Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple
Abstract
:1. Introduction
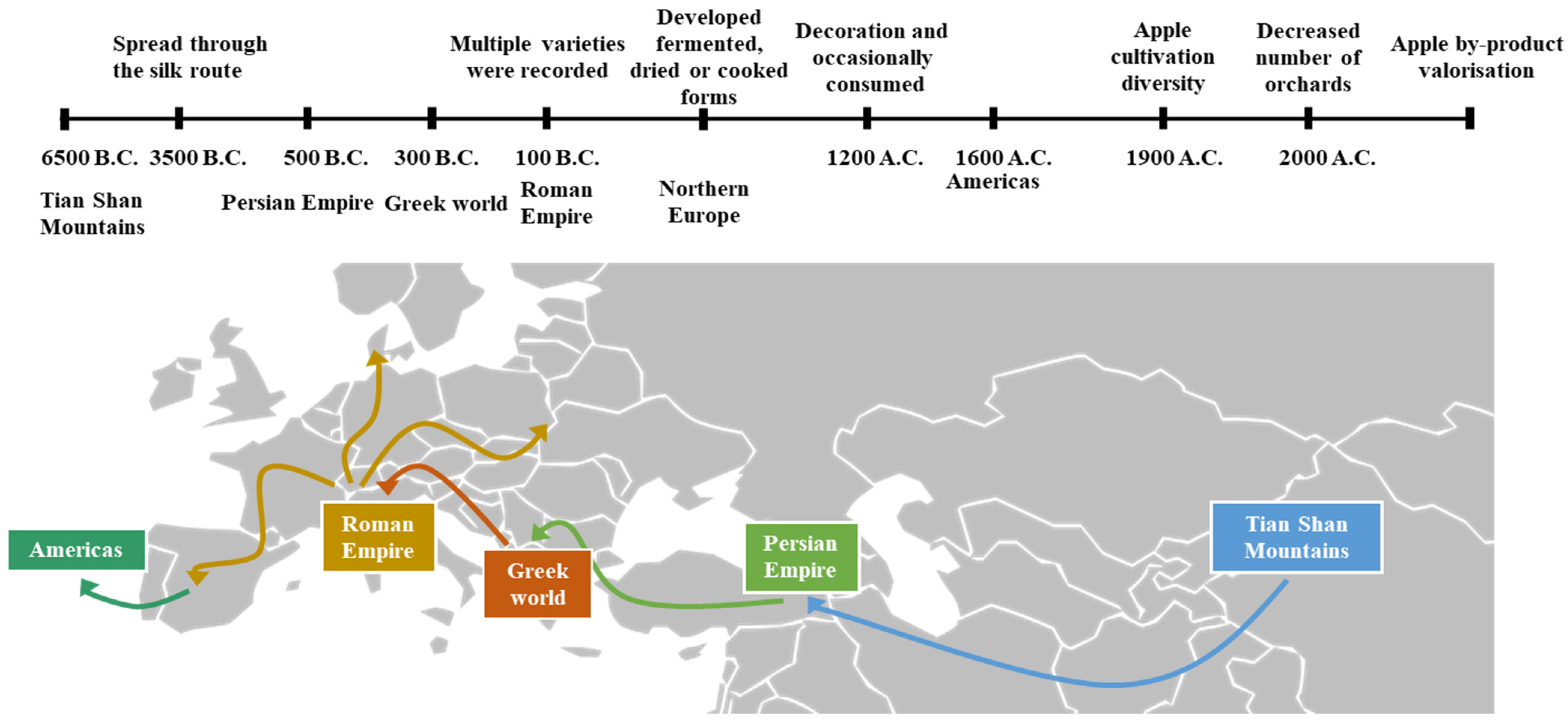
2. History of the Apple
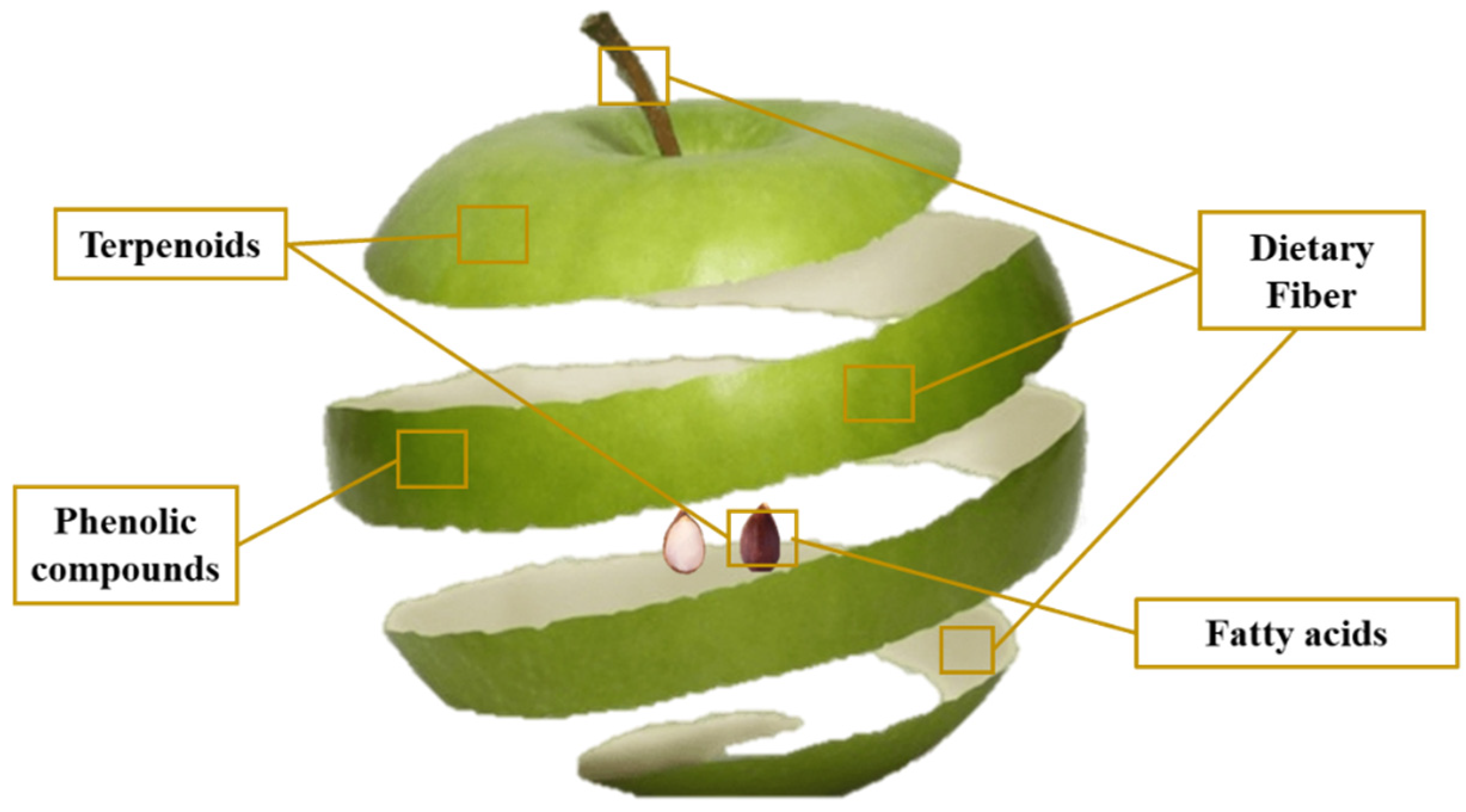
3. Apple By-Product
4. Valorisation Procedures
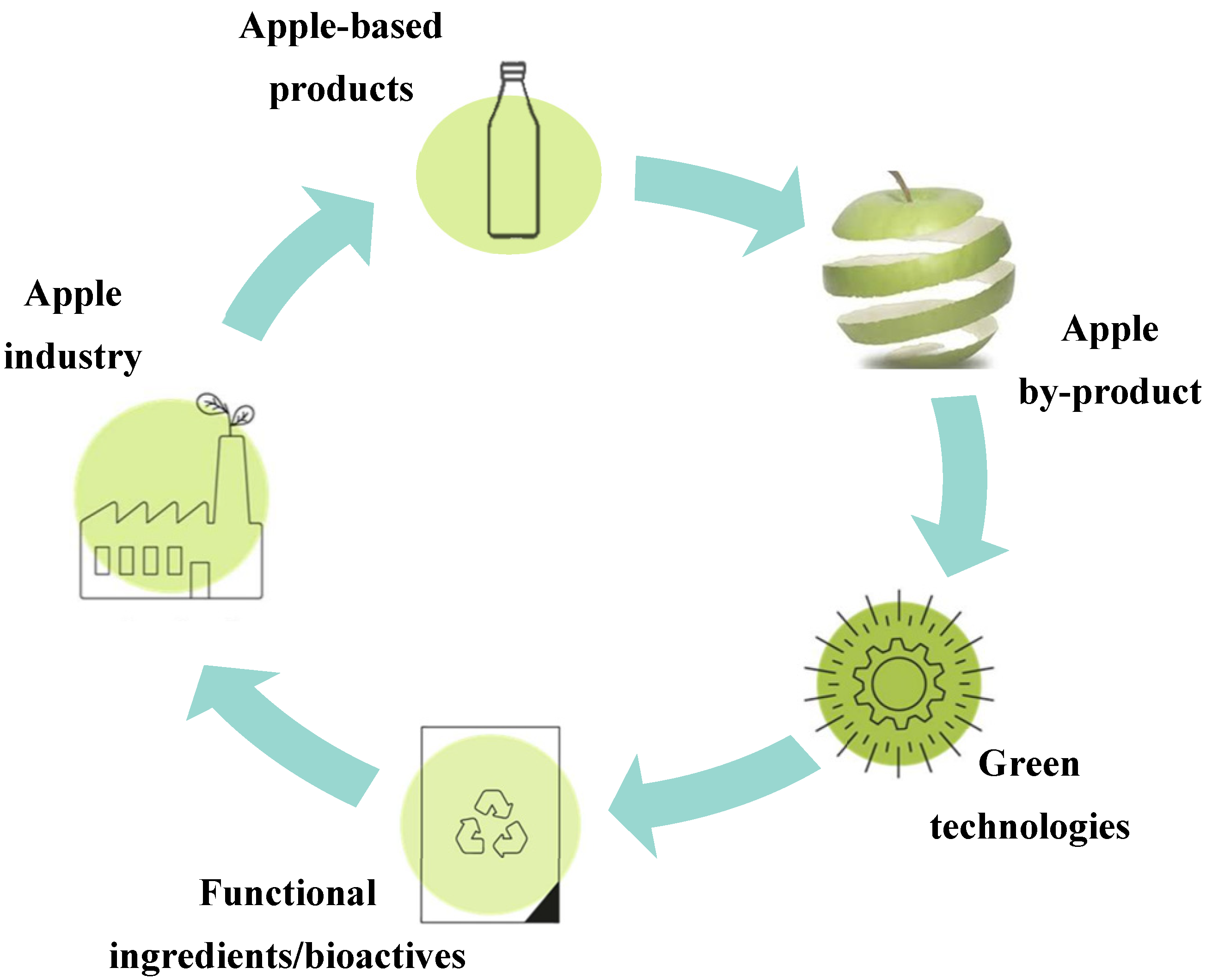
5. Innovation for Apple By-Product Valorisation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2016. [Google Scholar]
- United Nations. Sustainable Development Goals; United Nations: New York, NY, USA, 2015. [Google Scholar]
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High Value-Added Compounds from Fruit and Vegetable by-Products–Characterization, Bioactivities, and Application in the Development of Novel Food Products. Crit. Rev. Food. Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Aschemann-Witzel, J. Waste Not, Want Not, Emit Less. Science 2016, 352, 408–409. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Foof Losses and Food Waste—Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies, Industrial Techniques, and Applications; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food By-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef] [Green Version]
- Campos, D.A.; Ricardo, G.; Vilas-boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.; Bhushan, B. An Overview of the Recent Trends on the Waste Valorization Techniques for Food Wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple Fruit Quality: Overview on Pre-Harvest Factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 2019. [Google Scholar]
- Cornille, A.; Antolín, F.; Garcia, E.; Vernesi, C.; Fietta, A.; Brinkkemper, O.; Kirleis, W.; Schlumbaum, A.; Roldán-Ruiz, I. A Multifaceted Overview of Apple Tree Domestication. Trends Plant Sci. 2019, 24, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of Bioactive Compounds in Fruit Pomace from Agro-Fruit Industries: Present Insights and Future Challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Cornille, A.; Giraud, T.; Smulders, M.J.M.; Roldán-Ruiz, I.; Gladieux, P. The Domestication and Evolutionary Ecology of Apples. Trends Genet. 2014, 30, 57–65. [Google Scholar] [CrossRef]
- Ferre, D.C.; Warrington, I.J. (Eds.) Apples Botany, Production and Uses; CABI: Cambrigde, MA, USA, 2003. [Google Scholar]
- Juniper, B.E.; Watkins, R.; Harris, S.A. The Origin of the Apple. Acta Hortic. 1998, 484, 27–33. [Google Scholar] [CrossRef]
- Cornille, A.; Gladieux, P.; Smulders, M.J.M.; Roldán-Ruiz, I.; Laurens, F.; le Cam, B.; Nersesyan, A.; Clavel, J.; Olonova, M.; Feugey, L.; et al. New Insight into the History of Domesticated Apple: Secondary Contribution of the European Wild Apple to the Genome of Cultivated Varieties. PLoS Genet. 2012, 8, e1002703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, N.; Bai, Y.; Sun, H.; Wang, N.; Ma, Y.; Li, M.; Wang, X.; Jiao, C.; Legall, N.; Mao, L.; et al. Genome Re-Sequencing Reveals the History of Apple and Supports a Two-Stage Model for Fruit Enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- OECD. Assessment of Foods and Feeds Derived from Transgenic Crops, Volume 3: Common Bean, Rice, Cowpea and Apple Compositional Considerations; OECD: Paris, France, 2019; Volume 3. [Google Scholar]
- Awasthi, M.K.; Ferreira, J.A.; Sirohi, R.; Sarsaiya, S.; Khoshnevisan, B.; Baladi, S.; Sindhu, R.; Binod, P.; Pandey, A.; Juneja, A.; et al. A Critical Review on the Development Stage of Biorefinery Systems towards the Management of Apple Processing-Derived Waste. Renew. Sustain. Energy Rev. 2021, 143, 110972. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and Functional Compounds in Apple Pomace from Juice and Cider Manufacturing: Potential Use in Dermal Formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of Pomace from Apple Processing Industries: A Review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Liu, S.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.; Yang, B.; Laaksonen, O. Effect of Saccharomyces Cerevisiae and Schizosaccharomyces Pombe Strains on Chemical Composition and Sensory Quality of Ciders Made from Finnish Apple Cultivars. Food Chem. 2021, 345, 128833. [Google Scholar] [CrossRef]
- Costa, J.M.; Ampese, L.C.; Ziero, H.D.D.; Sganzerla, W.G.; Forster-Carneiro, T. Apple Pomace Biorefinery: Integrated Approaches for the Production of Bioenergy, Biochemicals, and Value-Added Products—An Updated Review. J. Environ. Chem. Eng. 2022, 10, 108358. [Google Scholar] [CrossRef]
- Global Cider Market: Industry Analysis and Forecast (2021-2027) by Product, Packaging Type and Distribution Channel. Available online: https://www.maximizemarketresearch.com/market-report/global-cider-market/27300/ (accessed on 28 June 2022).
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.M.; Tou, J.C. A Comprehensive Analysis of the Composition, Health Benefits, and Safety of Apple Pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Bortolini, D.G.; Benvenutti, L.; Demiate, I.M.; Nogueira, A.; Alberti, A.; Zielinski, A.A.F. A New Approach to the Use of Apple Pomace in Cider Making for the Recovery of Phenolic Compounds. LWT 2020, 126, 109316. [Google Scholar] [CrossRef]
- Vukušić, J.L.; Millenautzki, T.; Cieplik, R.; Obst, V.; Saaid, A.M.; Clavijo, L.; Zlatanovic, S.; Hof, J.; Mösche, M.; Barbe, S. Reshaping Apple Juice Production Into a Zero Discharge Biorefinery Process. Waste Biomass Valorization 2021, 12, 3617–3627. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I. Food Waste Recovery. Processing Technologies, Industrial Techniques, and Applications. In Plant-based by-products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 367–397. [Google Scholar]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Molina-García, A.D.; Rupérez, P.; Mateos-Aparicio, I. Novel Rich-in-Soluble Dietary Fiber Apple Ingredient Obtained from the Synergistic Effect of High Hydrostatic Pressure Aided by Celluclast®. LWT 2021, 146, 111421. [Google Scholar] [CrossRef]
- De la Peña Armada, R.; Bronze, M.R.; Matias, A.; Mateos-Aparicio, I. Triterpene-Rich Supercritical CO2 Extracts from Apple By-Product Protect Human Keratinocytes Against ROS. Food Bioproc. Tech. 2021, 14, 909–919. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of Polyphenols in Apple Pomace: A Comparative Study of Different Extraction and Hydrolysis Procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Nile, S.H.; Nile, A.; Liu, J.; Kim, D.H.; Kai, G. Exploitation of Apple Pomace towards Extraction of Triterpenic Acids, Antioxidant Potential, Cytotoxic Effects, and Inhibition of Clinically Important Enzymes. Food Chem. Toxicol. 2019, 131, 110563. [Google Scholar] [CrossRef]
- Poirier, B.C.; Buchanan, D.A.; Rudell, D.R.; Mattheis, J.P. Differential Partitioning of Triterpenes and Triterpene Esters in Apple Peel. J. Agric. Food Chem. 2018, 66, 1800–1806. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit Cuticular Waxes as a Source of Biologically Active Triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [Green Version]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Ind. Crops Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Klein, B.; Thewes, F.R.; Rogério de Oliveira, A.; Brackmann, A.; Barin, J.S.; Cichoski, A.J.; Wagner, R. Development of Dispersive Solvent Extraction Method to Determine the Chemical Composition of Apple Peel Wax. Food Res. Int. 2019, 116, 611–619. [Google Scholar] [CrossRef]
- Montañés, F.; Catchpole, O.J.; Tallon, S.; Mitchell, K.A.; Scott, D.; Webby, R.F. Extraction of Apple Seed Oil by Supercritical Carbon Dioxide at Pressures up to 1300 bar. J. Supercrit. Fluids 2018, 141, 128–136. [Google Scholar] [CrossRef]
- Walia, M.; Rawat, K.; Bhushan, S.; Padwad, Y.S.; Singh, B. Fatty Acid Composition, Physicochemical Properties, Antioxidant and Cytotoxic Activity of Apple Seed Oil Obtained from Apple Pomace. J. Sci. Food Agric. 2014, 94, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.L.; Zhan, P.; Li, K.X. Analysis of Components and Study on Antioxidant and Antimicrobial Activities of Oil in Apple Seeds. Int. J. Food Sci. Nutr. 2010, 61, 395–403. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; de la Peña Armada, R.; Pérez-Cózar, M.L.; Rupérez, P.; Redondo-Cuenca, A.; Villanueva-Suárez, M.J. Apple By-Product Dietary Fibre Exhibits Potential Prebiotic and Hypolipidemic Effectsin High-Fat Fed Wistar Rats. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100219. [Google Scholar] [CrossRef]
- Davies, S.J.; Guroy, D.; Hassaan, M.S.; El-Ajnaf, S.M.; El-Haroun, E. Evaluation of Co-Fermented Apple-Pomace, Molasses and Formic Acid Generated Sardine Based Fish Silages as Fishmeal Substitutes in Diets for Juvenile European Sea Bass (Dicentrachus Labrax) Production. Aquaculture 2020, 521, 735087. [Google Scholar] [CrossRef]
- Lu, Z.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. Micronized Apple Pomace as a Novel Emulsifier for Food O/W Pickering Emulsion. Food Chem. 2020, 330, 127325. [Google Scholar] [CrossRef]
- Kırbaş, Z.; Kumcuoglu, S.; Tavman, S. Effects of Apple, Orange and Carrot Pomace Powders on Gluten-Free Batter Rheology and Cake Properties. J. Food Sci. Technol. 2019, 56, 914–926. [Google Scholar] [CrossRef]
- Wang, S.; Gu, B.J.; Ganjyal, G.M. Impacts of the Inclusion of Various Fruit Pomace Types on the Expansion of Corn Starch Extrudates. LWT 2019, 110, 223–230. [Google Scholar] [CrossRef]
- Macagnan, F.T.; dos Santos, L.R.; Roberto, B.S.; de Moura, F.A.; Bizzani, M.; da Silva, L.P. Biological Properties of Apple Pomace, Orange Bagasse and Passion Fruit Peel as Alternative Sources of Dietary Fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, Y.B.; Hwang, K.E.; Song, D.H.; Ham, Y.K.; Kim, H.W.; Sung, J.M.; Kim, C.J. Effect of Apple Pomace Fiber and Pork Fat Levels on Quality Characteristics of Uncured, Reduced-Fat Chicken Sausages. Poult. Sci. 2016, 95, 1465–1471. [Google Scholar] [CrossRef]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; de Jesús Zazueta-Morales, J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; de Jesús Ruiz-Anchondo, T. Polyphenolic Compound Stability and Antioxidant Capacity of Apple Pomace in an Extruded Cereal. LWT—Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fiber and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Figuerola, F.; Hurtado, M.L.; Estévez, A.M.; Chiffelle, I.; Asenjo, F. Fibre Concentrates from Apple Pomace and Citrus Peel as Potential Fibre Sources for Food Enrichment. Food Chem. 2005, 91, 395–401. [Google Scholar] [CrossRef]
- Skinner, R.C.; Warren, D.C.; Naveed, M.; Agarwal, G.; Benedito, V.A.; Tou, J.C. Apple Pomace Improves Liver and Adipose Inflammatory and Antioxidant Status in Young Female Rats Consuming a Western Diet. J. Funct. Foods 2019, 61, 103471. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Sabater, C.; Antón, M.J.; Moreno, F.J.; Riestra, S.; Margolles, A.; Ruiz, L. Prebiotic Potential of Apple Pomace and Pectins from Different Apple Varieties: Modulatory Effects on Key Target Commensal Microbial Populations. Food Hydrocoll. 2022, 133, 107958. [Google Scholar] [CrossRef]
- Gorjanović, S.; Micić, D.; Pastor, F.; Tosti, T.; Kalušević, A.; Ristić, S.; Zlatanovic, S. Evaluation of Apple Pomace Flour Obtained Industrially by Dehydration as a Source of Biomolecules with Antioxidant, Antidiabetic and Antiobesity Effects. Antioxidants 2020, 9, 413. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Krath, B.N.; Markowski, J.; Poulsen, M.; Hansen, M.; Kołodziejczyk, K.; Kosmala, M.; Dragsted, L.O. Apple Pomace Improves Gut Health in Fisher Rats Independent of Seed Content. Food Funct. 2018, 9, 2931–2941. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.D.; Han, C.K.; Lee, B.H. Loss of Body Weight and Fat and Improved Lipid Profiles in Obese Rats Fed Apple Pomace or Apple Juice Concentrate. J. Med. Food 2013, 16, 823–830. [Google Scholar] [CrossRef] [Green Version]
- De la Peña-Armada, R.; Villanueva, A.M.J.; Mateos, S.I. High Hydrostatic Pressure Processing Enhances Pectin Solubilisation on Apple by—Product Improving Techno—Functional Properties. Eur. Food Res. Technol. 2020, 146, 111421. [Google Scholar] [CrossRef]
- Gray, J. Dietary Fibre Definition, Analysis, Physiology Health; Champ, M., Ed.; ILSI Europe: Brussels, Belgium, 2006. [Google Scholar]
- Singh, R.P.; Tingirikari, J.M.R. Agro Waste Derived Pectin Poly and Oligosaccharides: Synthesis and Functional Characterization. Biocatal. Agric. Biotechnol. 2021, 31, 101910. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; dos Santos Pereira, E.; de Oliveira Raphaelli, C.; Radünz, M.; Camargo, T.M.; da Rocha Concenço, F.I.G.; Cantillano, R.F.F.; Fiorentini, Â.M.; Nora, L. Application of Prebiotics in Apple Products and Potential Health Benefits. J. Food Sci. Technol. 2022, 59, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastall, R.A.; Gibson, G.R. Recent Developments in Prebiotics to Selectively Impact Beneficial Microbes and Promote Intestinal Health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Włodarczyk, M.; Śliżewska, K. Obesity as the 21st Century’s Major Disease: The Role of Probiotics and Prebiotics in Prevention and Treatment. Food Biosci. 2021, 42, 101115. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorisation of Food Agro-Industrial by-Products: From the Past to the Present and Perspectives. J. Environ. Manag. 2021, 299, 113571. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green Extraction of Natural Products. Origins, Current Status, and Future Challenges. TrAC—Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Matias, A. Food Industry Processing By-Products in Foods; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Marić, M.; Nin, A.; Zhu, Z.; Barba, F.J.; Brn, M.; Rimac, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Arshadi, M.; Attard, T.M.; Lukasik, R.M.; Brncic, M.; da Costa Lopes, A.M.; Finell, M.; Geladi, P.; Gerschenson, L.N.; Gogus, F.; Herrero, M.; et al. Pre-Treatment and Extraction Techniques for Recovery of Added Value Compounds from Wastes throughout the Agri-Food Chain. Green Chem. 2016, 18, 6160–6204. [Google Scholar] [CrossRef] [Green Version]
- Chizoba Ekezie, F.G.; Sun, D.W.; Han, Z.; Cheng, J.H. Microwave-Assisted Food Processing Technologies for Enhancing Product Quality and Process Efficiency: A Review of Recent Developments. Trends Food Sci. Technol. 2017, 67, 58–69. [Google Scholar] [CrossRef]
- Singla, M.; Sit, N. Application of Ultrasound in Combination with Other Technologies in Food Processing: A Review. Ultrason. Sonochem. 2021, 73, 105506. [Google Scholar] [CrossRef] [PubMed]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Kirk, O.; Borchert, T.V.; Fuglsang, C.C. Industrial Enzyme Applications. Curr. Opin. Biotechnol. 2002, 13, 345–351. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-Conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Bélafi-Bakó, K. Enzymatic Extraction and Fermentation for the Recovery of Food Processing Products; Woodhead Publishing Limited: Sawston, UK, 2007; Volume 1. [Google Scholar]
- Rodríguez Madrera, R.; Pando Bedriñana, R.; Suárez Valles, B. Enhancement of the Nutritional Properties of Apple Pomace by Fermentation with Autochthonous Yeasts. LWT—Food Sci. Technol. 2017, 79, 27–33. [Google Scholar] [CrossRef]
- Yadav, M.; Ahmadi, Y.; Chiu, F.-C. Handbook of Polymer Nanocomposites for Industrial Applications. In Food and Bioprocessing Industry; Mustansar Hussain, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple Pomace as a Potential Valuable Resource for Full-Components Utilization: A Review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Leonel, L.V.; Sene, L.; da Cunha, M.A.A.; Dalanhol, K.C.F.; de Almeida Felipe, M.d.G. Valorization of Apple Pomace Using Bio-Based Technology for the Production of Xylitol and 2G Ethanol. Bioprocess Biosyst. Eng. 2020, 43, 2153–2163. [Google Scholar] [CrossRef]
- Huang, J.; Liao, J.; Qi, J.; Jiang, W.; Yang, X. Structural and Physicochemical Properties of Pectin-Rich Dietary Fiber Prepared from Citrus Peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging Technologies for the Production of Nutraceuticals from Agricultural By-Products: A Viewpoint of Opportunities and Challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Tsevdou, M.; Gogou, E.; Taoukis, P. High Hydrostatic Pressure Processing of Foods; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Pérez-lópez, E.; Mateos-aparicio, I.; Rupérez, P. High Hydrostatic Pressure Aided by Food-Grade Enzymes as a Novel Approach for Okara Valorization. Innov. Food Sci. Emerg. Technol. 2017, 42, 197–203. [Google Scholar] [CrossRef]
- Ghosh, P.; Rama, C.P. Exposition on History and Potential of Supercritical Fluid Processing. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–521. [Google Scholar]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and Supercritical Fluid Extraction of Bioactive Compounds from Plants, Food-by-Products, Seaweeds and Microalgae—An Update. TrAC—Trends Anal. Chem. 2019, 116, 116–213. [Google Scholar] [CrossRef]
- Rodrigues, L.; Silva, I.; Poejo, J.; Serra, A.T.; Matias, A.A.; Simplício, A.L.; Bronze, M.R.; Duarte, C.M.M. Recovery of Antioxidant and Antiproliferative Compounds from Watercress Using Pressurized Fluid Extraction. RSC Adv. 2016, 6, 30905–30918. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Vegetable Matrices: Applications, Trends and Future Perspectives of a Convincing Green Technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Perussello, C.A.; Zhang, Z.; Marzocchella, A.; Tiwari, B.K. Valorization of Apple Pomace by Extraction of Valuable Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 776–796. [Google Scholar] [CrossRef] [Green Version]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of Apple Pomaces for Biofuel Production: A Biorefinery Approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Ma, Q.; Bi, J.; Yi, J.; Wu, X.; Li, X.; Zhao, Y. Stability of Phenolic Compounds and Drying Characteristics of Apple Peel as Affected by Three Drying Treatments. Food Sci. Hum. Wellness 2021, 10, 174–182. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding Apple Pomace as a Functional Ingredient in Stirred-Type Yogurt and Yogurt Drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the Glycemic Index of Short Dough Biscuits by Using Apple Pomace as a Functional Ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Yadav, S.; Malik, A.; Pathera, A.; Islam, R.U.; Sharma, D. Development of Dietary Fibre Enriched Chicken Sausages by Incorporating Corn Bran, Dried Apple Pomace and Dried Tomato Pomace. Nutr. Food Sci. 2016, 46, 16–29. [Google Scholar] [CrossRef]
- Chandrasekar, V.; Martín-González, M.F.S.; Hirst, P.; Ballard, T.S. Optimizing Microwave-Assisted Extraction of Phenolic Antioxidants from Red Delicious and Jonathan Apple Pomace. J. Food Process Eng. 2015, 38, 571–582. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of Microwave-Assisted Extraction of Polyphenols from Apple Pomace Using Response Surface Methodology and HPLC Analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef]
- Dranca, F.; Vargas, M.; Oroian, M. Physicochemical Properties of Pectin from Malus Domestica ‘Fălticeni’ Apple Pomace as Affected by Non-Conventional Extraction Techniques. Food Hydrocoll. 2020, 100, 105383. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Tiwari, B.K. Comparison of Selected Clean and Green Extraction Technologies for Biomolecules from Apple Pomace. Electrophoresis 2018, 39, 1934–1945. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Le Bourvellec, C.; Renard, C.M.G.C.; Chemat, F. Lab and Pilot-Scale Ultrasound-Assisted Water Extraction of Polyphenols from Apple Pomace. J. Food Eng. 2012, 111, 73–81. [Google Scholar] [CrossRef]
- Egüés, I.; Hernandez-Ramos, F.; Rivilla, I.; Labidi, J. Optimization of Ultrasound Assisted Extraction of Bioactive Compounds from Apple Pomace. Molecules 2021, 26, 3783. [Google Scholar] [CrossRef]
- Pollini, L.; Cossignani, L.; Juan, C.; Mañes, J. Extraction of Phenolic Compounds from Fresh Apple Pomace by Different Non-Conventional Techniques. Molecules 2021, 26, 4272. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple Pomaces Derived from Mono-Varietal Asturian Ciders Production Are Potential Source of Pectins with Appealing Functional Properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Starzyńska-Janiszewska, A.; Stodolak, B. Endo-Xylanase and Endo-Cellulase-Assisted Extraction of Pectin from Apple Pomace. Carbohydr. Polym. 2016, 142, 199–205. [Google Scholar] [CrossRef]
- Gullón, B.; Falqué, E.; Alonso, J.L.; Parajó, J.C. Evaluation of Apple Pomace as a Raw Material for Alternative Applications in Food Industries. Food Technol. Biotechnol. 2007, 45, 426–433. [Google Scholar]
- Rodríguez Madrera, R.; Pando Bedriñana, R.; Suárez Valles, B. Application of Central Composite Design in the Fermentation of Apple Pomace to Optimize Its Nutritional and Functional Properties. Acta Aliment. 2018, 47, 324–332. [Google Scholar] [CrossRef]
- Ferrentino, G.; Morozova, K.; Mosibo, O.K.; Ramezani, M.; Scampicchio, M. Biorecovery of Antioxidants from Apple Pomace by Supercritical Fluid Extraction. J. Clean. Prod. 2018, 186, 253–261. [Google Scholar] [CrossRef]
- Woźniak, L.; Szakiel, A.; Paczkowski, C.; Marszałek, K.; Skapska, S.; Kowalska, H.; Jȩdrzejczak, R. Extraction of Triterpenic Acids and Phytosterols from Apple Pomace with Supercritical Carbon Dioxide: Impact of Process Parameters, Modelling of Kinetics, and Scaling-up Study. Molecules 2018, 23, 2790. [Google Scholar] [CrossRef] [Green Version]
- Ferrentino, G.; Giampiccolo, S.; Morozova, K.; Haman, N.; Spilimbergo, S.; Scampicchio, M. Supercritical Fluid Extraction of Oils from Apple Seeds: Process Optimization, Chemical Characterization and Comparison with a Conventional Solvent Extraction. Innov. Food Sci. Emerg. Technol. 2020, 64, 102428. [Google Scholar] [CrossRef]
- De la Peña-Armada, R.; Villanueva-Suárez, M.J.; Rupérez, P.; Mateos-Aparicio, I. High Hydrostatic Pressure Assisted by Celluclast® Releases Oligosaccharides from Apple By-Product. Foods 2020, 9, 1058. [Google Scholar] [CrossRef]

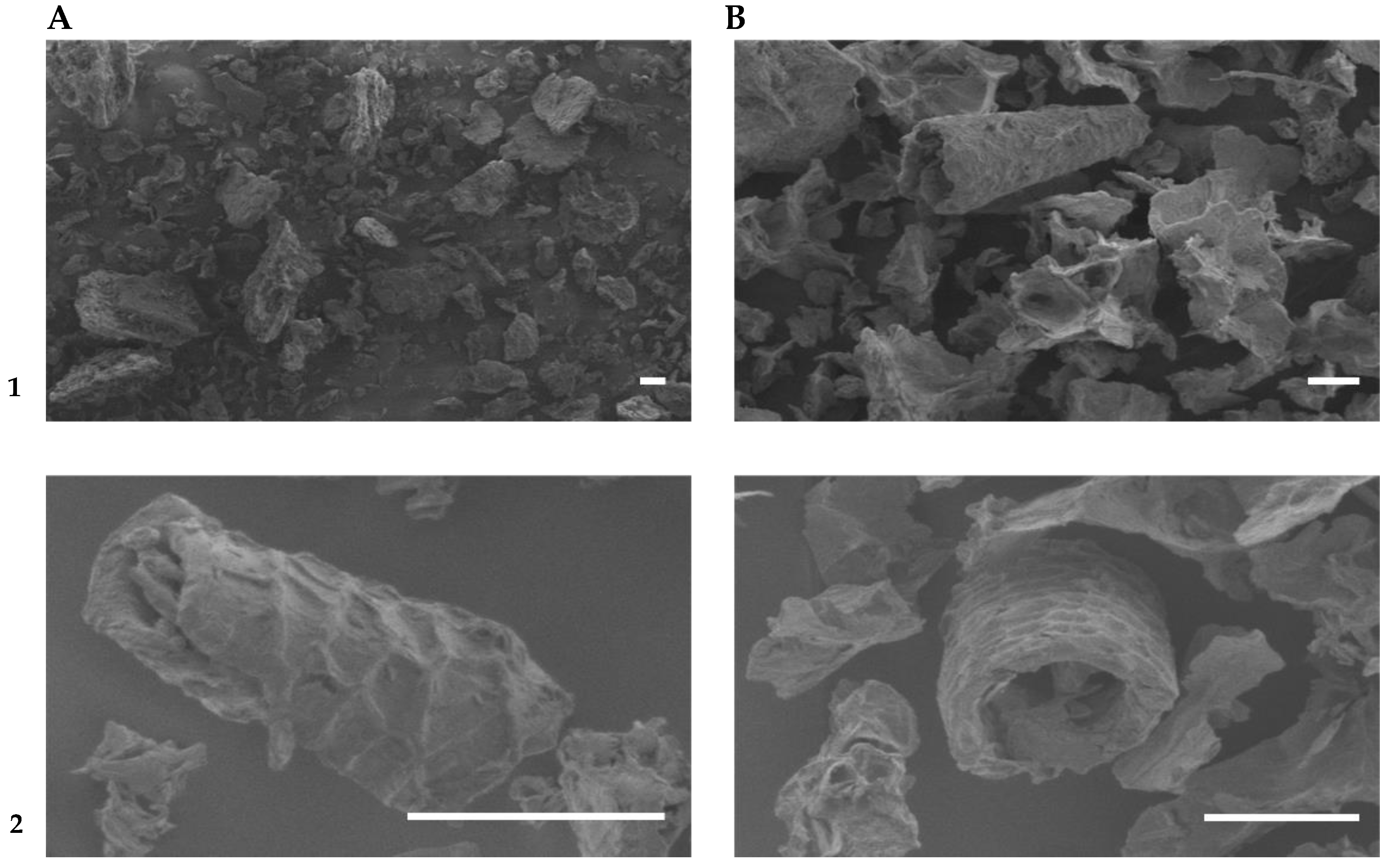
| TDF | SDF | IDF | Non-Fibrous Carbohydrates | Protein | Lipids | Ashes | Reference |
|---|---|---|---|---|---|---|---|
| 55.48 ± 0.7 | 11.06 ± 0.1 | 43.58 ± 0.6 | 28.48 ± 1.1 | 6.25 ± 0.1 | 6.58 ± 0.1 | 1.56 ± 0.3 | [45] |
| - | - | - | - | 3.8 ± 0.13 | 1.2 ± 0.10 | 1.5 ± 0.02 | [46] |
| - | 20.63 ± 2.00 | 62.08 ± 0.77 | - | 4.36 ± 0.24 | 7.36 ± 0.74 | - | [47] |
| 64.84 ± 1.78 | 20.27 ± 0.09 | 44.57 ± 0.24 | - | 3.57 ± 0.08 | - | 4.29 ± 0.06 | [48] |
| 53.1 ± 0.7 | 6.1 ± 0.2 | 47.0 ± 0.8 | - | 0.5 ± 0.0 | - | 1.8 ± 0.0 | [49] |
| 76.84 ± 1.24 | 18.97 ± 0.92 | 57.87 ± 0.33 | 6.72 ± 1.54 | 6.98 ± 0.32 | 8.19 ± 0.05 | 1.26 ± 0.12 | [50] |
| 60.48 | - | - | - | 2.28 | 3.84 | 6.67 | [51] |
| 8.86 ± 0.50 | 74.44 ± 0.15 | 3.42 ± 0.94 | 0.26 ± 0.13 | 1.68 ± 0.74 | [52] | ||
| 51.10 ± 1.86 | 14.60 ± 0.14 | 36.50 ± 1.14 | - | 2.06 ± 0.05 | 2.70 ± 0.10 | 0.50 ± 0.05 | [53] |
| 78.2 ± 0.60 | 14.33 ± 0.61 | 63.9 ± 0.16 | 13.3 | 3.12 ± 0.07 | 1.57 ± 0.08 | 1.88 ± 0.11 | [54] |
| Study Characteristics | Results | References |
|---|---|---|
| Female Sprague–Dawley rats randomly assigned: standard purified rodent diet or AIN-93G, AIN-93G with freeze-dried apple pomace, or Western diet, or Western diet with freeze-dried apple pomace. | Improved liver and adipose inflammatory and antioxidant status: Rats consuming Western/AP downregulated hepatic and adipose proinflammatory cytokine gene expression and improved antioxidant status compared to rats consuming a Western diet. | [55] |
| In vitro digested apple pomace and pectin fractions derived from three apple varieties were subjected to faecal batch fermentation by using samples from healthy donors and from patients of Crohn’s disease. | Prebiotic potential: Growth of Akkermansia, Lachnospiraceae UCG-010, Prevotella, Sucinivibrio, and Turicibacter on samples from healthy donors, whereas Blautia, Lachnospiraceae CAG-56, Dialister, Eubacterium eligens, and Intestinimonas were stimulated in fermentations from inflammatory bowel disease patients. | [56] |
| Male C57BL/6J mice were exposed to a high-fat and sucrose diet without and with the addition of 10 mg apple pomace flower per day whereas the control groups were fed with standard pellet rodent diet without. | Antioxidant, antidiabetic, and antiobesity effects: Long-term supplementation with apple pomace flower was shown to decrease glycemia, significantly improve glucose tolerance, and decrease body weight gain in mice exposed to a high-fat and sucrose diet. | [57] |
| Wistar Hannover rats were fed with high-fat diets for 5 weeks and randomised in two groups (control and supplemented with apple by-product). Diets were prepared from a commercial diet AIN-210 enriched with fat. | Prebiotic and hypolipidemic effect: Butyrate was increased 3-fold in this in vivo assay indicating that butyrate bacteria producers can use apple by-product. Apple by-product enriched diet increased HDL and diminished trygliceridemia and hepatic total lipids. | [45] |
| Male F344 rats were fed a control feed or the same feed with 2.1%, or 6.5% dry apple pomace, with or without seeds for 4 weeks. | Hypocholesterolemic effect and improved gut health: Pomace feeding decreased total-, LDL- and IDL-cholesterol concentrations, increased production of SCFA and increased excretion of total- and primary bile acids. No hepatotoxic or other effects were derived from apple seeds. | [58] |
| Male Sprague–Dawley rats were assigned to four groups: the normal diet group, the high-fat diet group, and high-fat diet group containing either apple pomace or apple juice concentrate. | Loss of body weight and fat and improved lipid profiles: Body weight gain, white adipose tissue, weight, serum total cholesterol, LDL cholesterol, and triglyceride concentrations, epididymal adipocyte size, and lesion scores were significantly lower and serum HDL cholesterol concentration and brown adipose tissue weights were significantly higher in the apple pomace and apple juice concentrate groups compared with the high-fat diet group. | [59] |
| Extraction Methodology | Application | Conditions Tested | References |
|---|---|---|---|
| Microwave | Antioxidant compound recovery | Solvent type: 70% acetone and 60% ethanol Microwave power: 100–900 W Solvent volume to sample ratio: 4–12 mL/g dry pomace. Extraction time: 30–180 s | [100] |
| Polyphenol extraction | Microwave power: 500–700 W Extraction time: 40–60 s Ethanol concentration: 50–70% Ratio of solvent to raw material: 10:1–30:1 mL/g | [101] | |
| Pectin extraction | Microwave power: 560 W Extraction time: 120 s | [102] | |
| Phenolic compound extraction | Microwave power: 400, 600, 1000 W Extraction time: 60, 90 s | [103] | |
| Ultrasound | Polyphenol extraction | UI: 0.431–0.764 W/cm2 Temperature: 16–40 °C Sonication time: 5–55 min | [104] |
| Extraction of antioxidant compounds | Amplitude: 50–70% Temperature: 40–90 °C Sonication time: 5–20 min | [105] | |
| Phenolic compounds extraction | EtOH:H2O ratios: 50:50, 70:30, 30:70, v/v Liquid/solid ratio: 1:10 g/mL Time: 60 min Temperature: 60 °C | [106] | |
| Pectin extraction | Mode: bath, probe Time: 30, 60 min Frequency ultrasonic bath: 45 kHz Temperature: 60 °C Amplitude probe mode: 30%, 50% | [107] | |
| Phenolic compound extraction | Time: 2, 5, 10, 20, 30 min Temperature: 25 °C Sonication powers: 7.8, 49.5 W | [103] | |
| Phenolic compound extraction | Time: 2, 5, 10, 20, 30 min Temperature: 25 °C Sonication powers: 7.8, 49.5 W Enzyme: pectinase | [103] | |
| Enzymatic | Pectin recovery | Endo-xylanase and endo-cellulase Extraction time: 10 h Temperature: 40 °C pH 5.0 | [108] |
| Raw material | Enzyme concentrates: Celluclast 1.5 L and b-glucosidase Temperature: 48.5 °C Orbital agitation: 150 rpm pH: 4.85 | [109] | |
| Nutritional composition and phenolic compounds | Three yeast strains: S. cerevisiae; S. bayanus, and H. uvarum. Time: 7 days Temperature: 25 °C | [81] | |
| Nutritional and functional properties | Yeast strain: S. cerevisiae Time: 4.9 days Temperature: 29.5 °C | [110] | |
| Supercritical CO2 | Extraction of antioxidants | Pressure: 20–30 bar Temperature: 45–55 °C Co-solvent: ethanol (5%) | [111] |
| Extraction of Triterpenic acids and phytosterols | Pressure: 20–30 bar Temperature: 60–80 °C Flow rate: 4.17–12,5 *10−4 L s−1 | [112] | |
| Recover bioactive lipophilic compounds | Pressure: 300–550 bar Temperature: 37–55 °C Flow rate: 10 g/min | [35] | |
| Oil extraction | Pressure: 10–30 bar Temperature: 40–60 °C Flow rate: 1–8 L/h | [113] | |
| Oil extraction | Pressure: 300–1300 bar Temperature: 316–336 K Flow rate: 6–10 mL/min | [42] | |
| High hydrostatic pressure | Pectin recovery | Pressure: 200–600 MPa Temperature: 50 °C Time: 15–30 min | [60] |
| Pectin and oligosaccharides recovery | Pressure: 200–600 MPa Enzyme: 92 EGU Celluclast® Temperature: 50 °C Time: 15–30 min | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Peña-Armada, R.; Mateos-Aparicio, I. Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple. Molecules 2022, 27, 6937. https://doi.org/10.3390/molecules27206937
De la Peña-Armada R, Mateos-Aparicio I. Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple. Molecules. 2022; 27(20):6937. https://doi.org/10.3390/molecules27206937
Chicago/Turabian StyleDe la Peña-Armada, Rocío, and Inmaculada Mateos-Aparicio. 2022. "Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple" Molecules 27, no. 20: 6937. https://doi.org/10.3390/molecules27206937
APA StyleDe la Peña-Armada, R., & Mateos-Aparicio, I. (2022). Sustainable Approaches Using Green Technologies for Apple By-Product Valorisation as A New Perspective into the History of the Apple. Molecules, 27(20), 6937. https://doi.org/10.3390/molecules27206937







