Chemical and Synthetic Biology Approaches for Cancer Vaccine Development
Abstract
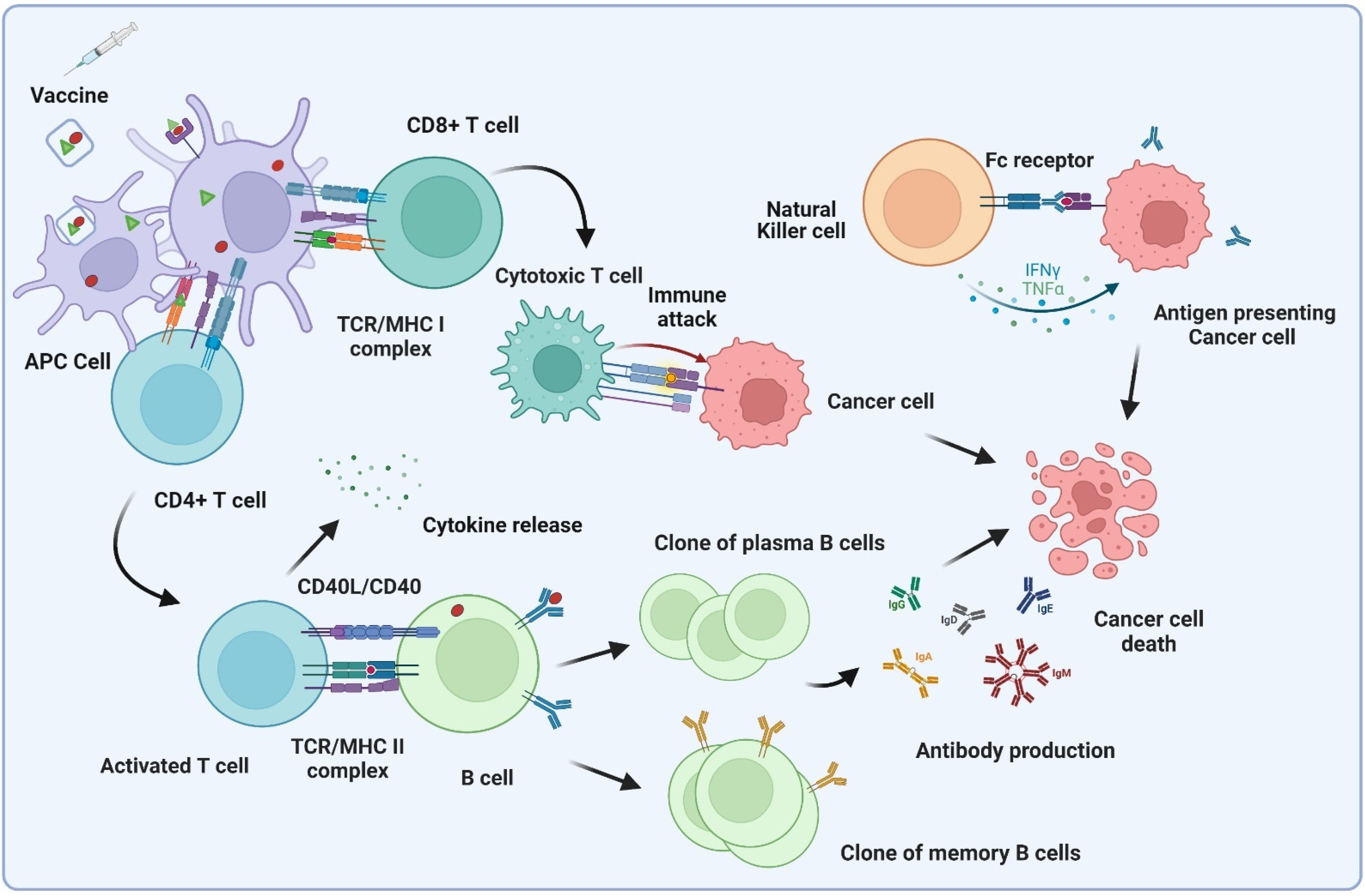
:1. Introduction
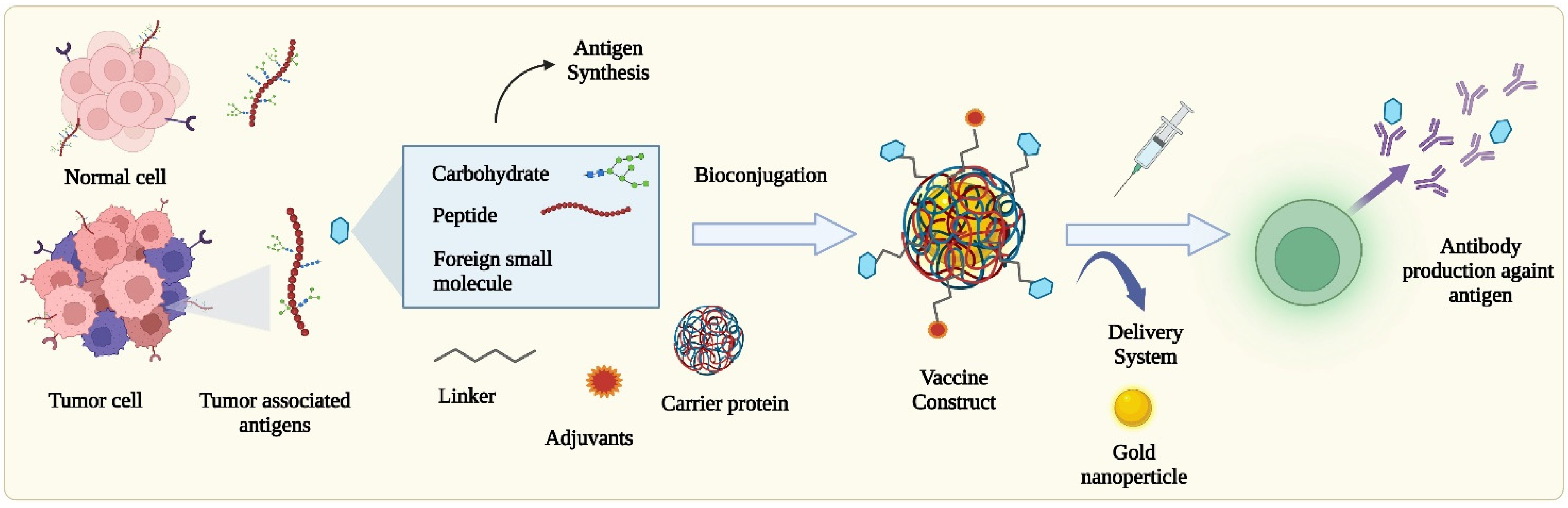
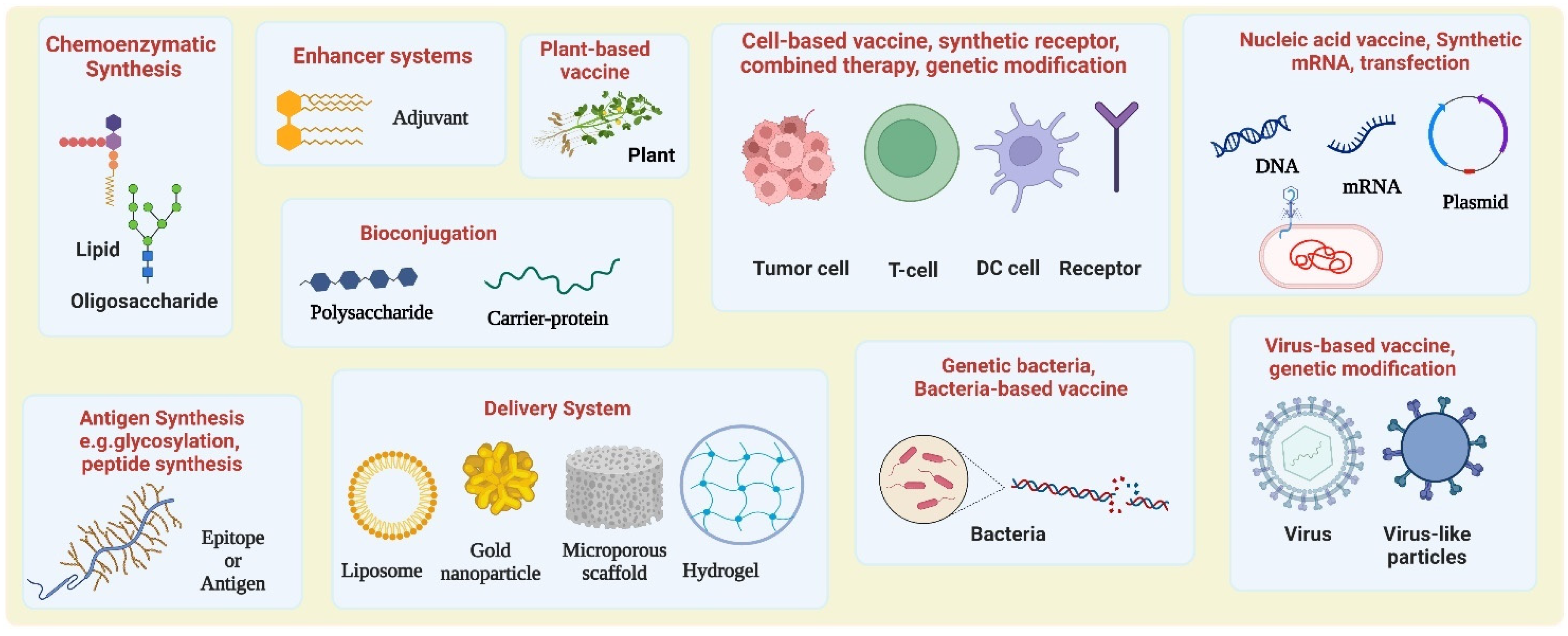
2. Chemical Approaches for the Construction of Tumor Antigens, Vaccines, and Improving Potency
2.1. Carbohydrate Antigens
2.1.1. Glycosylation/Glycal Assembly
2.1.2. Chemoenzymatic Synthesis
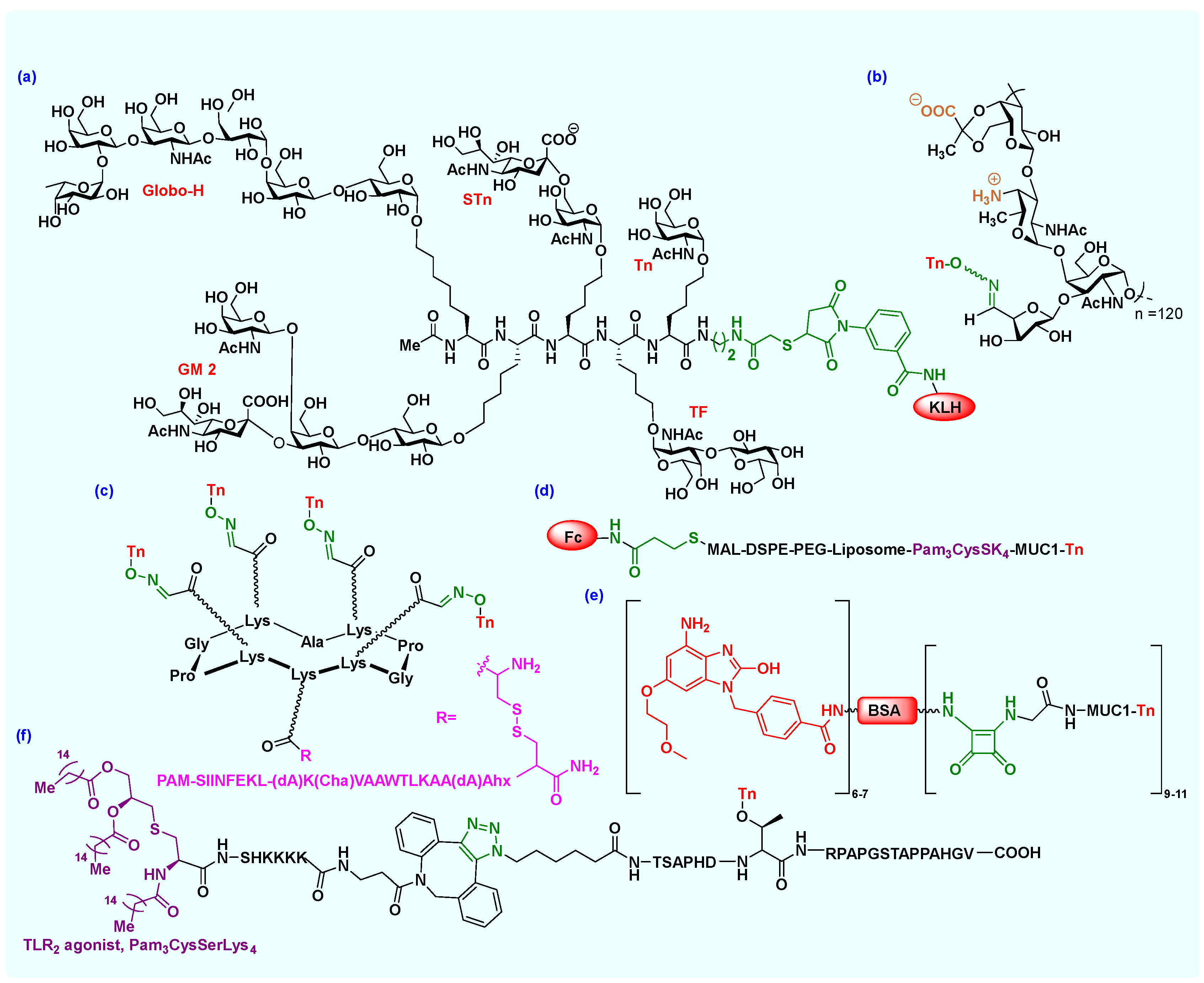
2.2. Carbohydrate-Based Vaccines
Bioconjugation
2.3. Protein/Peptide-Based Epitopes and Vaccines
2.3.1. Peptide Synthesis
2.3.2. Bioconjugation
2.3.3. Click Chemistry
2.4. Immunostimulant Adjuvants
3. Synthetic Biology Approaches for Vaccine Construction and Improving the Potency
3.1. Cell-Based Cancer Vaccine
3.2. Virus-Based Vaccines and Virus-like Particles
3.3. Nucleic Acid-Based Vaccines
3.4. Bacteria and Plant-Based Vaccine
4. Biomaterial Scaffolds as Vaccine Delivery Systems
5. Outlook and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Jones, L.H. Recent Advances in the Molecular Design of Synthetic Vaccines. Nat. Chem. 2015, 7, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Andreana, P.R. Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blass, E.; Ott, P.A. Advances in the Development of Personalized Neoantigen-Based Therapeutic Cancer Vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Li, Y.-M. Chemical Strategies to Boost Cancer Vaccines. ACS Chem. Rev. 2020, 120, 11420–11478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Billingsley, M.M.; Mitchell, M.J. Biomaterials for Vaccine-Based Cancer Immunotherapy. J. Control. Release 2018, 292, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Buskas, T.; Thompson, P.; Boons, G.J. Immunotherapy for Cancer: Synthetic Carbohydrate-Based Vaccines. Chem. Commun. 2009, 36, 5335–5349. [Google Scholar] [CrossRef] [Green Version]
- Feng, D.; Shaikh, A.S.; Wang, F. Recent Advance in Tumor-Associated Carbohydrate Antigens (Tacas)-Based Antitumor Vaccines. ACS Chem. Biol. 2016, 11, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Nativi, C.; Renaudet, O. Recent Progress in Antitumoral Synthetic Vaccines. ACS Med. Chem. Lett. 2014, 5, 1176–1178. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Kleski, K.A.; Trabbic, K.R.; Bourgault, J.-P.; Andreana, P.R. Sialyl-Tn Polysaccharide A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc. 2016, 138, 14264–14272. [Google Scholar] [CrossRef]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.J. Immune Recognition of Tumor-Associated Mucin Muc1 Is Achieved by a Fully Synthetic Aberrantly Glycosylated Muc1 Tripartite Vaccine. Proc. Natl. Acad. Sci. USA 2012, 109, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Andrianantoandro, E.; Basu, S.; Karig, D.K.; Weiss, R. Synthetic Biology: New Engineering Rules for an Emerging Discipline. Mol. Syst. Biol. 2006, 2, 2006-0028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Letendre, J.H.; Collins, J.J.; Wong, W.W. Synthetic Biology in the Clinic: Engineering Vaccines, Diagnostics, and Therapeutics. Cell 2021, 184, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines as Promising Immuno-Therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, W.; Pi, R.; Zhao, X.; Wang, W. Genetically Modified Cancer Vaccines: Current Status and Future Prospects. Med. Res. Rev. 2022, 42, 1492–1517. [Google Scholar] [CrossRef] [PubMed]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef] [PubMed]
- Sabado, R.L.; Bhardwaj, N. Directing Dendritic Cell Immunotherapy Towards Successful Cancer Treatment. Immunotherapy 2010, 2, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-Based Therapeutic Cancer Vaccine: Current Trends in Clinical Application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic Cancer Vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Abudula, T.; Bhatt, K.; Eggermont, L.J.; O’Hare, N.; Memic, A.; Bencherif, S.A. Supramolecular Self-Assembled Peptide-Based Vaccines: Current State and Future Perspectives. Front. Chem. 2020, 8, 598160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Geng, Y.; Yue, B.; Lo, P.C.; Huang, J.; Jin, H. Injectable Hydrogel as a Unique Platform for Antitumor Therapy Targeting Immunosuppressive Tumor Microenvironment. Front. Immunol. 2021, 12, 832942. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human Tumor Antigens Tn and Sialyl Tn Arise from Mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, T.; Mo, K.-F.; Boons, G.-J. Stereoselective Assembly of Complex Oligosaccharides Using Anomeric Sulfonium Ions as Glycosyl Donors. J. Am. Chem. Soc. 2012, 134, 7545–7552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleski, K.A.; Shi, M.; Lohman, M.; Hymel, G.T.; Gattoji, V.K.; Andreana, P.R. Synthesis of an Aminooxy Derivative of the Gm3 Antigen and Its Application in Oxime Ligation. J. Org. Chem. 2020, 85, 16207–16217. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Nishat, S.; Ghosh, S.; Boga, S.; Hymel, G.T.; Andreana, P.R. Synthesis of Glycoimmunogen Tn-Thr-Ps A1 Via Hydrazone Bond and Stability Optimization of Ps A1 Monosaccharide Mimics under Vaccine Development Conditions. J. Carbohydr. Chem. 2020, 39, 107–129. [Google Scholar] [CrossRef]
- Ghosh, S.; Andreana, P.R. Synthesis of an Aminooxy Derivative of the Trisaccharide Globotriose Gb3. J. Carbohydr. Chem. 2014, 33, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Ollmann, I.R.; Ye, X.-S.; Wischnat, R.; Baasov, T.; Wong, C.-H. Programmable One-Pot Oligosaccharide Synthesis. J. Am. Chem. Soc. 1999, 121, 734–753. [Google Scholar] [CrossRef]
- Burkhart, F.; Zhang, Z.; Wacowich-Sgarbi, S.; Wong, C.-H. Synthesis of the Globo H Hexasaccharide Using the Programmable Reactivity-Based One-Pot Strategy. Angew. Chem. Int. Ed. 2001, 40, 1274–1277. [Google Scholar] [CrossRef]
- Huang, C.Y.; Thayer, D.A.; Chang, A.Y.; Best, M.D.; Hoffmann, J.; Head, S.; Wong, C.H. Carbohydrate Microarray for Profiling the Antibodies Interacting with Globo H Tumor Antigen. Proc. Natl. Acad. Sci. USA 2006, 103, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Ye, J.; De Laitsch, A.T.; Rashidijahanabad, Z.; Lang, S.; Kakeshpour, T.; Zhao, Y.; Ramadan, S.; Saavedra, P.V.; Yuzbasiyan-Gurkan, V.; et al. Chemoenzymatic Synthesis of 9nhac-Gd2 Antigen to Overcome the Hydrolytic Instability of O-Acetylated-Gd2 for Anticancer Conjugate Vaccine Development. Angew. Chem. Int. Ed. 2021, 60, 24179–24188. [Google Scholar] [CrossRef]
- Galonić, D.P.; Gin, D.Y. Chemical Glycosylation in the Synthesis of Glycoconjugate Antitumour Vaccines. Nature 2007, 446, 1000–1007. [Google Scholar] [CrossRef]
- Yu, H.; Santra, A.; Li, Y.; McArthur, J.B.; Ghosh, T.; Yang, X.; Wang, P.G.; Chen, X. Streamlined Chemoenzymatic Total Synthesis of Prioritized Ganglioside Cancer Antigens. Org. Biomol. Chem. 2018, 16, 4076–4080. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, H.; Karpel, R.; Chen, X. Chemoenzymatic Synthesis of Cmp-Sialic Acid Derivatives by a One-Pot Two-Enzyme System: Comparison of Substrate Flexibility of Three Microbial Cmp-Sialic Acid Synthetases. Bioorg. Med. Chem. 2004, 12, 6427–6435. [Google Scholar] [CrossRef] [PubMed]
- Sorieul, C.; Papi, F.; Carboni, F.; Pecetta, S.; Phogat, S.; Adamo, R. Recent Advances and Future Perspectives on Carbohydrate-Based Cancer Vaccines and Therapeutics. Pharmacol. Ther. 2022, 235, 108158. [Google Scholar] [CrossRef]
- Xiong, A.W.; Fang, J.M.; Ren, S.X.; Li, W.; Wang, J.; Zhao, Y.; Chen, G.Y.; Xu, Q.; Zhou, C.C. A Novel Combined Conjugate Therapeutic Cancer Vaccine, Recombinant Egf-Crm197, in Patients with Advanced Solid Tumors: A Phase I Clinical Study. Front. Oncol. 2021, 11, 745699. [Google Scholar] [CrossRef] [PubMed]
- Danishefsky, S.J.; Allen, J.R. From the Laboratory to the Clinic: A Retrospective on Fully Synthetic Carbohydrate-Based Anticancer Vaccines Frequently Used Abbreviations Are Listed in the Appendix. Angew. Chem. Int. Ed. 2000, 39, 836–863. [Google Scholar] [CrossRef]
- Ragupathi, G.; Koide, F.; Livingston, P.O.; Cho, Y.S.; Endo, A.; Wan, Q.; Spassova, M.K.; Keding, S.J.; Allen, J.; Ouerfelli, O.; et al. Preparation and Evaluation of Unimolecular Pentavalent and Hexavalent Antigenic Constructs Targeting Prostate and Breast Cancer: A Synthetic Route to Anticancer Vaccine Candidates. J. Am. Chem. Soc. 2006, 128, 2715–2725. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Hung, J.-T.; Cheung, S.K.C.; Lee, H.-Y.; Chu, K.-C.; Li, S.-T.; Lin, Y.-C.; Ren, C.-T.; Cheng, T.-J.R.; Hsu, T.-L.; et al. Carbohydrate-Based Vaccines with a Glycolipid Adjuvant for Breast Cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 2517–2522. [Google Scholar] [CrossRef] [Green Version]
- Hevey, R.; Ling, C.C. Recent Advances in Developing Synthetic Carbohydrate-Based Vaccines for Cancer Immunotherapies. Futur. Med. Chem. 2012, 4, 545–584. [Google Scholar] [CrossRef]
- Wang, Q.; McLoughlin, R.M.; Cobb, B.A.; Charrel-Dennis, M.; Zaleski, K.J.; Golenbock, D.; Tzianabos, A.O.; Kasper, D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-Like Receptor 2. J. Exp. Med. 2006, 203, 2853–2863. [Google Scholar] [CrossRef] [Green Version]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef]
- Ghosh, S.; Trabbic, K.R.; Shi, M.; Nishat, S.; Eradi, P.; Kleski, K.A.; Andreana, P.R. Chemical Synthesis and Immunological Evaluation of Entirely Carbohydrate Conjugate Globo H-Ps A1. Chem. Sci. 2020, 11, 13052–13059. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.J.; Burgess-Brown, N.A.; Jiang, S. Beyond Just Peptide Antigens: The Complex World of Peptide-Based Cancer Vaccines. Front. Immunol. 2021, 12, 696791. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Patronov, A.; Doytchinova, I. T-Cell Epitope Vaccine Design by Immunoinformatics. Open Biol. 2013, 3, 120139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfert, M.A.; Boons, G.-J. Adaptive Immune Activation: Glycosylation Does Matter. Nat. Chem. Biol. 2013, 9, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Apostolopoulos, V.; Yu, M.; Corper, A.L.; Teyton, L.; Pietersz, G.A.; McKenzie, I.F.; Wilson, I.A.; Plebanski, M. Crystal Structure of a Non-Canonical Low-Affinity Peptide Complexed with Mhc Class I: A New Approach for Vaccine Design. J. Mol. Biol. 2002, 318, 1293–1305. [Google Scholar] [CrossRef]
- Hossain, M.K.; Wall, K.A. Immunological Evaluation of Recent Muc1 Glycopeptide Cancer Vaccines. Vaccines 2016, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Supekar, N.T.; Lakshminarayanan, V.; Capicciotti, C.J.; Sirohiwal, A.; Madsen, C.S.; Wolfert, M.A.; Cohen, P.A.; Gendler, S.J.; Boons, G.J. Synthesis and Immunological Evaluation of a Multicomponent Cancer Vaccine Candidate Containing a Long Muc1 Glycopeptide. Chembiochem 2018, 19, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, C.; Deriaud, E.; Mimic, V.; van der Werf, S. Identification of a T-Cell Epitope Adjacent to Neutralization Antigenic Site 1 of Poliovirus Type 1. J. Virol. 1991, 65, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kutubuddin, M.; Simons, J.; Chow, M. Poliovirus-Specific Major Histocompatibility Complex Class I-Restricted Cytolytic T-Cell Epitopes in Mice Localize to Neutralizing Antigenic Regions. J. Virol. 1992, 66, 5967–5974. [Google Scholar] [CrossRef]
- Onodi, F.; Maherzi-Mechalikh, C.; Mougel, A.; Ben Hamouda, N.; Taboas, C.; Gueugnon, F.; Tran, T.; Nozach, H.; Marcon, E.; Gey, A.; et al. High Therapeutic Efficacy of a New Survivin Lsp-Cancer Vaccine Containing Cd4+ and Cd8+ T-Cell Epitopes. Front. Oncol. 2018, 8, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, Y.; Sunazuka, T.; Nagai, K.; Hirose, T.; Namatame, M.; Ishiyama, A.; Otoguro, K.; Ōmura, S. Solid-Phase Synthesis and Biological Activity of Malformin C and Its Derivatives. J. Antibiot. 2009, 62, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Wu, J.-C.; Yu, H.-M.; Hsu, H.-T.; Wu, Y.-T.; Yu, A.L.-T.; Yu, C.-D.T.; Wong, C.-H. Design and Synthesis of Glyco-Peptides as Anti-Cancer Agents Targeting Thrombin-Protease Activated Receptor-1 Interaction. Chem. Commun. 2020, 56, 5827–5830. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.; Nishat, S.; Andreana, P.R. Synthesis of Malformin-A1, C, a Glycan, and an Aglycon Analog: Potential Scaffolds for Targeted Cancer Therapy. Pept. Sci. 2022, 114, e24260. [Google Scholar] [CrossRef]
- McDermott, J.R.; Benoiton, N.L. N-Methylamino Acids in Peptide Synthesis. Iii. Racemization During Deprotection by Saponification and Acidolysis. Can. J. Chem. 1973, 51, 2555–2561. [Google Scholar] [CrossRef]
- Mandal, P.K.; McMurray, J.S. Pd−C-Induced Catalytic Transfer Hydrogenation with Triethylsilane. J. Org. Chem. 2007, 72, 6599–6601. [Google Scholar] [CrossRef] [PubMed]
- Sungsuwan, S.; Yin, Z.; Huang, X. Lipopeptide-Coated Iron Oxide Nanoparticles as Potential Glycoconjugate-Based Synthetic Anticancer Vaccines. ACS Appl. Mater. Interfaces 2015, 7, 17535–17544. [Google Scholar] [CrossRef] [Green Version]
- Renaudet, O.; BenMohamed, L.; Dasgupta, G.; Bettahi, I.; Dumy, P. Towards a Self-Adjuvanting Multivalent B and T Cell Epitope Containing Synthetic Glycolipopeptide Cancer Vaccine. ChemMedChem 2008, 3, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Vartak, A.; Sucheck, S.J.; Wall, K.A. Liposomal Fc Domain Conjugated to a Cancer Vaccine Enhances Both Humoral and Cellular Immunity. ACS Omega 2019, 4, 5204–5208. [Google Scholar] [CrossRef] [PubMed]
- Du, J.-J.; Wang, C.-W.; Xu, W.-B.; Zhang, L.; Tang, Y.-K.; Zhou, S.-H.; Gao, X.-F.; Yang, G.-F.; Guo, J. Multifunctional Protein Conjugates with Built-in Adjuvant (Adjuvant-Protein-Antigen) as Cancer Vaccines Boost Potent Immune Responses. iScience 2020, 23, 100935. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Hossain, M.K.; Vartak, A.; Karmakar, P.; Sucheck, S.J.; Wall, K.A. Augmenting Vaccine Immunogenicity through the Use of Natural Human Anti-Rhamnose Antibodies. ACS Chem. Biol. 2018, 13, 2130–2142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.N.; Delaveris, C.S.; Kramer, J.R.; Kenkel, J.A.; Engleman, E.G.; Bertozzi, C.R. N-Carboxyanhydride Polymerization of Glycopolypeptides That Activate Antigen-Presenting Cells through Dectin-1 and Dectin-2. Angew. Chem. Int. Ed. Engl. 2018, 57, 3137–3142. [Google Scholar] [CrossRef] [PubMed]
- Beal, D.M.; Albrow, V.E.; Burslem, G.; Hitchen, L.; Fernandes, C.; Lapthorn, C.; Roberts, L.R.; Selby, M.D.; Jones, L.H. Click-Enabled Heterotrifunctional Template for Sequential Bioconjugations. Org. Biomol. Chem. 2012, 10, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Wang, P.; Navarro-Villalobos, M.; Rohde, B.D.; DerryBerry, J.; Gin, D.Y. Synthetic Studies of Complex Immunostimulants from Quillaja Saponaria: Synthesis of the Potent Clinical Immunoadjuvant Qs-21aapi. J. Am. Chem. Soc. 2006, 128, 11906–11915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khong, H.; Overwijk, W.W. Adjuvants for Peptide-Based Cancer Vaccines. J. Immunother. Cancer 2016, 4, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.L.; Williams, G.M.; Verdon, D.J.; Dunbar, P.R.; Brimble, M.A. Synthesis and Evaluation of Novel Tlr2 Agonists as Potential Adjuvants for Cancer Vaccines. J. Med. Chem. 2020, 63, 2282–2291. [Google Scholar] [CrossRef]
- Kensil, C.R.; Patel, U.; Lennick, M.; Marciani, D. Separation and Characterization of Saponins with Adjuvant Activity from Quillaja Saponaria Molina Cortex. J. Immunol. 1991, 146, 431–437. [Google Scholar]
- Wright, T.H.; Brooks, A.E.; Didsbury, A.J.; MacIntosh, J.D.; Williams, G.M.; Harris, P.W.; Dunbar, P.R.; Brimble, M.A. Direct Peptide Lipidation through Thiol-Ene Coupling Enables Rapid Synthesis and Evaluation of Self-Adjuvanting Vaccine Candidates. Angew. Chem. Int. Ed. Engl. 2013, 52, 10616–10619. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, G.; Crall, B.M.; Lewis, T.C.; Day, T.P.; Balakrishna, R.; Warshakoon, H.J.; Malladi, S.S.; David, S.A. Structure–Activity Relationships in Toll-Like Receptor 2-Agonists Leading to Simplified Monoacyl Lipopeptides. J. Med. Chem. 2011, 54, 8148–8160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.L.; Benencia, F.; Coukos, G. Whole Tumor Antigen Vaccines. Semin. Immunol. 2010, 22, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Yamano, T.; Kubo, S.; Fukumoto, M.; Yano, A.; Mawatari-Furukawa, Y.; Okamura, H.; Tomita, N. Whole Cell Vaccination Using Immunogenic Cell Death by an Oncolytic Adenovirus Is Effective against a Colorectal Cancer Model. Mol. Ther. Oncolytics 2016, 3, 16031. [Google Scholar] [CrossRef]
- Gamrekelashvili, J.; Greten, T.F.; Korangy, F. Immunogenicity of Necrotic Cell Death. Cell. Mol. Life Sci. 2015, 72, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Wang-Bishop, L.; Wehbe, M.; Shae, D.; James, J.; Hacker, B.C.; Garland, K.; Chistov, P.P.; Rafat, M.; Balko, J.M.; Wilson, J.T. Potent Sting Activation Stimulates Immunogenic Cell Death to Enhance Antitumor Immunity in Neuroblastoma. J. Immunother. Cancer 2020, 8, e000282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thi, V.A.D.; Jeon, H.M.; Park, S.M.; Lee, H.; Kim, Y.S. Cell-Based Il-15:Il-15rα Secreting Vaccine as an Effective Therapy for Ct26 Colon Cancer in Mice. Mol. Cells 2019, 42, 869–883. [Google Scholar] [PubMed]
- Gardner, T.A.; Elzey, B.D.; Hahn, N.M. Sipuleucel-T (Provenge) Autologous Vaccine Approved for Treatment of Men with Asymptomatic or Minimally Symptomatic Castrate-Resistant Metastatic Prostate Cancer. Hum. Vaccines Immunother. 2012, 8, 534–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filin, I.Y.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Recent Advances in Experimental Dendritic Cell Vaccines for Cancer. Front. Oncol. 2021, 11, 730824. [Google Scholar] [CrossRef] [PubMed]
- Jansen, Y.; Kruse, V.; Corthals, J.; Schats, K.; van Dam, P.J.; Seremet, T.; Heirman, C.; Brochez, L.; Kockx, M.; Thielemans, K.; et al. A Randomized Controlled Phase Ii Clinical Trial on Mrna Electroporated Autologous Monocyte-Derived Dendritic Cells (Trimixdc-Mel) as Adjuvant Treatment for Stage Iii/Iv Melanoma Patients Who Are Disease-Free Following the Resection of Macrometastases. Cancer Immunol. Immunother. 2020, 69, 2589–2598. [Google Scholar] [CrossRef]
- Korell, F.; Laier, S.; Sauer, S.; Veelken, K.; Hennemann, H.; Schubert, M.L.; Sauer, T.; Pavel, P.; Mueller-Tidow, C.; Dreger, P.; et al. Current Challenges in Providing Good Leukapheresis Products for Manufacturing of Car-T Cells for Patients with Relapsed/Refractory Nhl or All. Cells 2020, 9, 1225. [Google Scholar] [CrossRef]
- Ramachandran, M.; Dimberg, A.; Essand, M. The Cancer-Immunity Cycle as Rational Design for Synthetic Cancer Drugs: Novel Dc Vaccines and Car T-Cells. Semin. Cancer Biol. 2017, 45, 23–35. [Google Scholar] [CrossRef]
- Goto, T. Radiation as an in Situ Auto-Vaccination: Current Perspectives and Challenges. Vaccines 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.K.; Tan, W.S.; Ho, K.L. Virus Like Particles as a Platform for Cancer Vaccine Development. PeerJ 2017, 5, e4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J. Adenovirus Vectors: Excellent Tools for Vaccine Development. Immune Netw. 2021, 21, e6. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical Use of Lentiviral Vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Liu, J.; Zhong, J.F.; Zhang, X. Engineering Car-T Cells. Biomark. Res. 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.M.; Kim, M.C.; Vartabedian, V.F.; Lee, Y.N.; He, S.; Song, J.M.; Choi, H.J.; Yamanaka, S.; Amaram, N.; Lukacher, A.; et al. Protein Transfer-Mediated Surface Engineering to Adjuvantate Virus-Like Nanoparticles for Enhanced Anti-Viral Immune Responses. Nanomedicine 2015, 11, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Jahanafrooz, Z.; Baradaran, B.; Mosafer, J.; Hashemzaei, M.; Rezaei, T.; Mokhtarzadeh, A.; Hamblin, M.R. Comparison of DNA and Mrna Vaccines against Cancer. Drug Discov. Today 2020, 25, 552–560. [Google Scholar] [CrossRef]
- Lopes, A.; Bastiancich, C.; Bausart, M.; Ligot, S.; Lambricht, L.; Vanvarenberg, K.; Ucakar, B.; Gallez, B.; Préat, V.; Vandermeulen, G. New Generation of DNA-Based Immunotherapy Induces a Potent Immune Response and Increases the Survival in Different Tumor Models. J. Immunother. Cancer 2021, 9, e001243. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Huang, L. Mrna Vaccine for Cancer Immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA Vaccines: Current Preclinical and Clinical Developments and Future Perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Reinhard, K.; Rengstl, B.; Oehm, P.; Michel, K.; Billmeier, A.; Hayduk, N.; Klein, O.; Kuna, K.; Ouchan, Y.; Wöll, S.; et al. An Rna Vaccine Drives Expansion and Efficacy of Claudin-Car-T Cells against Solid Tumors. Science 2020, 367, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kandalai, S.; Hossain, F.; Zheng, Q. Tumor Microbiome Metabolism: A Game Changer in Cancer Development and Therapy. Front. Oncol. 2022, 12, 933407. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-Targeting Bacteria Engineered to Fight Cancer. Nat. Rev. Cancer 2018, 18, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Cheng, Y.; Liu, W.; Luo, W.; Zhou, P.; Qian, D. Bacteria-Based Synergistic Therapy in the Backdrop of Synthetic Biology. Front. Oncol. 2022, 12, 845346. [Google Scholar] [CrossRef] [PubMed]
- Sfakianos, J.P.; Salome, B.; Daza, J.; Farkas, A.; Bhardwaj, N.; Horowitz, A. Bacillus Calmette-Guerin (Bcg): Its Fight against Pathogens and Cancer. Urol. Oncol. 2021, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Chen, J.; Yao, H.; Wang, Y.; Jia, Y.; Ling, Z.; Feng, Y.; Pan, Z.; Yin, Y.; Jiao, X. Enhanced Therapeutic Efficacy of Listeria-Based Cancer Vaccine with Codon-Optimized Hpv16 E7. Hum. Vaccin. Immunother. 2021, 17, 1568–1577. [Google Scholar] [CrossRef]
- Niethammer, A.G.; Lubenau, H.; Mikus, G.; Knebel, P.; Hohmann, N.; Leowardi, C.; Beckhove, P.; Akhisaroglu, M.; Ge, Y.; Springer, M.; et al. Double-Blind, Placebo-Controlled First in Human Study to Investigate an Oral Vaccine Aimed to Elicit an Immune Reaction against the Vegf-Receptor 2 in Patients with Stage Iv and Locally Advanced Pancreatic Cancer. BMC Cancer 2012, 12, 361. [Google Scholar] [CrossRef] [Green Version]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2021, 12, 805695. [Google Scholar] [CrossRef]
- Hu, Q.; Wu, M.; Fang, C.; Cheng, C.; Zhao, M.; Fang, W.; Chu, P.K.; Ping, Y.; Tang, G. Engineering Nanoparticle-Coated Bacteria as Oral DNA Vaccines for Cancer Immunotherapy. Nano Lett. 2015, 15, 2732–2739. [Google Scholar] [CrossRef]
- Chowdhury, S.; Castro, S.; Coker, C.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Programmable Bacteria Induce Durable Tumor Regression and Systemic Antitumor Immunity. Nat. Med. 2019, 25, 1057–1063. [Google Scholar] [CrossRef]
- Wang, C.Z.; Kazmierczak, R.A.; Eisenstark, A. Strains, Mechanism, and Perspective: Salmonella-Based Cancer Therapy. Int. J. Microbiol. 2016, 2016, 5678702. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Hahn-Löbmann, S.; Stephan, A.; Schulz, S.; Giritch, A.; Naumann, M.; Kleinschmidt, M.; Tusé, D.; Gleba, Y. Plant-Made Salmonella bacteriocins Salmocins for Control of Salmonella Pathovars. Sci. Rep. 2018, 8, 4078. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, N.; Miraei, H.R.; Amiri, A.; Ebrahimi, M.S.; Nahand, J.S.; Tarrahimofrad, H.; Hamblin, M.R.; Khan, H.; Mirzaei, H. Plant-Based Vaccines and Cancer Therapy: Where Are We Now and Where Are We Going? Pharmacol. Res. 2021, 169, 105655. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ko, K. Production of Recombinant Anti-Cancer Vaccines in Plants. Biomol. Ther. 2017, 25, 345–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabbic, K.R.; Kleski, K.A.; Barchi, J.J. Stable Gold-Nanoparticle-Based Vaccine for the Targeted Delivery of Tumor-Associated Glycopeptide Antigens. ACS Bio Med Chem Au 2021, 1, 31–43. [Google Scholar] [CrossRef]
- Li, J.; Luo, Y.; Li, B.; Xia, Y.; Wang, H.; Fu, C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020, 8, 612950. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, P.; Huang, P.; Zhang, C.; Kong, D.; Wang, W. Injectable Polypeptide Hydrogel-Based Co-Delivery of Vaccine and Immune Checkpoint Inhibitors Improves Tumor Immunotherapy. Theranostics 2019, 9, 2299–2314. [Google Scholar] [CrossRef]

| NCT Number (Status) | Antigens or Biological | Cancer Type | Clinical Phase |
|---|---|---|---|
| NCT00003871 (Completed) | fowlpox virus, recombinant vaccinia prostate-specific antigen | Prostate cancer | II |
| NCT00128661 (Completed) | human papillomavirus 16/18 L1 virus-like particle/AS04, hepatitis A inactivated virus vaccine | Cervical cancer (pre-cancerous) | III |
| NCT01031719 (Completed) | adjuvanted A (H1N1) influenza vaccine, non-adjuvanted A (H1N1) influenza vaccine | Invasive solid tumors | III |
| NCT01263327 (Completed) | HPV 16/18 | Cervical cancer | I |
| NCT00579423 (Completed) | Globo H, Lewisy and GM2, and glycosylated MUC-1, Tn, and TF | Prostate cancer | II |
| NCT01248273 (Completed) | Globo-H-GM2-sTn-TF-Tn-KLH conjugate & QS-21 | Ovarian cancer, Peritoneal cancer | I |
| NCT01141491 (Completed) | GM2, GD2, GD3 & OPT-821 | Sarcoma | II |
| NCT00911560 (Active, not recruiting) | GD2L and GD3L conjugated to KLH & OPT-821. Oral β-glucan | Neuroblastoma | I/II |
| NCT00854789 (Completed) | E75+GM-CSF | Breast cancer | I |
| NCT00892567 (Completed) | Her-2/neu, CEA& CTA | Breast cancer | I |
| NCT05013216 (Recruiting) | KRAS Peptide | Pancreatic cancer | I |
| NCT02019524 (Completed) | E39 & J65 peptide | Breast cancer, Ovarian cancer | I |
| NCT04024800 (Active, not recruiting) | AE37 peptide, Pembrolizumab | Triple-negative breast cancer | II |
| NCT01789099 (Active, not recruiting) | UV1 synthetic peptide vaccine, GM-CSF | Non-small cell lung cancer | I/II |
| NCT04270149 (Recruiting) | ESR1 peptide vaccine | Breast cancer | I |
| NCT05479045 (Not yet recruiting) | NY-ESO-1 peptide vaccine | Ovarian cancer | II |
| NCT00681252 (Completed) | URLC10, VEGFR1, VEGFR2 | Gastric cancer | I/II |
| NCT00681330 (Completed) | URC10, TTK, KOC1 | Esophageal cancer | I/II |
| NCT00655785 (Completed) | VEGFR1-1084, VEGFR2-169 | Pancreatic cancer | I/II |
| NCT00433745 (Completed) | WT1 peptide vaccine | Acute myeloid leukemia (AML), Chronic myeloid leukemia (CML) | II |
| NCT01232712 (Completed) | ImMucin, hGM-CSF | Multiple myeloma | I/II |
| NCT00254397 (Completed) | GP100: 209-217(210M), MAGE-3 peptide | Melanoma | II |
| NCT00499577 (Completed) | CMV pp65, hTERT I540/R572Y/D988Y multi-peptide, pneumococcal polyvalent vaccine, survivin (Sur1M2) vaccine | Multiple myeloma, Plasma cell neoplasm | I/II |
| NCT00019929 (Completed) | mutant p53 peptide-pulsed dendritic cell vaccine | Lung cancer | II |
| NCT00299728 (Completed) | NY-ESO-1 protein | Various cancers | I |
| NCT01522820 (Completed) | DEC-205/NY-ESO-1 Fusion protein CDX-1401 | Various cancers | I |
| NCT00705835 (Completed) | rsPSMA protein & Alhydrogel vaccine | Prostate cancer | I |
| NCT00503568 (Completed) | Ad100-gp96Ig-HLA A1 | Lung cancer | I |
| NCT00072085 (Completed) | Aldesleukin, gp100 antigen, incomplete Freund’s adjuvant | Melanoma | II |
| NCT00142454 (Completed) | NY-ESO-1 protein & Imiquimod | Melanoma (malignant) | I |
| NCT04521764 (Recruiting) | Oncolytic Measles Virus encoding Helicobacter pylori neutrophil-activating protein | Breast cancer, stage IV | I |
| NCT00343109 (Completed) | HER-2/neu | Breast cancer, stages III and IV | II |
| NCT00204516 (Completed) | mRNA coding for melanoma-associated antigens & GM-CSF | Malignant melanoma | I/II |
| NCT04847050 (Recruiting) | mRNA-1273 | Solid tumor malignancy, Leukemia, Lymphoma, Multiple myeloma | II |
| NCT03897881 (Active, not recruiting) | mRNA-4157 & Pembrolizumab | Melanoma | II |
| NCT01995708 (Completed) | CT7, MAGE-A3, and WT1 mRNA-electroporated Langerhans cells | Multiple myeloma | I |
| NCT04573140 (Recruiting) | Autologous total tumor mRNA and pp65 full length lysosomal associated membrane protein (LAMP), mRNA loaded DOTAP liposome vaccine administered intravenously (RNA loaded lipid particles, RNA-LPs) | Glioblastoma | I |
| NCT03688178 (Recruiting) | Human CMV pp65-LAMP mRNA-pulsed autologous DCs | Glioblastoma | II |
| NCT00807781 (Completed) | Mammaglobin-A DNA vaccine | Metastatic breast cancer | I |
| NCT02348320 (Completed) | Personalized polyepitope DNA vaccine | Breast cancer | I |
| NCT00859729 (Completed) | pVAXrcPSAv53l (DNA encoding rhesus PSA) | Prostate cancer | I/II |
| NCT01706458 (Completed) | Sipuleucel-T | Prostate cancer | II |
| NCT02018458 (Completed) | LA TNBC; ER+/HER2-BC | Breast cancer | I/II |
| NCT04348747 (Recruiting) | Anti-HER2/HER3 DC vaccine, Pembrolizumab | Breast cancer, stage IV | II |
| NCT02061423 (Active, not recruiting) | HER-2 pulsed DC vaccine | Breast cancer | I |
| NCT01730118 (Completed) | AdHER2/neu DC vaccine | Breast cancer, Adenocarcinomas, Metastatic solid tumors | I |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, F.; Kandalai, S.; Zhou, X.; Zhang, N.; Zheng, Q. Chemical and Synthetic Biology Approaches for Cancer Vaccine Development. Molecules 2022, 27, 6933. https://doi.org/10.3390/molecules27206933
Hossain F, Kandalai S, Zhou X, Zhang N, Zheng Q. Chemical and Synthetic Biology Approaches for Cancer Vaccine Development. Molecules. 2022; 27(20):6933. https://doi.org/10.3390/molecules27206933
Chicago/Turabian StyleHossain, Farzana, Shruthi Kandalai, Xiaozhuang Zhou, Nan Zhang, and Qingfei Zheng. 2022. "Chemical and Synthetic Biology Approaches for Cancer Vaccine Development" Molecules 27, no. 20: 6933. https://doi.org/10.3390/molecules27206933
APA StyleHossain, F., Kandalai, S., Zhou, X., Zhang, N., & Zheng, Q. (2022). Chemical and Synthetic Biology Approaches for Cancer Vaccine Development. Molecules, 27(20), 6933. https://doi.org/10.3390/molecules27206933







