AgCu NP Formation by the Ag NP Catalysis of Cu Ions at Room Temperature and Their Antibacterial Efficacy: A Kinetic Study
Abstract
:1. Introduction
2. Results
2.1. Appearance Change
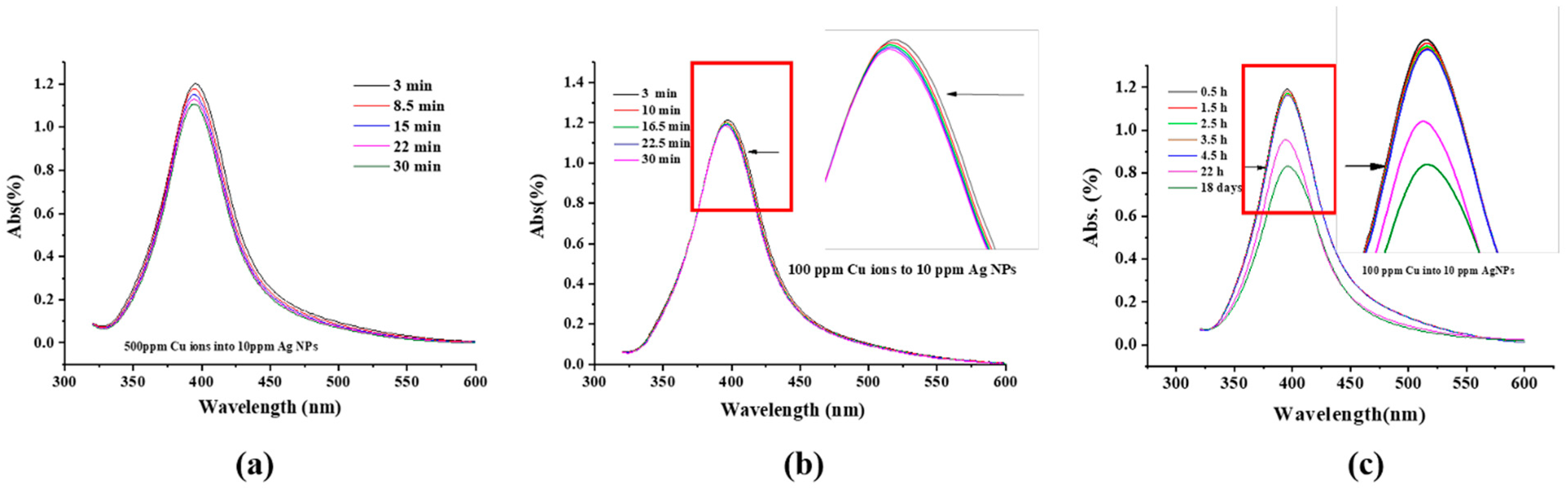
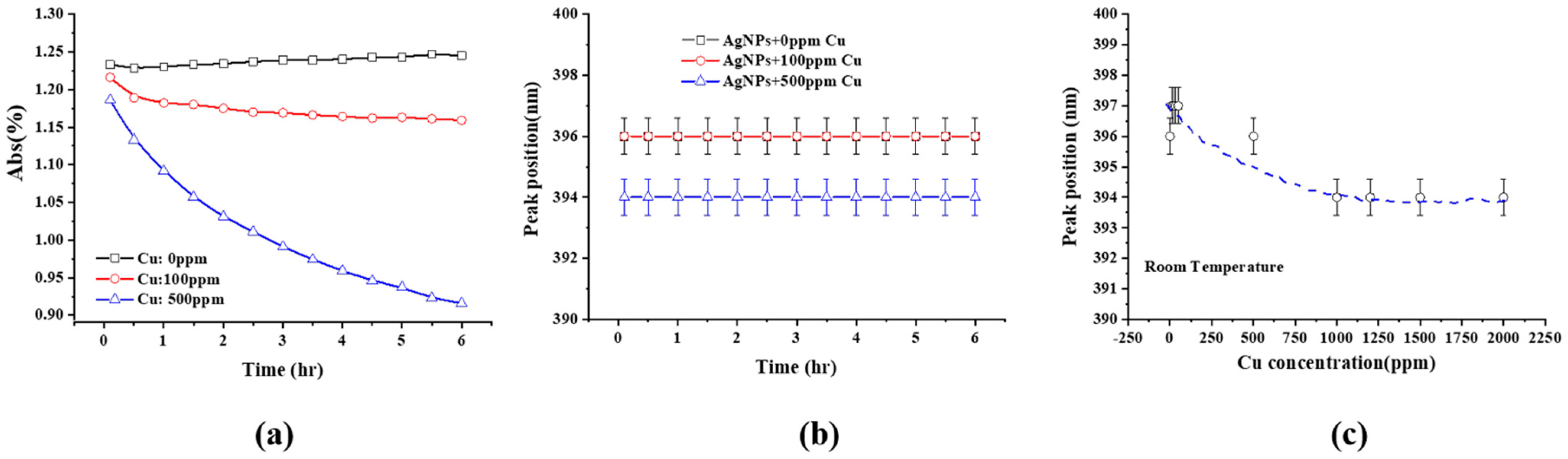
2.2. UV-Visible Spectra
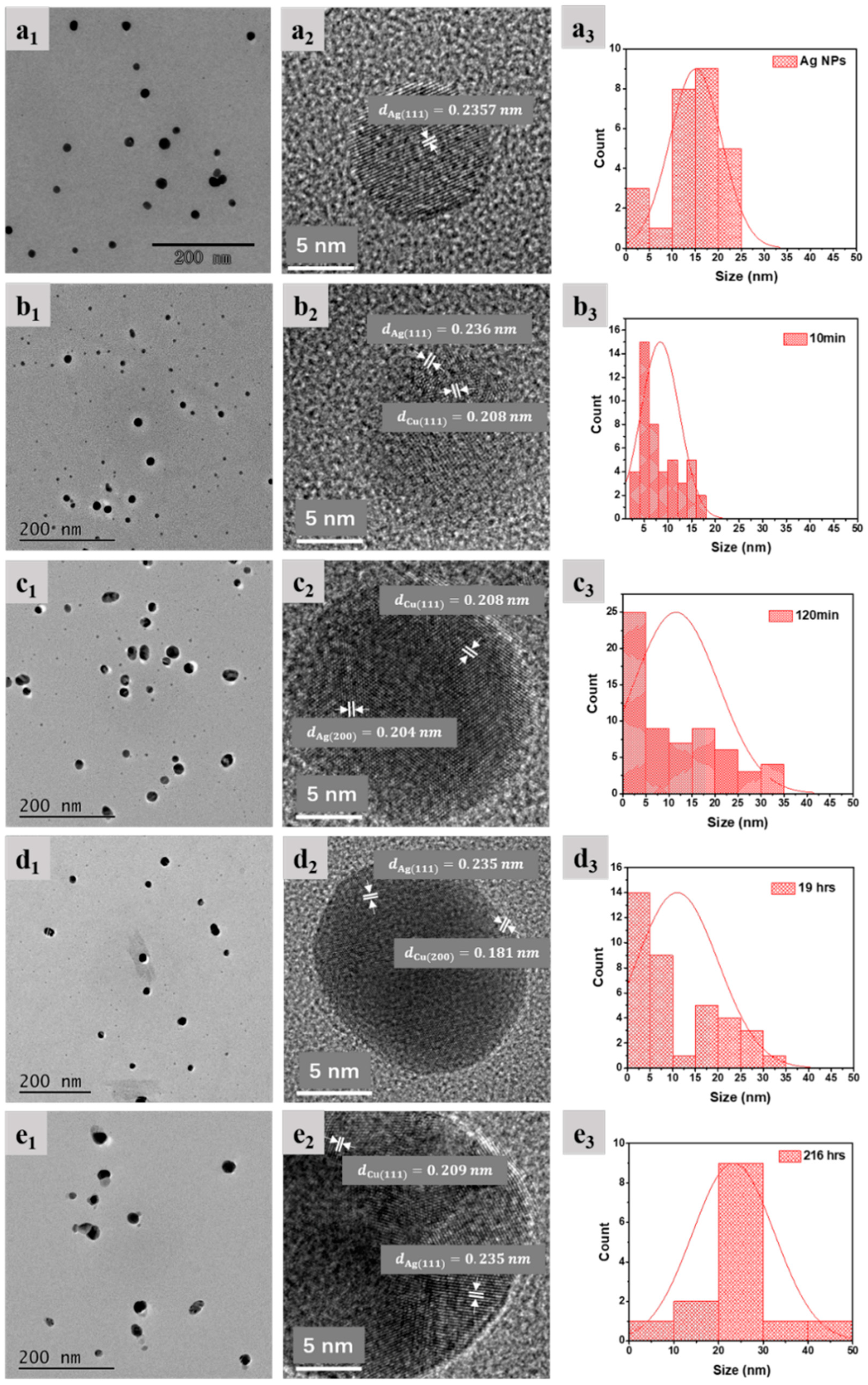
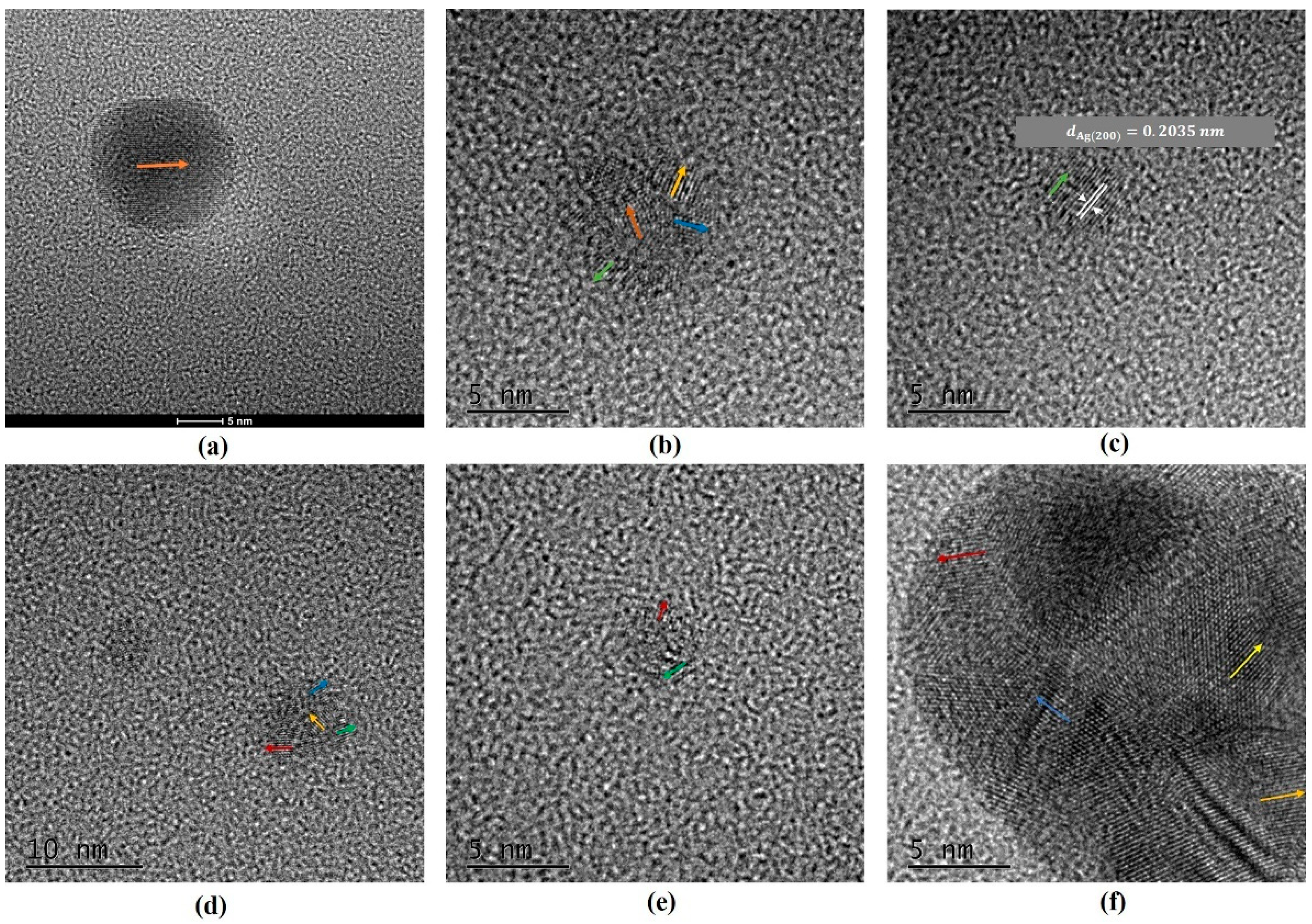
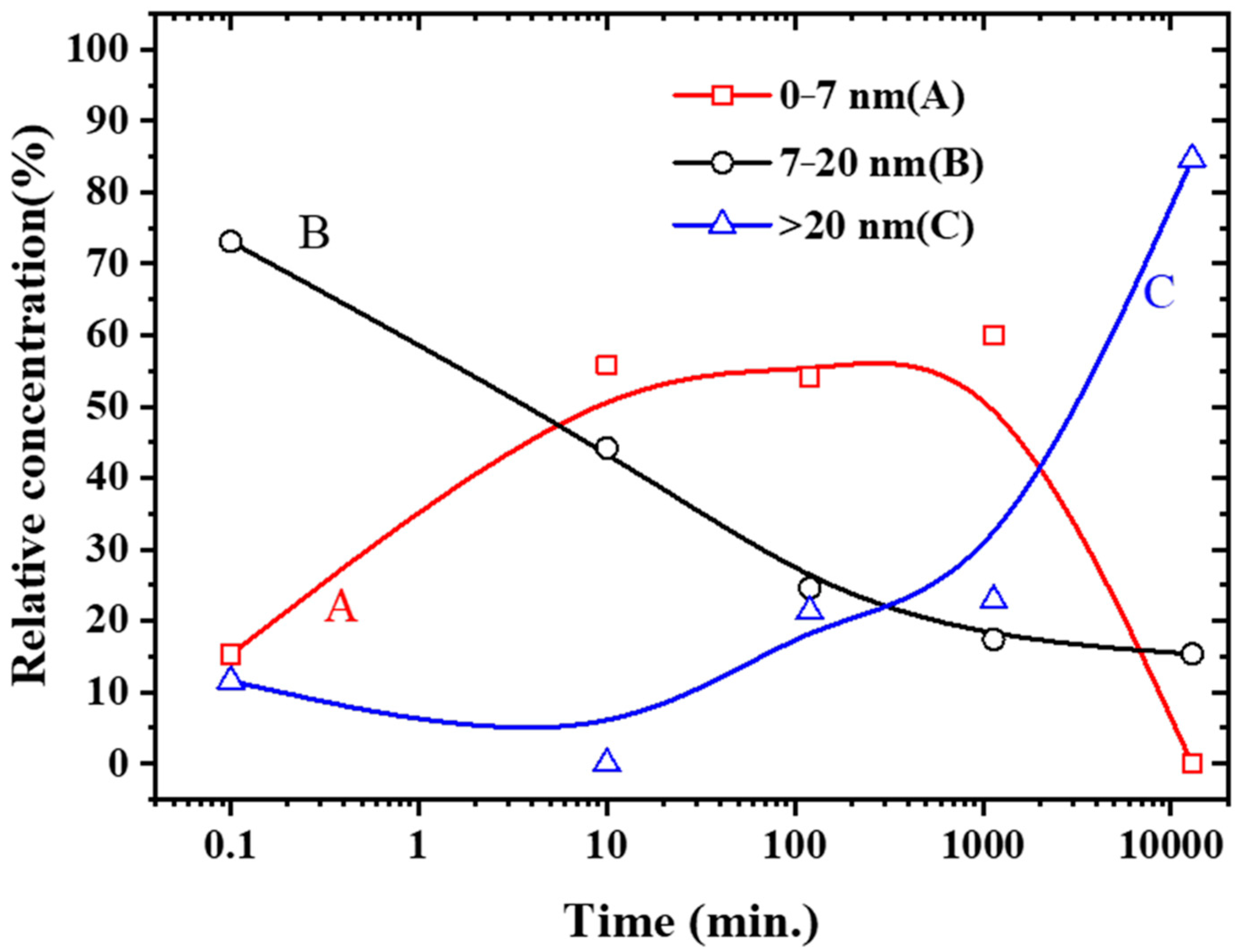
2.3. Morphologic Change of Nanoparticles
2.4. Antibacterial Activity Testing
3. Discussion
3.1. Color Change and UV-Visible Spectra
- (1)
- The peak intensity decreases with time, which can be caused by either the deposition of an outer layer of Cu or a size reduction in the Ag NPs. Both effectively reduce the Ag NP surface area, and the SPR peak intensity of the Ag NPs is directly proportional to its surface area [40];
- (2)
- (3)
- The initial SPR peak narrowing at the high wavelength side and the later narrowing at the low wavelength side, in Figure 1a,b, are attributed, respectively, to the NP size decrease and AgCu alloying; alloying NPs will lead to an SPR peak red shift [1,5,44]. This is consistent with the TEM data and will be discussed later.
- (4)
- (5)
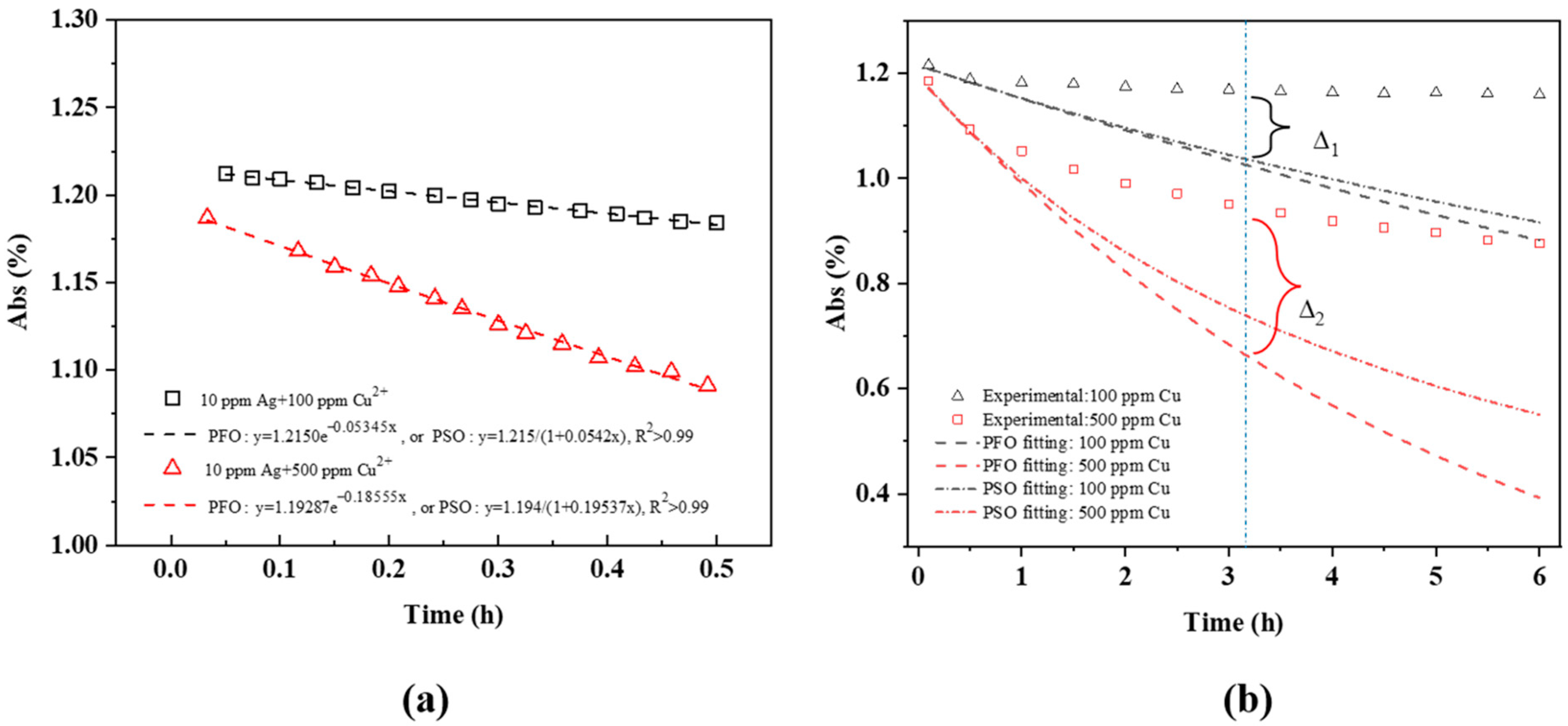
- The dependence of the SPR peak intensity on time and Cu2+ concentration (Figure 2a and Figure 3) indicates that the greater the Cu concentration, the greater the SPR intensity decrease. It may indicate that the greater the Cu2+ concentration, the faster it is deposited onto the Ag NPs, reducing the uncovered Ag NP area, and diminishing the SPR peak intensity. This will be discussed in Section 3.3.
3.2. Morphologic Changes
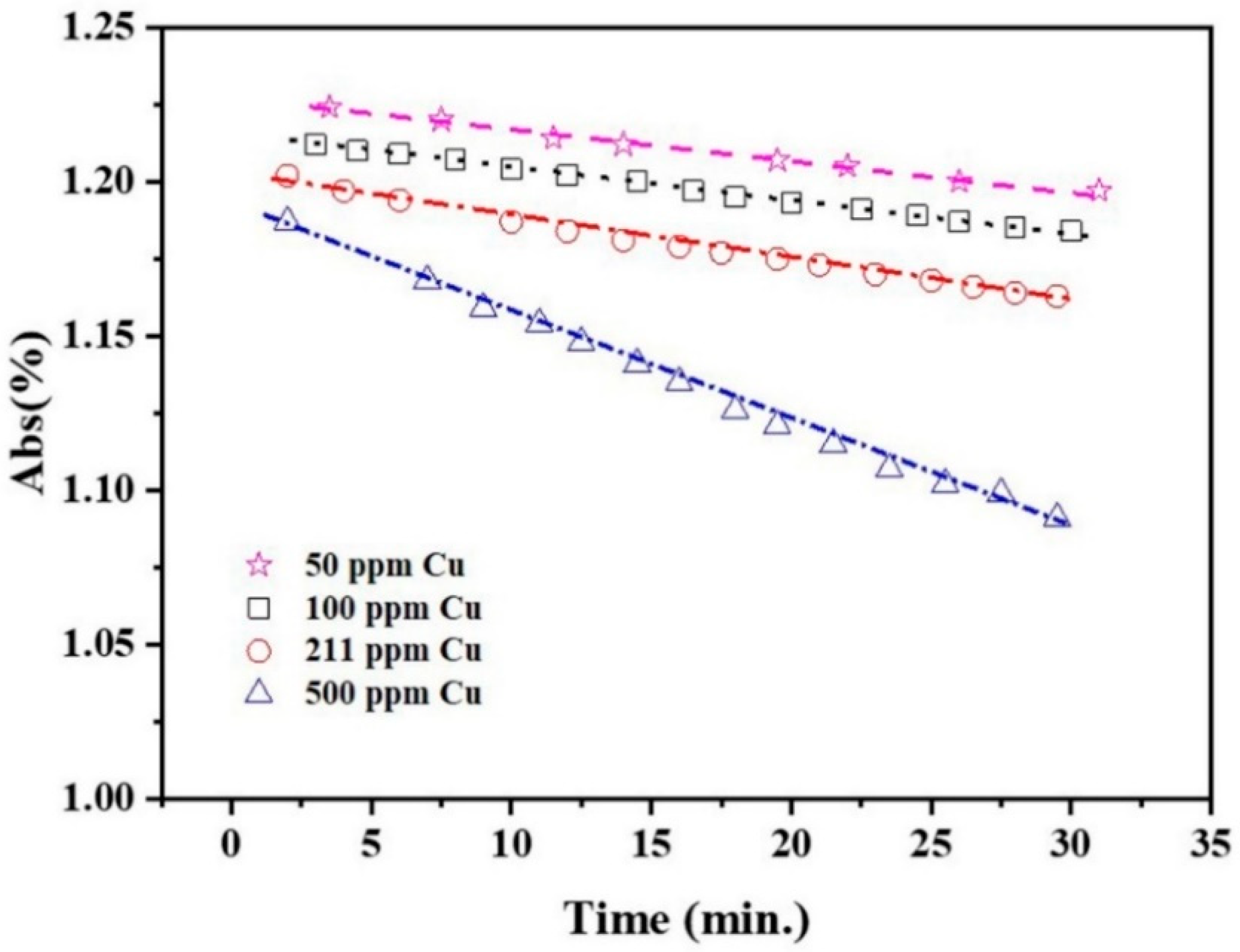
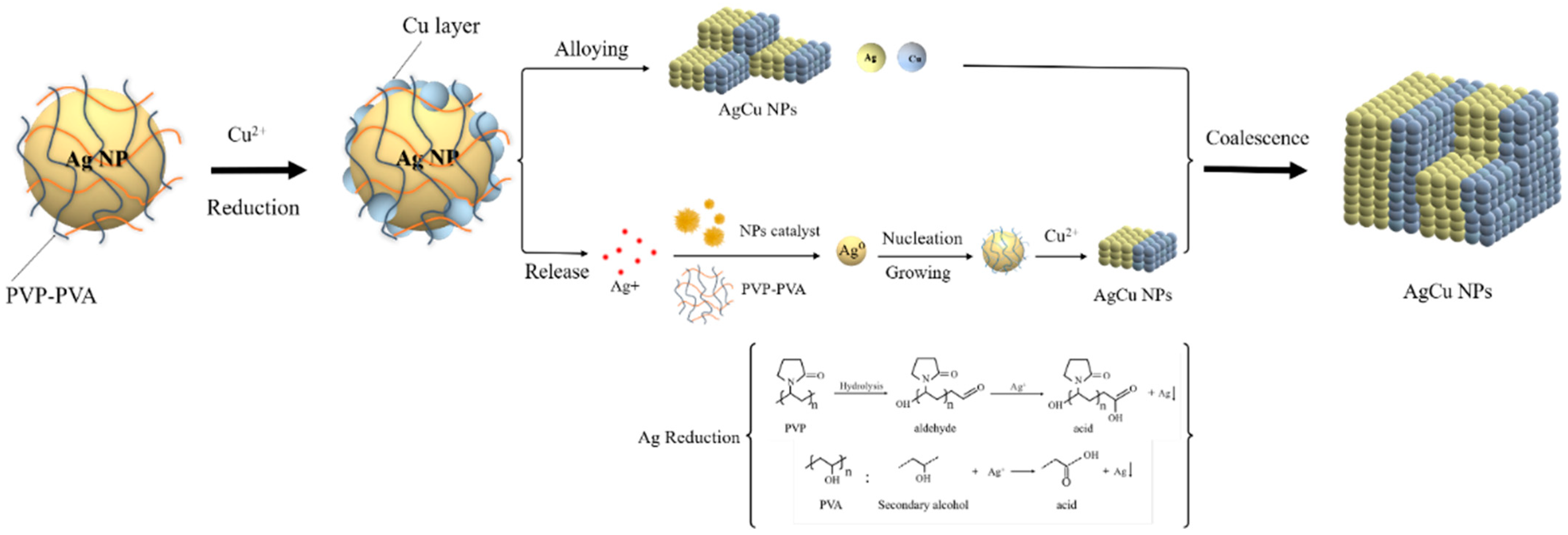
3.3. AgCu NA Formation Kinetics
3.4. Antibacterial Efficacy Enhancement of AgCu NAs
4. Materials and Methods
4.1. Materials
4.2. Characterization
4.3. Antibacterial Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Taner, M.; Sayar, N.; Yulug, I.G.; Suzer, S. Synthesis, characterization and antibacterial investigation of silver–copper nanoalloys. J. Mater. Chem. 2011, 21, 13150–13154. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Gołąbiewska, A.; Rajski, Ł.; Kowal, E.; Sajdak, A.; Zaleska-Medynska, A. Synthesis and characterization of monometallic (Ag, Cu) and bimetallic Ag-Cu particles for antibacterial and antifungal applications. J. Nanomater. 2016, 2016, 2187940. [Google Scholar] [CrossRef] [Green Version]
- Długosz, O.; Sochocka, M.; Ochnik, M.; Banach, M. Metal and bimetallic nanoparticles: Flow synthesis, bioactivity and toxicity. J. Colloid Interface Sci. 2021, 586, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yahia, L.H.; Sacher, E. Antimicrobial properties of the Ag, Cu nanoparticle system. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Zhou, F.; Zhu, Y.; Yang, L.; Yang, D.-Q.; Sacher, E. Ag NP catalysis of Cu ions in the preparation of AgCu NPs and the mechanism of their enhanced antibacterial efficacy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127831. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Chen, Y.-C.; Kang, L.; Yu, J.; Wang, Y.; Lu, C.; Mashimo, T.; Yoshiasa, A.; Lin, C.-H. Homogeneously alloyed nanoparticles of immiscible Ag–Cu with ultrahigh antibacterial activity. Colloids Surf. B Biointerfaces 2019, 180, 466–472. [Google Scholar] [CrossRef]
- Thakore, S.I.; Nagar, P.S.; Jadeja, R.N.; Thounaojam, M.; Devkar, R.V.; Rathore, P.S. Sapota fruit latex mediated synthesis of Ag, Cu mono and bimetallic nanoparticles and their in vitro toxicity studies. Arab. J. Chem. 2019, 12, 694–700. [Google Scholar] [CrossRef] [Green Version]
- Al Tamimi, S.; Ashraf, S.; Abdulrehman, T.; Parray, A.; Mansour, S.A.; Haik, Y.; Qadri, S. Synthesis and analysis of silver–copper alloy nanoparticles of different ratios manifest anticancer activity in breast cancer cells. Cancer Nanotechnol. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Ashraf, S.; Qadri, S.; Akbar, S.; Parray, A.; Haik, Y. Biogenesis of Exosomes Laden with Metallic Silver–Copper Nanoparticles Liaised by Wheat Germ Agglutinin for Targeted Delivery of Therapeutics to Breast Cancer. Adv. Biol. 2022, 6, 2200005. [Google Scholar] [CrossRef]
- Kushwah, M.; Gaur, M.; Berlina, A.N.; Arora, K. Biosynthesis of novel Ag@ Cu alloy NPs for enhancement of methylene blue photocatalytic activity and antibacterial activity. Mater. Res. Express 2019, 6, 116561. [Google Scholar] [CrossRef]
- Bello-Lopez, J.; Silva-Bermudez, P.; Prado, G.; Martínez, A.; Ibáñez-Cervantes, G.; Cureño-Díaz, M.A.; Rocha-Zavaleta, L.; Manzo-Merino, J.; Almaguer-Flores, A.; Ramos-Vilchis, C. Biocide effect against SARS-CoV-2 and ESKAPE pathogens of a noncytotoxic silver–copper nanofilm. Biomed. Mater. 2021, 17, 015002. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F. Optimization of the synthesis of fungus-mediated bi-metallic Ag-Cu nanoparticles. Appl. Sci. 2022, 12, 1384. [Google Scholar] [CrossRef]
- Kim, N.R.; Shin, K.; Jung, I.; Shim, M.; Lee, H.M.J. Ag–Cu bimetallic nanoparticles with enhanced resistance to oxidation: A combined experimental and theoretical study. J. Phys. Chem. C 2014, 118, 26324–26331. [Google Scholar] [CrossRef]
- Gao, X.; Zou, C.; Zhou, H.; Yuan, C.; He, J.; Luo, X. Enhanced nonlinear optical properties of alloyed AgCu glassy nanoparticles. J. Alloy. Compd. 2020, 819, 153003. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, F. Facile preparation of Ag-Cu bifunctional electrocatalysts for zinc-air batteries. Electrochim. Acta 2015, 158, 437–445. [Google Scholar] [CrossRef]
- Shin, K.; Kim, D.H.; Lee, H.M. Catalytic characteristics of AgCu bimetallic nanoparticles in the oxygen reduction reaction. ChemSusChem 2013, 6, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Blas, M.; Maldonado-Luna, N.M.; Rivera-Quiñones, C.M.; Vega-Avila, A.L.; Roman-Velázquez, F.R.; Perales-Perez, O.J. Single Step Microwave Assisted Synthesis and Antimicrobial Activity of Silver, Copper and Silver-Copper Nanoparticles. J. Mater. Sci. Chem. Eng. 2020, 8, 13. [Google Scholar] [CrossRef]
- Shih, C.-Y.; Chen, C.; Rehbock, C.; Tymoczko, A.; Wiedwald, U.; Kamp, M.; Schuermann, U.; Kienle, L.; Barcikowski, S.; Zhigilei, L.V. Limited elemental mixing in nanoparticles generated by ultrashort pulse laser ablation of AgCu bilayer thin films in a liquid environment: Atomistic modeling and experiments. J. Phys. Chem. C 2021, 125, 2132–2155. [Google Scholar] [CrossRef]
- Swiatkowska-Warkocka, Z.; Pyatenko, A.; Krok, F.; Jany, B.R.; Marszalek, M. Synthesis of new metastable nanoalloys of immiscible metals with a pulse laser technique. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Abdul Salam, A.; Singaravelan, R.; Vasanthi, P.; Bangarusudarsan Alwar, S. Electrochemical fabrication of Ag–Cu nano alloy and its characterization: An investigation. J. Nanostruct. Chem. 2015, 5, 383–392. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Paszkiewicz, O.; Michalkiewicz, B.; Kamińska, A.; Wróbel, R.J. Fabrication of Antibacterial Metal Surfaces Using Magnetron-Sputtering Method. Materials 2021, 14, 7301. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, properties, and biological applications of metallic alloy nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Rehbock, C.; Hühn, D.; Carrillo-Carrion, C.; de Aberasturi, D.J.; Merk, V.; Barcikowski, S.; Parak, W.J. Interaction of colloidal nanoparticles with their local environment: The (ionic) nanoenvironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface 2014, 11, 20130931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla-Cruz, A.; Garza-Cervantes, J.; Vasto-Anzaldo, X.; García-Rivas, G.; León-Buitimea, A.; Morones-Ramírez, J. Synthesis and design of Ag–Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens. Sci. Rep. 2021, 11, 1–10. [Google Scholar]
- Al-Haddad, J.; Alzaabi, F.; Pal, P.; Rambabu, K.; Banat, F.J.C.T.; Policy, E. Green synthesis of bimetallic copper–silver nanoparticles and their application in catalytic and antibacterial activities. Clean Technol. Environ. Policy 2020, 22, 269–277. [Google Scholar] [CrossRef]
- Escudero-Escribano, M.; Jensen, K.D.; Jensen, A.W. Recent advances in bimetallic electrocatalysts for oxygen reduction: Design principles, structure-function relations and active phase elucidation. Curr. Opin. Electrochem. 2018, 8, 135–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Z.; Yuan, W.; Xu, H.; You, X.; Liao, X. Green and rapid synthesis of cysteine-directed novel AgCu nanocluster hydrogel with good antibacterial activity. Materialia 2021, 20, 101232. [Google Scholar] [CrossRef]
- Providence, B.A.; Chinyere, A.A.; Ayi, A.A.; Charles, O.O.; Elijah, T.A.; Ayomide, H.L. Green synthesis of silver monometallic and copper-silver bimetallic nanoparticles using Kigelia africana fruit extract and evaluation of their antimicrobial activities. Int. J. Phys. Sci. 2018, 13, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Sasireka, K.S.; Lalitha, P. Biogenic synthesis of bimetallic nanoparticles and their applications. Rev. Inorg. Chem. 2021, 41, 223–244. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, F.; Hu, J.; Yang, L.; Yang, D.-Q.; Sacher, E. A facile route to prepare colorless Ag-Cu nanoparticle dispersions with elevated antibacterial effects. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127116. [Google Scholar] [CrossRef]
- Dou, Q.; Li, Y.; Wong, K.W.; Ng, K.M. Facile synthesis of nearly monodisperse AgCu alloy nanoparticles with synergistic effect against oxidation and electromigration. J. Mater. Res. 2019, 34, 2095–2104. [Google Scholar] [CrossRef]
- Mock, J.; Barbic, M.; Smith, D.; Schultz, D.; Schultz, S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Gonzalez, A.L.; Noguez, C.; Beranek, J.; Barnard, A.S. Size, Shape, Stability, and Color of Plasmonic Silver Nanoparticles. J. Phys. Chem. C 2014, 118, 9128–9136. [Google Scholar] [CrossRef]
- Stockman, M.I.; Halas, N.; Huser, T. Plasmonics: Metallic Nanostructures and Their Optical Properties III; SPIE: Bellingham, WA, USA, 2005. [Google Scholar]
- Underwood, S.; Mulvaney, P. Effect of the solution refractive index on the color of gold colloids. Langmuir 1994, 10, 3427–3430. [Google Scholar] [CrossRef]
- Yang, L.; Yang, D.-Q.; Sacher, E. Colors and surface plasmon resonances of low concentrations of silver nanoparticles in various liquid media. Explor. Mater. Sci. Res. 2022, 3, 83–91. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Sikder, M.; Lead, J.R.; Chandler, G.T.; Baalousha, M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by UV–Vis. Sci. Total Environ. 2018, 618, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Lodeiro, P.; Achterberg, E.P.; El-Shahawi, M.S. Detection of silver nanoparticles in seawater at ppb levels using UV–visible spectrophotometry with long path cells. Talanta 2017, 164, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Gómez, L.A.; de Araújo, C.B.; Silva, A.B.; Galembeck, A. Influence of stabilizing agents on the nonlinear susceptibility of silver nanoparticles. JOSA B 2007, 24, 2136–2140. [Google Scholar] [CrossRef]
- Peng, S.; McMahon, J.M.; Schatz, G.C.; Gray, S.K.; Sun, Y. Reversing the size-dependence of surface plasmon resonances. Proc. Natl. Acad. Sci. USA 2010, 107, 14530–14534. [Google Scholar] [CrossRef] [Green Version]
- Mock, J.J.; Smith, D.R.; Schultz, S. Local refractive index dependence of plasmon resonance spectra from individual nanoparticles. Nano Lett. 2003, 3, 485–491. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Characterization of the optical properties of heavy metal ions using surface plasmon resonance technique. Opt. Photonics J. 2011, 1, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Wang, Z.; Sun, Q.; Wang, J.; Zhang, C.; Chen, D.; Li, X. High-performance Ag–Cu nanoalloy catalyst for the selective catalytic oxidation of ammonia. ACS Appl. Mater. Interfaces 2019, 11, 46875–46885. [Google Scholar] [CrossRef] [PubMed]
- Ebisawa, M.; Yamaguchi, T.; Teranishi, Y.; Isoda, K. Color modulation of Ag nanoparticle dispersion by light-induced aggregation. IEEJ Trans. Electr. Electron. Eng. 2017, 12, S125–S126. [Google Scholar] [CrossRef]
- Hu, J.; Yang, L.; Zhu, Y.; Yang, D.-Q.; Sacher, E. Destabilization of PVA-stabilized Ag NPs: Color changes at low aqueous concentrations, induced by aggregation and coalescence. Mater. Res. Express 2020, 7, 025003. [Google Scholar] [CrossRef]
- Alqadi, M.; Abo Noqtah, O.; Alzoubi, F.; Alzouby, J.; Aljarrah, K. pH effect on the aggregation of silver nanoparticles synthesized by chemical reduction. Mater. Sci.-Pol. 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Haaf, F.; Sanner, A.; Straub, F. Polymers of N-vinylpyrrolidone: Synthesis, characterization and uses. Polym. J. 1985, 17, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Dong, K. Cu ions suppression effects of PVA reduction Ag ions and how to overcome it. 2023; paper in preparation. [Google Scholar]
- Lu, Y.; Chen, W. Size effect of silver nanoclusters on their catalytic activity for oxygen electro-reduction. J. Power Sources 2012, 197, 107–110. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Chen, H.-W.; Chien, P.-S.; Chiou, C.-S.; Liu, C.-C. Application of bifunctional magnetic adsorbent to adsorb metal cations and anionic dyes in aqueous solution. J. Hazard. Mater. 2011, 185, 1124–1130. [Google Scholar] [CrossRef]
- Hao, Y.-M.; Man, C.; Hu, Z.-B. Effective removal of Cu (II) ions from aqueous solution by amino-functionalized magnetic nanoparticles. J. Hazard. Mater. 2010, 184, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Kowanga, K.D.; Gatebe, E.; Mauti, G.O.; Mauti, E.M. Kinetic, sorption isotherms, pseudo-first-order model and pseudo-second-order model studies of Cu (II) and Pb (II) using defatted Moringa oleifera seed powder. J. Phytopharm. 2016, 5, 71–78. [Google Scholar] [CrossRef]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328–7340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kurvet, I.; Kahru, A. Particle-cell contact enhances antibacterial activity of silver nanoparticles. PLoS ONE 2013, 8, e64060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Dong, Y.; Yang, Y.; Yu, P.; Zhang, Y.; Hu, J.; Li, T.; Zhang, X.; Liu, X.; Xu, Q. Rational design of silver gradient for studying size effect of silver nanoparticles on contact killing. ACS Biomater. Sci. Eng. 2018, 5, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F. The enhanced stability and antibacterial efficacy, and the reduced cytotoxicity, of AgCu nanoparticles Incubated with bacteria. Biomater. Adv. 2022. submitted. [Google Scholar]
- Amsterdam, D. Antibiotics in Laboratory Medicine, 6th ed.; LWW: Philadelphia, AR, USA, 26 September 2014. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Mb, N.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Parvekar, P.; Palaskar, J.N.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Zhou, F.; Wang, K.; Yang, D.; Sacher, E. AgCu NP Formation by the Ag NP Catalysis of Cu Ions at Room Temperature and Their Antibacterial Efficacy: A Kinetic Study. Molecules 2022, 27, 6951. https://doi.org/10.3390/molecules27206951
Tao Y, Zhou F, Wang K, Yang D, Sacher E. AgCu NP Formation by the Ag NP Catalysis of Cu Ions at Room Temperature and Their Antibacterial Efficacy: A Kinetic Study. Molecules. 2022; 27(20):6951. https://doi.org/10.3390/molecules27206951
Chicago/Turabian StyleTao, Yujie, Fang Zhou, Kaixin Wang, Dequan Yang, and Edward Sacher. 2022. "AgCu NP Formation by the Ag NP Catalysis of Cu Ions at Room Temperature and Their Antibacterial Efficacy: A Kinetic Study" Molecules 27, no. 20: 6951. https://doi.org/10.3390/molecules27206951
APA StyleTao, Y., Zhou, F., Wang, K., Yang, D., & Sacher, E. (2022). AgCu NP Formation by the Ag NP Catalysis of Cu Ions at Room Temperature and Their Antibacterial Efficacy: A Kinetic Study. Molecules, 27(20), 6951. https://doi.org/10.3390/molecules27206951







