Effects of Salicylic Acid Concentration and Post-Treatment Time on the Direct and Systemic Chemical Defense Responses in Maize (Zea mays L.) Following Exogenous Foliar Application
Abstract
1. Introduction
2. Results
2.1. The Direct Effect of SA Concentration and Post-Treatment Time on Chemical Defense Responses in Treated Leaves
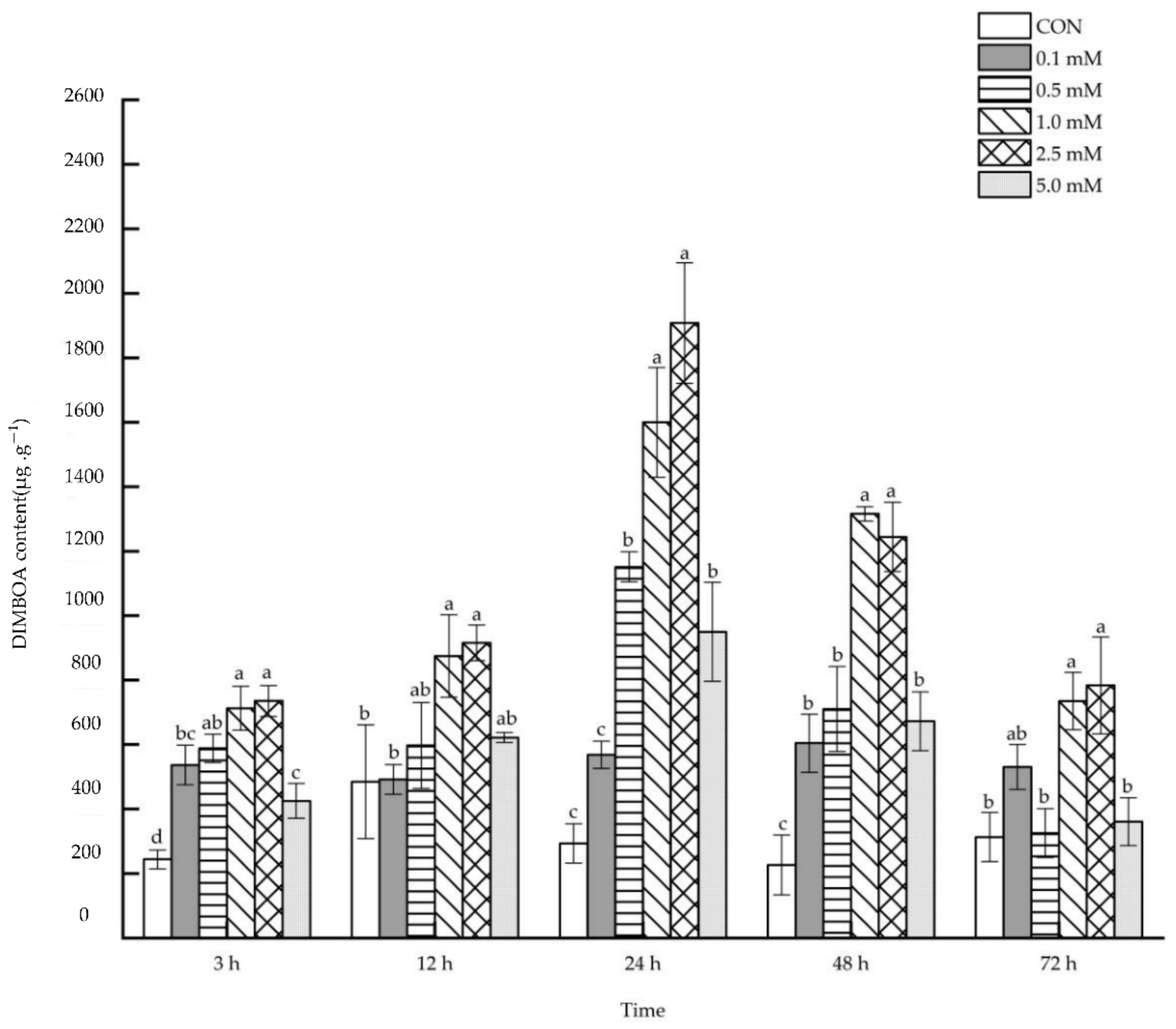
2.1.1. DIMBOA Content
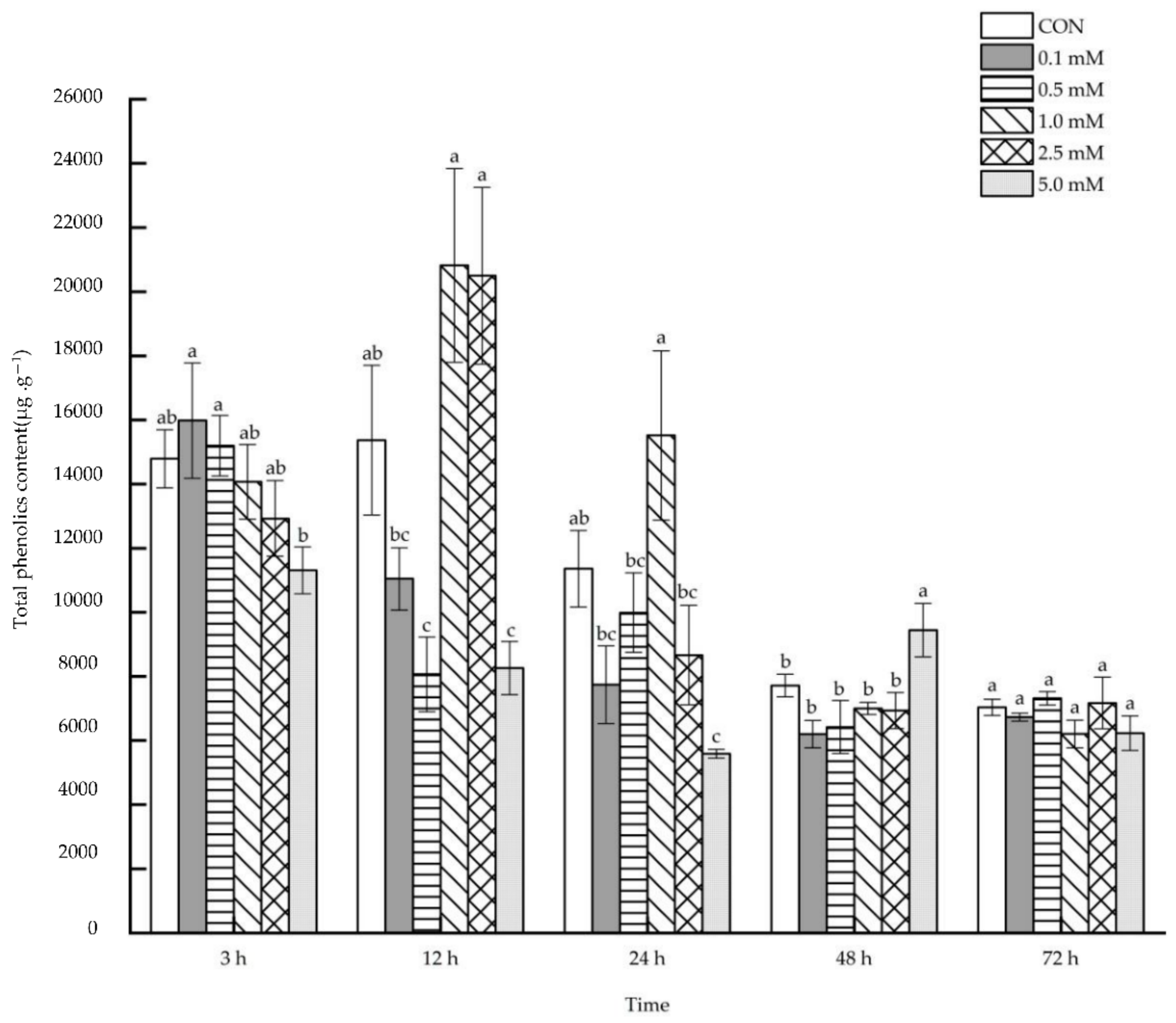
2.1.2. Total Phenolics Content
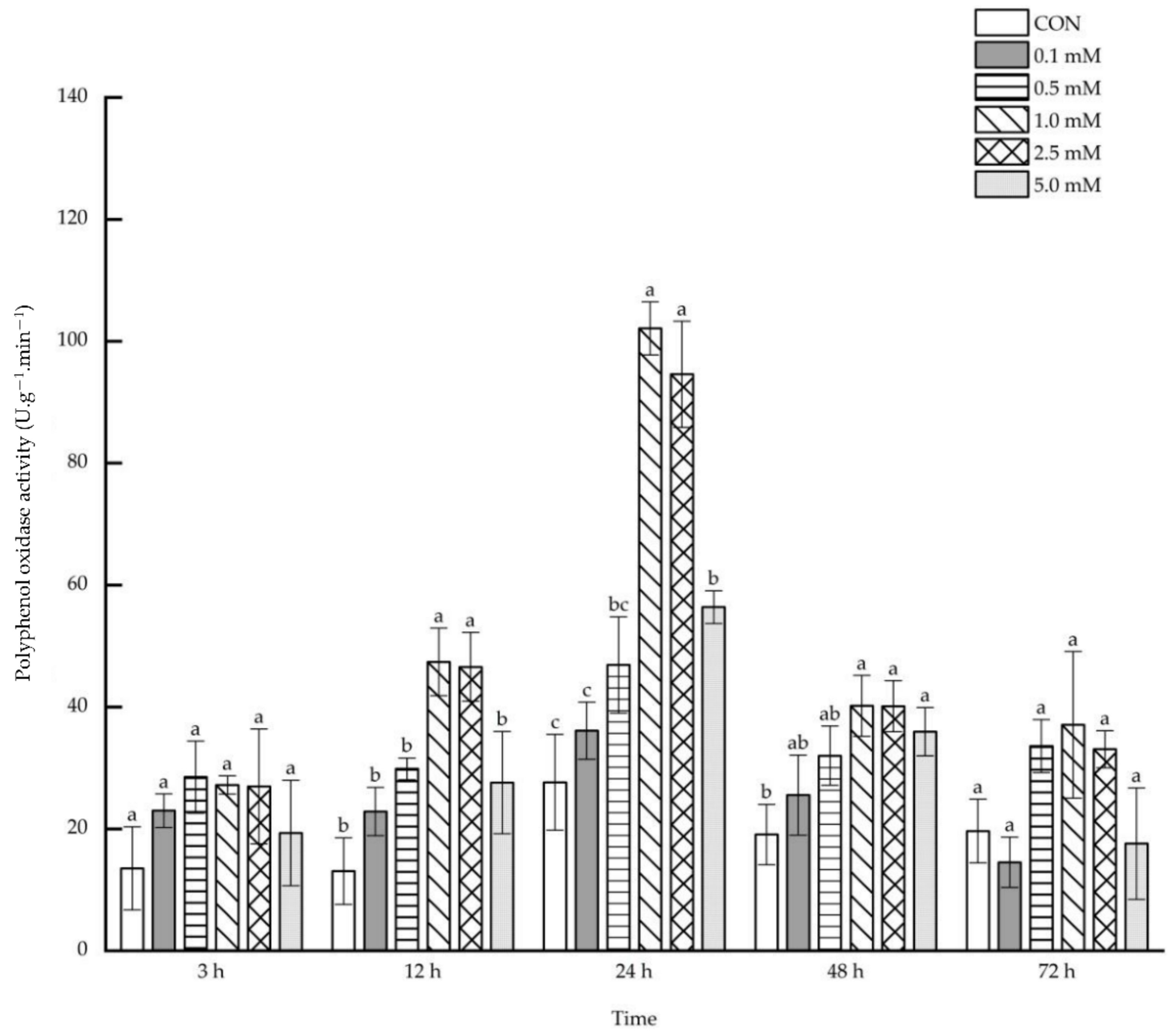
2.1.3. Polyphenol Oxidase Activity
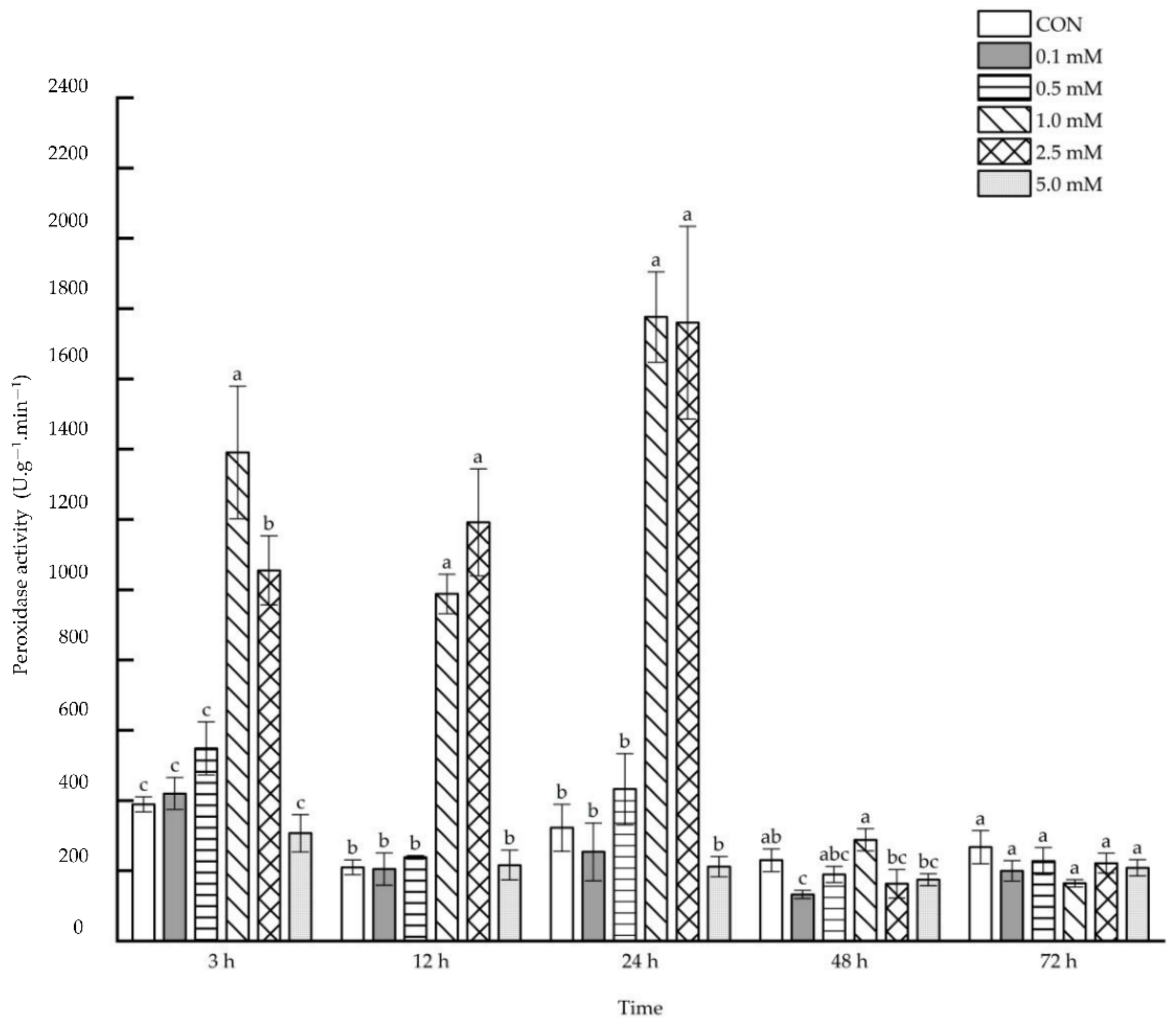
2.1.4. Peroxidase Activity
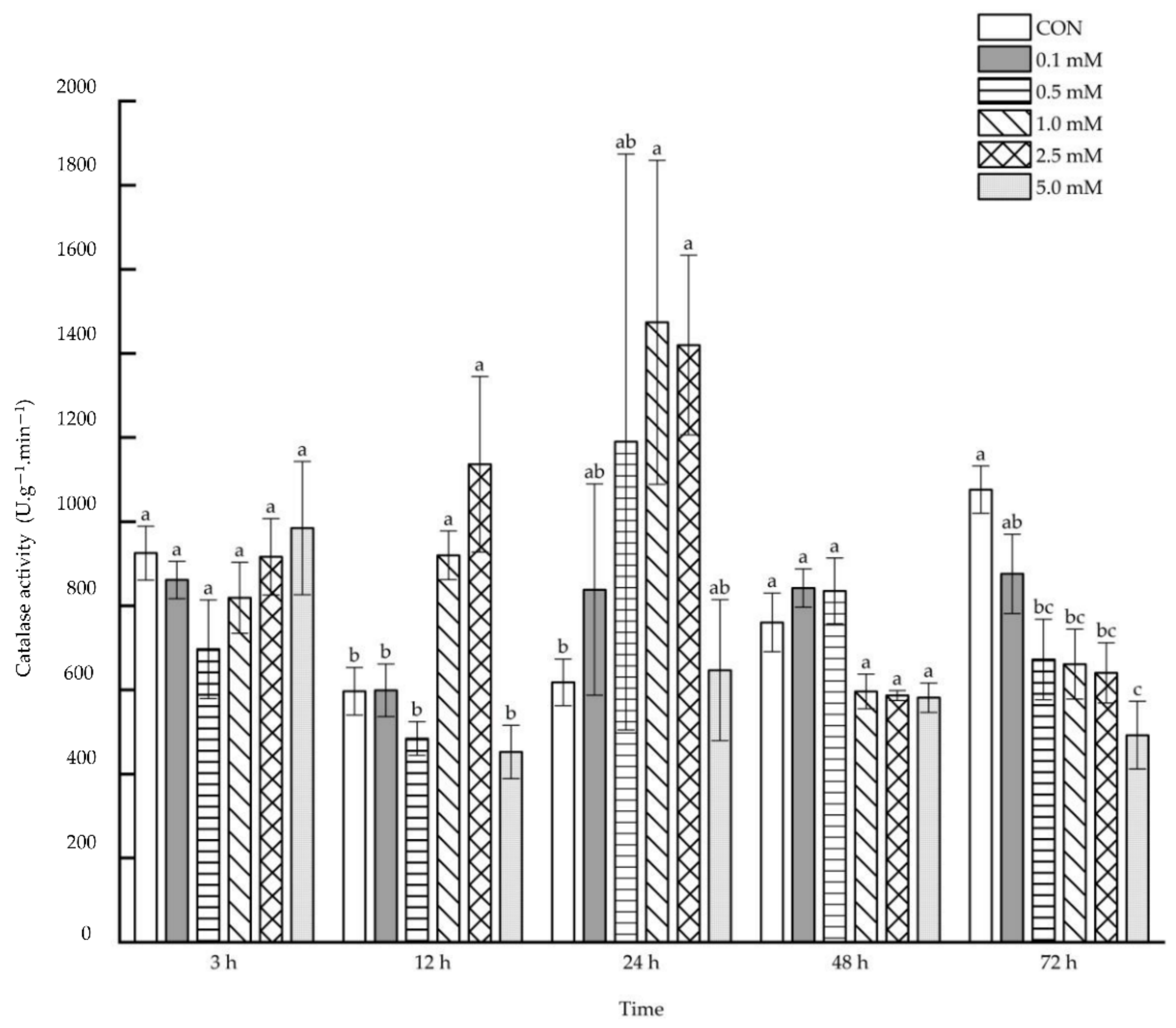
2.1.5. Catalase Activity
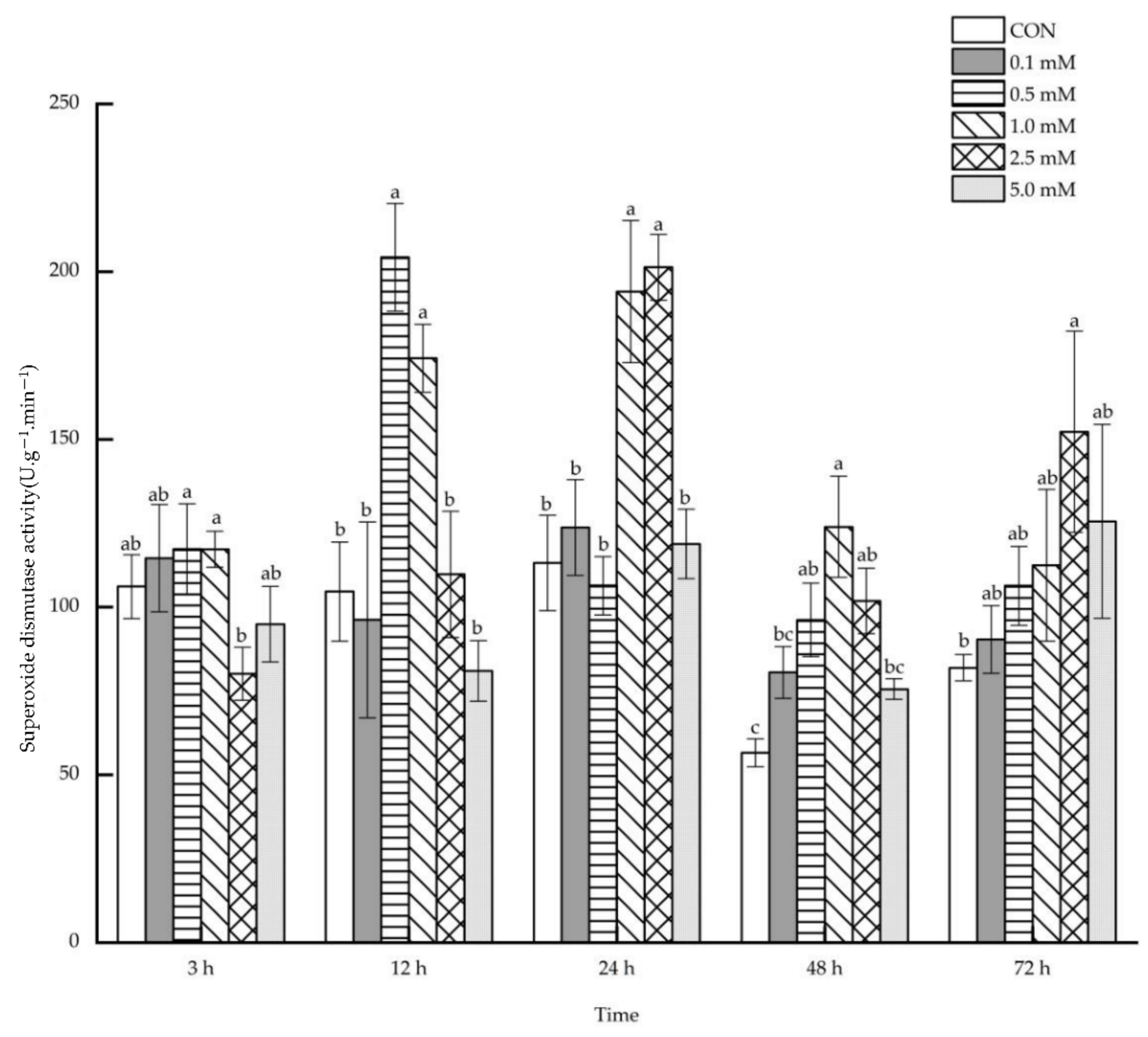
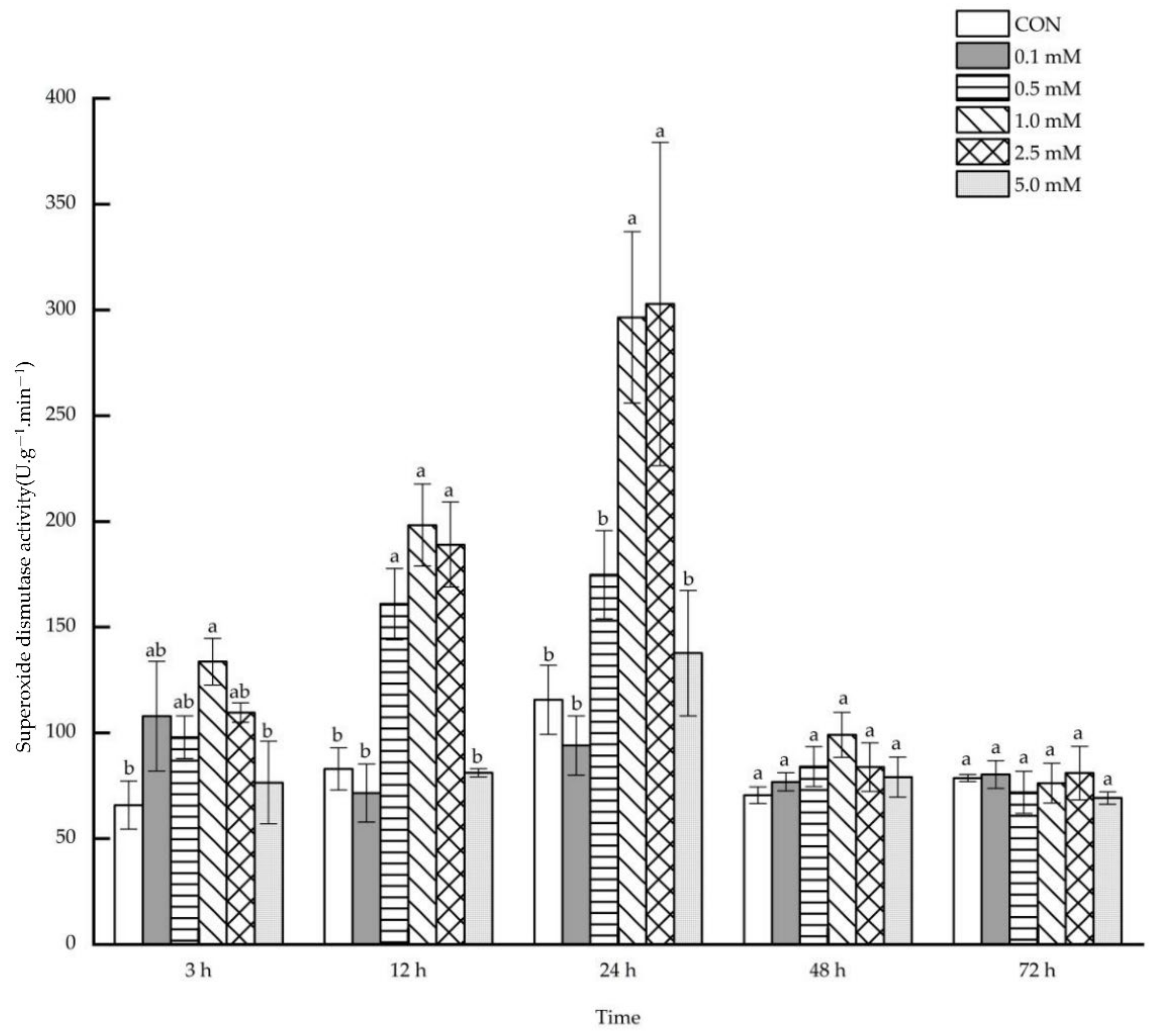
2.1.6. Superoxide Dismutase Activity
2.2. The Systemic Effect of SA Concentration and Post-Treatment Time on Chemical Defense Responses in Non-Treated Roots
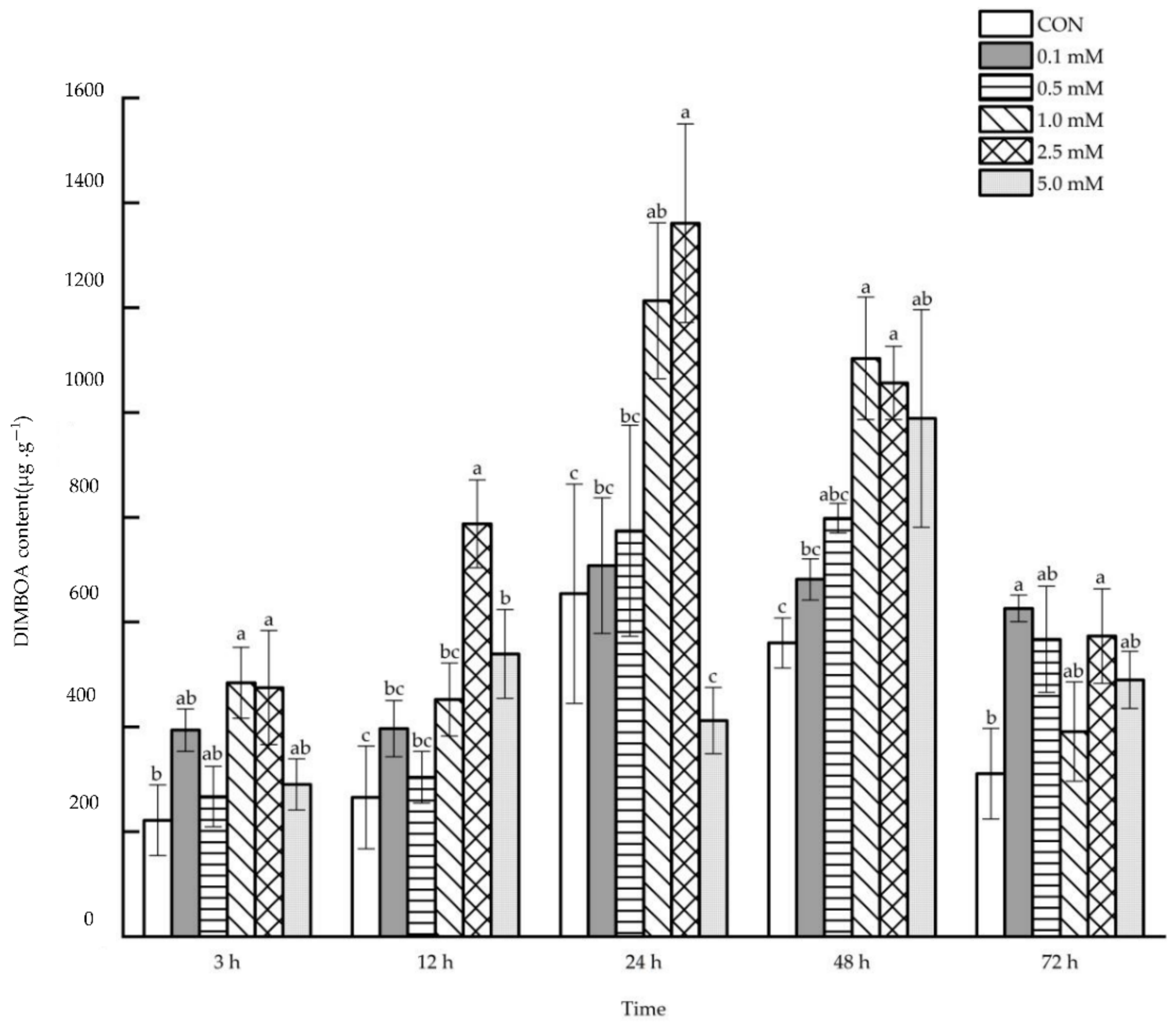
2.2.1. DIMBOA Content
2.2.2. Total Phenolics Content
2.2.3. Polyphenol Oxidase Activity
2.2.4. Peroxidase Activity
2.2.5. Catalase Activity
2.2.6. Superoxide Dismutase Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Analysis of DIMBOA
4.4. Analysis of Total Phenolics
4.5. Determination of Polyphenol Oxidase Activity
4.6. Determination of Peroxidase Activity
4.7. Determination of Catalase Activity
4.8. Determination of Superoxide Dismutase Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous glutathione attenuates lead-induced oxidative stress in wheat by improving antioxidant defense and physiological mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Khalil, N.; Fekry, M.; Bishr, M.; Zalabani, S.E.; Salama, O. Foliar spraying of salicylic acid induced accumulation of phenolics, increased radical scavenging activity and modified the composition of the essential oil of water stressed Thymus vulgaris L. Plant Physiol. Biochem. 2018, 123, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Robbins, P.S.; Niedz, R.; McCollum, G.; Alessandro, R. Exogenous application of the plant signalers methyl jasmonate and salicylic acid induces changes in volatile emissions from citrus foliage and influences the aggregation behavior of Asian citrus psyllid (Diaphorina citri), vector of Huanglongbing. PLoS ONE 2018, 13, e0193724. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Wang, J.; Shi, S.H.; Wang, D.Y.; Sun, Y.; Zhu, M.; Li, F.H. Exogenous salicylic acid ameliorates waterlogging stress damages and improves photosynthetic efficiency and antioxidative defense system in waxy corn. Photosynthetic 2021, 59, 84–94. [Google Scholar] [CrossRef]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef]
- Métraux, J.P. Systemic acquired resistance and salicylic acid: Current state of knowledge. Eur. J. Plant Pathol. 2001, 107, 13–18. [Google Scholar] [CrossRef]
- Ding, P.T.; Ding, Y.L. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic acid biosynthesis in plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Pokotylo, I.; Hodges, M.; Kravets, V.; Ruelland, E. A ménage à trois: Salicylic acid, growth inhibition, and immunity. Trends Plant Sci. 2022, 27, 460–471. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Singh, H.; Singh, A.; Hussain, I.; Singh, N.B. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress in maize (Zea mays L.) grown under cinnamic acid stress. Russ. Agric. Sci. 2018, 44, 9–17. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Iqbal, N.; Irfan, M.; Sehar, Z.; Khan, N.A. Crosstalk of plant growth regulators protects photosynthetic performance from arsenic damage by modulating defense systems in rice. Ecotox. Environ. Saf. 2021, 222, 112535. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Xi, M.; Li, Y.; Cheng, Z.W.; Wang, S.; Kong, F. Improvement in salt tolerance of Iris pseudacorus L. in constructed wetland by exogenous application of salicylic acid and calcium chloride. J. Environ. Manag. 2021, 300, 13703. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Fayez, K.A.; Mahmoud, S.Y.; Lu, G.Q. Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol. Plant 2010, 32, 891–904. [Google Scholar] [CrossRef]
- Song, Y.L.; Dong, Y.J.; Kong, J.; Tian, X.Y.; Bai, X.Y.; Xu, L.L. Effects of root addition and foliar application of nitric oxide and salicylic acid in alleviating iron deficiency induced chlorosis of peanut seedlings. J. Plant Nutr. 2017, 40, 63–81. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Ahanger, M.A.; Egamberdieva, D.; Wijaya, L.; Alam, P. Salicylic acid (SA) Iinduced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russ. J. Plant Physiol. 2018, 65, 104–114. [Google Scholar] [CrossRef]
- Methenni, K.; Abdallah, M.B.; Nouairi, I.; Smaoui, A.; Ammar, W.B.; Zarrouk, M.; Youssef, N.B. Salicylic acid and calcium pretreatments alleviate the toxic effect of salinity in the Oueslati olive variety. Sci. Hortic. 2018, 233, 349–358. [Google Scholar] [CrossRef]
- Somboon, T.; Chayjarung, P.; Pilaisangsuree, V.; Keawracha, P.; Tonglairoum, P.; Kongbangkerd, A.; Wongkrajang, K.; Limmongkon, A. Methyl jasmonate and cyclodextrin-mediated defense mechanism and protective effect in response to paraquat-induced stress in peanut hairy root. Phytochemistry 2019, 163, 11–22. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Schubert, B.; Pieper, K.; Ihmig, S.; Hilgenberg, W. Glucosinolate content in susceptible and resistant chinese cabbage varieties during development of clubroot disease. Phytochemistry 1997, 44, 407–417. [Google Scholar] [CrossRef]
- Feng, Y.J.; Wang, J.W.; Luo, S.M.; Jin, Q.; Fan, H.Z.; Su, Y.J.; Liu, Y.H. Effects of exogenous application of jasmomic acid and salicylic acid on the leaf and root induction of chemical defence in maize (Zea mays L.). Allelopath. J. 2010, 25, 133–146. [Google Scholar]
- Guo, B.; Zhang, Y.Q.; Li, S.L.; Lai, T.; Yang, L.; Chen, J.N.; Ding, W. Extract from maize (Zea mays L.): Antibacterial activity of DIMBOA and its derivatives against ralstonia solanacearum. Molecules 2016, 21, 1397. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.M.; Ren, H.K.; Xue, Y.; Meng, H.; Li, M.H. Increased antioxidant activity and polyphenol metabolites in methyl jasmonate treated mung bean (Vigna radiata) sprouts. Food Sci. Technol. 2017, 37, 411–417. [Google Scholar] [CrossRef][Green Version]
- Maity, A.; Sharma, J.; Sarkar, A.; More, A.K.; Pal, R.K.; Nagane, V.P.; Maity, A. Salicylic acid mediated multi-pronged strategy to combat bacterial blight disease (Xanthomonas axonopodis pv. punicae) in pomegranate. Eur. J. Plant Pathol. 2018, 150, 923–937. [Google Scholar] [CrossRef]
- Mageroy, M.H.; Wilkinson, S.W.; Tengs, T.; Cross, H.; Almvik, M.; Pétriacq, P.; Vivian Smith, A.; Zhao, T.; Fossdal, C.G.; Krokene, P. Molecular underpinnings of methyl jasmonate-induced resistance in Norway spruce. Plant Cell Environ. 2020, 43, 1827–1843. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhang, Q.M. Exogenous salicylic acid alleviates the toxicity of chlorpyrifos in wheat plants (Triticum aestivum). Ecotox. Environ. Saf. 2017, 137, 218–224. [Google Scholar] [CrossRef]
- Xi, D.D.; Li, X.F.; Gao, L.; Zhang, Z.H.; Zhu, Y.Y.; Zhu, H.F. Application of exogenous salicylic acid reduces disease severity of Plasmodiophora brassicae in pakchoi (Brassica campestris ssp. chinensis Makino). PLoS ONE 2021, 16, e248648. [Google Scholar]
- Manzoor, K.; Ilyas, N.; Batool, N.; Ahmad, B.; Arshad, M. Effect of salicylic acid on the growth and physiological characteristics of maize under stress conditions. J. Chem. Soc. Pak. 2015, 37, 588–593. [Google Scholar]
- Khanna, P.; Kaur, K.; Gupta, A.K. Salicylic acid induces differential antioxidant response in spring maize under high temperature stress. Indian J. Exp. Biol. 2016, 54, 386–393. [Google Scholar]
- Du, Y.P.; Ji, X.L.; Jiang, E.S.; Cui, L.J.; Zhai, H. Phylloxera resistance induced by salicylic and jasmonic acids in Kyoho grapevine. Acta Entomol. Sin. 2014, 47, 443–448. [Google Scholar]
- Feng, Y.J.; Wang, J.W.; Luo, S.M. Effects of exogenous jasmonic acid on concentrations of direct defense chemicals and expression of related genes in Bt (Bacillus thuringiensis) corn (Zea mays). Sci. Agric. Sin. 2007, 40, 2481–2487. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Arce, C.C.M.; McClure, M.A.; Baldwin, I.T.; Erb, M. Aboveground herbivory-induced jasmonates disproportionately reduce plant reproductive potential by facilitating root nematode infestation. Plant Cell Environ. 2018, 41, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Castano-Duque, L.; Luthe, D.S. Protein networks reveal organ-specific defense strategies in maize in response to an aboveground herbivore. Arthropod-Plant Interact. 2018, 12, 147–175. [Google Scholar] [CrossRef]
- Vaughan, M.M.; Block, A.; Christensen, S.A.; Allen, L.H.; Schmelz, E.A. The effects of climate change associated abiotic stresses on maize phytochemical defenses. Phytochem. Rev. 2018, 17, 37–49. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef]
- Manila, S.; Nelson, R. Biochemical changes induced in tomato as a result of arbuscular mycorrhizal fungal colonization and tomato wilt pathogen infection. Asian J. Plant Sci. R. 2014, 4, 62–68. [Google Scholar]
- Lokhandwala, S.; Kareliya, N.; Patel, T.; Rana, M. Phenol production and anti-microbial activity of fulleniformis mosseae inoculated allium opea roots. Int. J. Recent Biotechnol. 2014, 2, 35–37. [Google Scholar]
- Aghababaei, F.; Raiesi, F. Mycorrhizal fungi and earthworms reduce antioxidant enzyme activities in maize and sunflower plants grown in Cd-polluted soils. Soil Biol. Biochem. 2015, 86, 87–97. [Google Scholar] [CrossRef]
- Hemanth-Kumar, N.K.; Kumari, M.S.; Singh, M.V.; Jagannath, S. Oxidative stress and antioxidant metabolic enzymes response of maize (Zea mays L.) seedlings to a biotic stress (Alachlor) condition. Int. J. Agric. Sci. 2016, 8, 2227–2231. [Google Scholar]
- Cueto-Ginzo, I.A.; Serrano, L.; Sin, E.; Rodríguez, R.; Morales, J.G.; Lade, S.B.; Medina, V.; Achon, M.A. Exogenous salicylic acid treatment delays initial infection and counteracts alterations induced by Maize dwarf mosaic virus in the maize proteome. Physiol. Mol. Plant Pathol. 2016, 96, 47–59. [Google Scholar] [CrossRef]
- Ali, B. Salicylic acid induced antioxidant system enhances the tolerence to aluminium in mung bean (Vigna radiata L. Wilczek) plants. Indian J. Plant Physiol. 2017, 22, 178–189. [Google Scholar] [CrossRef]
- Moharramnejad, S.; Azam, A.T.; Panahandeh, J.; Dehghanian, Z.; Ashraf, M. Effect of methyl jasmonate and salicylic acid on in vitro growth, stevioside production, and oxidative defense system in stevia rebaudiana. Sugar Technol. 2019, 21, 1031–1038. [Google Scholar] [CrossRef]
- Hussain, I.; Rasheed, R.; Ashraf, M.A.; Mohsin, M.; Shah, S.M.A.; Rashid, D.A.; Akram, M.; Nisar, J.; Riaz, M. Foliar applied acetylsalicylic acid induced growth and key-biochemical changes in chickpea (Cicer arietinum L.) under drought stress. Dose-Response 2020, 18, 500552896. [Google Scholar] [CrossRef] [PubMed]
- Taherbahrani, S.; Zoufan, P.; Zargar, B. Modulation of the toxic effects of zinc oxide nanoparticles by exogenous salicylic acid pretreatment in Chenopodium murale L. Environ Sci. Pollut. R. 2021, 28, 65644–65654. [Google Scholar] [CrossRef]
- Van Dam, N.M.; Harvey, J.A.; Wäckers, F.L.; Bezemer, T.M.; Van der Putten, W.H.; Vet, L.E.M. Interactions between aboveground and belowground induced responses against phytophages. Basic Appl. Ecol. 2003, 4, 63–77. [Google Scholar] [CrossRef]
- Bhar, A.; Chatterjee, M.; Gupta, S.; Das, S. Salicylic acid regulates systemic defense signaling in chickpea during Fusarium oxysporum f. sp. ciceri race 1 infection. Plant Mol. Biol. Rep. 2018, 36, 162–175. [Google Scholar] [CrossRef]
- Dabrowski, Z.T.; Bielak, B. Effect of some plant chemical compounds on the behaviour and reproduction of spider mites (Acarina: Tetranychidae). Entomol. Exp. Appl. 1978, 24, 317–326. [Google Scholar] [CrossRef]
- Tzin, V.; Lindsay, P.L.; Christensen, S.A.; Meihls, L.N.; Blue, L.B.; Jander, G. Genetic mapping shows intraspecific variation and transgressive segregation for caterpillar-induced aphid resistance in maize. Mol. Ecol. 2015, 24, 5739–5750. [Google Scholar] [CrossRef]
- Chen, N.L.; Hu, M.; Dai, C.Y.; Yang, S.M. The effects of inducing treatments on phenolic metabolism of melon leaves. Acta Hortic. Sin. 2010, 37, 1759–1766. [Google Scholar]
- Kundu, S.; Chakraborty, D.; Pal, A. Proteomic analysis of salicylic acid induced resistance to Mungbean Yellow Mosaic India Virus in Vigna mungo. J. Proteomics 2011, 74, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Azooz, M.M.; Youssef, A.M.; Ahmad, P. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Int. J. Plant Physiol. Biochem. 2011, 3, 253–264. [Google Scholar]
- Sharhrtash, M.; Mohsenzadeh, S.; Mohabatkar, H. Salicylic acid alleviates paraquat oxidative damage in maize seedling. Asian J. Exp. Biol. Sci. 2011, 2, 377–382. [Google Scholar]
- Bowles, D.J. Defense-related proteins in higher plants. Annu. Rev. Biochem. 1990, 59, 873–907. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Recognition of herbivory associated molecular patterns. Plant Physiol. 2008, 146, 825–831. [Google Scholar] [CrossRef]
- Hegde, D.R.; Nelson, S.J.; Natarajan, N.; Kumar, S.M.; Arumugam, T. Effect of cis-jasmone and salicylic acid on the induction of defensive enzymes in Brinjal against Brinjal shoot and fruit borer (Leucinodes orbonalis Guenee). J. Pharmacogn. Phytochem. 2018, 7, 3125–3131. [Google Scholar]
- Zhang, Q.; Li, D.M.; Wang, Q.; Song, X.Y.; Wang, Y.B.; Yang, X.L.; Qin, D.L.; Xie, T.L.; Yang, D.G. Exogenous salicylic acid improves chilling tolerance in maize seedlings by improving plant growth and physiological characteristics. Agronomy 2021, 11, 1341. [Google Scholar] [CrossRef]
- He, S.L.; Lin, W.X.; Chen, R.K. Induction of sesquiterpene cyclase gene expression and antioxidant enzymes regulated by exogenous salicylic acid in leaves of Capsicum annuum. Chin. J. Appl. Ecol. 2002, 13, 569–572. [Google Scholar]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Feng, Y.J.; Jin, Q.; Wang, J.W. Systemic induced effects of machanical wounding on the chemical defense of Bt corn (Zea mays). Chin. J. Plant Ecol. 2010, 34, 695–703. [Google Scholar]
- Kamal, A.H.M.; Komatsu, S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J. Proteomics 2016, 133, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.Y.; Zhang, S.S.; Kang, H.M.; Yang, W.Z. Effect of salicylic acid on high quality seedling forming and physiological index of pinus wangii. Chin. Agric. Sci. Bull. 2017, 33, 29–32. [Google Scholar]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R.; Zhang, Y.; Martin, J.; Smart, L. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 2011, 157, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Mechanism of damage-induced alkaloid production in wild tobacco. J. Chem. Ecol. 1989, 15, 1661–1680. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates Publishers: Sunderland, MA, USA, 1998. [Google Scholar]
- López-Orenes, A.; Martínez-Pérez, A.; Calderón, A.A.; Ferrer, M.A. Pb-induced responses in Zygophyllum fabago plants are organ-dependent and modulated by salicylic acid. Plant Physiol. Biochem. 2014, 84, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.X.; Gill, M.B.; Mao, B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ. Sci. Pollut. R. 2016, 23, 20483–20496. [Google Scholar] [CrossRef]
- Gharbi, E.; Lutts, S.; Dailly, H.; Quinet, M. Comparison between the impacts of two different modes of salicylic acid application on tomato (Solanum lycopersicum) responses to salinity. Plant Signal. Behav. 2018, 13, e1469361. [Google Scholar] [CrossRef]
- Poór, P.; Takács, Z.; Bela, K.; Czékus, Z.; Szalai, G.; Tari, I. Prolonged dark period modulates the oxidative burst and enzymatic antioxidant systems in the leaves of salicylic acid-treated tomato. J. Plant Physiol. 2017, 213, 216–226. [Google Scholar] [CrossRef]
- Colak, N.; Kurt-Celebi, A.; Fauzan, R.; Torun, H.; Ayaz, F.A. The protective effect of exogenous salicylic and gallic acids ameliorates the adverse effects of ionizing radiation stress in wheat seedlings by modulating the antioxidant defence system. Plant Physiol. Biochem. 2021, 168, 526–545. [Google Scholar] [CrossRef]
- Cueto-Ginzo, A.I.; Serrano, L.; Bostock, R.M.; Ferrio, J.P.; Rodríguez, R.; Arcal, L.; Achon, M.; Falcioni, T.; Luzuriaga, W.P.; Medina, V. Salicylic acid mitigates physiological and proteomic changes induced by the SPCP1 strain of Potato virus X in tomato plants. Physiol. Mol. Plant Pathol. 2016, 93, 1–11. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, W.; Han, X.; Wu, J.; Lyu, B.; Chen, F.; Caplan, A.; Li, C.; Wu, J.; Wang, W.; et al. Isochorismate-based salicylic acid biosynthesis confers basal resistance to Fusarium graminearum in barley. Mol. Plant Pathol. 2018, 19, 1995–2010. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Quisenberry, S.S. Comparison of DIMBOA concentrations among wheat isolines and corresponding plant introduction lines. Entomol. Appl. 2000, 96, 275–279. [Google Scholar] [CrossRef]
- Randhir, R.; Shetty, K. Developmental stimulation of total phenolics and related antioxidant activity in light-and dark-germinated corn by natural elicitors. Process Biochem. 2005, 40, 1724–1732. [Google Scholar] [CrossRef]
- Sivakumar, G.; Sharma, R.C. Induced biochemical changes due to seed bacterization by Pseudomonas fluorescens in maize plants. Indian Phytopathol. 2003, 56, 134–137. [Google Scholar]
- Gao, J.F. Plant Physiology Experimental Guidance; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2003. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Wang, X.; Du, T.; Shu, Y.; Tan, F.; Wang, J. Effects of Salicylic Acid Concentration and Post-Treatment Time on the Direct and Systemic Chemical Defense Responses in Maize (Zea mays L.) Following Exogenous Foliar Application. Molecules 2022, 27, 6917. https://doi.org/10.3390/molecules27206917
Feng Y, Wang X, Du T, Shu Y, Tan F, Wang J. Effects of Salicylic Acid Concentration and Post-Treatment Time on the Direct and Systemic Chemical Defense Responses in Maize (Zea mays L.) Following Exogenous Foliar Application. Molecules. 2022; 27(20):6917. https://doi.org/10.3390/molecules27206917
Chicago/Turabian StyleFeng, Yuanjiao, Xiaoyi Wang, Tiantian Du, Yinghua Shu, Fengxiao Tan, and Jianwu Wang. 2022. "Effects of Salicylic Acid Concentration and Post-Treatment Time on the Direct and Systemic Chemical Defense Responses in Maize (Zea mays L.) Following Exogenous Foliar Application" Molecules 27, no. 20: 6917. https://doi.org/10.3390/molecules27206917
APA StyleFeng, Y., Wang, X., Du, T., Shu, Y., Tan, F., & Wang, J. (2022). Effects of Salicylic Acid Concentration and Post-Treatment Time on the Direct and Systemic Chemical Defense Responses in Maize (Zea mays L.) Following Exogenous Foliar Application. Molecules, 27(20), 6917. https://doi.org/10.3390/molecules27206917





