Y.; Yao, Y. Pillar[5]arene-derived covalent organic materials with pre-encoded molecular recognition for targeted and synergistic cancer photo- and chemotherapy. Chem. Commun. 2022, 58, 1689–1692. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, J.; Bian, X.; Yao, H.; Yuan, X.; Han, Y.; Yan, C. A microenvironment sensitive pillar[5]arene-based fluorescent probe for cell imaging and drug delivery. Chin. Chem. Lett. 2022, 33, 1979–1982. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, Y.; Zhang, Z.; Wang, J.; Yuan, X.; Zhao, Q.; Ding, Y.; Yao, Y. CO2 and photo-controlled reversible conversion of supramolecular assemblies based on water soluble pillar[5]arene and coumarin-containing guest. Chin. Chem. Lett. 2021, 32, 349–352. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, X.; Liu, C.; Piao, Y.; Sun, Y.; Diao, G. Water-soluble inclusion complex of fullerene with γ-cyclodextrin polymer for photodynamic therapy. J. Mater. Chem. B 2014, 2, 5107–5115. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, J.; Li, X.; Chen, T.; Park, S.; Bae, M.; Jung, D.; Lin, L.; Paek, S.H.; Piao, Y. Anticancer pH-Responsive Supramolecular Vesicles Fabricated Using Water-Soluble Pillar[5]arene and Curcumin Derivative. Mater. Des. 2022, 222, 111084. [Google Scholar] [CrossRef]




| Title 1 | OH-2 | OH-6 | H-a | H-b, c |
|---|---|---|---|---|
| DPG | - | - | 6.88 | 7.22 |
| β-CDP | 5.72 | 4.48 | - | - |
| DPG-β-CDP | 5.80 | 4.47 | 6.87 | 7.20 |
| Δδ | 0.08 | −0.01 | −0.01 | −0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Lin, L.; Wang, Y.; Zhang, W.; Diao, G.; Piao, Y. Supramolecular Design Strategy of a Water-Soluble Diphenylguanidine-Cyclodextrin Polymer Inclusion Complex. Molecules 2022, 27, 6919. https://doi.org/10.3390/molecules27206919
Guo J, Lin L, Wang Y, Zhang W, Diao G, Piao Y. Supramolecular Design Strategy of a Water-Soluble Diphenylguanidine-Cyclodextrin Polymer Inclusion Complex. Molecules. 2022; 27(20):6919. https://doi.org/10.3390/molecules27206919
Chicago/Turabian StyleGuo, Junqiang, Liwei Lin, Yuping Wang, Wang Zhang, Guowang Diao, and Yuanzhe Piao. 2022. "Supramolecular Design Strategy of a Water-Soluble Diphenylguanidine-Cyclodextrin Polymer Inclusion Complex" Molecules 27, no. 20: 6919. https://doi.org/10.3390/molecules27206919
APA StyleGuo, J., Lin, L., Wang, Y., Zhang, W., Diao, G., & Piao, Y. (2022). Supramolecular Design Strategy of a Water-Soluble Diphenylguanidine-Cyclodextrin Polymer Inclusion Complex. Molecules, 27(20), 6919. https://doi.org/10.3390/molecules27206919





