Integration of Mn-ZnFe2O4 with S-g-C3N4 for Boosting Spatial Charge Generation and Separation as an Efficient Photocatalyst
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Synthesis of Chromium-Doped Zinc Ferrites
2.3. Synthesis of S-g-C3N4
2.4. Synthesis of Mn-ZnFe2O4/S-g-C3N4
2.5. Photocatalytic Activity
2.6. Characterization
3. Results and Discussion
3.1. TEM and EDX Analyses
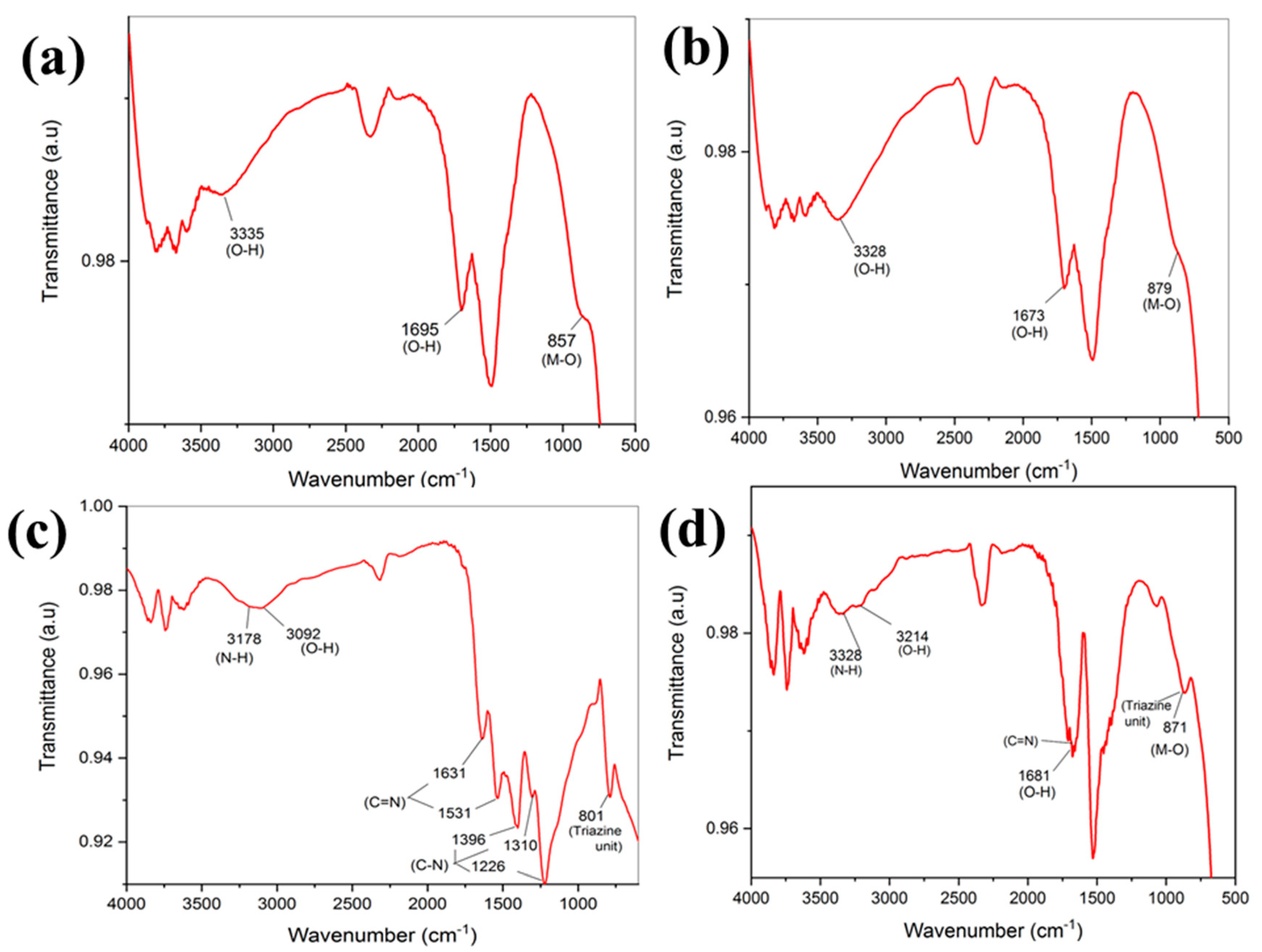
3.2. FTIR Analysis
3.3. XRD Analysis
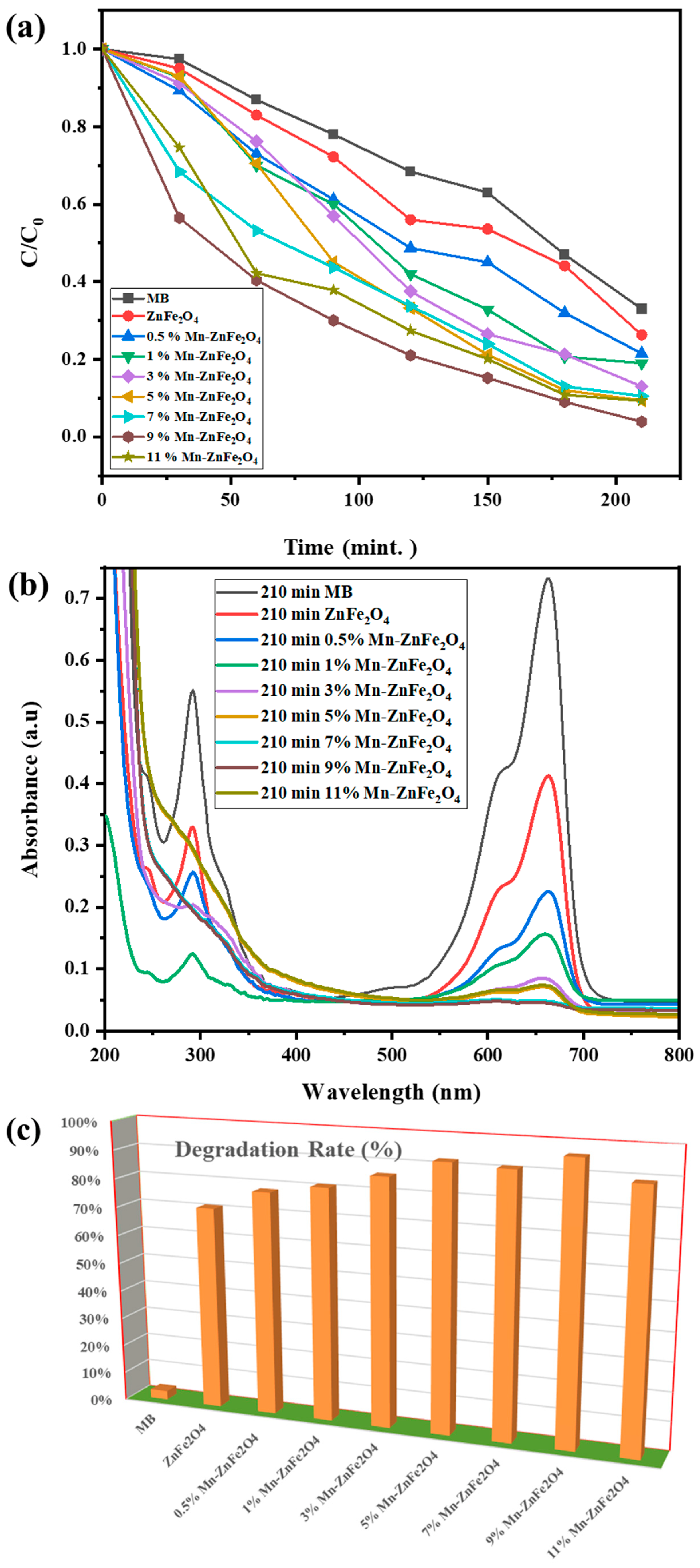
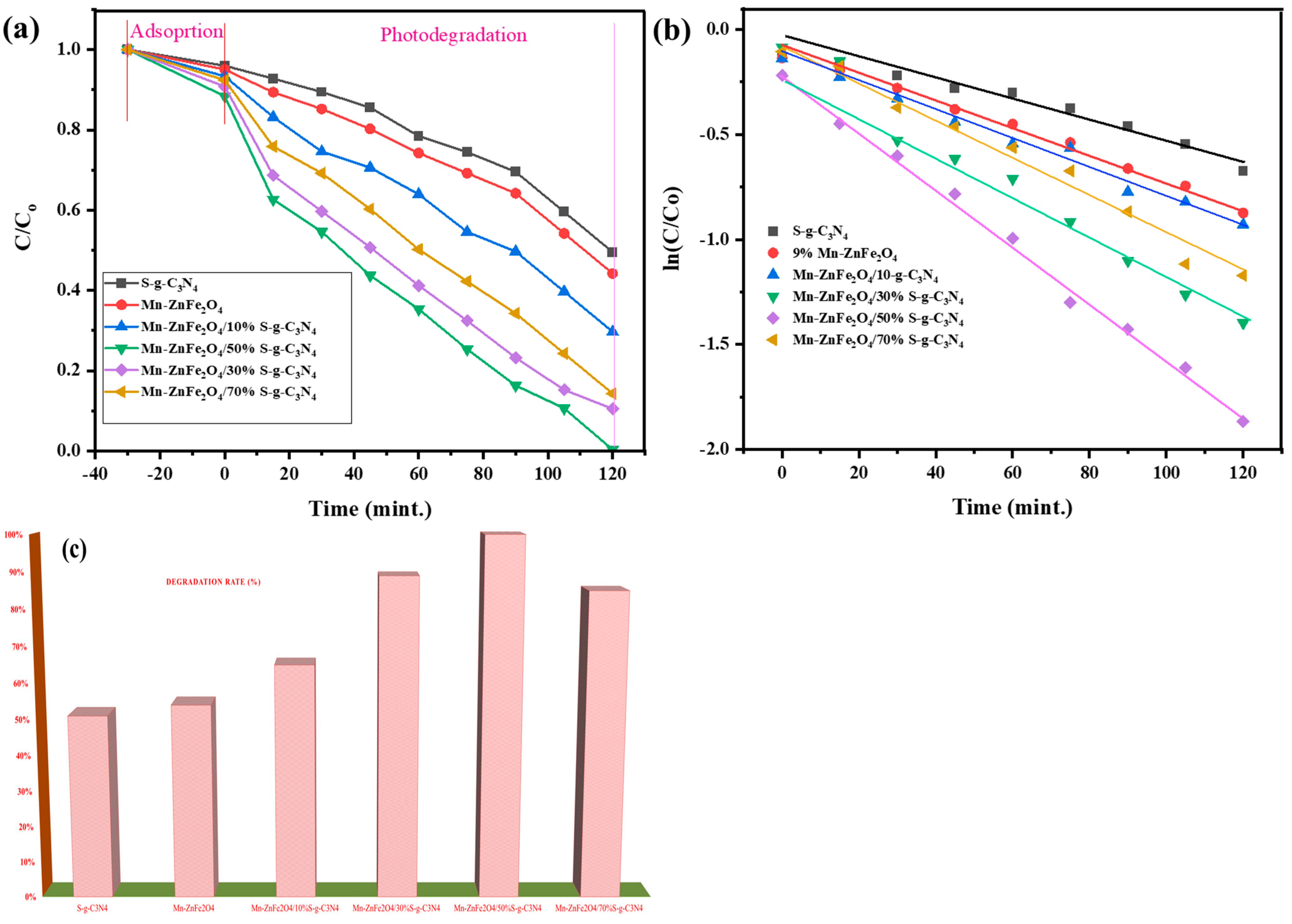
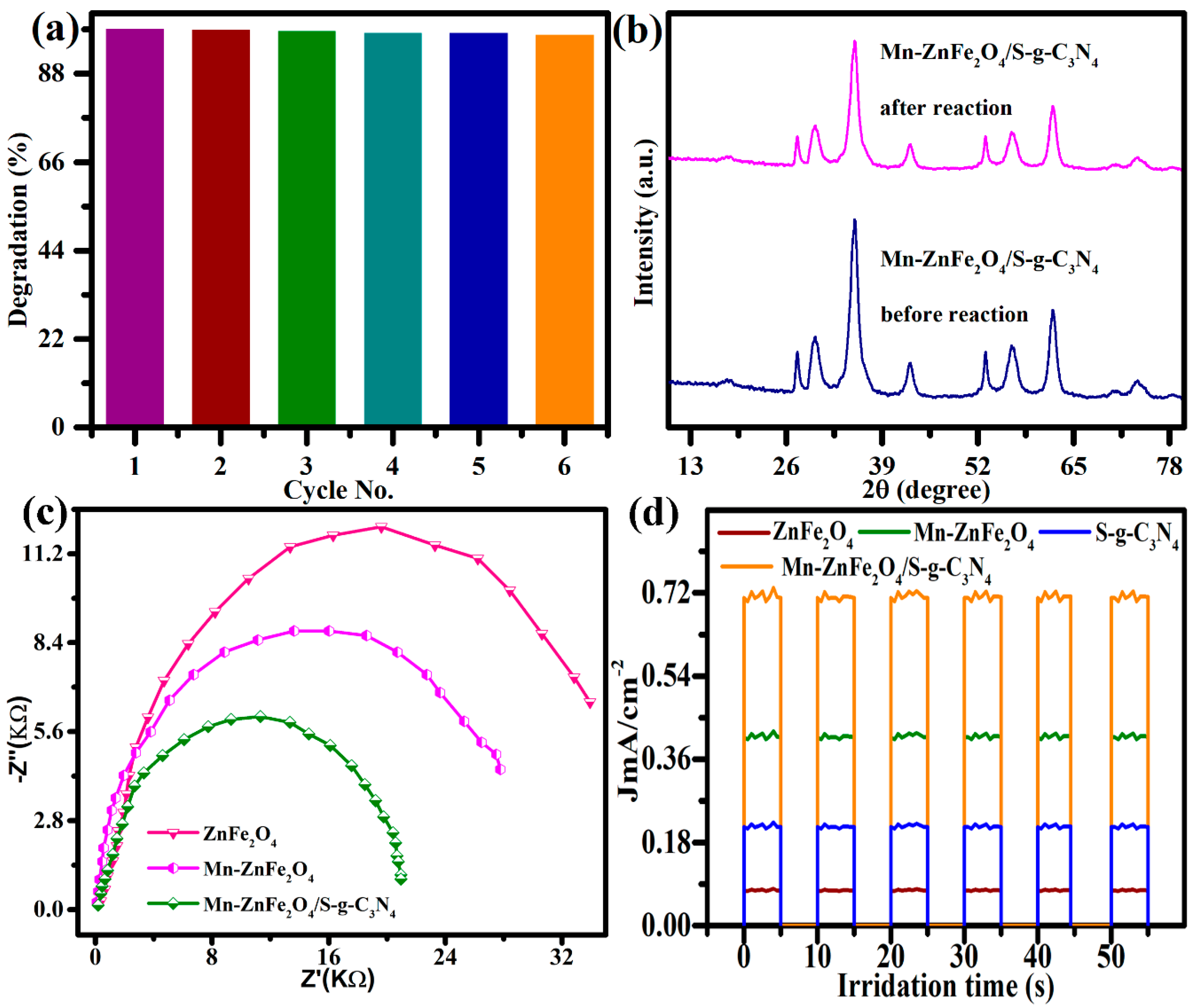
3.4. Photocatalytic Degradation Study
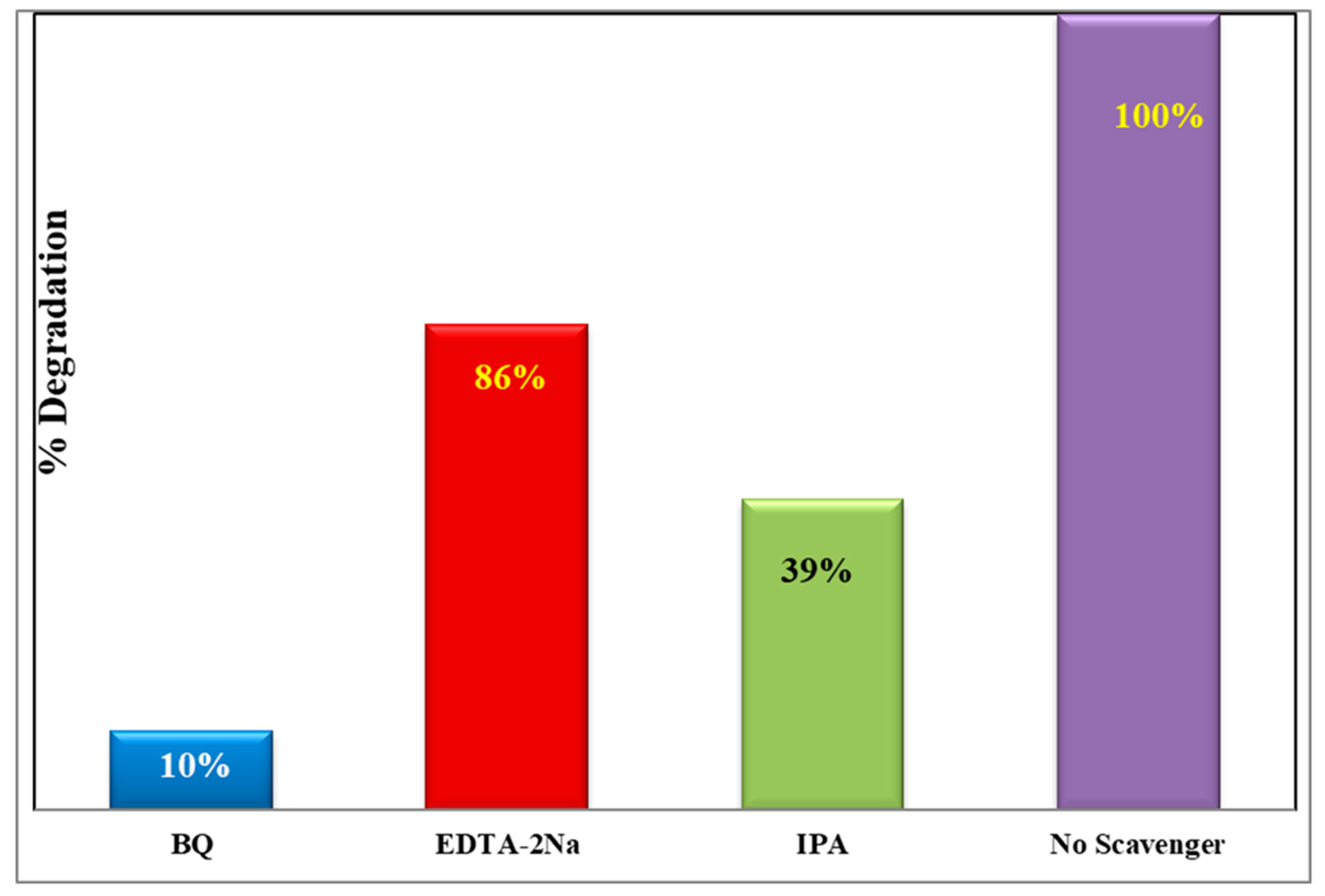
3.5. Scavenging Activity
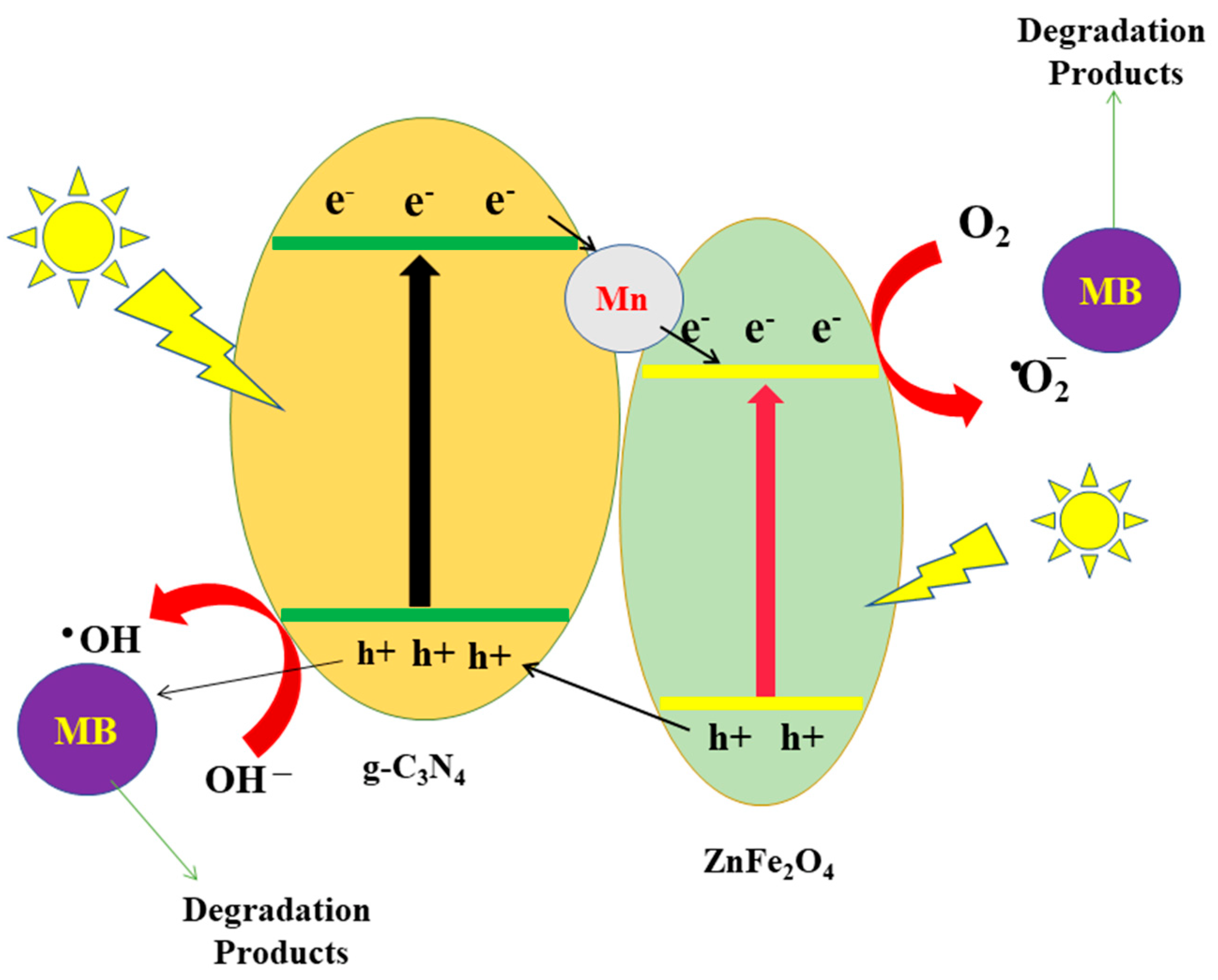
4. Photocatalytic Degradation Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Wang, Y.; Di, A.; Yang, X.; Chen, G. Enhanced photocatalytic performance of hierarchical ZnFe2O4/g-C3N4 heterojunction composite microspheres. Catal. Lett. 2018, 148, 2179–2189. [Google Scholar] [CrossRef]
- Rehman, K.; Shahzad, T.; Sahar, A.; Hussain, S.; Mahmood, F.; Siddique, M.H.; Siddique, M.A.; Rashid, M.I. Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling. PeerJ 2018, 6, e4802. [Google Scholar] [CrossRef] [PubMed]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Abd El-Monaem, E.M.; Omer, A.M.; Khalifa, R.E.; Abd El-Latif, M.M.; El-Subruiti, G.M. Efficient removal of toxic methylene blue (MB) dye from aqueous solution using a metal-organic framework (MOF) MIL-101 (Fe): Isotherms, kinetics, and thermodynamic studies. Desalin. Water Treat. 2020, 189, 395–407. [Google Scholar] [CrossRef]
- Kuang, M.; Zhang, J.; Wang, W.; Chen, J.; Liu, R.; Xie, S.; Wang, J.; Ji, Z. Synthesis of octahedral-like ZnO/ZnFe2O4 heterojunction photocatalysts with superior photocatalytic activity. Solid State Sci. 2019, 96, 105901. [Google Scholar] [CrossRef]
- Kang, S.G.; Choe, T.H.; Ryom, C.U.; Ri, M.C. Research on synthesis and photocatalytic activity of ZnFe2O4/Ag/g-C3N4 nanosheets composites. Compos. Interfaces 2021, 28, 223–235. [Google Scholar] [CrossRef]
- Dhasmana, A.; Uniyal, S.; Kumar, V.; Gupta, S.; Kesari, K.K.; Haque, S.; Lohani, M.; Pandey, J. Scope of nanoparticles in environmental toxicant remediation. In Environmental Biotechnology: For Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; pp. 31–44. [Google Scholar]
- Sharma, S.; Dutta, V.; Raizada, P.; Hosseini-Bandegharaei, A.; Thakur, V.; Nguyen, V.-H.; VanLe, Q.; Singh, P. An overview of heterojunctioned ZnFe2O4 photocatalyst for enhanced oxidative water purification. J. Environ. Chem. Eng. 2021, 9, 105812. [Google Scholar]
- Yao, Y.; Cai, Y.; Lu, F.; Qin, J.; Wei, F.; Xu, C.; Wang, S. Magnetic ZnFe2O4–C3N4 hybrid for photocatalytic degradation of aqueous organic pollutants by visible light. Ind. Eng. Chem. Res. 2014, 53, 17294–17302. [Google Scholar] [CrossRef]
- Sun, J.; Wu, T.; Liu, Z.; Shao, B.; Liang, Q.; He, Q.; Luo, S.; Pan, Y.; Zhao, C.; Huang, D. Peroxymonosulfate activation induced by spinel ferrite nanoparticles and their nanocomposites for organic pollutants removal: A review. J. Clean. Prod. 2022, 346, 131143. [Google Scholar] [CrossRef]
- Amin, A.M.; Rayan, D.A.; Ahmed, Y.M.; El-Shall, M.S.; Abdelbasir, S.M. Zinc ferrite nanoparticles from industrial waste for Se (IV) elimination from wastewater. J. Environ. Manag. 2022, 312, 114956. [Google Scholar] [CrossRef]
- Patil, S.; Bhojya Naik, H.; Nagaraju, G.; Viswanath, R.; Rashmi, S. Synthesis of visible light active Gd3+-substituted ZnFe2O4 nanoparticles for photocatalytic and antibacterial activities. Eur. Phys. J. Plus 2017, 132, 328. [Google Scholar] [CrossRef]
- Ajithkumar, P.; Mohana, S.; Sumathi, S. Synthesis, characterization, optical and photocatalytic activity of yttrium and copper co-doped zinc ferrite under visible light. J. Mater. Sci. Mater. Electron. 2020, 31, 1168–1182. [Google Scholar] [CrossRef]
- Fan, G.; Tong, J.; Li, F. Visible-light-induced photocatalyst based on cobalt-doped zinc ferrite nanocrystals. Ind. Eng. Chem. Res. 2012, 51, 13639–13647. [Google Scholar] [CrossRef]
- Savunthari, K.V.; Shanmugam, S. Effect of co-doping of bismuth, copper and cerium in zinc ferrite on the photocatalytic degradation of bisphenol A. J. Taiwan Inst. Chem. Eng. 2019, 101, 105–118. [Google Scholar] [CrossRef]
- Chen, Y.; Su, F.; Xie, H.; Wang, R.; Ding, C.; Huang, J.; Xu, Y.; Ye, L. One-step construction of S-scheme heterojunctions of N-doped MoS2 and S-doped g-C3N4 for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 404, 126498. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zong, R.; Zhu, Y. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods. J. Phys. Chem. C 2013, 117, 9952–9961. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006–15012. [Google Scholar] [CrossRef]
- Akintunde, O.O.; Yu, L.; Hu, J.; Kibria, M.G.; Hubert, C.R.; Pogosian, S.; Achari, G. Disinfection and Photocatalytic Degradation of Organic Contaminants Using Visible Light-Activated GCN/Ag2CrO4 Nanocomposites. Catalysts 2022, 12, 943. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S.; Sher, M. Fabrication of g-C3N4/transition metal (Fe, Co, Ni, Mn and Cr)-doped ZnO ternary composites: Excellent visible light active photocatalysts for the degradation of organic pollutants from wastewater. Mater. Res. Bull. 2022, 147, 111630. [Google Scholar] [CrossRef]
- Javed, M.; Qamar, M.A.; Iqbal, S.; Aljazzar, S.O.; Iqbal, S.; Khan, H.; Abourehab, M.A.; Elkaeed, E.B.; Alharthi, A.I.; Awwad, N.S. Synergistic Influences of Doping Techniques and Well-Defined Heterointerface Formation to Improve the Photocatalytic Ability of the S-ZnO/GO Nanocomposite. ChemistrySelect 2022, 7, e202201913. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, J.; Yu, Y.; Zuo, S.; Li, B. A simple fabrication for sulfur doped graphitic carbon nitride porous rods with excellent photocatalytic activity degrading RhB dye. Appl. Surf. Sci. 2017, 391, 360–368. [Google Scholar] [CrossRef]
- Guan, K.; Li, J.; Lei, W.; Wang, H.; Tong, Z.; Jia, Q.; Zhang, H.; Zhang, S. Synthesis of sulfur doped g-C3N4 with enhanced photocatalytic activity in molten salt. J. Mater. 2021, 7, 1131–1142. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Y.; Bo, Q.; Xu, X.; Zhang, D. One-step preparation of sulfur-doped porous g-C3N4 for enhanced visible light photocatalytic performance. Dalton Trans. 2020, 49, 8041–8050. [Google Scholar] [CrossRef]
- Basaleh, A. Construction of mesoporous ZnFe2O4-g-C3N4 nanocomposites for enhanced photocatalytic degradation of acridine orange dye under visible light illumination adopting soft-and hard-template-assisted routines. J. Mater. Res. Technol. 2021, 11, 1260–1271. [Google Scholar] [CrossRef]
- Shanavas, S.; Ahamad, T.; Alshehri, S.M.; Acevedo, R.; Munusamy Anbarasan, P. Hydrothermal assisted synthesis of ZnFe2O4 embedded g-C3N4 nanocomposite with enhanced charge transfer ability for effective removal of nitrobenzene and Cr (VI). ChemistrySelect 2020, 5, 5117–5127. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Bahadur, A.; AL-Anazy, M.M.; Laref, A.; Li, D. Designing of highly active g-C3N4/Ni-ZnO photocatalyst nanocomposite for the disinfection and degradation of the organic dye under sunlight radiations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126176. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Sher, M.; Iqbal, S.; Bahadur, A.; Li, D. Fabricated novel g-C3N4/Mn doped ZnO nanocomposite as highly active photocatalyst for the disinfection of pathogens and degradation of the organic pollutants from wastewater under sunlight radiations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125863. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Ren, Y.; Wei, T. Elevated removal of di-n-butyl phthalate by catalytic ozonation over magnetic Mn-doped ferrospinel ZnFe2O4 materials: Efficiency and mechanism. Appl. Surf. Sci. 2020, 505, 144476. [Google Scholar] [CrossRef]
- Yu, Y.; An, H.; Zhao, Y.; Feng, J.; Wei, T.; Yu, S.; Ren, Y.; Chen, Y. MnFe2O4 decorated graphene as a heterogeneous catalyst for efficient degradation of di-n-butyl phthalate using catalytic ozonation in water. Sep. Purif. Technol. 2021, 259, 118097. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M. Synthesis of dynamic g-C3N4/Fe@ ZnO nanocomposites for environmental remediation applications. Ceram. Int. 2020, 46, 22171–22180. [Google Scholar] [CrossRef]
- Palanivel, B.; Maiyalagan, T.; Jayarman, V.; Ayyappan, C.; Alagiri, M. Rational design of ZnFe2O4/g-C3N4 nanocomposite for enhanced photo-Fenton reaction and supercapacitor performance. Appl. Surf. Sci. 2019, 498, 143807. [Google Scholar] [CrossRef]
- Saeed, H.; Nadeem, N.; Zahid, M.; Yaseen, M.; Noreen, S.; Jilani, A.; Shahid, I. Mixed metal ferrite (Mn0. 6Zn0. 4Fe2O4) intercalated g-C3N4 nanocomposite: Efficient sunlight driven photocatalyst for methylene blue degradation. Nanotechnology 2021, 32, 505714. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Liming, W.; Yong, S.; Lihui, X.; Mingrui, X. Preparation and photocatalytic properties of magnetic g-C3N4/MnZnFe2O4 composite. In Journal of Physics: Conference Series (Shanghai, China); IOP Publishing: Bistol, UK, 2021; p. 012075. [Google Scholar]
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Askari, M.B.; Salarizadeh, P.; Seifi, M.; Di Bartolomeo, A. ZnFe2O4 nanorods on reduced graphene oxide as advanced supercapacitor electrodes. J. Alloy. Compd. 2021, 860, 158497. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S. Designing and investigation of enhanced photocatalytic and antibacterial properties of 3d (Fe, Co, Ni, Mn and Cr) metal-doped zinc oxide nanoparticles. Opt. Mater. 2022, 126, 112211. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Facile one-pot synthesis of cerium oxide/sulfur-doped graphitic carbon nitride (g-C3N4) as efficient nanophotocatalysts under visible light irradiation. J. Colloid Interface Sci. 2017, 507, 59–73. [Google Scholar] [CrossRef]
- Kalisamy, P.; Lallimathi, M.; Suryamathi, M.; Palanivel, B.; Venkatachalam, M. ZnO-embedded S-doped gC3N4 heterojunction: Mediator-free Z-scheme mechanism for enhanced charge separation and photocatalytic degradation. RSC Adv. 2020, 10, 28365–28375. [Google Scholar] [CrossRef]
- Dai, Z.; Zhen, Y.; Sun, Y.; Li, L.; Ding, D. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium (VI) removal. Chem. Eng. J. 2021, 415, 129002. [Google Scholar] [CrossRef]
- Kuai, S.; Zhang, Z.; Nan, Z. Synthesis of Ce3+ doped ZnFe2O4 self-assembled clusters and adsorption of chromium (VI). J. Hazard. Mater. 2013, 250, 229–237. [Google Scholar] [CrossRef]
- Peng, L.; Fang, L.; Yang, X.; Li, Y.; Huang, Q.; Wu, F.; Kong, C. Effect of annealing temperature on the structure and optical properties of In-doped ZnO thin films. J. Alloy. Compd. 2009, 484, 575–579. [Google Scholar] [CrossRef]
- Sher, M.; Shahid, S.; Javed, M. Synthesis of a novel ternary (g-C3N4 nanosheets loaded with Mo doped ZnOnanoparticles) nanocomposite for superior photocatalytic and antibacterial applications. J. Photochem. Photobiol. B Biol. 2021, 219, 112202. [Google Scholar] [CrossRef]
- Vinoth, S.; Subramani, K.; Ong, W.-J.; Sathish, M.; Pandikumar, A. CoS2 engulfed ultra-thin S-doped g-C3N4 and its enhanced electrochemical performance in hybrid asymmetric supercapacitor. J. Colloid Interface Sci. 2021, 584, 204–215. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, S.; Qiu, C.; Xu, Y.; Zuo, J. Engineering carbon nitride with cyanide groups for efficient photocatalytic alcohol oxidation and H2O2 production-Utilization of photogenerated electrons and holes. Appl. Surf. Sci. 2022, 573, 151506. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.; Zhang, G.; Li, M.; Tang, Q.; Cao, J. Palladium modified ZnFe2O4/g-C3N4 nanocomposite as an efficiently magnetic recycling photocatalyst. J. Solid State Chem. 2020, 288, 121389. [Google Scholar] [CrossRef]
- Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Amin, S.; Guo, F.; Shi, W.; Li, Y. Engineering of 2D/3D architectures type II heterojunction with high-crystalline g-C3N4 nanosheets on yolk-shell ZnFe2O4 for enhanced photocatalytic tetracycline degradation. Mater. Res. Bull. 2022, 150, 111789. [Google Scholar] [CrossRef]
- Tamaddon, F.; Mosslemin, M.H.; Asadipour, A.; Gharaghani, M.A.; Nasiri, A. Microwave-assisted preparation of ZnFe2O4@ methyl cellulose as a new nano-biomagnetic photocatalyst for photodegradation of metronidazole. Int. J. Biol. Macromol. 2020, 154, 1036–1049. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Chang, C.; Fu, Y.; Chu, X. Preparation of magnetic composite photocatalyst Bi2WO6/CoFe2O4by two-step hydrothermal method and itsphotocatalytic degradation of bisphenol A. Catal. Commun. 2013, 37, 92–95. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, H.T.; Le, T.H.; Nguyen, L.T.; Nguyen, H.Q.; Pham, T.T.; Bui, N.D.; Tran, N.T.; Nguyen, D.T.C.; Lam, T.V. Enhanced photocatalytic activity of spherical Nd3+ substituted ZnFe2O4 nanoparticles. Materials 2021, 14, 2054. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Yin, Z.; Wang, X.; Ma, H.; Wang, L. Synergetic effect of facet junction and specific facet activation of ZnFe2O4 nanoparticles on photocatalytic activity improvement. ACS Appl. Mater. Interfaces 2019, 11, 29004–29013. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A ternary magnetic recyclable ZnO/Fe3O4/g-C3N4 composite photocatalyst for efficient photodegradation of monoazo dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.-M.; Sin, J.-C.; Zeng, H.; Mohamed, A.R. Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination. Sep. Purif. Technol. 2020, 236, 116195. [Google Scholar] [CrossRef]
- Mohamed, R.; Shawky, A. CNT supported Mn-doped ZnO nanoparticles: Simple synthesis and improved photocatalytic activity for degradation of malachite green dye under visible light. Appl. Nanosci. 2018, 8, 1179–1188. [Google Scholar] [CrossRef]
- Kulkarni, S.D.; Kumbar, S.; Menon, S.G.; Choudhari, K.; Santhosh, C. Magnetically separable core–shell ZnFe2O4@ ZnO nanoparticles for visible light photodegradation of methyl orange. Mater. Res. Bull. 2016, 77, 70–77. [Google Scholar] [CrossRef]



| Sr. No. | Nanocomposites | Manganese Chloride | Zinc Chloride | Ferric Chloride | S-Doped g-C3N4 |
|---|---|---|---|---|---|
| 1 | Mn-ZnFe2O4 | 0.185 g | 1.706 g | 2.435 g | - |
| 2 | S-g-C3N4 | - | - | - | 0.52 g |
| 3 | 9% Mn-ZnFe2O4/10S-g-C3N4 | 0.185 g | 1.706 g | 2.435 g | 0.17 g |
| 4 | 9% Mn-ZnFe2O4/30S-g-C3N4 | 0.185 g | 1.706 g | 2.435 g | 0.52 g |
| 5 | 9% Mn-ZnFe2O4/50S-g-C3N4 | 0.185 g | 1.706 g | 2.435 g | 0.87 g |
| 6 | 9% Mn-ZnFe2O4/70S-g-C3N4 | 0.185 g | 1.706 g | 2.435 g | 0.94 g |
| Sr. No. | Nanocomposites | S-g-C3N4 (wt.%) | % Degradation | k (min−1) |
|---|---|---|---|---|
| 1 | S-g-C3N4 | 100 | 51 | 0.00515 |
| 2 | ZnFe2O4 | - | 54 | 0.00673 |
| 3 | 9% Mn-ZnFe2O4/10%S-g-C3N4 | 10 | 65 | 0.00721 |
| 4 | 9% Mn-ZnFe2O4/30%S-g-C3N4 | 30 | 89 | 0.00838 |
| 5 | 9% Mn-ZnFe2O4/50% S-g-C3N4 | 50 | 100 | 0.0142 |
| 7 | 9% Mn-ZnFe2O4/70% S-g-C3N4 | 70 | 85 | 0.0096 |
| Sr. No. | Photocatalyst | Contaminant | Light Source | Radiation Time (min.) | Degradation % | Ref. |
|---|---|---|---|---|---|---|
| 1 | ZnFe2O4@metyle cellulose | Metronidazole | Xe lamp | 120 | 92.65 | [49] |
| 2 | Bi2WO6/CoFe2O4 | Bisphenol A | Solar | 120 | 92 | [50] |
| 3 | ZnNdxFe2−xO4 | Rhodamine B | Xe lamp | 180 | 98 | [51] |
| 4 | ZnFe2O4 | Toluene | Xe lamp | 300 | 57.2 | [52] |
| 5 | ZnO/Fe3O4/g-C3N4 | MO | Visible | 150 | 97.87 | [53] |
| 5 | Pt-BiFeO3 | MG | Solar | 240 | 96 | [54] |
| 7 | Mn–ZnO/RGO | RhB | Visible | 140 | 99 | [55] |
| 8 | ZnFe2O4@ZnO | MO | Visible | 240 | 240 | [56] |
| 9 | Mn-ZnFe2O4/S-g-C3N4 | MB | Solar | 120 | 100 | Present Work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, M.; Khalid, W.B.; Iqbal, S.; Qamar, M.A.; Alrbyawi, H.; Awwad, N.S.; Ibrahium, H.A.; Al-Anazy, M.M.; Elkaeed, E.B.; Pashameah, R.A.; et al. Integration of Mn-ZnFe2O4 with S-g-C3N4 for Boosting Spatial Charge Generation and Separation as an Efficient Photocatalyst. Molecules 2022, 27, 6925. https://doi.org/10.3390/molecules27206925
Javed M, Khalid WB, Iqbal S, Qamar MA, Alrbyawi H, Awwad NS, Ibrahium HA, Al-Anazy MM, Elkaeed EB, Pashameah RA, et al. Integration of Mn-ZnFe2O4 with S-g-C3N4 for Boosting Spatial Charge Generation and Separation as an Efficient Photocatalyst. Molecules. 2022; 27(20):6925. https://doi.org/10.3390/molecules27206925
Chicago/Turabian StyleJaved, Mohsin, Waleed Bin Khalid, Shahid Iqbal, Muhammad Azam Qamar, Hamad Alrbyawi, Nasser S. Awwad, Hala A. Ibrahium, Murefah Mana Al-Anazy, Eslam B. Elkaeed, Rami Adel Pashameah, and et al. 2022. "Integration of Mn-ZnFe2O4 with S-g-C3N4 for Boosting Spatial Charge Generation and Separation as an Efficient Photocatalyst" Molecules 27, no. 20: 6925. https://doi.org/10.3390/molecules27206925
APA StyleJaved, M., Khalid, W. B., Iqbal, S., Qamar, M. A., Alrbyawi, H., Awwad, N. S., Ibrahium, H. A., Al-Anazy, M. M., Elkaeed, E. B., Pashameah, R. A., Alzahrani, E., & Farouk, A.-E. (2022). Integration of Mn-ZnFe2O4 with S-g-C3N4 for Boosting Spatial Charge Generation and Separation as an Efficient Photocatalyst. Molecules, 27(20), 6925. https://doi.org/10.3390/molecules27206925










