Tripeptide Leu-Ser-Trp Regulates the Vascular Endothelial Cells Phenotype Switching by Mediating the Vascular Smooth Muscle Cells-Derived Small Extracellular Vesicles Packaging of miR-145
Abstract
:1. Introduction
2. Results
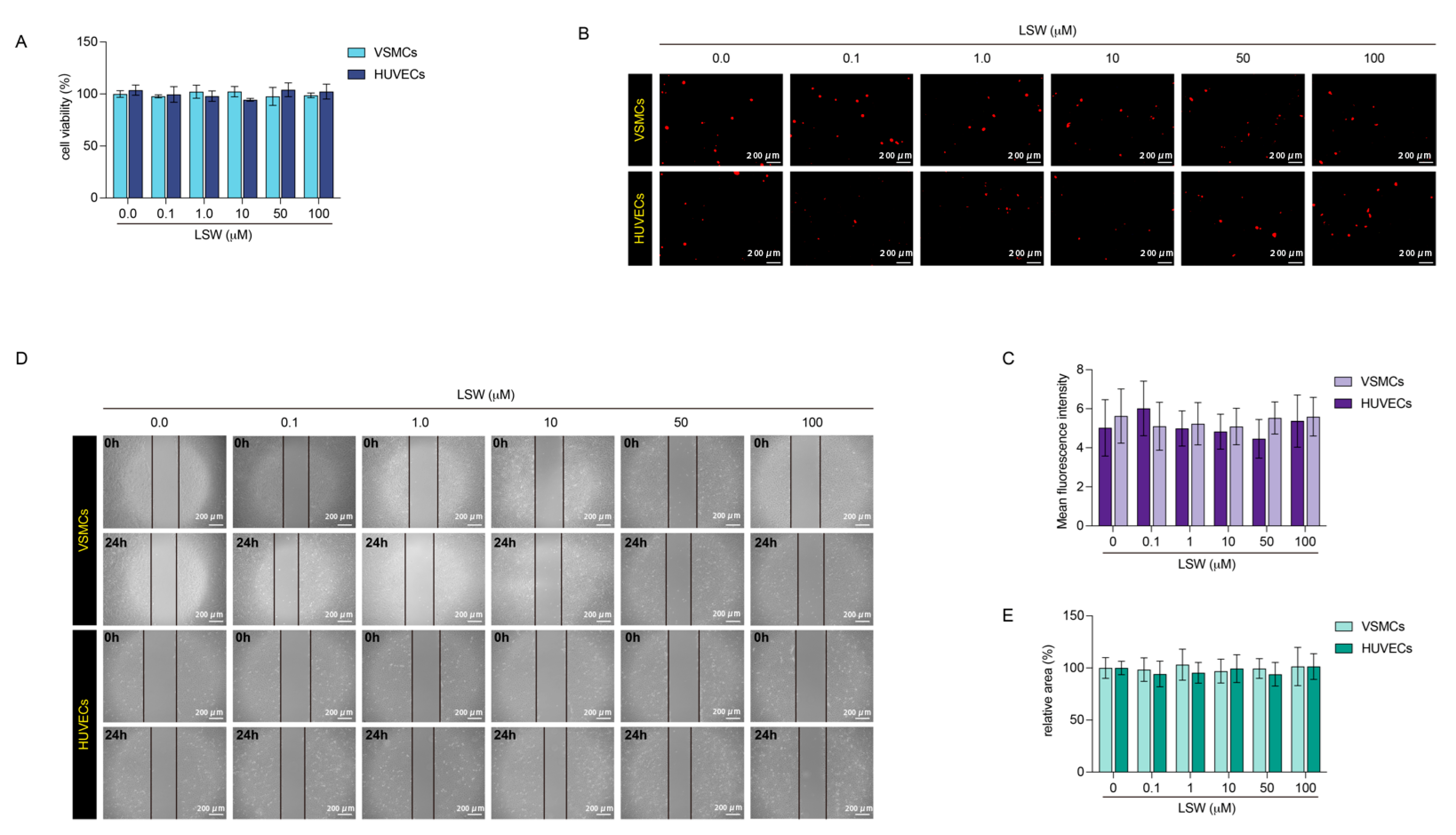
2.1. The Effects of Tripeptide LSW on VSMCs and HUVECs
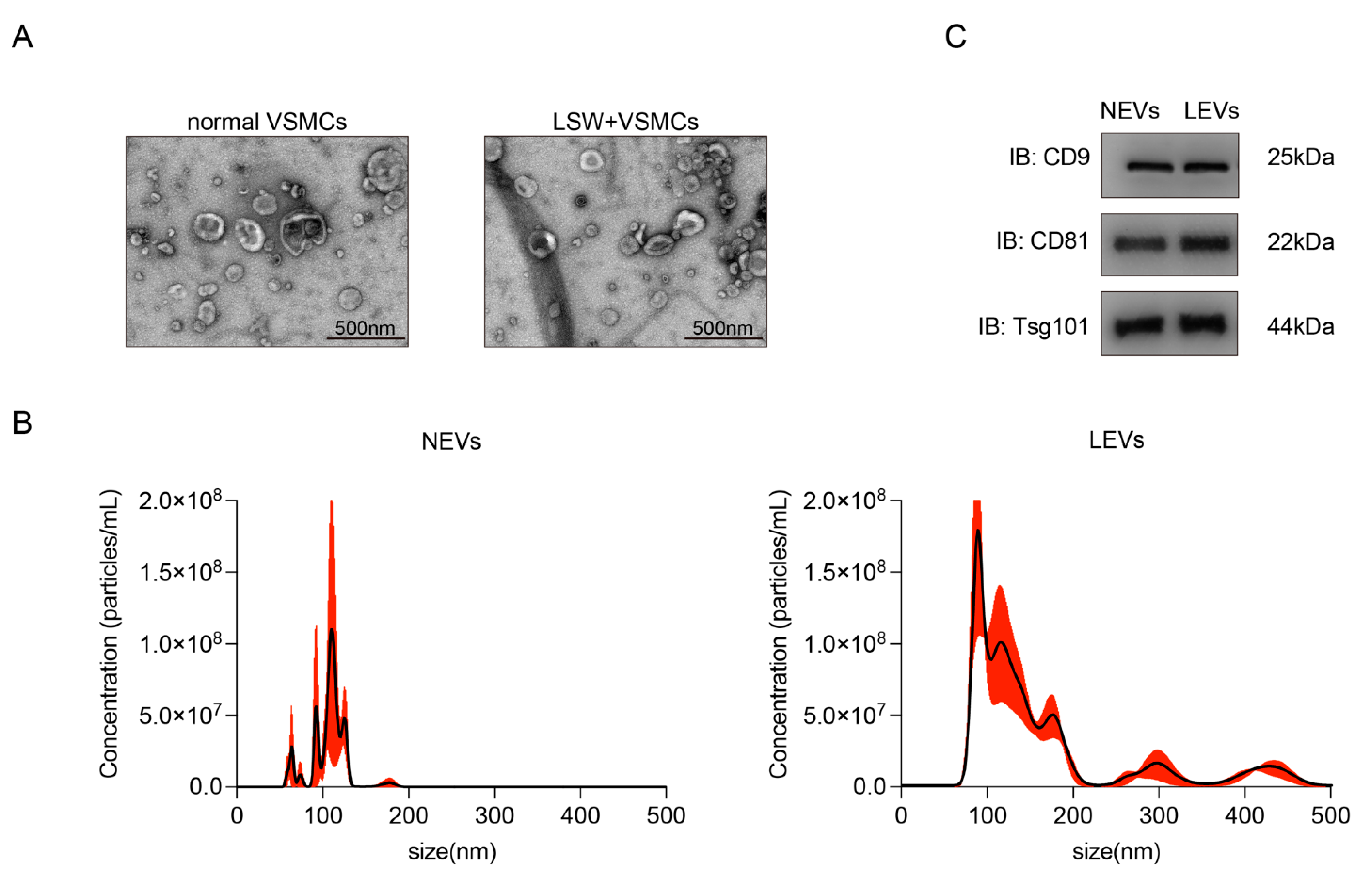
2.2. Characterization of EVs from VSMCs with or without LSW-Incubation
2.3. LEVs Attenuated the oxLDL-Induced Endothelial Dysfunction in HUVECs
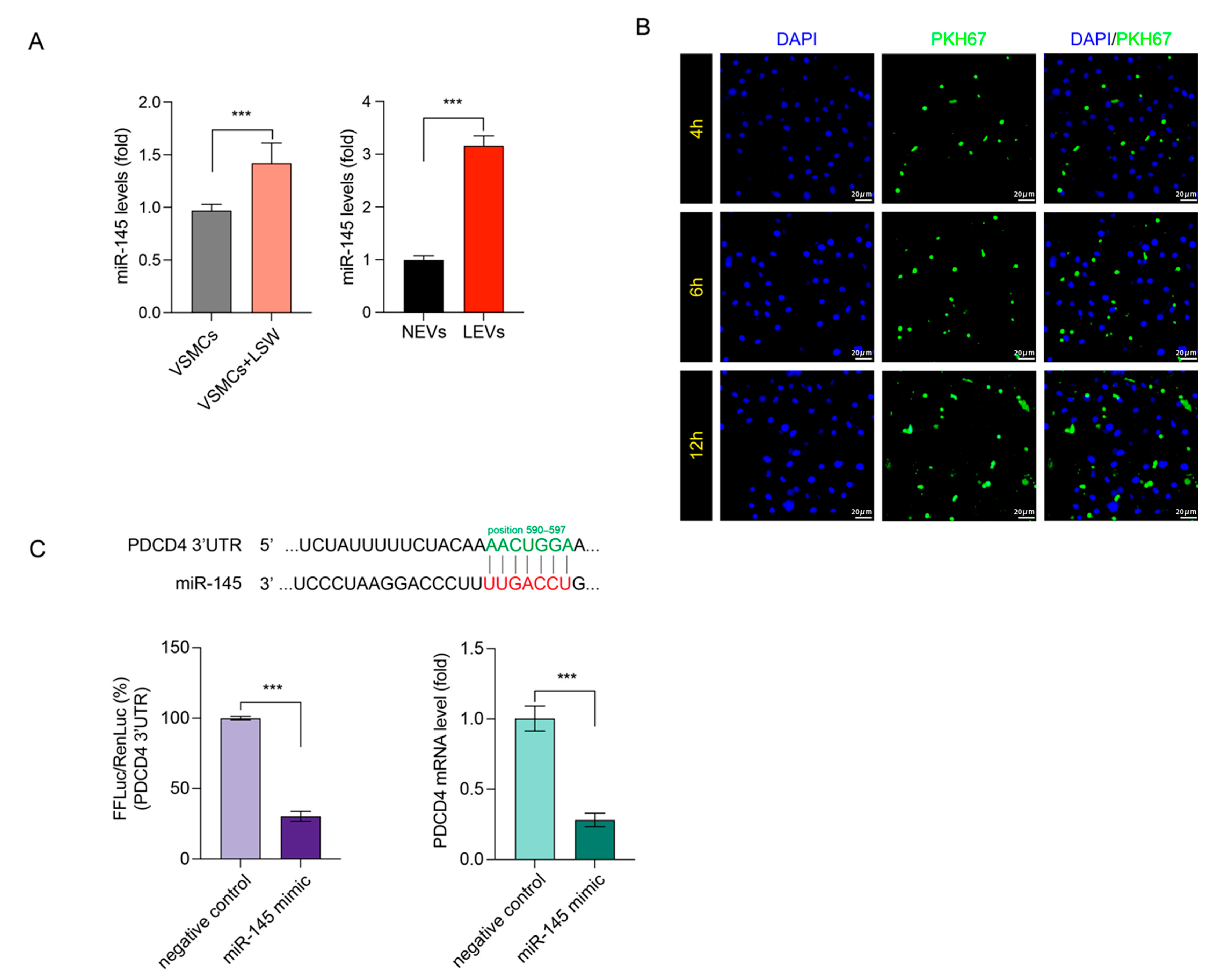
2.4. LSW Promoted miR-145 Loading in VSMCs-Derived EVs and Targeted PDCD4
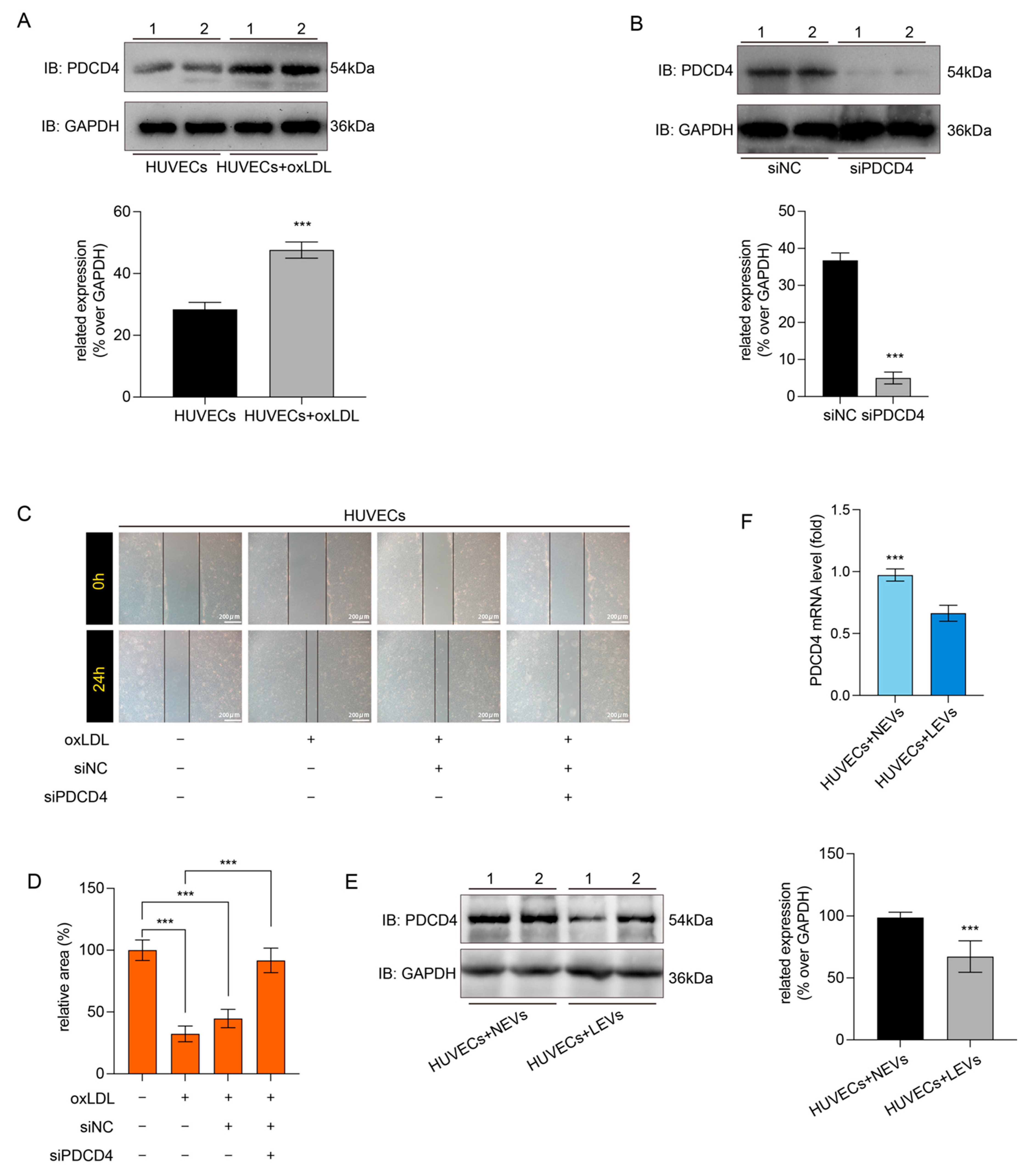
2.5. LEVs Improved Endothelial Migration by Reducing the oxLDL-Induced PDCD4 Overexpression in HUVECs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Dihydroethidium Staining and Wound Healing Assay
4.4. Adhesion of U937 Monocytes to HUVECs
4.5. Isolation and Characterization of Extracellular Vesicles
4.6. The EVs Labeling and Tracer Observations
4.7. Cell Transfection
4.8. Western Blot and qRT-PCR
4.9. Luciferase Activity Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jackson, A.O.; Regine, M.A.; Subrata, C.; Long, S. Molecular Mechanisms and Genetic Regulation in Atherosclerosis. IJC Heart Vasc. 2018, 21, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.Y.; Leening, M.J.G.; Norby, F.L.; Roetker, N.S.; Hofman, A.; Franco, O.H.; Pan, W.; Polak, J.F.; Witteman, J.C.M.; Kronmal, R.A.; et al. Carotid Intima-Media Thickness and Arterial Stiffness and the Risk of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study, Multi-Ethnic Study of Atherosclerosis (MESA), and the Rotterdam Study. J. Am. Heart Assoc. 2016, 5, e002907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bories, G.F.P.; Leitinger, N. Macrophage Metabolism in Atherosclerosis. FEBS Lett. 2017, 591, 3042–3060. [Google Scholar] [CrossRef] [Green Version]
- Méndez-Cruz, A.R.; Paez, A.; Jiménez-Flores, R.; Reyes-Reali, J.; Varela, E.; Cerbulo-Vazquez, A.; Rodriguez, E.; López-Marure, R.; Masso, F.A.; Flores-Romo, L.; et al. Increased Expression of Inflammation-Related Co-Stimulatory Molecules by HUVECs from Newborns with a Strong Family History of Myocardial Infarction Stimulated with TNF-α and OxLDL. Immunol. Lett. 2007, 111, 116–123. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and Atherosclerosis. Mediat. Inflamm. 2013, 2013, 12. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Huang, Y.; Zhou, Y.; Nie, W.; Pu, X.; Xu, X.; Zhu, J. Exosomes Derived from Oxidized LDL-Stimulated Macrophages Attenuate the Growth and Tube Formation of Endothelial Cells. Mol. Med. Rep. 2018, 17, 4605–4610. [Google Scholar] [CrossRef]
- Hu, N.; Zeng, X.; Tang, F.; Xiong, S. Exosomal Long Non-Coding RNA LIPCAR Derived from OxLDL-Treated THP-1 Cells Regulates the Proliferation of Human Umbilical Vein Endothelial Cells and Human Vascular Smooth Muscle Cells. Biochem. Biophys. Res. Commun. 2021, 575, 65–72. [Google Scholar] [CrossRef]
- Gao, W.; Liu, H.; Yuan, J.; Wu, C.; Huang, D.; Ma, Y.; Zhu, J.; Ma, L.; Guo, J.; Shi, H.; et al. Exosomes Derived from Mature Dendritic Cells Increase Endothelial Inflammation and Atherosclerosis via Membrane TNF-α Mediated NF-ΚB Pathway. J. Cell. Mol. Med. 2016, 20, 2318–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanan, W.; Yingyu, X.; Ao, Z.; Mingyang, W.; Zihan, F.; Junping, Z. Exosomes: An Emerging Factor in Atherosclerosis. Biomed. Pharmacother. 2019, 115, 108951. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Wu, H.; Xie, X.; Sun, Y.; Dai, M. Paeonol Attenuated Inflammatory Response of Endothelial Cells via Stimulating Monocytes-Derived Exosomal MicroRNA-223. Front. Pharmacol. 2018, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, S.; Oggero, S.; Bruen, R.; McCarthy, C.; Strowitzki, M.J.; Mahon, N.G.; Ryan, N.; Brennan, E.P.; Barry, M.; Perretti, M.; et al. MicroRNA-155 Is Decreased During Atherosclerosis Regression and Is Increased in Urinary Extracellular Vesicles During Atherosclerosis Progression. Front. Immunol. 2020, 11, 576516. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 Regulates Endothelial Expression of Vascular Cell Adhesion Molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef] [Green Version]
- Rangrez, A.Y.; Massy, Z.A.; Meuth, V.M.L.; Metzinger, L. MiR-143 and MiR-145 Molecular Keys to Switch the Phenotype of Vascular Smooth Muscle Cells. Circ. Cardiovasc. Genet. 2011, 4, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Elia, L.; Quintavalle, M.; Zhang, J.; Contu, R.; Cossu, L.; Latronico, M.V.G.; Peterson, K.L.; Indolfi, C.; Catalucci, D.; Chen, J.; et al. The Knockout of MiR-143 and -145 Alters Smooth Muscle Cell Maintenance and Vascular Homeostasis in Mice: Correlates with Human Disease. Cell Death Differ. 2009, 16, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Hergenreider, E.; Heydt, S.; Tréguer, K.; Boettger, T.; Horrevoets, A.J.G.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective Communication between Endothelial Cells and Smooth Muscle Cells through MiRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-Derived Bioactive Peptides and Their Health Promoting Effects: A Potential Role in Atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, S.; Wu, J. Bioactive Peptides on Endothelial Function. Food Sci. Hum. Wellness 2016, 5, 1–7. [Google Scholar]
- Montoya-Rodríguez, A.; de Mejía, E.G. Pure Peptides from Amaranth (Amaranthus Hypochondriacus) Proteins Inhibit LOX-1 Receptor and Cellular Markers Associated with Atherosclerosis Development in Vitro. Food Res. Int. 2015, 77, 204–214. [Google Scholar] [CrossRef]
- Song, T.; Lv, M.; Zhou, M.; Huang, M.; Zheng, L.; Zhao, M. Soybean-Derived Antihypertensive Peptide LSW (Leu-Ser-Trp) Antagonizes the Damage of Angiotensin II to Vascular Endothelial Cells through the Trans-Vesicular Pathway. J. Agric. Food Chem. 2021, 69, 10536–10549. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Liao, W.; Bai, J.; Wu, W.; Wu, J. Soy Protein-Derived ACE-Inhibitory Peptide LSW (Leu-Ser-Trp) Shows Anti-Inflammatory Activity on Vascular Smooth Muscle Cells. J. Funct. Foods 2017, 34, 248–253. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Niu, X.; Yu, J.; Xiao, X.; Zang, L.; Zhao, J.; Huang, Q.; Li, W. Imperatorin Alleviates the Abnormal Proliferation, Migration, and Foaming of Ox-LDL-Induced VSMCs through Regulating PI3K/Akt/MTOR Signaling Pathway. J. Funct. Foods 2020, 70, 103982. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Bezsonov, E.E.; Rafieian-Kopaei, M. Role of Lipid Accumulation and Inflammation in Atherosclerosis: Focus on Molecular and Cellular Mechanisms. Front. Cardiovasc. Med. 2021, 8, 707529. [Google Scholar] [CrossRef]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From Endothelial Dysfunction to Atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.; Ren, Y.; Zeng, Y.; Huang, Q.; Wang, C. Antihypertensive Effect of Soybean Bioactive Peptides: A Review. Curr. Opin. Pharmacol. 2022, 62, 74–81. [Google Scholar] [CrossRef]
- Chen, H.; Chen, S.; Zhang, W.; Xu, J.; Li, M.; Qian, M.; Kyler, K. Endothelial–Vascular Smooth Muscle Cells Interactions in Atherosclerosis. Atheroscler. Front. Cardiovasc. Med. 2018, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Giri, H.; Georgescu, A.; Xiang, D.; Li, Y.; Cao, Y.; Huang, Y.; Zhou, L.; Lin, X.; Qiao, Y.; et al. Different Effects of Endothelial Extracellular Vesicles and LPS-Induced Endothelial Extracellular Vesicles on Vascular Smooth Muscle Cells: Role of Curcumin and Its Derivatives. Front. Cardiovasc. Med. 2021, 1, 649352. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Zhong, Y.; Xiao, G.; Efferth, T.; Georgiev, M.I.; Vargas-De-La-Cruz, C.; Bajpai, V.K.; Caprioli, G.; Liu, J.; et al. Dendrobium Officinale Polysaccharide Alleviates Intestinal Inflammation by Promoting Small Extracellular Vesicle Packaging of MiR-433-3p. J. Agric. Food Chem. 2021, 69, 13510–13523. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, J. LC-MS/MS Coupled with QSAR Modeling in Characterising of Angiotensin I-Converting Enzyme Inhibitory Peptides from Soybean Proteins. Food Chem. 2013, 141, 2682–2690. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Xu, Q.; Bai, J.; Wu, W.; Hong, H.; Wu, J. Transport of Soybean Protein-Derived Antihypertensive Peptide LSW across Caco-2 Monolayers. J. Funct. Foods 2017, 39, 96–102. [Google Scholar] [CrossRef]
- Paone, S.; Baxter, A.A.; Hulett, M.D.; Poon, I.K.H. Endothelial Cell Apoptosis and the Role of Endothelial Cell-Derived Extracellular Vesicles in the Progression of Atherosclerosis. Cell. Mol. Life Sci. 2018, 76, 1093–1106. [Google Scholar] [CrossRef]
- Gao, Y.; Li, H.; Zhou, Y.; Lv, H.; Chen, Y. PDCD4 Expression in Coronary Atherosclerosis Rat Models and Its Mechanism. Exp. Ther. Med. 2019, 17, 3150–3154. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Xu, Z.; Yuan, M.; Zhang, Y.; Zhao, B.; Wang, J.; Zhang, A.; Li, G. MicroRNA-16 Suppresses the Activation of Inflammatory Macrophages in Atherosclerosis by Targeting PDCD4. Int. J. Mol. Med. 2016, 37, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Dong, Z.; Shang, Q.; Zhao, H.; Wang, L.; Guo, C.; Gao, F.; Zhang, L.; Wang, Q. Pdcd4 Is Involved in the Formation of Stress Granule in Response to Oxidized Low-Density Lipoprotein or High-Fat Diet. PLoS ONE 2016, 11, e0159568. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, Y.; Xu, Z.; Liang, X.; Wang, X.; Zhang, Y.; Yuan, M.; Ni, Y.; Liu, H.; Li, G. MiR-21 Suppresses Ox-LDL-Induced HUVECs Apoptosis by Targeting PDCD4. Int. J. Clin. Exp. Pathol. 2017, 10, 10075–10084. [Google Scholar]
- Song, T.; Lv, M.; Zhang, L.; Zhang, X.; Song, G.; Huang, M.; Zheng, L.; Zhao, M. The Protective Effects of Tripeptides VPP and IPP against Small Extracellular Vesicles from Angiotensin II-Induced Vascular Smooth Muscle Cells Mediating Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells. J. Agric. Food Chem. 2020, 68, 13730–13741. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Andaluz Aguilar, H.; Iliuk, A.B.; Chen, I.H.; Tao, W.A. Sequential Phosphoproteomics and N-Glycoproteomics of Plasma-Derived Extracellular Vesicles. Nat. Protoc. 2020, 15, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac Progenitor Cell-Derived Exosomes Prevent Cardiomyocytes Apoptosis through Exosomal MiR-21 by Targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Gao, C.; Zhou, K.; Liu, W.; Zhang, Y.; Zhao, Y. MicroRNA-532-5p-Programmed Cell Death Protein 4 (PDCD4) Axis Regulates Angiotensin II-Induced Human Umbilical Vein Endothelial Cell Apoptosis and Proliferation. Microvasc. Res. 2021, 138, 104195. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Zhou, M.; Li, W.; Zheng, L.; Wu, J.; Zhao, M. Tripeptide Leu-Ser-Trp Regulates the Vascular Endothelial Cells Phenotype Switching by Mediating the Vascular Smooth Muscle Cells-Derived Small Extracellular Vesicles Packaging of miR-145. Molecules 2022, 27, 7025. https://doi.org/10.3390/molecules27207025
Song T, Zhou M, Li W, Zheng L, Wu J, Zhao M. Tripeptide Leu-Ser-Trp Regulates the Vascular Endothelial Cells Phenotype Switching by Mediating the Vascular Smooth Muscle Cells-Derived Small Extracellular Vesicles Packaging of miR-145. Molecules. 2022; 27(20):7025. https://doi.org/10.3390/molecules27207025
Chicago/Turabian StyleSong, Tianyuan, Minzhi Zhou, Wen Li, Lin Zheng, Jianping Wu, and Mouming Zhao. 2022. "Tripeptide Leu-Ser-Trp Regulates the Vascular Endothelial Cells Phenotype Switching by Mediating the Vascular Smooth Muscle Cells-Derived Small Extracellular Vesicles Packaging of miR-145" Molecules 27, no. 20: 7025. https://doi.org/10.3390/molecules27207025
APA StyleSong, T., Zhou, M., Li, W., Zheng, L., Wu, J., & Zhao, M. (2022). Tripeptide Leu-Ser-Trp Regulates the Vascular Endothelial Cells Phenotype Switching by Mediating the Vascular Smooth Muscle Cells-Derived Small Extracellular Vesicles Packaging of miR-145. Molecules, 27(20), 7025. https://doi.org/10.3390/molecules27207025







