Preparation of Mixed Bis-N-Heterocyclic Carbene Rhodium(I) Complexes
Abstract
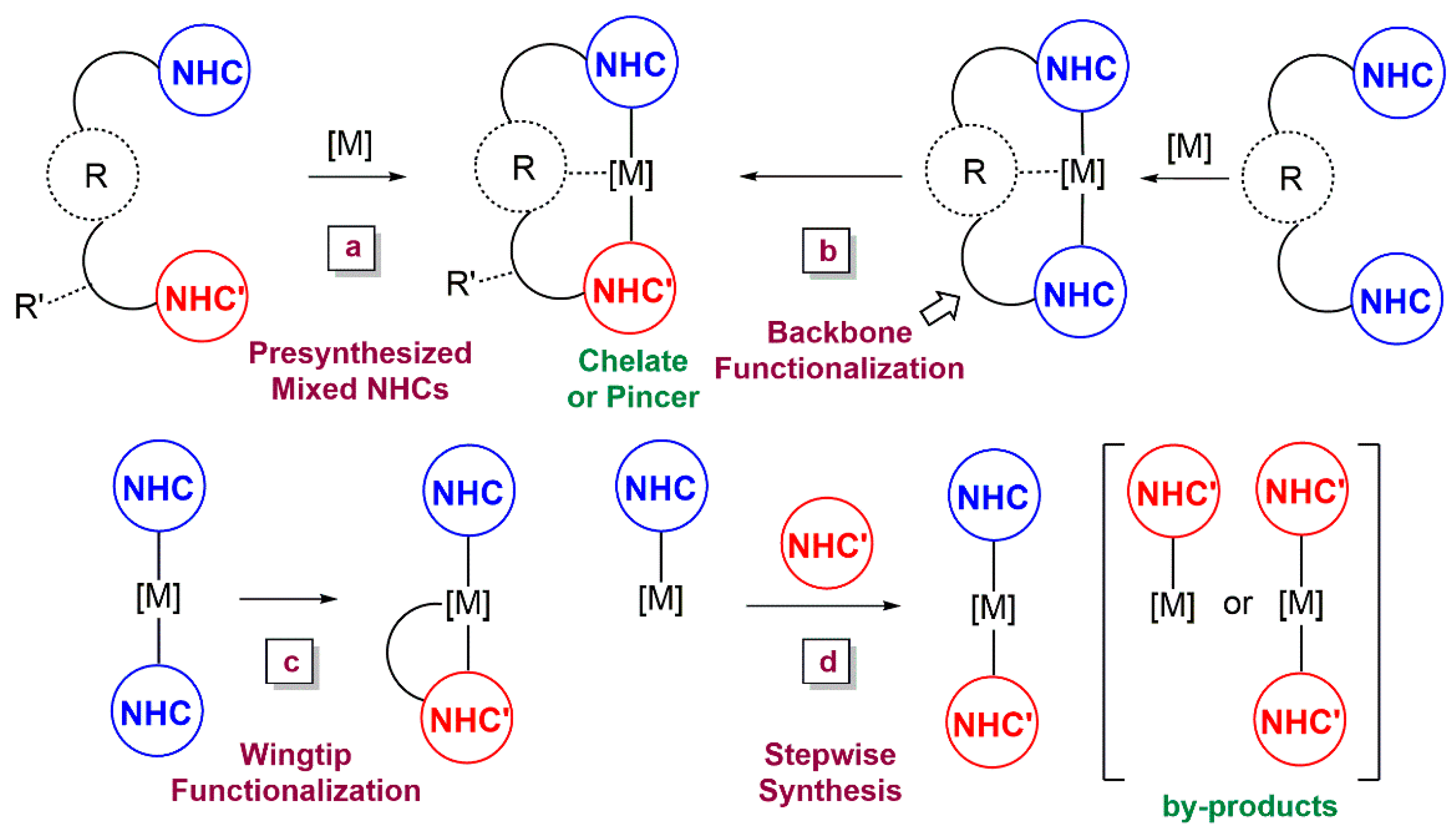
1. Introduction
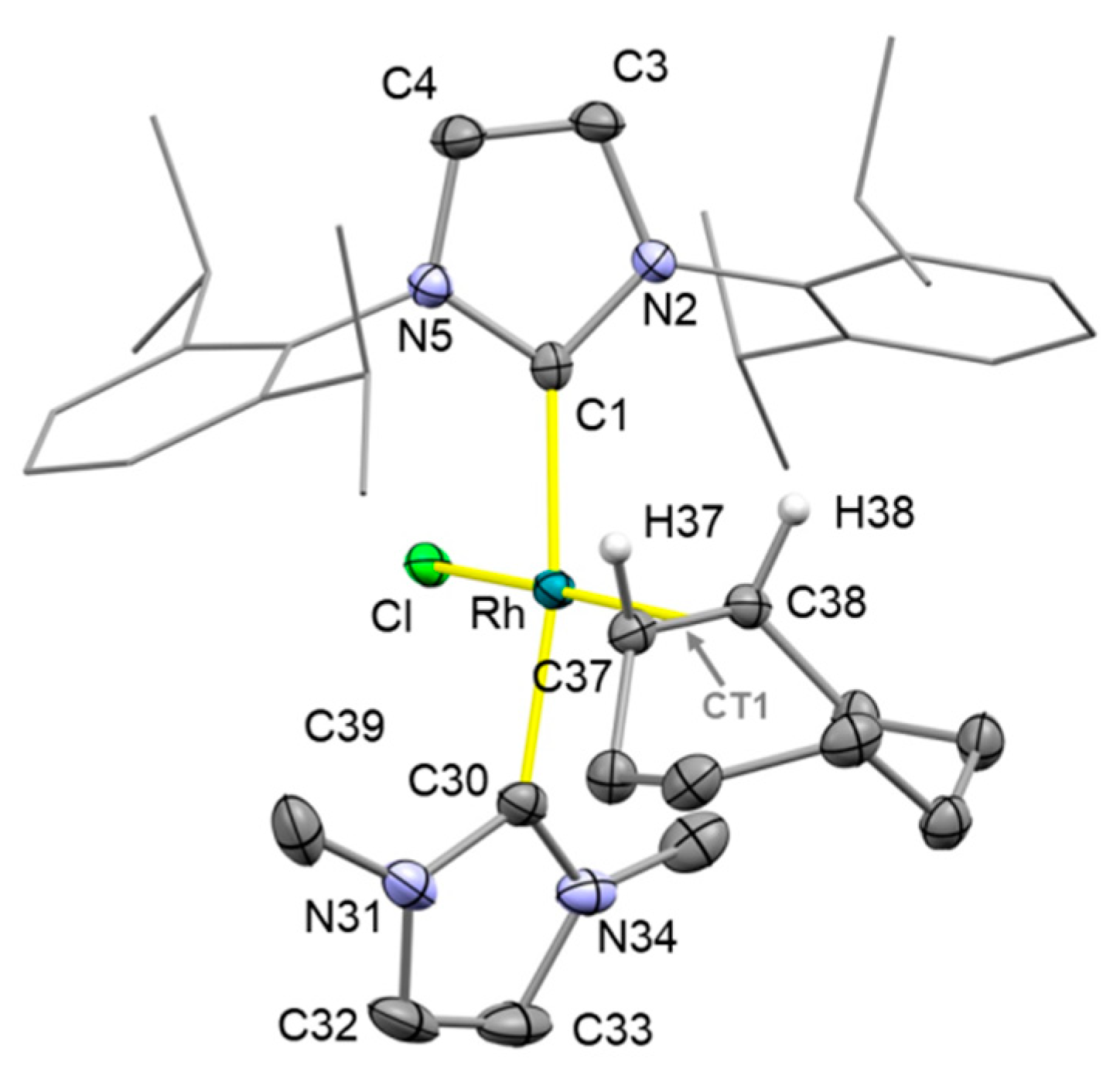
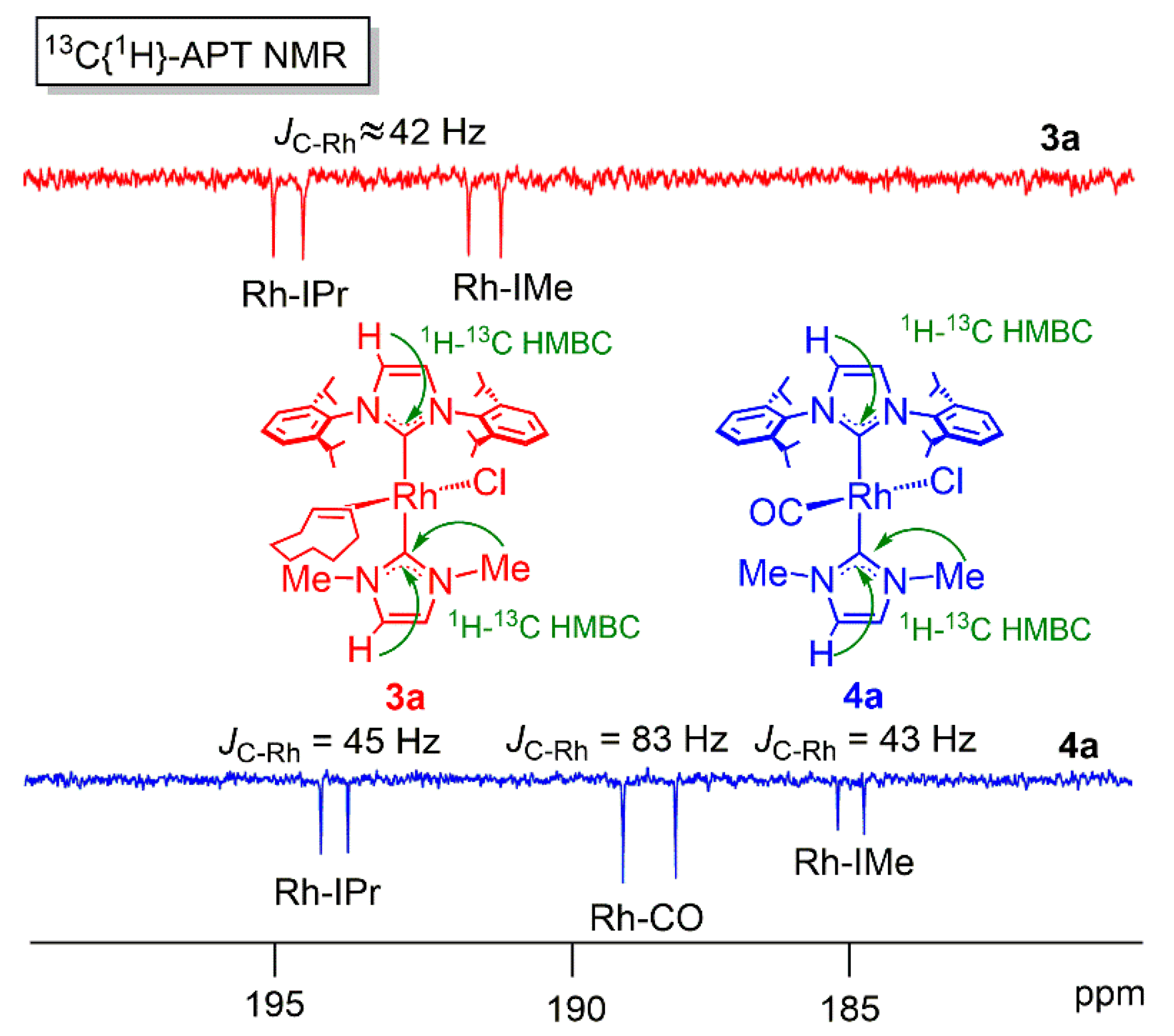
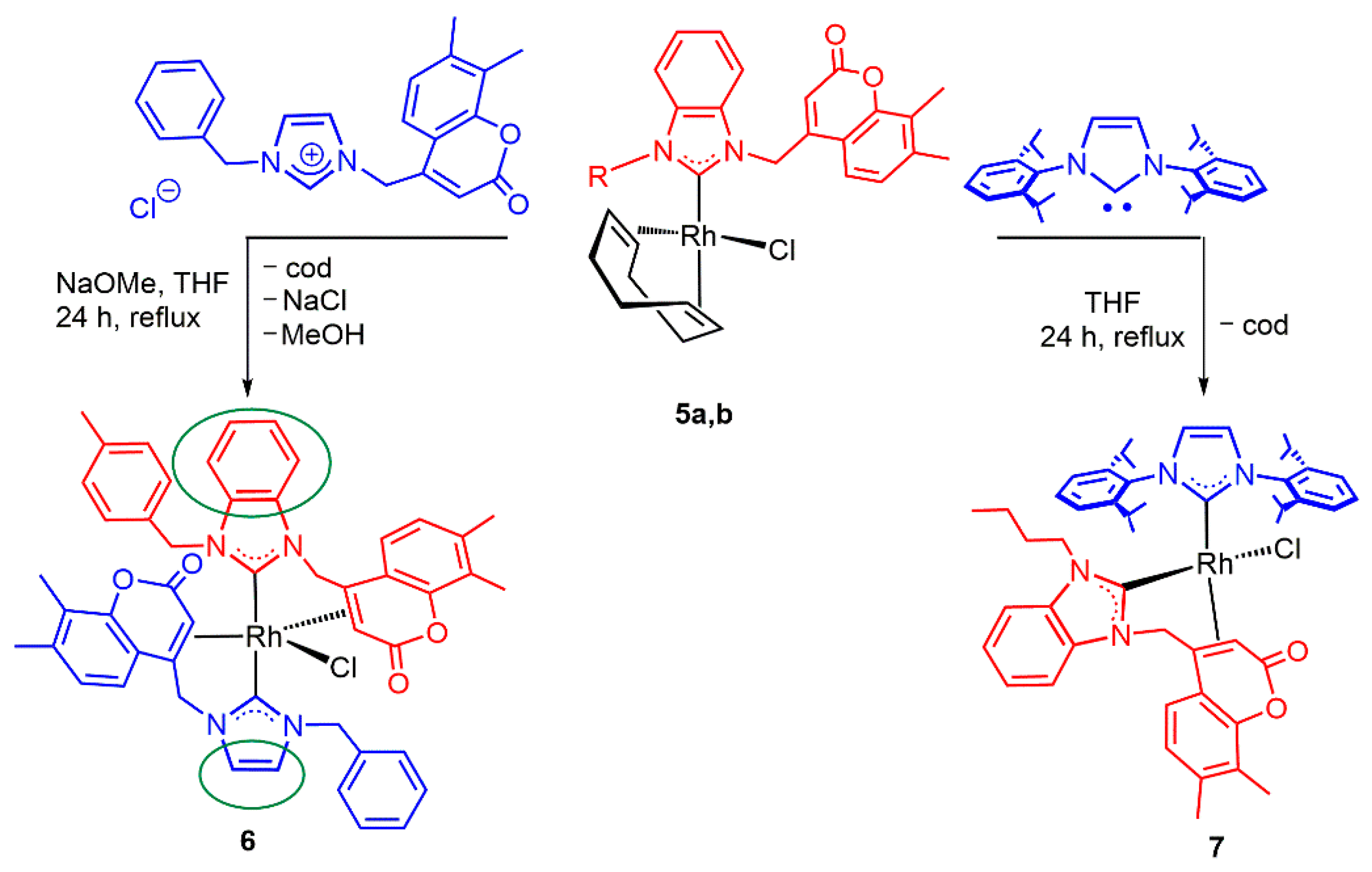
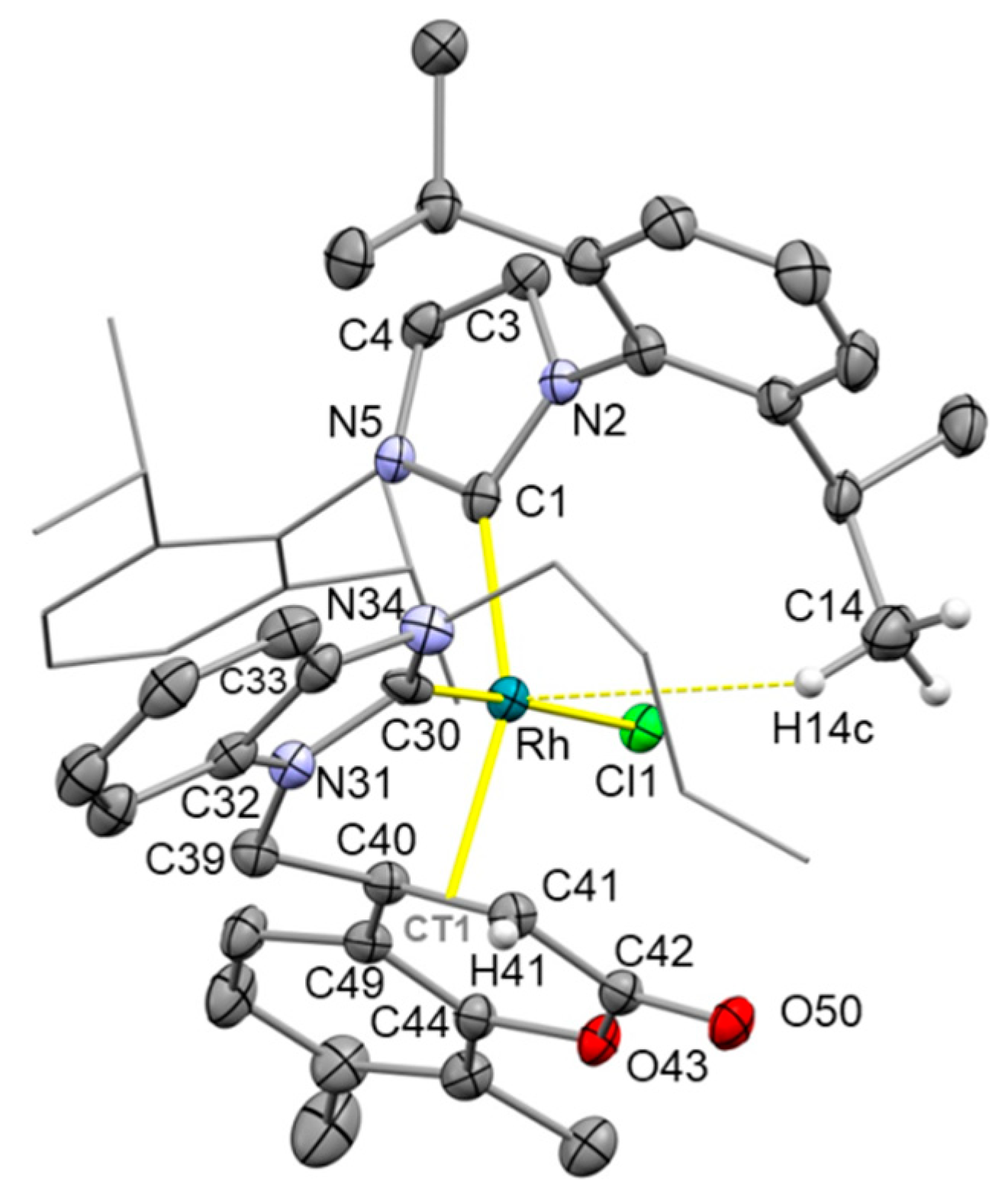
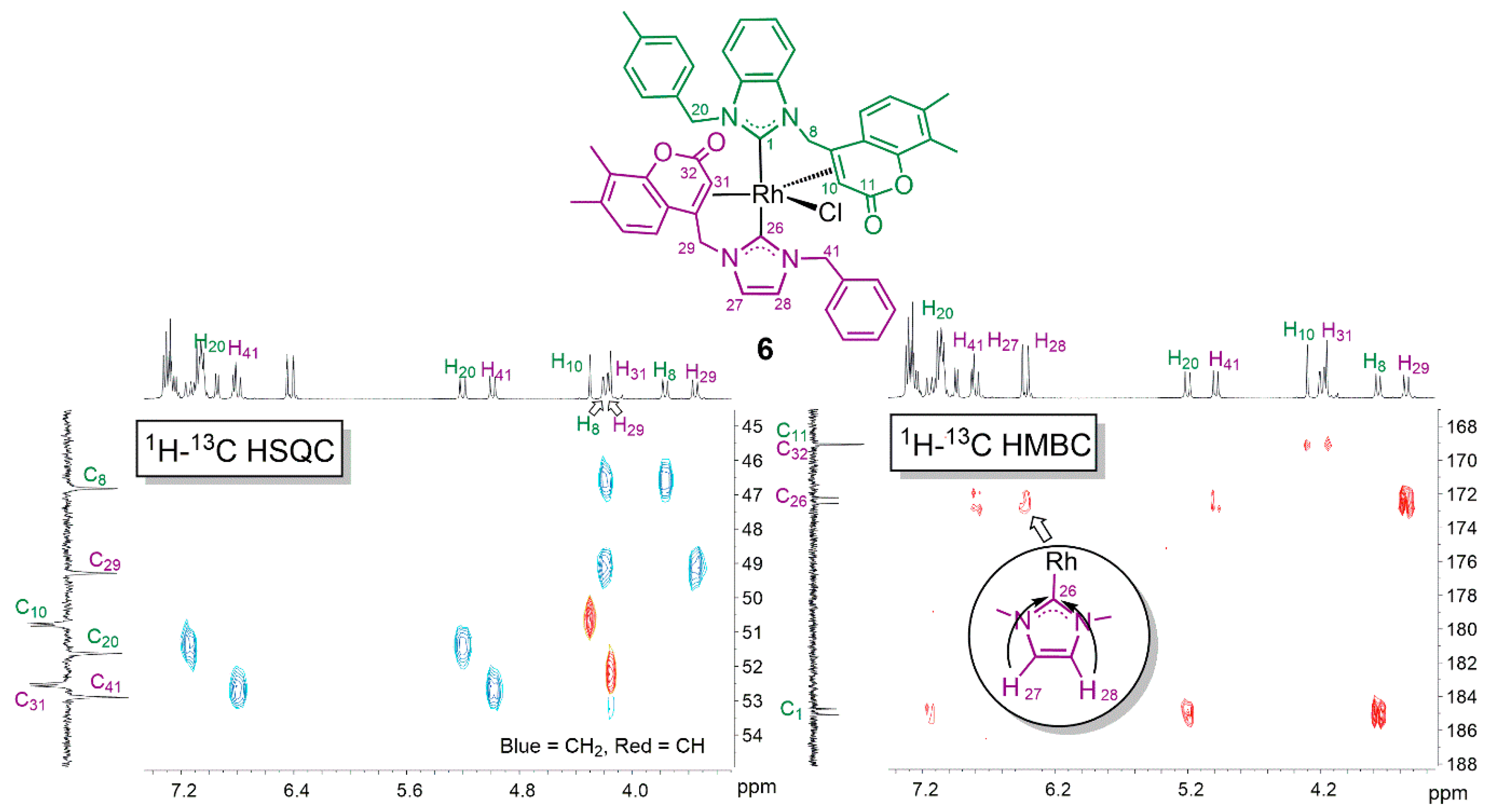
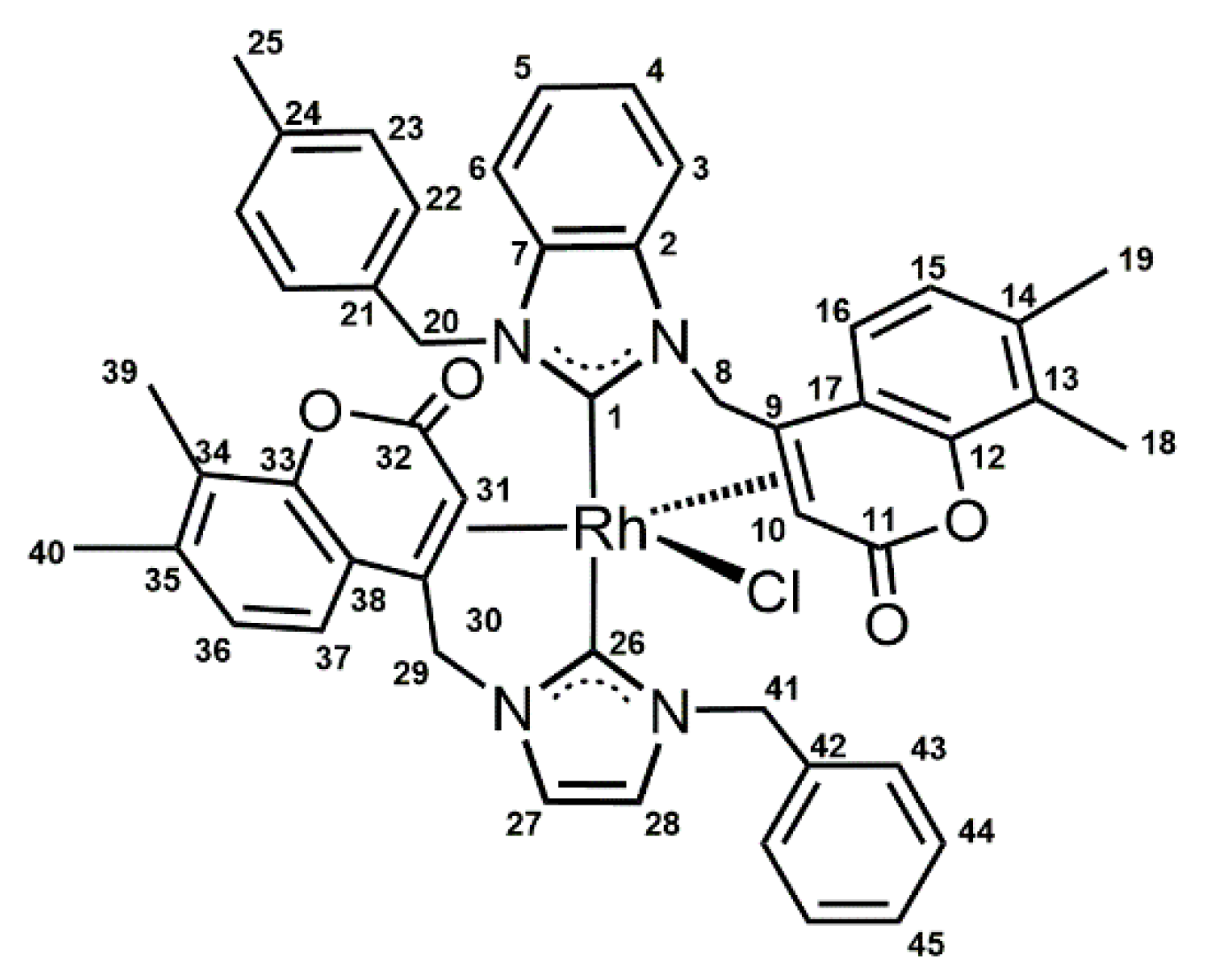
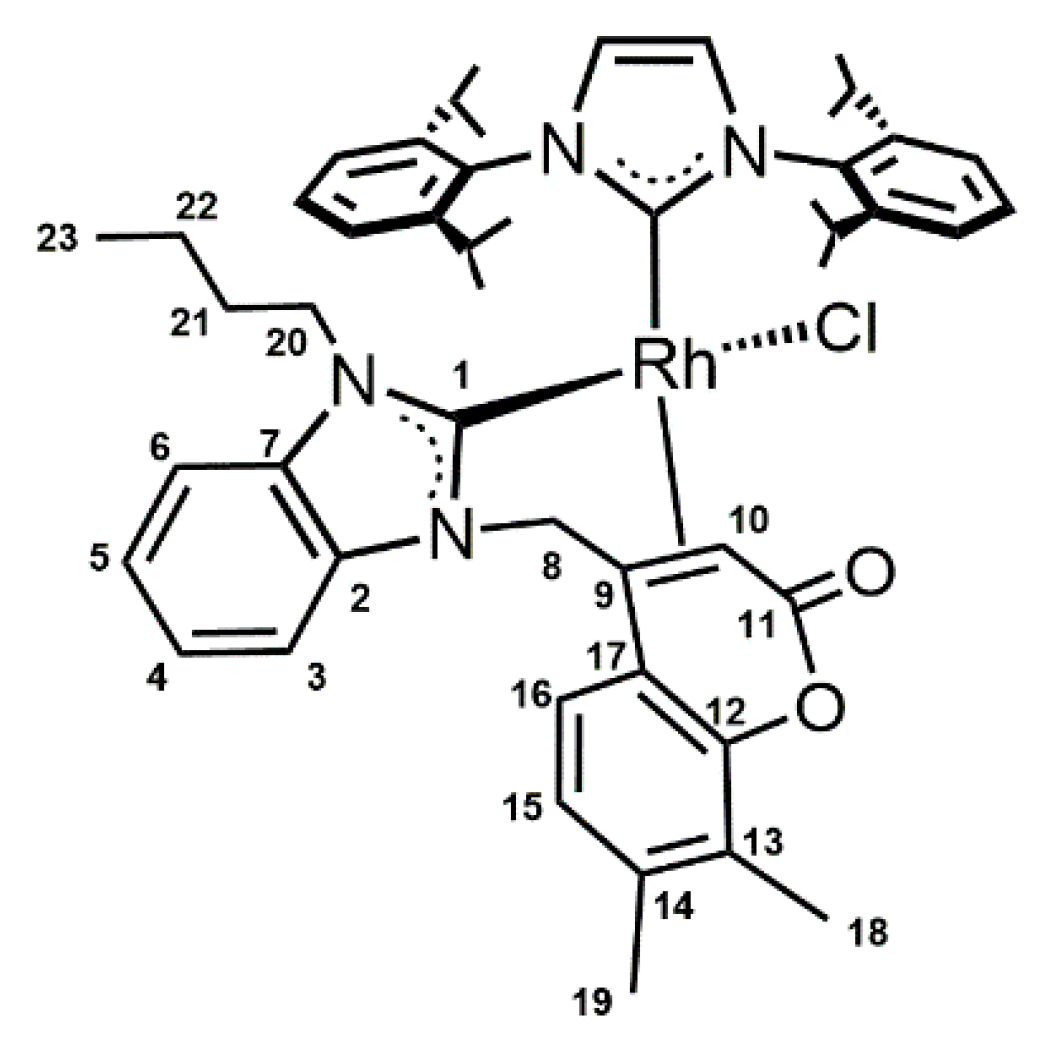
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Öfele, K. 1,3-Dimethyl-4-imidazolinyliden-(2)-pentacarbonylchrom ein neuer übergangsmetall-carben-komplex. J. Organomet. Chem. 1968, 12, P42–P43. [Google Scholar] [CrossRef]
- Wanzlick, H.-W.; Schönherr, H.-J. Direct synthesis of a mercury salt-carbene complex. Angew. Chem. Int. Ed. 1968, 7, 141–142. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Harlow, R.L.; Kline, M.A. Stable crystalline carbene. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Herrmann, W.A. N-Heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Visbal, R.; Gimeno, M.C. N-heterocyclic carbene metal complexes: Photoluminescence and applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.-H.; Crudden, C.M. N-Heterocyclic carbenes in materials chemistry. Chem. Rev. 2018, 119, 4986–5056. [Google Scholar] [CrossRef]
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. Adv. Inorg. Chem. 2020, 75, 121–148. [Google Scholar] [CrossRef]
- Bellotti, P.; Koy, M.; Hopkinson, M.N.; Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Chem. Rev. 2021, 5, 711–725. [Google Scholar] [CrossRef]
- Benhamou, L.; Chardon, E.; Lavigne, G.; Bellemin-Laponnaz, S.; César, V. Synthetic routes to N-heterocyclic carbene precursors. Chem. Rev. 2011, 111, 2705–2733. [Google Scholar] [CrossRef]
- Gardinier, M.G.; Ho, C.C. Recent advances in bidentate bis(N-heterocyclic carbene) transition metal complexes and their applications in metal-mediated reactions. Coord. Chem. Rev. 2018, 375, 373–388. [Google Scholar] [CrossRef]
- Yamashita, M.; Goto, K.; Kawashima, T. Fixation of both O2 and CO2 from air by a crystalline palladium complex bearing N-heterocyclic carbene ligands. J. Am. Chem. Soc. 2005, 127, 7294–7295. [Google Scholar] [CrossRef] [PubMed]
- Hickey, J.L.; Ruhayel, R.A.; Barnard, P.J.; Murray, V.; Baker, M.V.; Berners-Price, S.J.; Filipovska, A. Mitochondria-targeted chemotherapeutics: The rational design of Gold(I) N-heterocyclic carbene complexes that are selectively toxic to cancer cells and target protein selenols in preference to thiols. J. Am. Chem. Soc. 2008, 130, 12570–12571. [Google Scholar] [CrossRef] [PubMed]
- Henwood, A.F.; Lesieur, M.; Bansal, A.K.; Lemaur, V.; Beljonne, D.; Thompson, D.G.; Graham, D.; Slawin, A.M.Z.; Samuel, I.D.W.; Cazin, C.S.J.; et al. Palladium(0) NHC complexes: A new avenue to highly efficient phosphorescence. Chem. Sci. 2015, 6, 3248–3261. [Google Scholar] [CrossRef] [PubMed]
- Viciano, M.; Feliz, M.; Corberán, R.; Mata, J.A.; Clot, E.; Peris, E. Aliphatic versus aromatic C-H activation in the formation of abnormal carbenes with iridium: A combined experimental and theoretical study. Organometallics 2007, 26, 5304–5314. [Google Scholar] [CrossRef]
- Gardiner, M.G.; Ho, C.C.; Mackay, F.M.; McGuinness, D.S.; Tucker, M. Selective and adaptable access to N,N’-asymmetrically substituted imidazol-2-ylidene bis-NHC ligands: Pd(II) complexes featuring wide variation in N-alkyl and aryl steric bulk. Dalton Trans. 2013, 42, 7447–7457. [Google Scholar] [CrossRef]
- Aznarez, F.; Sanz Miguel, P.J.; Tan, T.T.Y.; Hahn, F.E. Preparation of rhodium(III) di-NHC chelate complexes featuring two different NHC donors via a mild NaOAc-assisted C−H activation. Organometallics 2016, 35, 410–419. [Google Scholar] [CrossRef]
- Tian, Y.; Jürgens, E.; Mill, K.; Jordan, R.; Maulbetsch, T.; Kunz, D. Nucleophilic isomerization of epoxides by pincer-rhodium catalysts: Activity increase and mechanistic insights. ChemCatChem 2019, 11, 4028–4035. [Google Scholar] [CrossRef]
- Donthireddy, S.N.R.; Illam, P.M.; Rit, A. Ruthenium(II) complexes of heteroditopic N-heterocyclic carbene ligands: Efficient catalysts for C−N bond formation via a hydrogen-borrowing strategy under solvent-free conditions. Inorg. Chem. 2020, 59, 1835–1847. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Tsoureas, N.; Wright, J.A.; Light, M.E. N-Heterocyclic pincer dicarbene complexes of iron(II): C-2 and C-5 metalated carbenes on the same metal center. Organometallic 2004, 23, 166–168. [Google Scholar] [CrossRef]
- Zuo, W.; Braunstein, P. N-Heterocyclic dicarbene iridium(III) pincer complexes featuring mixed NHC/abnormal NHC ligands and their applications in the transfer dehydrogenation of cyclooctane. Organometallics 2012, 31, 2606–2615. [Google Scholar] [CrossRef]
- Hermosilla, P.; García-Orduña, P.; Sanz Miguel, P.J.; Polo, V.; Casado, M.A. Nucleophilic reactivity at a =CH arm of a lutidine-based CNC/Rh system: Unusual alkyne and CO2 activation. Inorg. Chem. 2022, 61, 7120–7129. [Google Scholar] [CrossRef] [PubMed]
- Dorta, R.; Stevens, E.D.; Nolan, S.P. Double C-H activation in a Rh-NHC complex leading to the isolation of a 14-electron Rh(III) complex. J. Am. Chem. Soc. 2004, 126, 5054–5055. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Y.; Smith, W.; Vidovic, D.; Thompson, A.L.; Chaplin, A.B.; Aldridge, S. Sterically encumbered iridium Bis(N-heterocyclic carbene) systems: Multiple C-H activation processes and isomeric normal/abnormal carbene complexes. Organometallics 2009, 28, 3059–3063. [Google Scholar] [CrossRef]
- Zenkina, O.V.; Keske, E.C.; Wang, R.; Crudden, C.M. Double single-crystal-to-single-crystal transformation and small-molecule activation in rhodium NHC complexes. Angew. Chem. Int. Ed. 2011, 50, 8100–8104. [Google Scholar] [CrossRef]
- Clement, N.D.; Cavell, K.J.; Jones, C.; Elsevier, C.J. Oxidative addition of imidazolium salts to Ni0 and Pd0: Synthesis and structural characterization of unusually stable metal–hydride complexes. Angew. Chem. Int. Ed. 2004, 43, 1277–1279. [Google Scholar] [CrossRef]
- Burling, S.; Douglas, S.; Mahon, M.F.; Nama, D.; Pregosin, P.; Whittlesey, M.K. Cationic tris N-heterocyclic carbene rhodium carbonyl complexes: Molecular structures and solution NMR studies. Organometallics 2006, 25, 2642–2648. [Google Scholar] [CrossRef]
- Bittermann, A.; Herdtweck, E.; Härter, P.; Herrmann, W.A. Rhodium(I), a carbene-transfer transition-metal ion and a synthetic route to symmetrical and asymmetrical substituted trans-RhCl(CO)(NHC)(NHC) complexes. Organometallics 2009, 28, 6963–6968. [Google Scholar] [CrossRef]
- Sashuk, V.; Peeck, L.H.; Plenio, H. [(NHC)NHCewg)RuCl2(CHPh)] complexes with modified NHCewg ligands for efficient Ring-Closing Metathesis leading to tetrasubstituted olefins. Chem. Eur. J. 2010, 16, 3983–3993. [Google Scholar] [CrossRef]
- Gaillard, S.; Nun, P.; Slawin, A.M.Z.; Nolan, S.P. Expeditious synthesis of [Au(NHC)(L)](NHC = N-Heterocyclic Carbene; L = Phosphine or NHC) Complexes. Organometallics 2010, 29, 5402–5408. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Xu, Y.-C.; Xie, L.-Z.; Sun, H.-M.; Shen, Q.; Zhang, Y. Controlled synthesis of nickel(II) dihalides bearing two different or identical N-heterocyclic carbene ligands and the influence of carbene ligands on their structures and catalysis. Dalton Trans. 2011, 40, 4697–4706. [Google Scholar] [CrossRef]
- Lund, C.L.; Sgro, M.J.; Cariou, R.; Stephan, D.W. A cis-bis-mixed-carbene ruthenium hydride complex: An olefin-selective hydrogenation catalyst. Organometallics 2012, 31, 802–805. [Google Scholar] [CrossRef]
- Lazreg, F.; Slawin, A.M.Z.; Cazin, C.S.J. Heteroleptic bis(N-heterocyclic carbene)copper(I) complexes: Highly efficient systems for the [3+2] cycloaddition of azides and alkynes. Organometallics 2012, 31, 7969–7975. [Google Scholar] [CrossRef]
- César, V.; Barthes, C.; Farré, Y.C.; Cuisiat, S.V.; Vacher, B.Y.; Brousses, R.; Lugan, N.; Lavigne, G. Anionic and zwitterionic copper(I) complexes incorporating an anionic N-heterocyclic carbene decorated with a malonate backbone: Synthesis, structure and catalytic applications. Dalton Trans. 2013, 42, 7373–7385. [Google Scholar] [CrossRef]
- Gothe, Y.; Marzo, T.; Messori, L.; Metzler-Nolte, N. Iridium(I) compounds as prospective anticancer agents: Solution chemistry, antiproliferative profiles and protein interactions for a series of iridium(I) N-heterocyclic carbene complexes. Chem. Eur. J. 2016, 22, 12487–12494. [Google Scholar] [CrossRef] [PubMed]
- Rehm, T.; Rothemund, M.; Muenzner, J.K.; Noor, A.; Kempeb, R.; Schobert, R. Novel cis-[(NHC)1(NHC)2(L)Cl]platinum(II) complexes–synthesis, structures, and anticancer activities. Dalton Trans. 2016, 45, 15390–15398. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Yi, L.; Lynch, M.; Arumugan, K.; He, X.-P.; Sessler, J.L.; Arambula, J.F. Expanding the biological utility of bis-NHC gold(I) complexes through post synthetic carbamate conjugation. Chem. Commun. 2019, 55, 10627–10630. [Google Scholar] [CrossRef]
- Kumar, A.; Yuan, D.; Huynh, H.V. Stereoelectronic profiling of expanded-ring N-heterocyclic carbenes. Inorg. Chem. 2019, 58, 7545–7553. [Google Scholar] [CrossRef]
- Lebel, H.; Janes, M.K.; Charette, A.B.; Nolan, S.P. Structure and Reactivity of “Unusual” N-Heterocyclic Carbene (NHC) Palladium Complexes Synthesized from Imidazolium Salts. J. Am. Chem. Soc. 2004, 126, 5046–5047. [Google Scholar] [CrossRef]
- Appelhans, L.N.; Incarvito, C.D.; Crabtree, R.H. Synthesis of monodentate bis(N-heterocyclic carbene) complexes of iridium: Mixed complexes of abnormal NHCs, normal NHCs, and triazole NHCs. J. Organomet. Chem. 2008, 693, 2761–2766. [Google Scholar] [CrossRef]
- Mejuto, C.; Guisado-Barrios, G.; Gusev, D.; Peris, E. First homoleptic MIC and heteroleptic NHC–MIC coordination cages from 1,3,5-triphenylbenzenebridged tris-MIC and tris-NHC ligands. Chem. Commun. 2015, 51, 13914–13917. [Google Scholar] [CrossRef]
- Liberman-Martin, A.L.; Grubbs, R.H. Ruthenium olefin metathesis catalysts featuring a labile carbodicarbene ligand. Organometallics 2017, 36, 4091–4094. [Google Scholar] [CrossRef]
- Na, H.; Cañada, L.M.; Wen, Z.; Wu, J.I.-C.; Teets, T.S. Mixed-carbene cyclometalated iridium complexes with saturated blue luminescence. Chem. Sci. 2019, 10, 6254–6260. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Kumar, A.; Huynh, H.V. Stereoelectronic profiling of acyclic diamino carbenes (ADCs). Inorg. Chem. 2020, 59, 8451–8460. [Google Scholar] [CrossRef] [PubMed]
- Lazreg, F.; Cordes, D.B.; Slawin, A.M.Z.; Cazin, C.S.J. Synthesis of homoleptic and heteroleptic bis-N-heterocylic carbenegroup 11 complexes. Organometallics 2015, 34, 419–425. [Google Scholar] [CrossRef]
- Beillard, A.; Quintin, F.; Gatignol, J.; Retailleau, P.; Renaud, J.-L.; Gaillard, S.; Métro, T.-X.; Lamaty, F.; Bantreil, X. Solving the challenging synthesis of highly cytotoxic silver complexes bearing sterically hindered NHC ligands with mechanochemistry. Dalton Trans. 2020, 49, 12592–12598. [Google Scholar] [CrossRef]
- Biewend, M.; Neumann, S.; Michael, P.; Binder, W.H. Synthesis of polymer-linked copper(I) bis(N-heterocyclic carbene) complexes of linear and chain extended architecture. Polym. Chem. Trans. 2019, 10, 1078–1088. [Google Scholar] [CrossRef]
- Teng, Q.; Huynh, H.V. A unified ligand electronic parameter based on 13C NMR spectroscopy of N-heterocyclic carbene complexes. Dalton Trans. 2017, 46, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Azpíroz, R.; Rubio-Pérez, L.; Castarlenas, R.; Pérez-Torrente, J.J.; Oro, L.A. gem-selective cross-dimerization and cross-trimerization of alkynes with silylacetylenes promoted by a rhodium–pyridine–N-heterocyclic carbene catalyst. ChemCatChem 2014, 6, 2587–2592. [Google Scholar] [CrossRef]
- Palacios, L.; Di Giuseppe, A.; Castarlenas, R.; Lahoz, F.J.; Pérez-Torrente, J.J.; Oro, L.A. Pyridine versus acetonitrile coordination in rhodium–N-heterocyclic carbene square-planar complexes. Dalton Trans. 2015, 44, 5777–5789. [Google Scholar] [CrossRef]
- Palacios, L.; Meheut, Y.; Galiana-Cameo, M.; Artigas, M.J.; Di Giuseppe, A.; Lahoz, F.J.; Polo, V.; Castarlenas, R.; Pérez-Torrente, J.J.; Oro, L.A. Design of highly selective alkyne hydrothiolation RhI-NHC catalysts: Carbonyl-triggered non-oxidative mechanism. Organometallics 2017, 36, 2198–2207. [Google Scholar] [CrossRef]
- Galiana-Cameo, M.; Urriolabeitia, A.; Barrenas, E.; Passarelli, V.; Pérez-Torrente, J.J.; Di Giuseppe, A.; Polo, V.; Castarlenas, R. Metal−Ligand cooperative proton transfer as an efficient trigger for rhodium-NHC-pyridonato catalyzed gem-specific alkyne dimerization. ACS Catal. 2021, 11, 7553–7567. [Google Scholar] [CrossRef]
- Galiana-Cameo, M.; Romeo, R.; Urriolabeitia, A.; Passarelli, V.; Pérez-Torrente, J.J.; Polo, V.; Castarlenas, R. Rhodium-NHC-catalyzed gem-specific O-selective hydropyridonation of terminal alkynes. Angew. Chem. Int. Ed. 2022, 61, e202117006. [Google Scholar] [CrossRef] [PubMed]
- Karataş, M.O.; Di Giuseppe, A.; Passarelli, V.; Alıcı, B.; Pérez-Torrente, J.J.; Oro, L.A.; Özdemir, I.; Castarlenas, R. Pentacoordinated rhodium(I) complexes supported by coumarin-functionalized N-heterocyclic carbene ligands. Organometallics 2018, 37, 191–202. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Patrick, B.O.; James, B.R. Rhodium(III) peroxo complexes containing carbene and phosphine ligands. Organometallics 2006, 25, 4870–4877. [Google Scholar] [CrossRef]
- Palacios, L.; Di Giuseppe, A.; Artigas, M.J.; Polo, V.; Lahoz, F.J.; Castarlenas, R.; Pérez-Torrente, J.J.; Oro, L.A. Mechanistic insight into the pyridine enhanced α-selectivity in alkyne hydrothiolation catalysed by quinolinolate–rhodiumIJI)–N-heterocyclic carbene complexes. Catal. Sci. Technol. 2016, 6, 8548–8561. [Google Scholar] [CrossRef]
- Fooladi, E.; Dalhus, B.; Tilset, M. Synthesis and characterization of half-sandwich N-heterocyclic carbene complexes of cobalt and rhodium. Dalton Trans. 2004, 22, 3909–3917. [Google Scholar] [CrossRef]
- Di Giuseppe, A.; Castarlenas, R.; Pérez-Torrente, J.J.; Crucianelli, M.; Polo, V.; Sancho, R.; Lahoz, F.J.; Oro, L.A. Ligand-controlled regioselectivity in the hydrothiolation of alkynes by rhodium N-Heterocyclic carbene catalysts. J. Am. Chem. Soc. 2012, 134, 8171–8183. [Google Scholar] [CrossRef]
- Galiana-Cameo, M.; Passarelli, V.; Perez-Torrente, J.J.; Di Giuseppe, A.; Castarlenas, R. Variation on the π-acceptor ligand within a RhI-N-heterocyclic carbene framework: Divergent catalytic outcomes for phenylacetylene-methanol transformations. Eur. J. Inorg. Chem. 2021, 2021, 2947–2957. [Google Scholar] [CrossRef]
- Praetorius, J.M.; Wang, R.; Crudden, C.M. Structure and reactivity of dinitrogen rhodium complexes containing N-Heterocyclic carbene ligands. Eur. J. Inorg. Chem. 2009, 2009, 1746–1751. [Google Scholar] [CrossRef]
- Zenkina, O.V.; Keske, E.C.; Kochhar, G.S.; Wang, R.; Crudden, C.M. Heteroleptic rhodium NHC complexes with pyridine derived ligands: Synthetic accessibility and reactivity towards oxygen. Dalton Trans. 2013, 42, 2282–2293. [Google Scholar] [CrossRef]
- Chaplin, A.B. Rhodium(I) complexes of the conformationally rigid IBioxMe4 ligand: Preparation of mono-, bis-, and tris-ligated NHC complexes. Organometallics 2014, 33, 3069–3077. [Google Scholar] [CrossRef]
- Achar, G.; Shahini, C.R.; Patil, S.A.; Budagumpi, S. Synthesis, structural characterization, crystal structures and antibacterial potentials of coumarin-tethered N-heterocyclic carbene silver(I) complexes. J. Organomet. Chem. 2017, 833, 28–42. [Google Scholar] [CrossRef]
- Halter, O.; Plenio, H. Fluorescent dyes in organometallic chemistry: Coumarin-tagged NHC–metal complexes. Eur. J. Inorg. Chem. 2018, 25, 2935–2943. [Google Scholar] [CrossRef]
- Ruiz-Mendoza, F.J.; Mendoza-Espinosa, D.; Gonzalez-Montiel, S. Synthesis and catalytic activity of coumarin- and chrysin-Tethered Triazolylidene Gold(I) Complexes. Eur. J. Inorg. Chem. 2018, 42, 4622–4629. [Google Scholar] [CrossRef]
- Karataş, M.O.; Alıcı, B.; Passarelli, V.; Özdemir, I.; Pérez-Torrente, J.J.; Castarlenas, R. Iridium(I) complexes bearing hemilabile coumarin functionalised N-heterocyclic carbene ligands with application as alkyne hydrosilylation catalysts. Dalton Trans. 2021, 50, 11206–11215. [Google Scholar] [CrossRef]
- Akpunar, C.; Özdemir, N.; Karataş, M.O.; Alıcı, B.; Özdemir, I. Synthesis, crystal structures and catalytic activities of palladium complexes with coumarin-functionalised N-heterocyclic carbene ligands. Inorg. Chem. Commun. 2021, 131, 108755. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Frey, G.D.; Herdtweck, E.; Steinbeck, M. Synthesis and characterization of N-heterocyclic carbene substituted phosphine and phosphite rhodium Complexes and their Catalytic properties in hydrogenation reactions. Adv. Synth. Catal. 2007, 349, 1677–1691. [Google Scholar] [CrossRef]
- Ahmida, A.; Elmagbari, F.M.; Egold, H.; Flörke, U.; Henkel, G. NHC-phosphane rhodium complexes and their reaction with oxygen. Polyhedron 2020, 181, 114472. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals Radii of Elements. Inorg. Mater. 2001, 37, 871–885. [Google Scholar] [CrossRef]
- Brookhart, M.; Green, M.L.H.; Parkin, G. Agostic interactions in transition metal compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- SAINT+: Area-Detector Integration Software, version 6.01; Bruker AXS: Madison, WI, USA, 2001.
- Sheldrick, G.M. SADABS Program; University of Göttingen: Göttingen, Germany, 1999. [Google Scholar]
- Sheldrick, G.M. SHELXS 97, Program for the Solution of Crystal Structure; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azpíroz, R.; Karataş, M.O.; Passarelli, V.; Özdemir, I.; Pérez-Torrente, J.J.; Castarlenas, R. Preparation of Mixed Bis-N-Heterocyclic Carbene Rhodium(I) Complexes. Molecules 2022, 27, 7002. https://doi.org/10.3390/molecules27207002
Azpíroz R, Karataş MO, Passarelli V, Özdemir I, Pérez-Torrente JJ, Castarlenas R. Preparation of Mixed Bis-N-Heterocyclic Carbene Rhodium(I) Complexes. Molecules. 2022; 27(20):7002. https://doi.org/10.3390/molecules27207002
Chicago/Turabian StyleAzpíroz, Ramón, Mert Olgun Karataş, Vincenzo Passarelli, Ismail Özdemir, Jesús J. Pérez-Torrente, and Ricardo Castarlenas. 2022. "Preparation of Mixed Bis-N-Heterocyclic Carbene Rhodium(I) Complexes" Molecules 27, no. 20: 7002. https://doi.org/10.3390/molecules27207002
APA StyleAzpíroz, R., Karataş, M. O., Passarelli, V., Özdemir, I., Pérez-Torrente, J. J., & Castarlenas, R. (2022). Preparation of Mixed Bis-N-Heterocyclic Carbene Rhodium(I) Complexes. Molecules, 27(20), 7002. https://doi.org/10.3390/molecules27207002