High-Surface-Area-Activated Carbon Derived from Mango Peels and Seeds Wastes via Microwave-Induced ZnCl2 Activation for Adsorption of Methylene Blue Dye Molecules: Statistical Optimization and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MSMPAC Synthesis
2.3. Characterization
2.4. Experimental Design
2.5. Adsorption Study of MB on MSMPAC
3. Results and Discussion
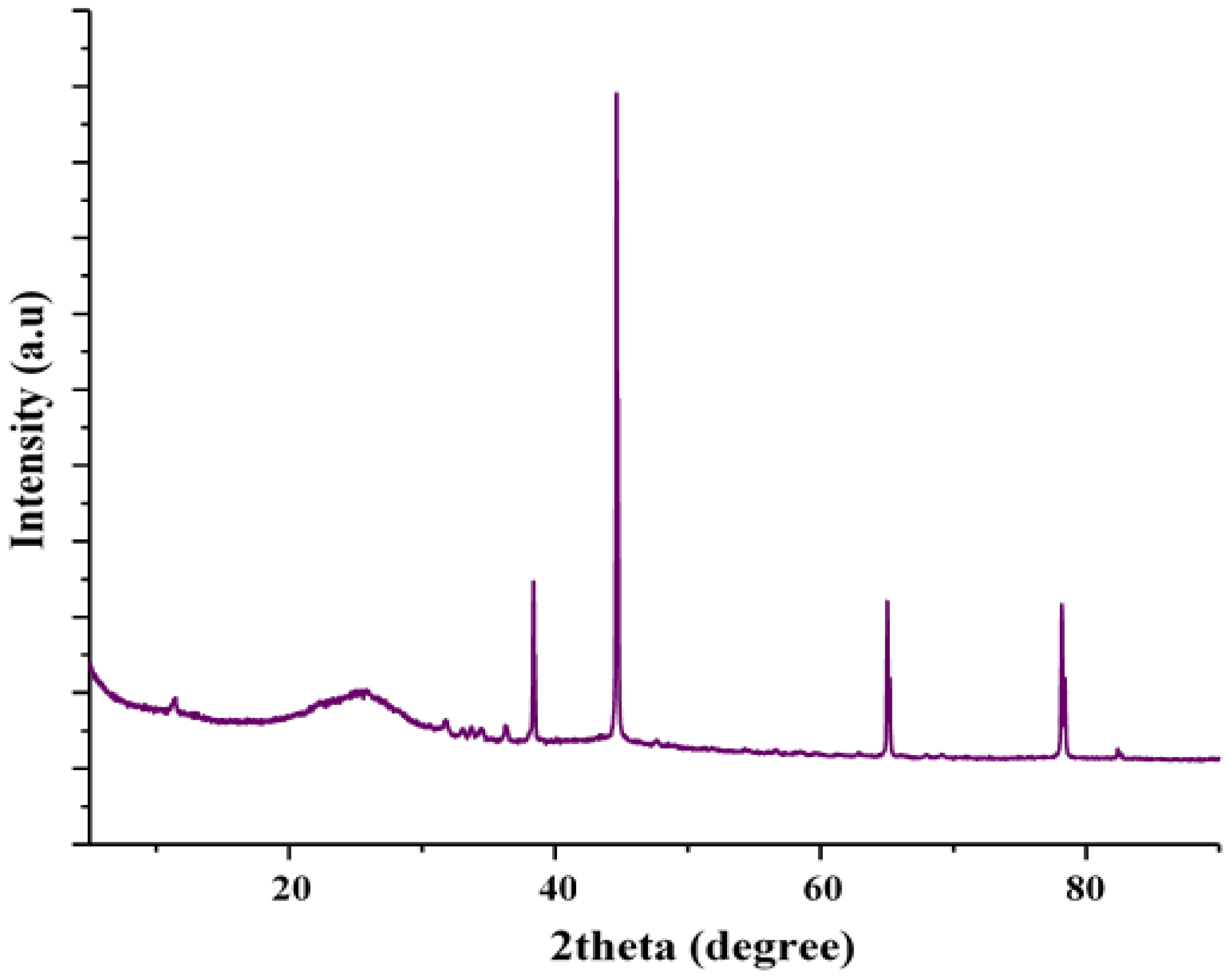
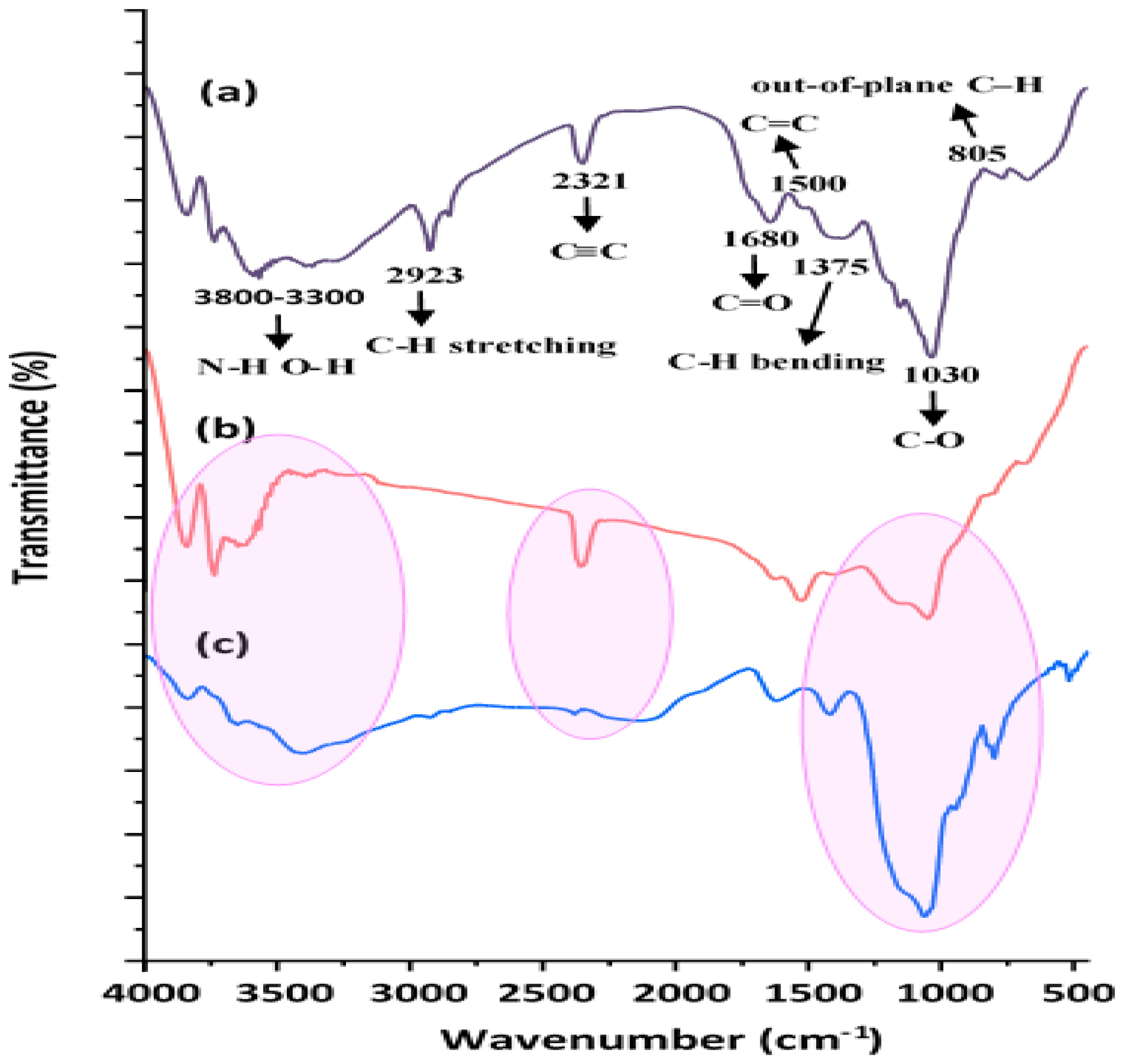
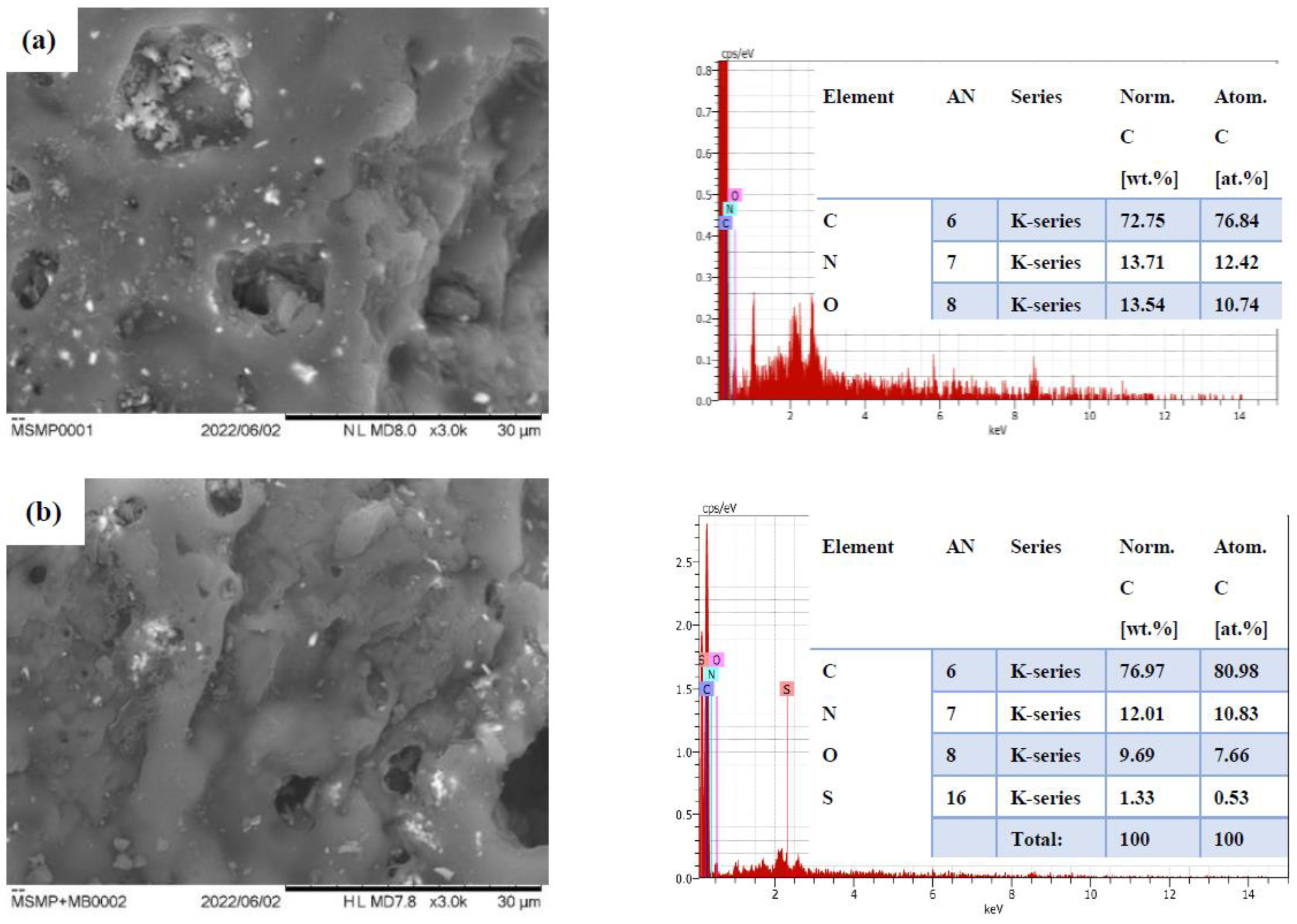
3.1. Characterization of MSMPAC
3.2. Model Fitting
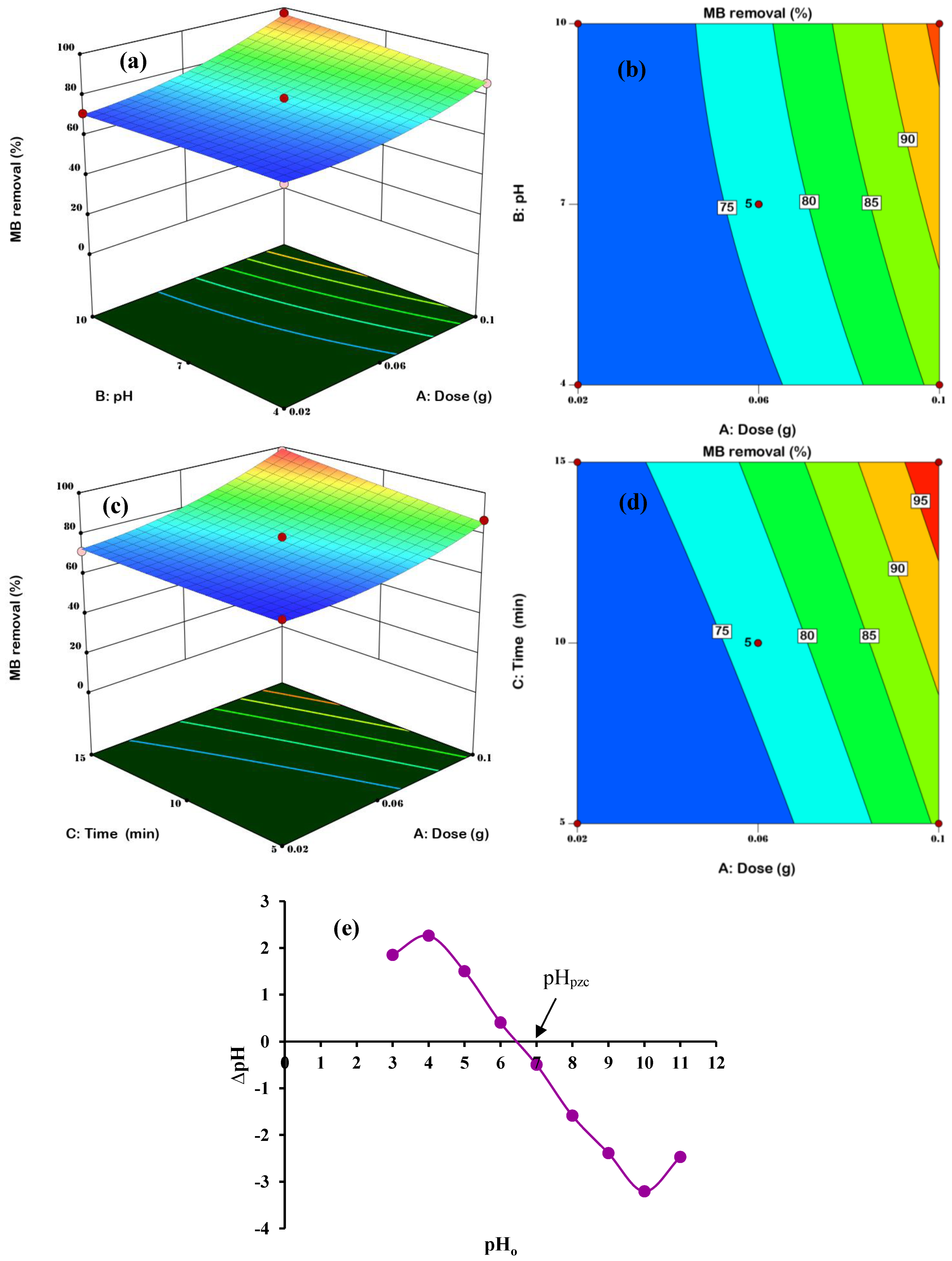
3.3. Effects of interactive variables
3.4. Optimization by the Desirability Functions
3.5. Adsorption Study
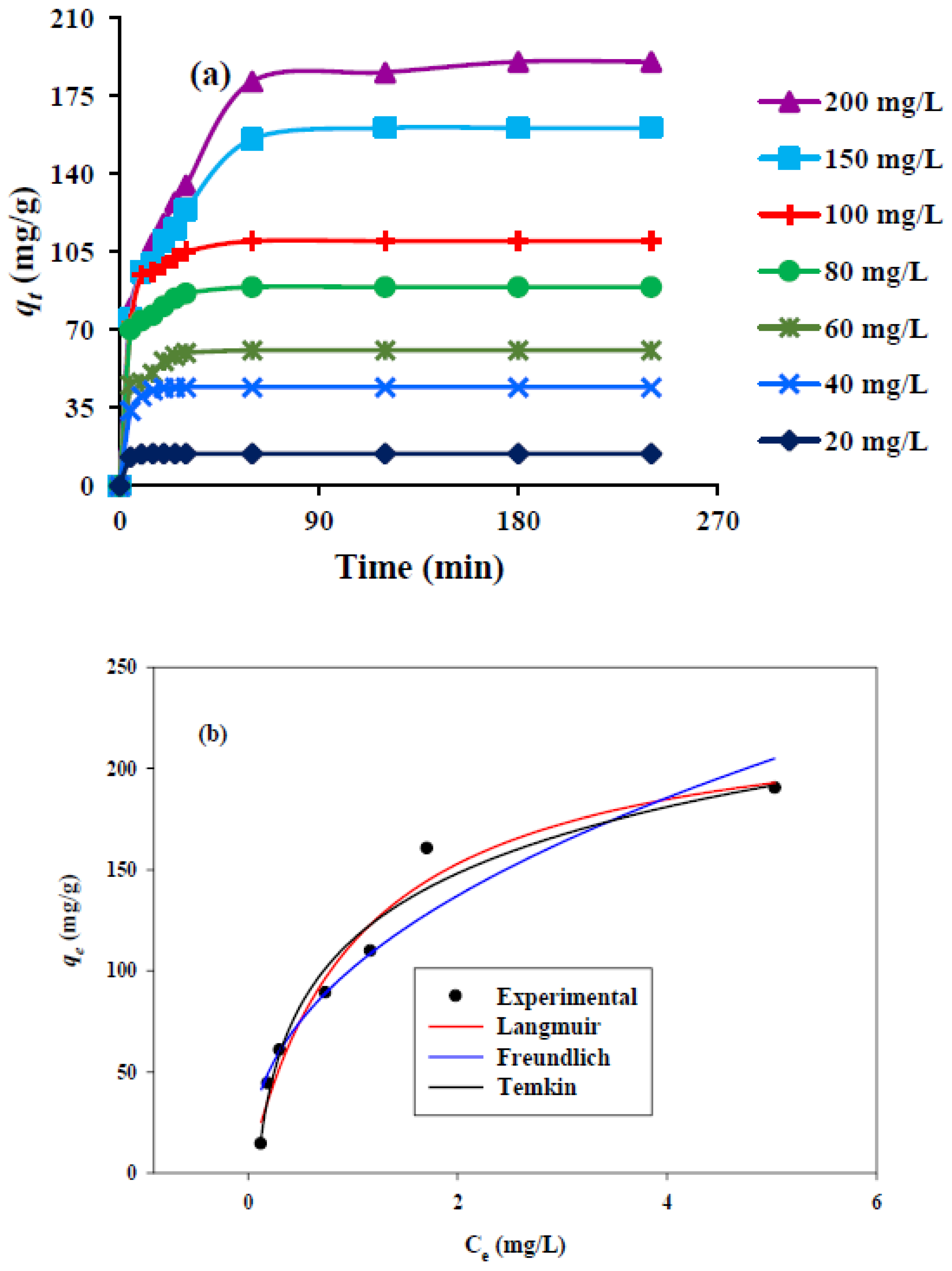
3.6. Adsorption Kinetics
3.7. Adsorption Isotherms
3.8. Thermodynamic Characteristics
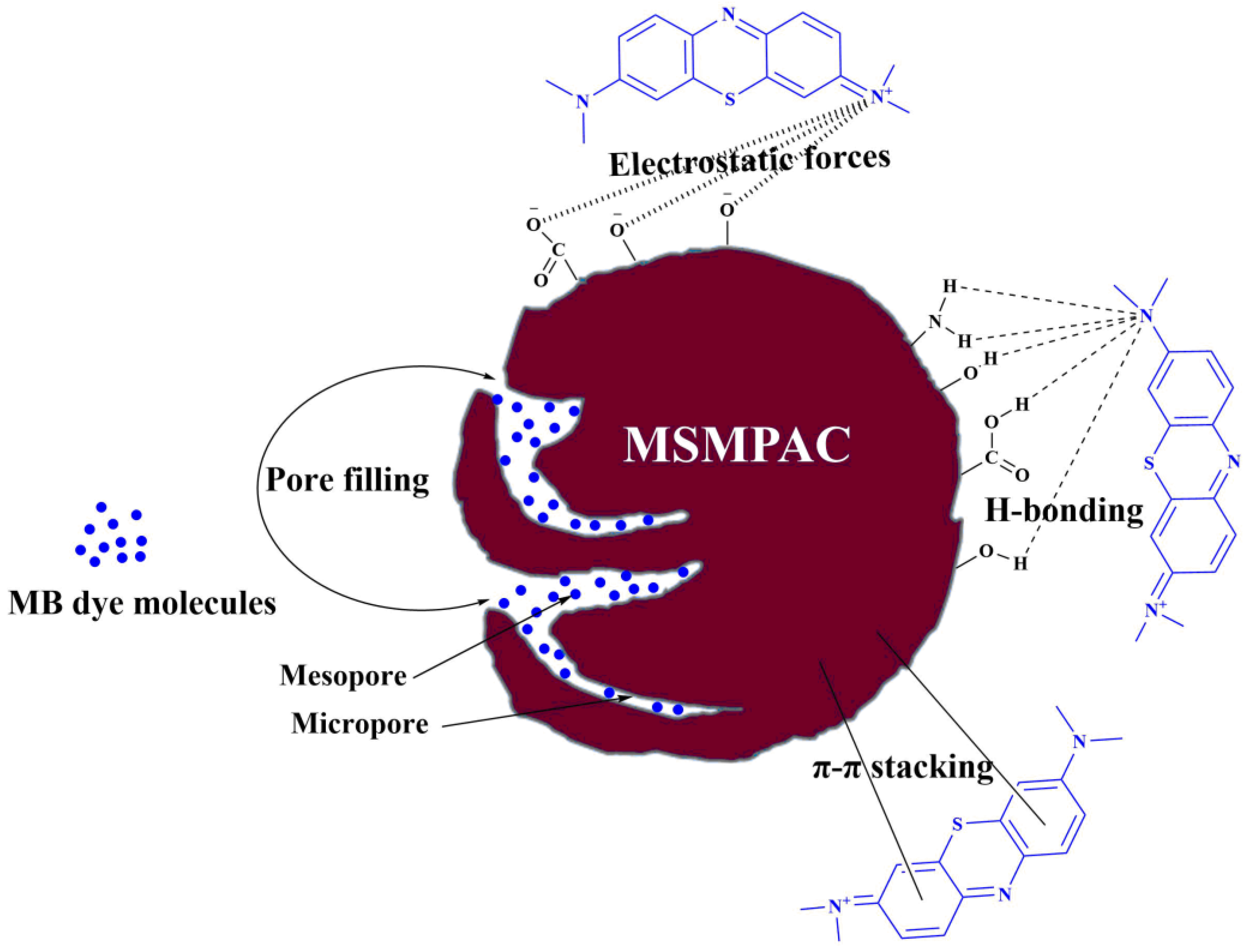
3.9. Adsorption Mechanism of MB Dye
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, D.; Wang, Z.; Zheng, X.; Tian, M. Activated carbon fiber derived from the seed hair fibers of Metaplexis japonica: Novel efficient adsorbent for methylene blue. Ind. Crops Prod. 2020, 148, 112319–112328. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, J.; Wu, L.; Ping, S. Fabrication of developed porous carbon derived from bluecoke powder by microwave-assisted KOH activation for simulative organic wastewater treatment. Diam. Relat. Mater. 2022, 124, 108929–108940. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Hanafiah, M.; ALOthman, Z.A.; Khan, M.R.; Surip, S. Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: Modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J. Chem. Eng. 2021, 38, 1499–1509. [Google Scholar] [CrossRef]
- Gayathiri, M.; Pulingam, T.; Lee, K.; Sudesh, K. Activated carbon from biomass waste precursors: Factors affecting production and adsorption mechanism. Chemosphere 2022, 294, 133764–133776. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.A.; Mohd Azlan, M.I.; Kasim, M.H. Adsorptive removal of methylene blue by commercial coconut shell sctivated carbon. Sci. Lett. 2018, 12, 1–25. [Google Scholar]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Activated carbons from biomass-based sources for CO2 capture applications. Chemosphere 2021, 282, 131111–131131. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Factors affecting the carbon yield and adsorption capability of the mangosteen peel activated carbon prepared by microwave assisted K2CO3 activation. Chem. Eng. J. 2012, 180, 66–74. [Google Scholar] [CrossRef]
- Nasrullah, A.; Saad, B.; Bhat, A.; Khan, A.S.; Danish, M.; Isa, M.H.; Naeem, A. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal. J. Clean. Prod. 2019, 211, 1190–1200. [Google Scholar] [CrossRef]
- Alacabey, İ. Antibiotic Removal from the Aquatic Environment with Activated Carbon Produced from Pumpkin Seeds. Molecules 2022, 27, 1380. [Google Scholar] [CrossRef] [PubMed]
- Zubrik, A.; Matik, M.; Hredzák, S.; Lovás, M.; Danková, Z.; Kováčová, M.; Briančin, J. Preparation of chemically activated carbon from waste biomass by single-stage and two-stage pyrolysis. J. Clean Prod. 2017, 143, 643–653. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Zhang, C.; Yang, W.; He, S. Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diam. Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- El-Nemr, M.A.; El Nemr, A.; Hassaan, M.A.; Ragab, S.; Tedone, L.; De Mastro, G.; Pantaleo, A. Microporous Activated Carbon from Pisum sativum Pods Using Various Activation Methods and Tested for Adsorption of Acid Orange 7 Dye from Water. Molecules 2022, 27, 4840. [Google Scholar] [CrossRef] [PubMed]
- Iradukunda, Y.; Wang, G.; Li, X.; Shi, G.; Hu, Y.; Luo, F.; Yi, K.; Albashir, A.I.M.; Niu, X.; Wu, Z. High performance of activated carbons prepared from mangosteen (Garcinia mangostana) peels using the hydrothermal process. J. Energy Storage 2021, 39, 102577–102587. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Jiang, H. A review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. J. Bioresour. Bioprod. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Pezoti, O., Jr.; Cazetta, A.L.; Souza, I.P.; Bedin, K.C.; Martins, A.C.; Silva, T.L.; Almeida, V.C. Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J. Ind. Eng. Chem. 2014, 20, 4401–4407. [Google Scholar] [CrossRef]
- Luo, X.; Cai, Y.; Liu, L.; Zeng, J. Cr (VI) adsorption performance and mechanism of an effective activated carbon prepared from bagasse with a one-step pyrolysis and ZnCl2 activation method. Cellulose 2019, 26, 4921–4934. [Google Scholar] [CrossRef]
- Zheng, N.-Y.; Lee, M.; Lin, Y.-L.; Samannan, B. Microwave-assisted wet co-torrefaction of food sludge and lignocellulose biowaste for biochar production and nutrient recovery. Process. Saf. Environ. Prot. 2020, 144, 273–283. [Google Scholar] [CrossRef]
- Razzak, S.A.; Farooque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168–100194. [Google Scholar] [CrossRef]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A review on recent advances in the treatment of dye-polluted wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Iqbal, Z.; Tanweer, M.S.; Alam, M. Recent advances in adsorptive removal of wastewater pollutants by chemically modified metal oxides: A review. J. Water Process. Eng. 2022, 46, 102641–102661. [Google Scholar] [CrossRef]
- Dao, M.U.; Le, H.S.; Hoang, H.Y.; Tran, V.A.; Doan, V.D.; Le, T.T.N.; Sirotkin, A. Natural core-shell structure activated carbon beads derived from Litsea glutinosa seeds for removal of methylene blue: Facile preparation, characterization, and adsorption properties. Environ. Res. 2021, 198, 110481–1104493. [Google Scholar] [CrossRef] [PubMed]
- Padmini, M.; Balaganapathi, T.; Thilakan, P. Rutile-TiO2: Post heat treatment and its influence on the photocatalytic degradation of MB dye. Ceram. Int. 2022, 48, 16685–16694. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Khan, A.; Yang, J.; Li, Q.; Wang, G. A highly efficient technique to simultaneously remove acidic and basic dyes using magnetic ion-exchange microbeads. J. Environ. Manag. 2022, 304, 114173–114180. [Google Scholar] [CrossRef] [PubMed]
- Bencheqroun, Z.; Sahin, N.E.; Soares, O.S.; Pereira, M.F.; Zaitan, H.; Nawdali, M.; Rombi, E.; Fonseca, A.M.; Parpot, P.; Neves, I.C. Fe (III)-exchanged zeolites as efficient electrocatalysts for Fenton-like oxidation of dyes in aqueous phase. J. Environ. Chem. Eng. 2022, 10, 107891–107904. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Wilson, L.D.; Hanafiah, M.A.K.M.; Nawawi, W.I.; ALOthman, Z.A.; Rizwan Khan, M. Fabrication of schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: Optimization using response surface methodology. J. Polym. Environ. 2021, 29, 2855–2868. [Google Scholar] [CrossRef]
- Subrahmanya, T.M.; Widakdo, J.; Mani, S.; Austria, H.F.M.; Hung, W.S.; Makari, H.K.; Lai, J.Y. An eco-friendly and reusable syringe filter membrane for the efficient removal of dyes from water via low pressure filtration assisted self-assembling of graphene oxide and SBA-15/PDA. J. Clean Prod. 2022, 349, 131425–131436. [Google Scholar] [CrossRef]
- Khan SAHussain, D.; Abbasi, N.; Khan, T.A. Deciphering the adsorption potential of a functionalized green hydrogel nanocomposite for aspartame from aqueous phase. Chemosphere 2022, 289, 133232. [Google Scholar] [CrossRef]
- Dalvand, A.; Nabizadeh, R.; Ganjali, M.R.; Khoobi, M.; Nazmara, S.; Mahvi, A.H. Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J. Magn. Magn. Mater. 2016, 404, 179–189. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Hu, L.; Peng, Y.; Wu, F.; Peng, S.; Li, J.; Liu, Z. Tubular activated carbons made from cotton stalk for dynamic adsorption of airborne toluene. J. Taiwan Inst. Chem. Eng. 2017, 80, 399–405. [Google Scholar] [CrossRef]
- Nasrullah, A.; Khan, A.S.; Bhat, A.; Din, I.U.; Inayat, A.; Muhammad, N.; Bakhsh, E.M.; Khan, S.B. Effect of short time ball milling on physicochemical and adsorption performance of activated carbon prepared from mangosteen peel waste. Renew. Energy 2021, 168, 723–733. [Google Scholar] [CrossRef]
- Hadi, S.; Taheri, E.; Amin, M.M.; Fatehizadeh, A.; Lima, E.C. Fabrication of activated carbon from pomegranate husk by dual consecutive chemical activation for 4-chlorophenol adsorption. Environ. Sci. Pollut. Res. 2021, 28, 13919–13930. [Google Scholar] [CrossRef] [PubMed]
- Kongsune, P.; Rattanapan, S.; Chanajaree, R. The removal of Pb2+ from aqueous solution using mangosteen peel activated carbon: Isotherm, kinetic, thermodynamic and binding energy calculation. Groundw. Sustain. Dev. 2021, 12, 100524–100535. [Google Scholar] [CrossRef]
- Kutluay, S.; Temel, F. Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125848–125862. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Jawad, A.H.; Ridwan, M.; Khadiran, T.; Wilson, L.D.; Yaseen, Z.M. Chitosan/Carbon-Doped TiO2 Composite for Adsorption of Two Anionic Dyes in Solution and Gaseous SO2 Capture: Experimental Modeling and Optimization. J. Polym. Environ. 2022, 1, 1–18. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, T.; Jiang, C.; Zhao, Y.; Ai, S. Renewable adsorbents from carboxylate-modified agro-forestry residues for efficient removal of methylene blue dye. J. Phys. Chem. Solids 2021, 149, 109811–109821. [Google Scholar] [CrossRef]
- Shengli, S.; Junping, L.; Qi, L.; Fangru, N.; Jia, F.; Shulian, X. Optimized preparation of Phragmites australis activated carbon using the Box-Behnken method and desirability function to remove hydroquinone. Ecotoxicol. Environ. Saf. 2018, 165, 411–422. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. Vet. Akad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Surip, S.N.; Sabar, S. Adsorptive performance of carbon modified chitosan biopolymer for cationic dye removal: Kinetic, isotherm, thermodynamic, and mechanism study. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Khan, S.A.; Abbasi, N.; Hussain, D.; Khan, T.A. Sustainable Mitigation of Paracetamol with a Novel Dual-Functionalized Pullulan/Kaolin Hydrogel Nanocomposite from Simulated Wastewater. Langmuir 2022, 38, 8280–8295. [Google Scholar] [CrossRef] [PubMed]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Temkin, M.I. Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Hoseinzadeh, H.; Hayati, B.; Ghaheh, F.S.; Seifpanahi-Shabani, K.; Mahmoodi, N.M. Development of room temperature synthesized and functionalized metal-organic framework/graphene oxide composite and pollutant adsorption ability. Mater. Res. Bullet 2021, 142, 111408. [Google Scholar] [CrossRef]
- El Nemr, A.; Shoaib, A.G.; El Sikaily, A.; Mohamed, A.E.-D.A.; Hassan, A.F. Evaluation of cationic Methylene Blue dye removal by high surface area mesoporous activated carbon derived from Ulva lactuca. Environ. Processes 2021, 8, 311–332. [Google Scholar] [CrossRef]
- Jaramillo-Martínez, D.; Buitrago-Sierra, R.; López, D. Use of Palm Oil Waste for Activated Carbons Production and Its Application in Methylene Blue Removal. ChemistrySelect 2022, 7, e202200791. [Google Scholar] [CrossRef]
- Mohd Ramli, M.R.; Shoparwe, N.F.; Ahmad, M.A. Methylene Blue Removal Using Activated Carbon Adsorbent from Jengkol Peel: Kinetic and Mass Transfer Studies. Arab. J. Sci. Eng. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Do, T.H.; Dung, N.Q.; Chu, M.N.; Van Kiet, D.; Ngan, T.T.K.; Van Tan, L. Study on methylene blue adsorption of activated carbon made from Moringa oleifera leaf. Mater. Today Proc. 2021, 38, 3405–3413. [Google Scholar] [CrossRef]
- Baytar, O.; Ceyhan, A.A.; Şahin, Ö. Production of activated carbon from Elaeagnus angustifolia seeds using H3PO4 activator and methylene blue and malachite green adsorption. Int. J. Phytoremediat. 2021, 23, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xu, D.; Gao, Y.; Chen, G.; Zhai, R.; Huang, X.; Xu, X.; Wang, J.; Yang, X.; Liu, G. Mussel-inspired triple bionic adsorbent: Facile preparation of layered double hydroxide@ polydopamine@ metal-polyphenol networks and their selective adsorption of dyes in single and binary systems. J. Hazard. Mater. 2021, 420, 126609. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wei, H.; Liu, Y.; Li, S.; Wang, G.; Han, H. Fabrication of novel starch-based composite hydrogel microspheres combining Diels-Alder reaction with spray drying for MB adsorption. J. Environ. Chem. Eng. 2021, 9, 105929–105942. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Bahrudin, N.N.; Hum, N.N.M.F.; Surip, S.N.; Syed-Hassan, S.S.A.; Sabar, S. Microporous activated carbon developed from KOH activated biomass waste: Surface mechanistic study of methylene blue dye adsorption. Water Sci. Technol. 2021, 84, 1858–1872. [Google Scholar] [CrossRef]
- Mahapatra, U.; Chatterjee, A.; Das, C.; Manna, A.K. Adsorptive removal of hexavalent chromium and methylene blue from simulated solution by activated carbon synthesized from natural rubber industry biosludge. Environ. Technol. Innov. 2021, 22, 101427–101448. [Google Scholar] [CrossRef]


| Codes | Variables | Level 1 (−1) | Level 2 (0) | Level 3 (+1) |
|---|---|---|---|---|
| A | MSMPAC dose (g) | 0.02 | 0.06 | 0.1 |
| B | pH | 4 | 7 | 10 |
| C | Time (min) | 5 | 10 | 15 |
| Run | A:Dose (g) | B:pH | C:Time (min) | MB Removal (%) |
|---|---|---|---|---|
| 1 | 0.02 | 4 | 10 | 70.1 |
| 2 | 0.1 | 4 | 10 | 85.9 |
| 3 | 0.02 | 10 | 10 | 71.2 |
| 4 | 0.1 | 10 | 10 | 97.1 |
| 5 | 0.02 | 7 | 5 | 71.4 |
| 6 | 0.1 | 7 | 5 | 86.9 |
| 7 | 0.02 | 7 | 15 | 71.7 |
| 8 | 0.1 | 7 | 15 | 97.8 |
| 9 | 0.06 | 4 | 5 | 70.7 |
| 10 | 0.06 | 10 | 5 | 72.6 |
| 11 | 0.06 | 4 | 15 | 79.1 |
| 12 | 0.06 | 10 | 15 | 85.2 |
| 13 | 0.06 | 7 | 10 | 74.1 |
| 14 | 0.06 | 7 | 10 | 75.9 |
| 15 | 0.06 | 7 | 10 | 78.8 |
| 16 | 0.06 | 7 | 10 | 76.5 |
| 17 | 0.06 | 7 | 10 | 78.7 |
| Parameter(s) | Value |
|---|---|
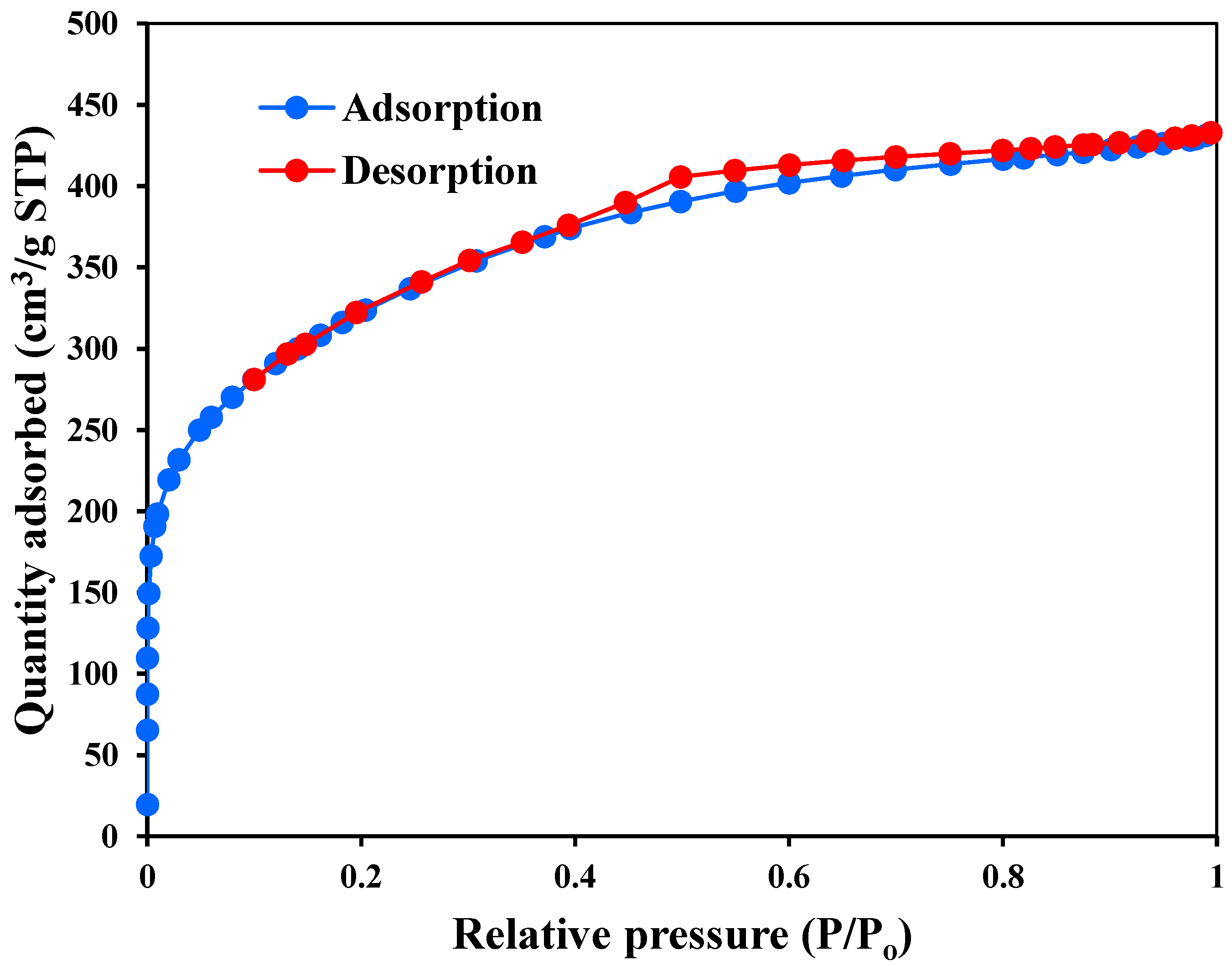
| BET surface area (m2/g) | 1151.6 |
| Langmuir surface area (m2/g) | 1585.4 |
| Total volume in pores ( cm3/g) | 0.614 |
| Mean pore diameter (nm) | 3.05 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Remarks |
|---|---|---|---|---|---|---|
| Model | 1200.32 | 9 | 133.37 | 31.00 | <0.0001 | Significant |
| A-Dose | 867.36 | 1 | 867.36 | 201.59 | <0.0001 | Significant |
| B-pH | 51.51 | 1 | 51.51 | 11.97 | 0.0105 | Significant |
| C-Time | 129.61 | 1 | 129.61 | 30.12 | 0.0009 | Significant |
| AB | 25.50 | 1 | 25.50 | 5.93 | 0.0451 | Significant |
| AC | 28.09 | 1 | 28.09 | 6.53 | 0.0378 | Significant |
| BC | 4.41 | 1 | 4.41 | 1.02 | 0.3450 | Not significant |
| A² | 91.53 | 1 | 91.53 | 21.27 | 0.0024 | Significant |
| B² | 0.6322 | 1 | 0.6322 | 0.1469 | 0.7128 | Not significant |
| C² | 1.00 | 1 | 1.00 | 0.2326 | 0.6443 | Not significant |
| Residual | 30.12 | 7 | 4.30 | |||
| Lack of Fit | 14.32 | 3 | 4.77 | 1.21 | 0.4143 | Not significant |
| Pure Error | 15.80 | 4 | 3.95 | |||
| Cor Total | 1230.44 | 16 |
| Models | Formula | Descriptions |
|---|---|---|
| Pseudo-first-order (PFO) | : pseudo-first-order rate constant (1/min) | |
| Pseudo-second-order (PSO) | : pseudo-second-order rate constant (g/mg min) | |
| Langmuir | : monolayer capacity (mg/g) KL : Langmuir constant (L/mg) | |
| Freundlich | KF : Freundlich constant (mg/g) (L/mg)1/n n: adsorption intensity | |
| Temkin | : Temkin constant (L/mg) : adsorption intensity(J/mol) |
| Concentration (mg/L) | qe exp (mg/g) | PFO | PSO | ||||
|---|---|---|---|---|---|---|---|
| qe cal (mg/g) | k1 (1/min) | R2 | qe cal (mg/g) | k2 × 10−2 (g/mg min) | R2 | ||
| 20 | 14.4 | 14.4 | 0.44 | 1 | 14.6 | 13.0 | 0.99 |
| 40 | 44.2 | 44.1 | 0.27 | 0.99 | 45.6 | 1.11 | 0.99 |
| 60 | 60.7 | 58.7 | 0.22 | 0.96 | 61.9 | 0.69 | 0.99 |
| 80 | 89.1 | 85.4 | 0.29 | 0.97 | 89.6 | 0.64 | 0.99 |
| 100 | 109.7 | 106.2 | 0.21 | 0.98 | 112.2 | 0.36 | 0.99 |
| 150 | 160.5 | 154.9 | 0.07 | 0.93 | 166.9 | 0.07 | 0.98 |
| 200 | 190.2 | 184.7 | 0.05 | 0.94 | 200.8 | 0.04 | 0.97 |
| Adsorption isotherm | Parameter | Value |
|---|---|---|
| Langmuir | qmax (mg/g) | 232.8 |
| Ka (L/mg) | 0.96 | |
| R2 | 0.97 | |
| Freundlich | Kf (mg/g) (L/mg)1/n | 101.4 |
| n | 2.29 | |
| R2 | 0.92 | |
| Temkin | KT (L/mg) | 2.44 |
| bT (J/mol) | 52.5 | |
| R2 | 0.96 |
| Adsorbents | qmax (mg/g) | References |
|---|---|---|
| MSMPAC | 232.8 | This study |
| Activated carbon from green algae Ulva lactuca | 344.83 | [48] |
| Activated carbon from palm oil waste | 188 | [49] |
| Jengkol peel-based activated carbon | 170.9 | [50] |
| Activated carbon from Moringa oleifera leaf | 136.99 | [51] |
| Activated carbon from Elaeagnus angustifolia seeds | 72 | [52] |
| T (K) | qe (mg/g) | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (kJ/mol·K) | |
|---|---|---|---|---|---|
| 298.15 | 92.3 | 4.06 | −3.48 | 18.2 | 0.074 |
| 308.15 | 102.0 | 7.92 | −5.30 | ||
| 318.15 | 103.7 | 8.23 | −5.58 | ||
| 328.15 | 103.8 | 8.34 | −5.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razali, N.S.; Abdulhameed, A.S.; Jawad, A.H.; ALOthman, Z.A.; Yousef, T.A.; Al-Duaij, O.K.; Alsaiari, N.S. High-Surface-Area-Activated Carbon Derived from Mango Peels and Seeds Wastes via Microwave-Induced ZnCl2 Activation for Adsorption of Methylene Blue Dye Molecules: Statistical Optimization and Mechanism. Molecules 2022, 27, 6947. https://doi.org/10.3390/molecules27206947
Razali NS, Abdulhameed AS, Jawad AH, ALOthman ZA, Yousef TA, Al-Duaij OK, Alsaiari NS. High-Surface-Area-Activated Carbon Derived from Mango Peels and Seeds Wastes via Microwave-Induced ZnCl2 Activation for Adsorption of Methylene Blue Dye Molecules: Statistical Optimization and Mechanism. Molecules. 2022; 27(20):6947. https://doi.org/10.3390/molecules27206947
Chicago/Turabian StyleRazali, Nur Shakinah, Ahmed Saud Abdulhameed, Ali H. Jawad, Zeid A. ALOthman, Tarek A. Yousef, Omar K. Al-Duaij, and Norah Salem Alsaiari. 2022. "High-Surface-Area-Activated Carbon Derived from Mango Peels and Seeds Wastes via Microwave-Induced ZnCl2 Activation for Adsorption of Methylene Blue Dye Molecules: Statistical Optimization and Mechanism" Molecules 27, no. 20: 6947. https://doi.org/10.3390/molecules27206947
APA StyleRazali, N. S., Abdulhameed, A. S., Jawad, A. H., ALOthman, Z. A., Yousef, T. A., Al-Duaij, O. K., & Alsaiari, N. S. (2022). High-Surface-Area-Activated Carbon Derived from Mango Peels and Seeds Wastes via Microwave-Induced ZnCl2 Activation for Adsorption of Methylene Blue Dye Molecules: Statistical Optimization and Mechanism. Molecules, 27(20), 6947. https://doi.org/10.3390/molecules27206947







