Multifunctional Characteristics of Carbon Fibers Modified with Imidazolium Ionic Liquids
Abstract
1. Introduction
2. Experiment
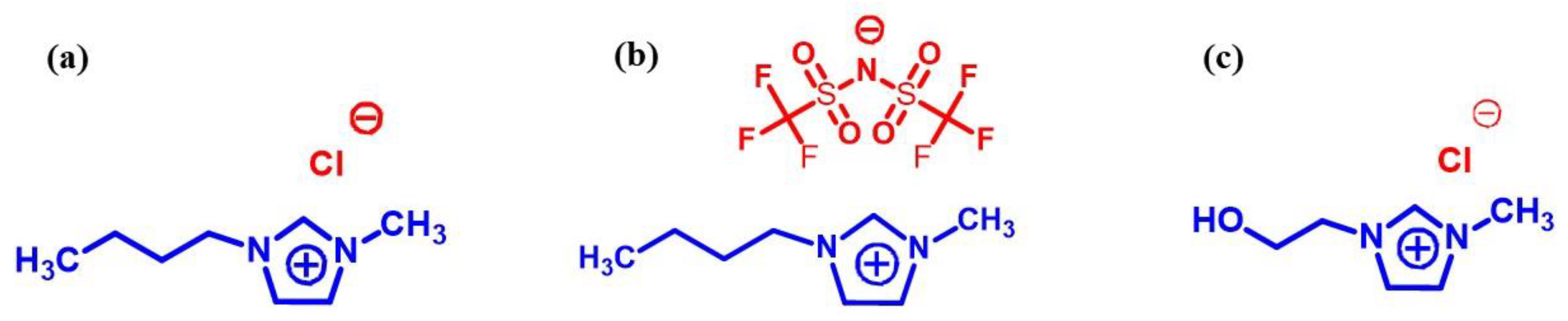
2.1. Materials
2.2. Fiber Treatment Process
2.3. Characterization Techniques
3. Results and Discussion
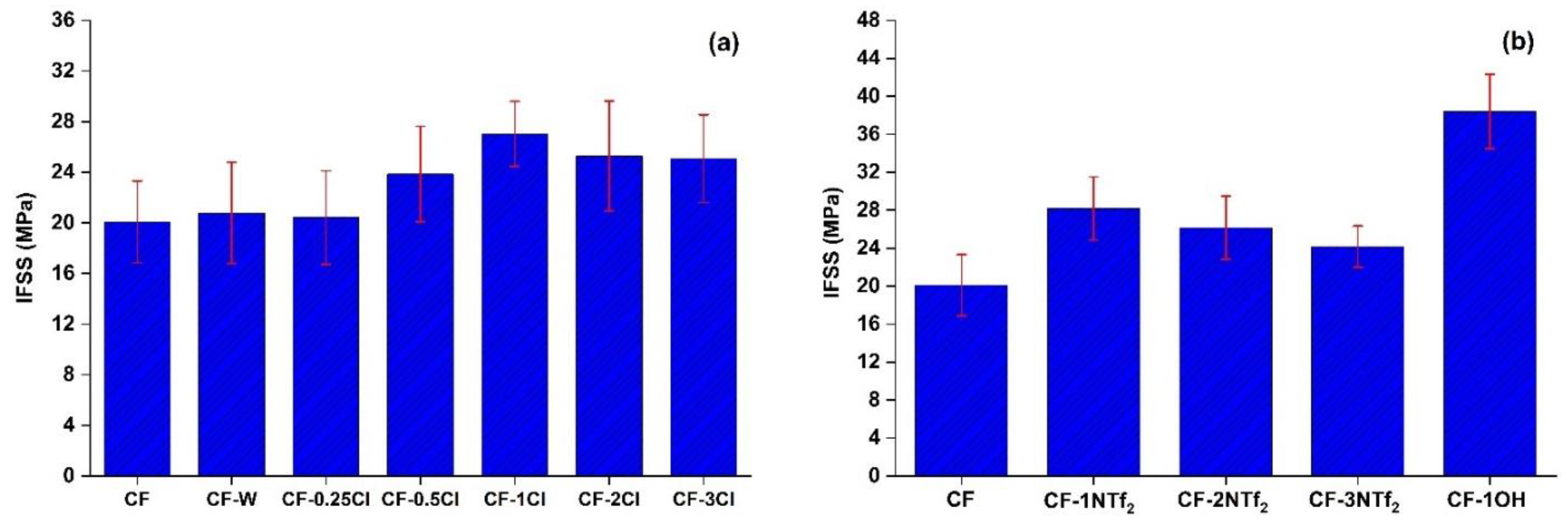
3.1. Interfacial Shear Strength
3.2. Wettability
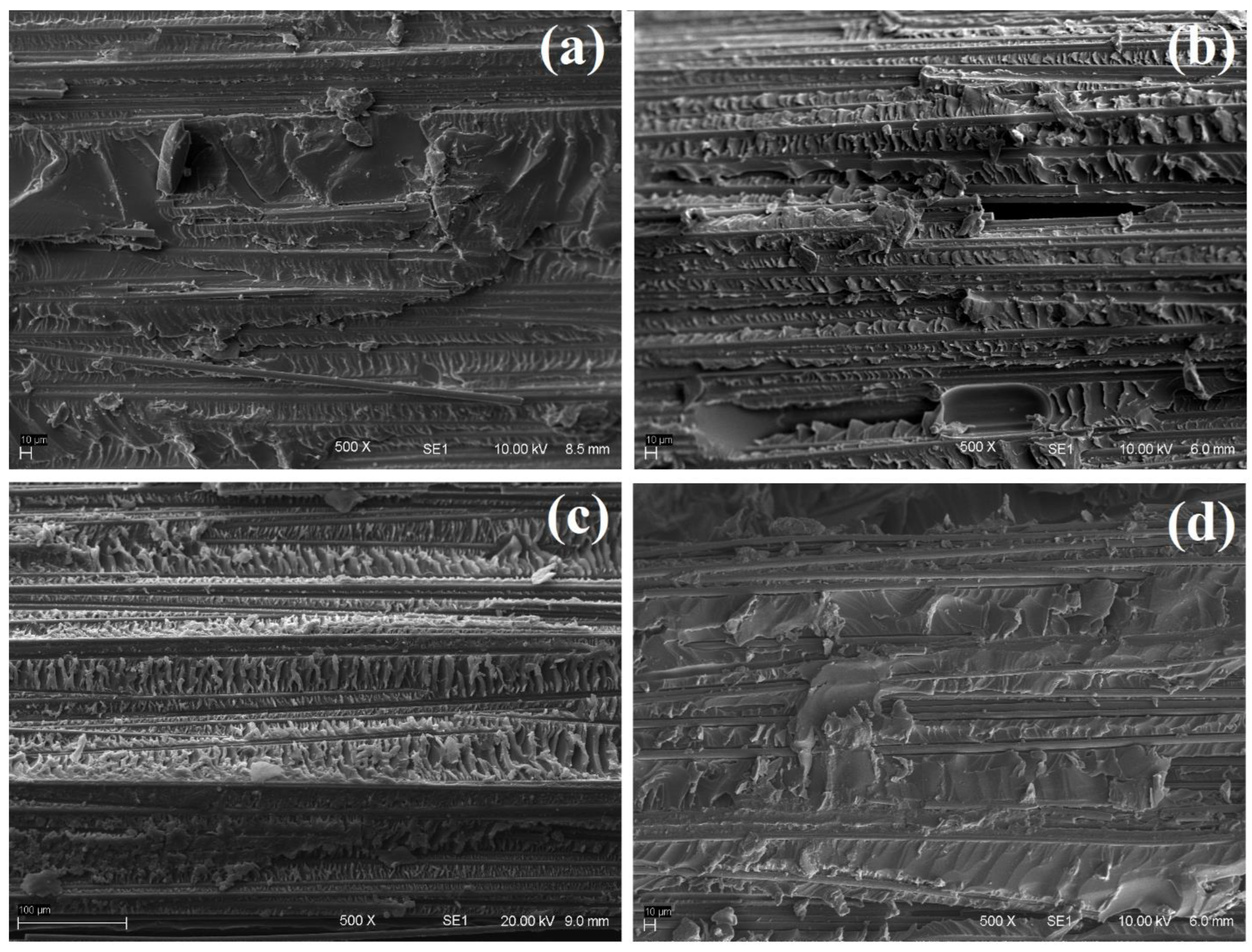
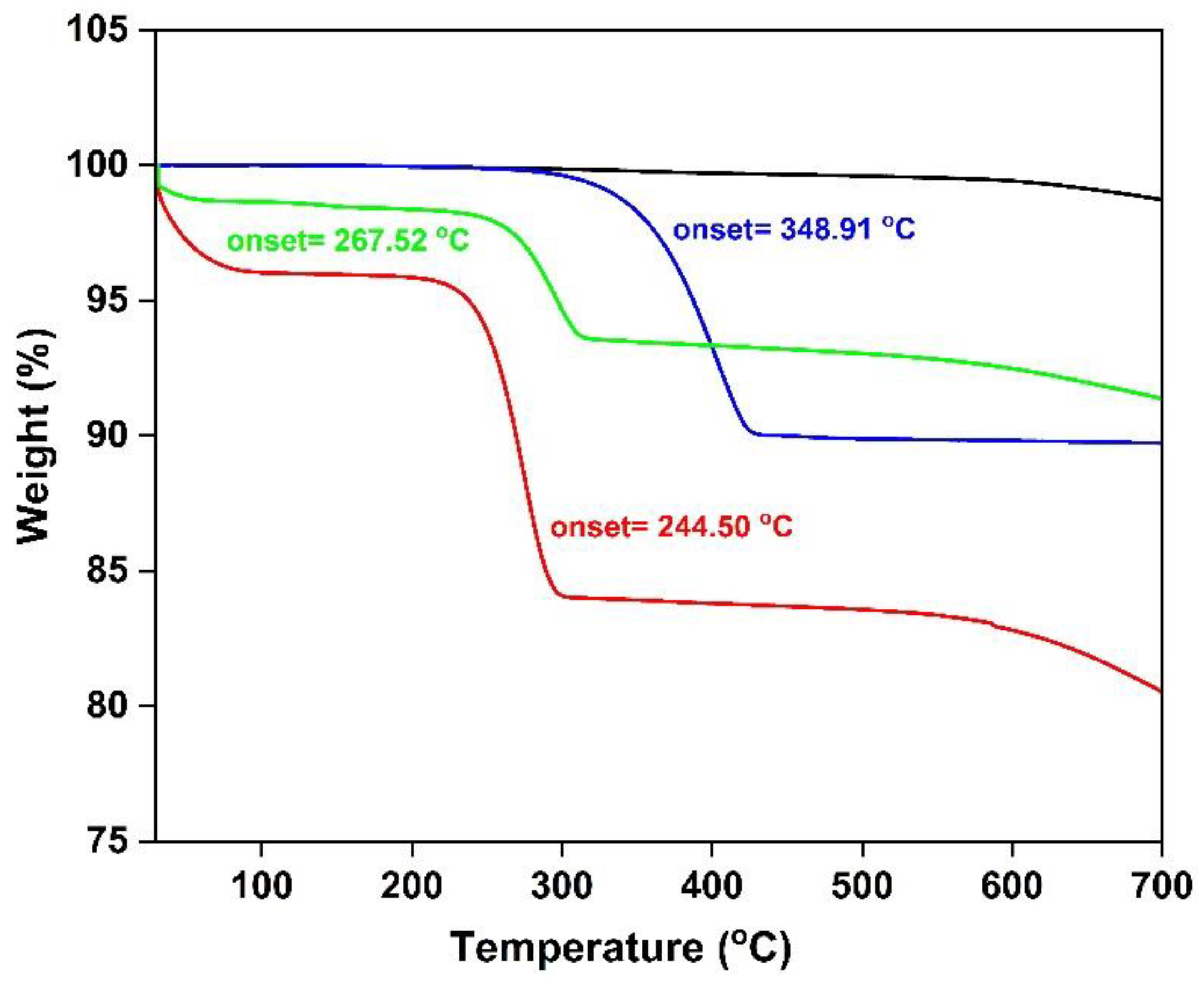
3.3. Surface Analysis
3.4. Electrical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kar, M.; Plechkova, N.V.; Seddon, K.R.; Pringle, J.M.; MacFarlane, D.R. Ionic liquids–further progress on the fundamental issues. Aust. J. Chem. 2018, 72, 3–10. [Google Scholar] [CrossRef]
- Kowsari, M.; Alavi, S.; Ashrafizaadeh, M.; Najafi, B. Molecular dynamics simulation of imidazolium-based ionic liquids. II. Transport coefficients. J. Chem. Phys. 2009, 130, 014703. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hu, Y.; Xu, W.; Wang, Y.; Guo, X. Recent progress in the assembly behavior of imidazolium-based ionic liquid surfactants. J. Mol. Liq. 2020, 319, 114354. [Google Scholar] [CrossRef]
- Izgorodina, E.I.; MacFarlane, D.R. Nature of hydrogen bonding in charged hydrogen-bonded complexes and imidazolium-based ionic liquids. J. Phys. Chem. B 2011, 115, 14659–14667. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xu, W.; Ding, Y.; Xue, P.; Sheng, P.; Qiao, H.; Wang, S.; Yu, Y. Mechanical and thermal properties of all-wood biocomposites through controllable dissolution of cellulose with ionic liquid. Polymers 2020, 12, 361. [Google Scholar] [CrossRef]
- May, D.; Goergen, C.; Friedrich, K. Multifunctionality of polymer composites based on recycled carbon fibers: A review. Adv. Ind. Eng. Polym. Res. 2021, 4, 70–81. [Google Scholar] [CrossRef]
- Abdou, T.R.; Junior, A.B.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of polymeric composites from industrial waste by pyrolysis: Deep evaluation for carbon fibers reuse. Waste Manag. 2021, 120, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, B.; Ye, D.; Liu, Z. Fully recyclable carbon fiber reinforced vanillin-based epoxy vitrimers. Eur. Polym. J. 2022, 162, 110927. [Google Scholar] [CrossRef]
- Pimenta, S.; Pinho, S.T. The effect of recycling on the mechanical response of carbon fibres and their composites. Compos. Struct. 2012, 94, 3669–3684. [Google Scholar] [CrossRef]
- Boulanghien, M.; R’Mili, M.; Bernhart, G.; Berthet, F.; Soudais, Y. Mechanical characterization of carbon fibres recycled by steam thermolysis: A statistical approach. Adv. Mater. Sci. Eng. 2018, 2018, 8630232. [Google Scholar] [CrossRef]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near-and supercritical solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Song, W.; Magid, A.; Li, D.; Lee, K.-Y. Application of recycled carbon-fibre-reinforced polymers as reinforcement for epoxy foams. J. Environ. Manag. 2020, 269, 110766. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, J.H.; Han, G.; Quagliato, L.; Kim, Y.; Choi, J.; Keum, T.; Kim, S.; Kim, N.; Lee, H. Design and numerical evaluation of recycled-carbon-fiber-reinforced polymer/metal hybrid engine cradle concepts. Int. J. Mech. Sci. 2019, 163, 105115. [Google Scholar] [CrossRef]
- Cai, G.; Wada, M.; Ohsawa, I.; Kitaoka, S.; Takahashi, J. Interfacial adhesion of recycled carbon fibers to polypropylene resin: Effect of superheated steam on the surface chemical state of carbon fiber. Compos. Part A Appl. Sci. Manuf. 2019, 120, 33–40. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.; Gadhamshetty, V.; Athanassiadis, D.; Tysklind, M.; Meng, F.; Pan, Q.; Cullen, J.M.; Yacout, D.M. Wind Turbine Blades Using Recycled Carbon Fibers: An Environmental Assessment. Environ. Sci. Technol. 2022, 56, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Luo, D.; Shi, X. Effect of chemically modified recycled carbon fiber composite on the mechanical properties of cementitious mortar. Compos. Part B Eng. 2019, 173, 106853. [Google Scholar] [CrossRef]
- Lee, H.; Ohsawa, I.; Takahashi, J. Effect of plasma surface treatment of recycled carbon fiber on carbon fiber-reinforced plastics (CFRP) interfacial properties. Appl. Surf. Sci. 2015, 328, 241–246. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.; Walker, G.; Wong, K.; Rudd, C. Surface characterisation of carbon fibre recycled using fluidised bed. Appl. Surf. Sci. 2008, 254, 2588–2593. [Google Scholar] [CrossRef]
- Jiang, G.Z.; Pickering, S.J. Recycled Carbon Fibres: Contact Angles and Interfacial Bonding with Thermoset Resins. In Materials Science Forum. 2012, pp. 255–261. Available online: https://www.scientific.net/MSF.714.255 (accessed on 30 August 2022).
- Feng, N.; Wang, X.; Wu, D. Surface modification of recycled carbon fiber and its reinforcement effect on nylon 6 composites: Mechanical properties, morphology and crystallization behaviors. Curr. Appl. Phys. 2013, 13, 2038–2050. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Varley, R.; Wang, X. Recycled carbon fiber nonwoven functionalized with fluorine-free superhydrophobic PDMS/ZIF-8 coating for efficient oil-water separation. J. Environ. Chem. Eng. 2021, 9, 106329. [Google Scholar] [CrossRef]
- Wong, K.; Mohammed, D.S.; Pickering, S.; Brooks, R. Effect of coupling agents on reinforcing potential of recycled carbon fibre for polypropylene composite. Compos. Sci. Technol. 2012, 72, 835–844. [Google Scholar] [CrossRef]
- Burn, D.; Harper, L.T.; Johnson, M.; Warrior, N.; Nagel, U.; Yang, L.; Thomason, J. The usability of recycled carbon fibres in short fibre thermoplastics: Interfacial properties. J. Mater. Sci. 2016, 51, 7699–7715. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, H.-M.; An, J.-H.; Chung, D.-C.; An, K.-H.; Kim, B.-J. Recycling and characterization of carbon fibers from carbon fiber reinforced epoxy matrix composites by a novel super-heated-steam method. J. Environ. Manag. 2017, 203, 872–879. [Google Scholar] [CrossRef]
- Palola, S.; Laurikainen, P.; García-Arrieta, S.; Goikuria Astorkia, E.; Sarlin, E. Towards Sustainable Composite Manufacturing with Recycled Carbon Fiber Reinforced Thermoplastic Composites. Polymers 2022, 14, 1098. [Google Scholar] [CrossRef] [PubMed]
- Van de Werken, N.; Reese, M.S.; Taha, M.R.; Tehrani, M. Investigating the effects of fiber surface treatment and alignment on mechanical properties of recycled carbon fiber composites. Compos. Part A Appl. Sci. Manuf. 2019, 119, 38–47. [Google Scholar] [CrossRef]
- Okajima, I.; Hiramatsu, M.; Shimamura, Y.; Awaya, T.; Sako, T. Chemical recycling of carbon fiber reinforced plastic using supercritical methanol. J. Supercrit. Fluids 2014, 91, 68–76. [Google Scholar] [CrossRef]
- Sun, H.; Guo, G.; Memon, S.A.; Xu, W.; Zhang, Q.; Zhu, J.-H.; Xing, F. Recycling of carbon fibers from carbon fiber reinforced polymer using electrochemical method. Compos. Part A Appl. Sci. Manuf. 2015, 78, 10–17. [Google Scholar] [CrossRef]
- Alves, S.M.C.; da Silva, F.S.; Donadon, M.V.; Garcia, R.R.; Corat, E.J. Process and characterization of reclaimed carbon fiber composites by pyrolysis and oxidation, assisted by thermal plasma to avoid pollutants emissions. J. Compos. Mater. 2018, 52, 1379–1398. [Google Scholar] [CrossRef]
- Ghafoor, B.; Schrekker, H.S.; Morais, J.; Amico, S.C. Surface modification of carbon fiber with imidazolium ionic liquids. Compos. Interfaces 2022, 29, 915–927. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Servinis, L.; Scheffler, C.; Wölfel, E.; Demir, B.; Walsh, T.R.; Henderson, L.C. Synergistic interfacial effects of ionic liquids as sizing agents and surface modified carbon fibers. J. Mater. Chem. A 2018, 6, 4504–4514. [Google Scholar] [CrossRef]
- Beggs, K.M.; Perus, M.D.; Servinis, L.; O’Dell, L.A.; Fox, B.L.; Gengenbach, T.R.; Henderson, L.C. Rapid surface functionalization of carbon fibres using microwave irradiation in an ionic liquid. RSC Adv. 2016, 6, 32480–32483. [Google Scholar] [CrossRef]
- Prabhakara, M.; Maiti, B. Ionic liquid-immobilized proline (s) organocatalyst-catalyzed one-pot multi-component Mannich reaction under solvent-free condition. Res. Chem. Intermed. 2020, 46, 2381–2401. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Y.; Li, N.; Hao, X.; Liu, C. Interfacial shear strength of microwave processed carbon fiber/epoxy composites characterized by an improved fiber-bundle pull-out test. Compos. Sci. Technol. 2016, 133, 173–183. [Google Scholar] [CrossRef]
- Zhang, F.-H.; Wang, R.-G.; He, X.-D.; Wang, C.; Ren, L.-N. Interfacial shearing strength and reinforcing mechanisms of an epoxy composite reinforced using a carbon nanotube/carbon fiber hybrid. J. Mater. Sci. 2009, 44, 3574–3577. [Google Scholar] [CrossRef]
- He, D.; Soo, V.K.; Stojcevski, F.; Lipiński, W.; Henderson, L.C.; Compston, P.; Doolan, M. The effect of sizing and surface oxidation on the surface properties and tensile behaviour of recycled carbon fibre: An end-of-life perspective. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106072. [Google Scholar] [CrossRef]
- He, M.; Qi, P.; Xu, P.; Cai, Q.; Li, P.; Jia, X.; Yang, X. Establishing a phthalocyanine-based crosslinking interphase enhances the interfacial performances of carbon fiber/epoxy composites at elevated temperatures. Compos. Sci. Technol. 2019, 173, 24–32. [Google Scholar] [CrossRef]
- Fonseca, E.; da Silva, V.D.; Klitzke, J.S.; Schrekker, H.S.; Amico, S.C. Imidazolium ionic liquids as fracture toughening agents in DGEBA-TETA epoxy resin. Polym. Test. 2020, 87, 106556. [Google Scholar] [CrossRef]
- Soares, B.G.; Ferreira, S.C.; Livi, S. Modification of anionic and cationic clays by zwitterionic imidazolium ionic liquid and their effect on the epoxy-based nanocomposites. Appl. Clay Sci. 2017, 135, 347–354. [Google Scholar] [CrossRef]
- Song, W.; Gu, A.; Liang, G.; Yuan, L. Effect of the surface roughness on interfacial properties of carbon fibers reinforced epoxy resin composites. Appl. Surf. Sci. 2011, 257, 4069–4074. [Google Scholar] [CrossRef]
- Arnold, C.L.; Beggs, K.M.; Eyckens, D.J.; Stojcevski, F.; Servinis, L.; Henderson, L.C. Enhancing interfacial shear strength via surface grafting of carbon fibers using the Kolbe decarboxylation reaction. Compos. Sci. Technol. 2018, 159, 135–141. [Google Scholar] [CrossRef]
- Matthews, R.P.; Ashworth, C.; Welton, T.; Hunt, P.A. The impact of anion electronic structure: Similarities and differences in imidazolium based ionic liquids. J. Phys. Condens. Matter 2014, 26, 284112. [Google Scholar] [CrossRef]
- Deng, J.; Xu, L.; Zhang, L.; Peng, J.; Guo, S.; Liu, J.; Koppala, S. Recycling of carbon fibers from CFRP waste by microwave thermolysis. Processes 2019, 7, 207. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lu, D.N. Surface energy and wettability of plasma-treated polyacrylonitrile fibers. Plasma Chem. Plasma Process. 2006, 26, 119–126. [Google Scholar] [CrossRef]
- Prasad, G.; Reddy, K.M.; Padamasuvarna, R.; Mohan, T.M.; Krishna, T.V.; Kumare, V.R. Thermophysical properties of 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide with 2-ethoxyethanol from T = (298.15 to 323.15) K at atmospheric pressure. J. Mol. Liq. 2018, 251, 335–344. [Google Scholar] [CrossRef]
- Yu, L.; Pizio, B.S.; Vaden, T.D. Conductivity and spectroscopic investigation of bis (trifluoromethanesulfonyl) imide solution in ionic liquid 1-butyl-3-methylimidazolium bis (trifluoromethanesulfonyl) imide. J. Phys. Chem. B 2012, 116, 6553–6560. [Google Scholar] [CrossRef]
- Akai, N.; Kawai, A.; Shibuya, K. First observation of the matrix-isolated FTIR spectrum of vaporized ionic liquid: An example of EmimTFSI, 1-Ethyl-3-methylimidazolium Bis (trifluoromethanesulfonyl) imide. Chem. Lett. 2008, 37, 256–257. [Google Scholar] [CrossRef]
- Ou, R.; Xie, Y.; Shen, X.; Yuan, F.; Wang, H.; Wang, Q. Solid biopolymer electrolytes based on all-cellulose composites prepared by partially dissolving cellulosic fibers in the ionic liquid 1-butyl-3-methylimidazolium chloride. J. Mater. Sci. 2012, 47, 5978–5986. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Han, F.; Dong, Z.; Yuan, G.; Ma, G.; Westwood, A.; He, K. The effect of pitch-based carbon fiber microstructure and composition on the formation and growth of SiC whiskers via reaction of such fibers with silicon sources. Carbon 2016, 99, 174–185. [Google Scholar] [CrossRef]
- Dobiášová, L.; Starý, V.; Glogar, P.; Valvoda, V. Analysis of carbon fibers and carbon composites by asymmetric X-ray diffraction technique. Carbon 1999, 37, 421–425. [Google Scholar] [CrossRef]
- Guimarães, C.J.B.; Aguiar, A.P.d.; Castro, A.T.d. Accurate measurement of pitch-based carbon fiber electrical resistivity. Polímeros 2021, 31. [Google Scholar] [CrossRef]
- Osti, N.C.; Mamontov, E. Microscopic dynamics in room-temperature ionic liquids confined in materials for supercapacitor applications. Sustain. Energy Fuels 2020, 4, 1554–1576. [Google Scholar] [CrossRef]
- Salanne, M. Ionic liquids for supercapacitor applications. In Ionic Liquids II; Springer: Cham, Switzerland, 2017; pp. 29–53. [Google Scholar]
- Daguenet, C.; Dyson, P.J.; Krossing, I.; Oleinikova, A.; Slattery, J.; Wakai, C.; Weingärtner, H. Dielectric response of imidazolium-based room-temperature ionic liquids. J. Phys. Chem. B 2006, 110, 12682–12688. [Google Scholar] [CrossRef] [PubMed]
- Kowsari, M.; Alavi, S.; Ashrafizaadeh, M.; Najafi, B. Molecular dynamics simulation of imidazolium-based ionic liquids. I. Dynamics and diffusion coefficient. J. Chem. Phys. 2008, 129, 224508. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cheng, Z. Thermal stability of ionic liquids: Current status and prospects for future development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]




| Resistivity (μAm) | Contact Resistance (Ω) | |
|---|---|---|
| CF | 11.30 ± 0.14 | 325 |
| CF-1Cl | 8.39 ± 3.68 | 244 |
| CF-1NTF2 | 3.33 ± 0.64 | 297 |
| CF-1OH | 2.84 ± 0.28 | 284 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafoor, B.; Schrekker, H.S.; Amico, S.C. Multifunctional Characteristics of Carbon Fibers Modified with Imidazolium Ionic Liquids. Molecules 2022, 27, 7001. https://doi.org/10.3390/molecules27207001
Ghafoor B, Schrekker HS, Amico SC. Multifunctional Characteristics of Carbon Fibers Modified with Imidazolium Ionic Liquids. Molecules. 2022; 27(20):7001. https://doi.org/10.3390/molecules27207001
Chicago/Turabian StyleGhafoor, Bilal, Henri Stephan Schrekker, and Sandro Campos Amico. 2022. "Multifunctional Characteristics of Carbon Fibers Modified with Imidazolium Ionic Liquids" Molecules 27, no. 20: 7001. https://doi.org/10.3390/molecules27207001
APA StyleGhafoor, B., Schrekker, H. S., & Amico, S. C. (2022). Multifunctional Characteristics of Carbon Fibers Modified with Imidazolium Ionic Liquids. Molecules, 27(20), 7001. https://doi.org/10.3390/molecules27207001









