Comparison of the Effect of Cold Plasma with Conventional Preservation Methods on Red Wine Quality Using Chemometrics Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.1.1. Determination of pH and Color Measurement
2.1.2. Polyphenolic Content
2.1.3. Antioxidant Activity
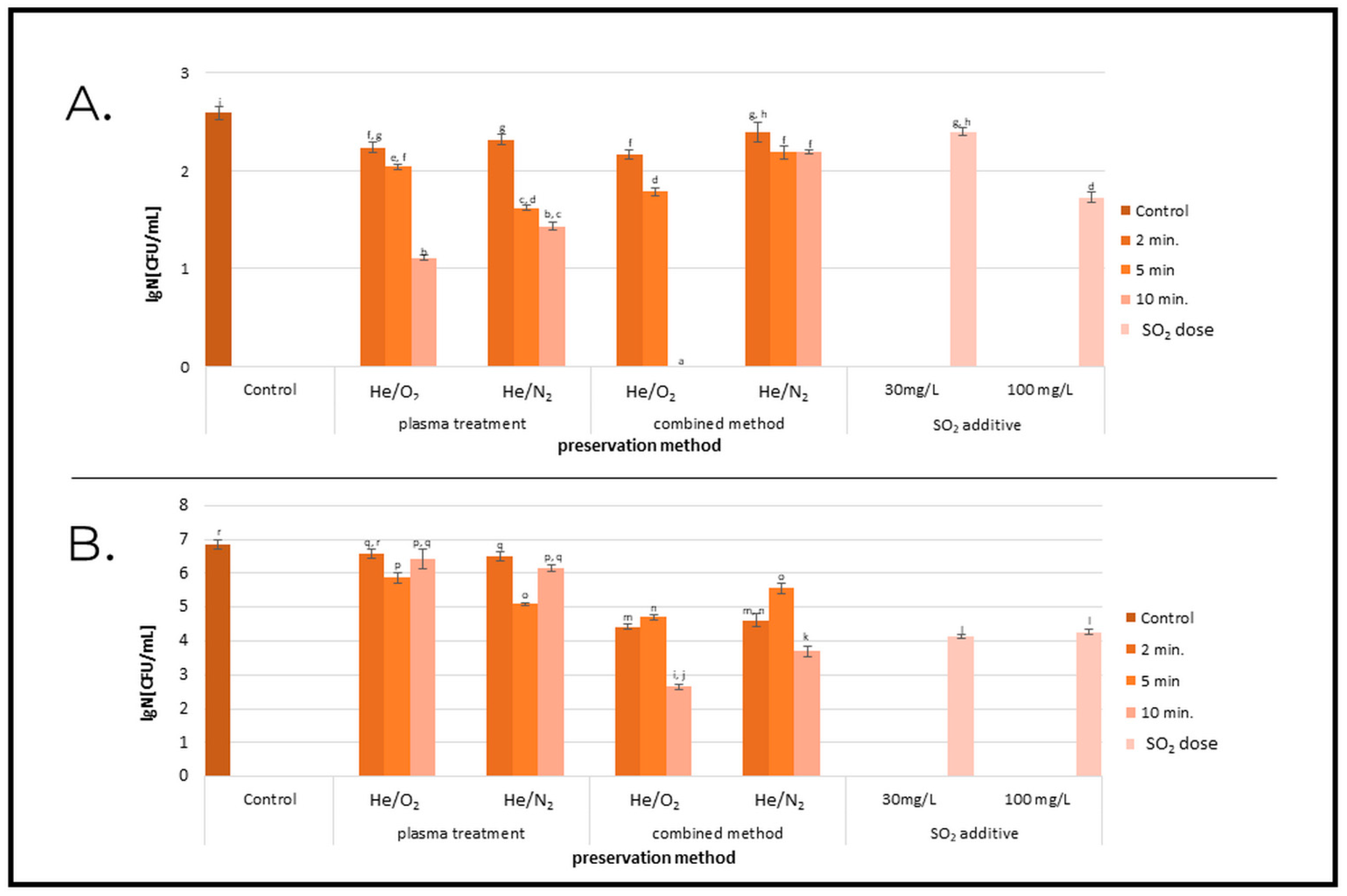
2.2. Microbial Contamination
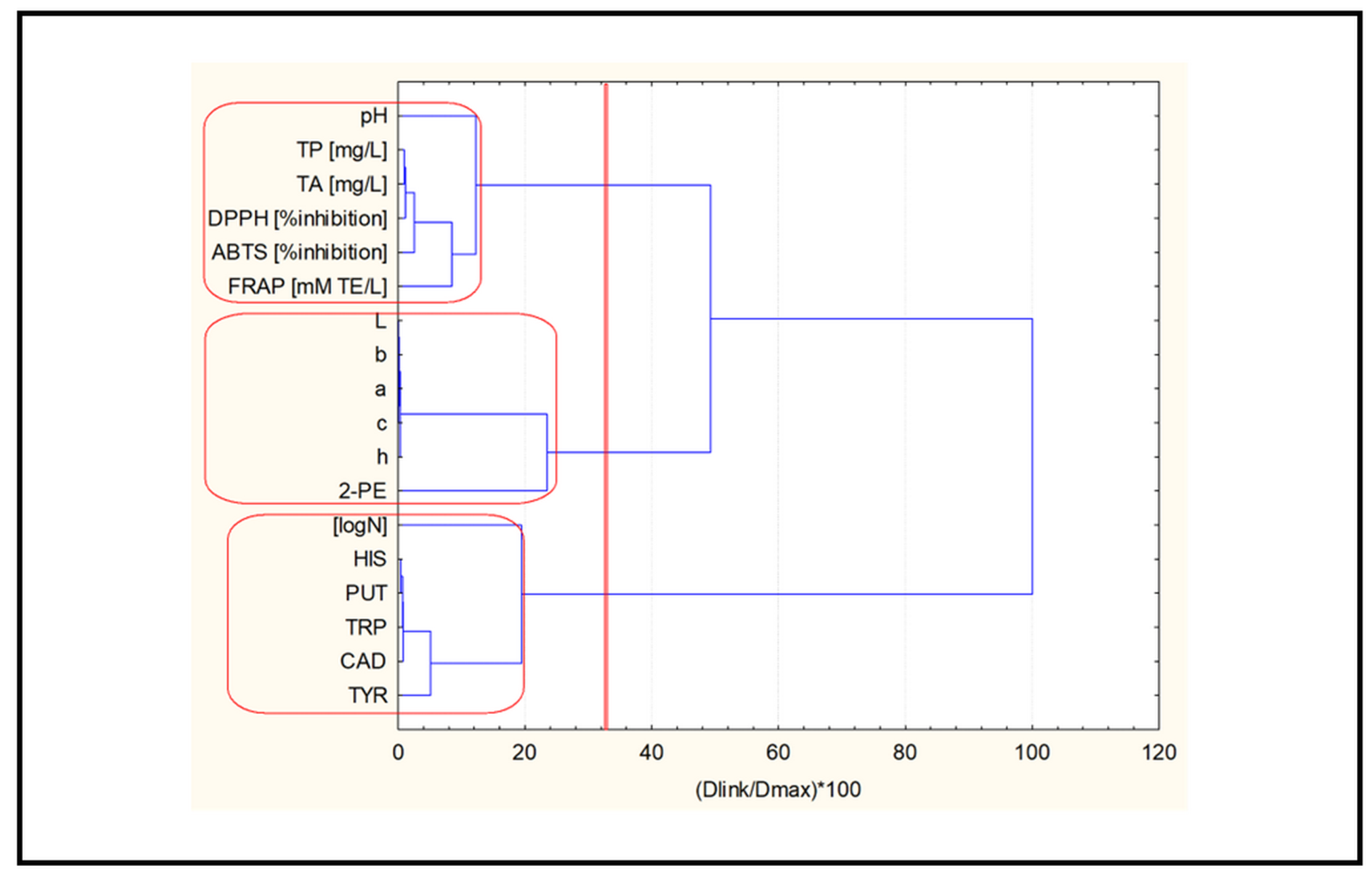
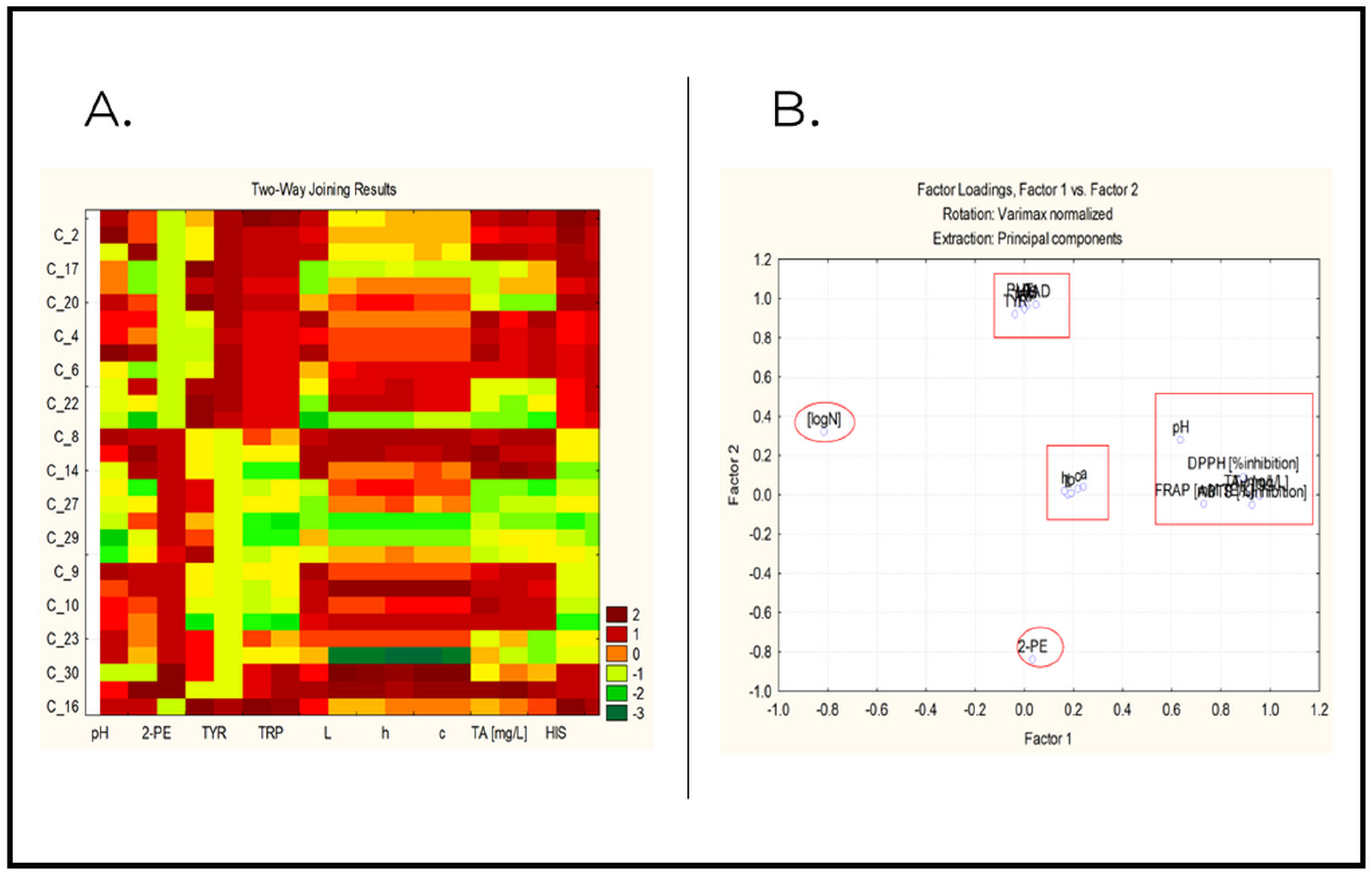
2.3. Chemometric Analysis
- Cluster analysis.
- Factor analysis and principal components analysis.
2.3.1. Cluster Analysis
2.3.2. Factor Analysis and Principal Component Analysis
3. Materials and Methods
3.1. Wine
3.2. Cold Plasma Treatment
3.3. Determination of pH and Color Measurement
3.4. Determination of Polyphenolic Compounds
3.4.1. Total Phenolic Content
3.4.2. Total Anthocyanin Content
3.5. Determination of Antioxidant Activity
3.5.1. DPPH Inhibition
3.5.2. ABTS
3.5.3. FRAP
3.6. Microbiological Analysis
3.7. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffith, M.P. Ancient wine: The search for the origins of viniculture. Econ. Bot. 2004, 58, 488. [Google Scholar] [CrossRef]
- Niedźwiedź, I.; Płotka-Wasylka, J.; Kapusta, I.; Simeonov, V.; Stój, A.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The impact of cold plasma on the phenolic composition and biogenic amine content of red wine. Food Chem. 2022, 381, 132257. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; López de Lerma, N. Alternative Winemaking Techniques to Improve the Content of Phenolic and Aromatic Compounds in Wines. Agriculture 2021, 11, 233. [Google Scholar] [CrossRef]
- Robles, A.D.; Fabjanowicz, M.; Płotka-Wasylka, J.; Konieczka, P. Organic acids and polyphenols determination in Polish wines by ultrasound-assisted solvent extraction of porous membrane-packed liquid samples. Molecules 2019, 24, 4376. [Google Scholar] [CrossRef] [Green Version]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Filho, D.W.; Pedrosav, R.C.; Bordignon-Luiz, M.T. Phenolic profile and effect of regular consumption of Brazilian redwines on in vivo antioxidant activity. J. Food Compos. Anal. 2013, 31, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasines-Perea, Z.; Teissedre, P.L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Haunschild, R.; Marx, W. On Health Effects of Resveratrol in Wine. Int. J. Environ. Res. Public Health 2022, 19, 3110. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Raso, J.; Álvarez, I.; Delso, C.; Morata, A. Pulsed Electric Fields to improve the use of non-Saccharomyces starters in red wines. Foods 2021, 10, 1472. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Pérez-Nevado, F.; Martínez, A.; Martín, A.; de Guía Córdoba, M. Addition of Grape Skin and Stems Extracts in Wines during the Storage to Reduce the Sulfur Dioxide: Impact on Red Wine Quality. Int. J. Environ. Res. Public Health 2021, 18, 2783. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.Á.; Monagas, M.; Bartolomé, B.; Moreno-Arribas, M.V. Wine features related to safety and consumer health: An integrated perspective. Crit. Rev. Food Sci. Nutr. 2012, 52, 31–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, F.V.M.; van Wyk, S. Emerging Non-Thermal Technologies as Alternative to SO2 for the Production of Wine. Foods 2021, 10, 2175. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kahar, S.P.; Ranveer, R.C.; Annapure, U.S. Cold plasma an emerging nonthermal technology for milk and milk products: A review. Int. J. Dairy Technol. 2021, 74, 615–626. [Google Scholar] [CrossRef]
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The state of research on antimicrobial activity of cold plasma. Polish J. Microbiol. 2019, 68, 153. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Pankaj, S.K.; Segat, A.; Ishikawa, K. Cold plasma interactions with enzymes in foods and model systems. Trends Food Sci. Technol. 2016, 55, 39–47. [Google Scholar] [CrossRef]
- Starek, A.; Pawłat, J.; Chudzik, B.; Kwiatkowski, M.; Terebun, P.; Sagan, A.; Andrejko, D. Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci. Rep. 2019, 9, 8407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef]
- Mandal, R.; Singh, A.; Singh, A.P. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáenz-Navajas, M.P.; Echavarri, F.; Ferreira, V.; Fernández-Zurbano, P. Pigment composition and color parameters of commercial Spanish red wine samples: Linkage to quality perception. Eur. Food Res. Technol. 2011, 232, 877–887. [Google Scholar] [CrossRef]
- Sarangapani, C.K.; O’Toole, G.; Cullen, P.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Lukić, K.; Vukušić, T.; Tomašević, M.; Ćurko, N.; Gracin, L.; Ganić, K.K. The impact of high voltage electrical discharge plasma on the chromatic characteristics and phenolic composition of red and white wines. Innov. Food Sci. Emerg. Technol. 2019, 53, 70–77. [Google Scholar] [CrossRef]
- De Souza, J.F.; De Souza Nascimento, A.M.; Linhares, M.d.S.S.; Dutra, M.d.C.P.; Dos Santos Lima, M.; Pereira, G.E. Evolution of Phenolic Compound Profiles and Antioxidant Activity of Syrah Red and Sparkling Moscatel Wines Stored in Bottles of Different Colors. Beverages 2018, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Tkacz, K.; Modzelewska-Kapituła, M.; Więk, A.; Nogalski, Z. The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times. Appl. Sci. 2020, 10, 8215. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Alves Filho, E.G.; de Brito, E.S.; Rodrigues, S. Effects of cold plasma processing in food components. In Advances in Cold Plasma Applications for Food Safety and Preservation; Academic Press: Cambridge, MA, USA, 2020; pp. 253–268. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.H.; Khaneghah, A.M.; Barba, F.J.; Misra, N.N. A critical analysis of the cold plasma induced lipid oxidation in foods. Trends Food Sci. Technol. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [Green Version]
- Arjunan, K.P.; Sharma, V.K.; Ptasinska, S. Effects of atmospheric pressure plasmas on isolated and cellular DNA—A review. Int. J. Mol. Sci. 2015, 16, 2971–3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esparza, I.; Martínez-Inda, B.; Cimminelli, M.J.; Jimeno-Mendoza, M.C.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Reducing SO2 doses in red wines by using grape stem extracts as antioxidants. Biomolecules 2020, 10, 1369. [Google Scholar] [CrossRef]
- Rodríguez-Vaquero, M.J.; Vallejo, C.V.; Aredes-Fernández, P.A. Antibacterial, antioxidant and antihypertensive properties of polyphenols from argentinean red wines varieties. J. Pharmacol. Pharmacother. 2020, 5, 001–006. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.F.; Jing, S.S.; Ni, X.F.; Zhang, K.K.; Fang, Y.L.; Zhang, Z.W.; Ju, Y.L. Anthocyanin and phenolic acids contents influence the color stability and antioxidant capacity of wine treated with mannoprotein. Front. Nutr. 2021, 8, 691784. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, E.J.; Choi, E.H.; Kim, Y.J. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov. Food Sci. Emerg. Technol. 2014, 22, 124–130. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Effect of high voltage atmospheric cold plasma on white grape juice quality. J. Sci. Food and Agric. 2017, 97, 4016–4021. [Google Scholar] [CrossRef]
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Lee, E.J.; Kim, Y.J. A submerged dielectric barrier discharge plasma inactivation mechanism of biofilms produced by Escherichia coli O157: H7, Cronobacter sakazakii, and Staphylococcus aureus. Sci. Rep. 2016, 6, 37072. [Google Scholar] [CrossRef] [Green Version]
- Niedźwiedź, I.; Juzwa, W.; Skrzypiec, K.; Skrzypek, T.; Waśko, A.; Kwiatkowski, M.; Pawłat, J.; Polak-Berecka, M. Morphological and physiological changes in Lentilactobacillus hilgardii cells after cold plasma treatment. Sci. Rep. 2020, 10, 18882. [Google Scholar] [CrossRef]
- Choi, S.; Puligundla, P.; Mok, C. Corona discharge plasma jet for inactivation of Escherichia coli O157: H7 and Listeria monocytogenes on inoculated pork and its impact on meat quality attributes. Ann. Microbiol. 2016, 66, 685–694. [Google Scholar] [CrossRef]
- Tappi, S.; Gozzi, G.; Vannini, L.; Berardinelli, A.; Romani, S.; Ragni, L.; Rocculi, P. Cold plasma treatment for fresh-cut melon stabilization. Innov. Food Sci. Emerg. Technol. 2016, 33, 225–233. [Google Scholar] [CrossRef]
- Wang, R.X.; Nian, W.F.; Wu, H.Y.; Feng, H.Q.; Zhang, K.; Zhang, J.; Zhu, W.D.; Becker, K.H.; Fang, J. Atmospheric-pressure cold plasma treatment of contaminated fresh fruit and vegetable slices: Inactivation and physiochemical properties evaluation. Eur. Phys. J. D 2012, 66, 276. [Google Scholar] [CrossRef]
- Restuccia, D.; Loizzo, M.R.; Spizzirri, U.G. Accumulation of biogenic amines in wine: Role of alcoholic and malolactic fermentation. Fermentation 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Kukula-Koch, W.; Kowalik, K.; Polak-Berecka, M.; Waśko, A. Evolution of the anticholinesterase, antioxidant, and anti-inflammatory activity of Epilobium angustifolium L. infusion during in vitro digestion. J. Funct. Foods 2021, 85, 104645. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]

| Sample | Preservation Methods | pH | L* | a* | b* | C* | H* | ∆E* |
|---|---|---|---|---|---|---|---|---|
| BEFORE STORAGE | ||||||||
| 1. | no preservation | 3.54 ± 0.01 de | 11.23 ± 0.02 e | 41.68 ± 0.08 ef | 19.35 ± 0.07 g | 45.96 ± 0.13 ef | 24.91 ± 0.03 e | - |
| 2. | cold plasma (2 min *; He/O2 **) | 3.55 ± 0.02 e | 11.28 ± 0.03 ef | 41.84 ± 0.22 ef | 19.45 ± 0.11 g | 46.14 ± 0.05 fg | 24.93 ± 0.07 e | 0.20 ± 0.01 |
| 3. | cold plasma (5 min; He/O2) | 3.52 ± 0.01 bcde | 11.81 ± 0.07 g | 42.43 ± 0.07 fghij | 20.36 ± 0.02 h | 47.06 ± 0.07 i | 25.64 ± 0.12 f | 1.39 ± 0.09 |
| 4. | cold plasma (10 min; He/O2) | 3.52 ± 0.01 bcde | 12.56 ± 0.06 ij | 43.16 ± 0.17 jk | 21.65 ± 0.22 l | 48.29 ± 0.06 jk | 26.64 ± 0.09 ij | 3.04 ± 0.12 |
| 5. | cold plasma (2 min; He/N2) | 3.48 ± 0.01 abcd | 11.02 ± 0.05 e | 41.51 ± 0.16 e | 19.01 ± 0.03 f | 45.65 ± 0.0 e | 24.61 ± 0.10 e | 0.43 ± 0.02 |
| 6. | cold plasma (5 min; He/N2) | 3.49 ± 0.01 abcde | 13.26 ± 0.22 k | 44.01 ± 0.12 lmn | 22.87 ± 0.10 m | 49.60 ± 0.23 l | 27.46 ± 0.06 m | 4.68 ± 0.16 |
| 7. | cold plasma (10 min; He/N2) | 3.55 ± 0.02 e | 12.55 ± 0.52 ij | 43.29 ± 0.37 kl | 21.70 ± 0.10 l | 48.41 ± 0.19 jk | 26.62 ± 0.03 ij | 3.14 ± 0.09 |
| 8. | 30 mg/L potassium metabisulfite | 3.54 ± 0.01 de | 14.79 ± 012 n | 45.89 ± 0.04 qr | 25.24 ± 0.10 r | 52.37 ± 0.33 p | 28.82 ± 0.20 pq | 8.07 ± 0.22 |
| 9. | cold plasma (2 min; He/O2) and 30 mg/L potassium metabisulfite | 3.54 ± 0.01 de | 12.31 ± 0.10 hij | 43.05 ± 0.25 ijk | 21.23 ± 0.14 jk | 48.00 ± 0.14 j | 26.24 ± 0.15 h | 2.56 ± 0.18 |
| 10. | cold plasma (5 min; He/O2) and 30 mg/L potassium metabisulfite | 3.52 ± 0.00 bcde | 12.69 ± 0.14 j | 43.44 ± 1.27 klm | 21.87 ± 0.05 l | 48.64 ± 0.05 k | 26.73 ± 0.17 jk | 3.40 ± 0.10 |
| 11. | cold plasma (10 min; He/O2) and 30 mg/L potassium metabisulfite | 3.52 ± 0.01 bcde | 14.10 ± 0.06 m | 44.88 ± 0.12 op | 23.98 ± 0.14 o | 51.04 ± 0.14 n | 28.51 ± 0.12 op | 6.32 ± 0.13 |
| 12. | cold plasma (2 min; He/N2) and 30 mg/L potassium metabisulfite | 3.52 ± 0.02 bcde | 14.33 ± 0.08 m | 45.27 ± 0.11 pq | 24.62 ± 0.10 p | 51.54 ± 0.16 o | 28.54 ± 0.18 op | 7.09 ± 0.09 |
| 13. | cold plasma (5 min; He/N2) and 30 mg/L potassium metabisulfite | 3.51 ± 0.01 bcde | 15.13 ± 0.14 n | 46.08 ± 0.22 qr | 25.80 ± 0.16 r | 52.81 ± 0.12 q | 29.24 ± 0.13 rs | 8.73 ± 0.18 |
| 14. | cold plasma (10 min; He/N2) and 30 mg/L potassium metabisulfite | 3.48 ± 0.01 abcd | 12.13 ± 0.15 ghi | 42.75 ± 0.15 ghijk | 20.91 ± 0.07 i | 46.92 ± 0.11 i | 26.06 ± 0.13 gh | 2.09 ± 0.03 |
| 15. | 100 mg/L potassium metabisulfite | 3.52 ± 0.02 bcde | 14.94 ± 0.06 n | 46.16 ± 0.09 r | 25.44 ± 0.12 q | 52.79 ± 0.13 pq | 29.03 ± 0.12 qr | 8.42 ± 0.01 |
| AFTER STORAGE | ||||||||
| 16. | no preservation | 3.53 ± 0.01 cde | 11.71 ± 0.15 fg | 42.22 ± 0.04 efghi | 20.19 ± 0.14 h | 46.80 ± 0.08 hi | 25.55 ± 0.10 f | 1.11 ± 0.12 |
| 17. | cold plasma (2 min; He/O2) | 3.50 ± 0.02 abcde | 10.18 ± 0.15 d | 39.55 ± 0.05 cd | 17.56 ± 0.11 e | 43.27 ± 0.14 d | 23.93 ± 0.12 d | 2.97 ± 0.09 |
| 18. | cold plasma (5 min; He/O2) | 3.48 ± 0.01 abcd | 13.62 ± 0.09 kl | 44.21 ± 0.07 mno | 23.48 ± 0.09 n | 50.05 ± 0.08 m | 27.97 ± 0.10 n | 5.40 ± 0.08 |
| 19. | cold plasma (10 min; He/O2) | 3.47 ± 0.01 abc | 9.72 ± 0.09 c | 39.89 ± 0.12 d | 16.75 ± 0.09 d | 43.26 ± 0.12 d | 22.80 ± 0.09 c | 3.50 ± 0.10 |
| 20. | cold plasma (2 min; He/N2) | 3.53 ± 0.02 cde | 12.74 ± 0.08 j | 42.99 ± 0.11 ijk | 21.96 ± 0.09 l | 48.28 ± 0.10 jk | 27.05 ± 0.10 kl | 3.29 ± 0.10 |
| 21. | cold plasma (5 min; He/N2) | 3.50 ± 0.01 abcde | 11.74 ± 0.10 g | 42.13 ± 0.15 efgh | 20.24 ± 0.14 h | 46.74 ± 0.11 hi | 25.67 ± 0.10 f | 1.12 ± 0.12 |
| 22. | cold plasma (10 min; He/N2) | 3.48 ± 0.01 abcd | 13.91 ± 0.12 lm | 44.34 ± 0.13 no | 23.98 ± 0.12 o | 50.41 ± 0.06 m | 22.41 ± 0.09 o | 5.97 ± 0.08 |
| 23. | 30 mg/L potassium metabisulfite | 3.53 ± 0.01 cde | 12.35 ± 0.06 hij | 43.05 ± 0.11 jk | 21.30 ± 0.11 k | 48.03 ± 0.14 j | 26.32 ± 0.08 hi | 2.63 ± 0.09 |
| 24. | cold plasma (2 min; He/O2) and 30 mg/L potassium metabisulfite | 3.53 ± 0.02 cde | 6.6 ± 0.08 a | 34.28 ± 0.14 a | 11.39 ± 0.03 a | 36.13 ± 0.04 a | 18.37 ± 0.04 a | 11.81 ± 0.07 |
| 25. | cold plasma (5 min; He/O2) and 30 mg/L potassium metabisulfite | 3.49 ± 0.01 abcde | 12.73 ± 0.05 j | 42.76 ± 0.06 hijk | 21.97 ± 0.06 l | 48.07 ± 0.07 j | 27.18 ± 0.04 lm | 3.21 ± 0.06 |
| 26. | cold plasma (10 min; He/O2) and 30 mg/L potassium metabisulfite | 3.47 ± 0.01 abc | 8.94 ± 0.05 b | 38.39 ± 0.07 b | 15.41 ± 0.11 b | 41.37 ± 0.04 b | 21.87 ± 0.07 b | 5.62 ± 0.09 |
| 27. | cold plasma (2 min; He/N2) and 30 mg/L potassium metabisulfite | 3.48 ± 0.00 abcd | 12.03 ± 0.03 gh | 41.92 ± 0.06 efg | 20.73 ± 0.12 i | 46.77 ± 0.09 hi | 26.32 ± 0.04 hi | 1.61 ± 0.09 |
| 28. | cold plasma (5 min; He/N2) and 30 mg/L potassium metabisulfite | 3.46 ± 0.01 ab | 11.73 ± 0.08 g | 41.79 ± 0.05 ef | 20.22 ± 0.06 h | 46.43 ± 0.09 gh | 25.82 ± 0.05 fg | 1.01 ± 0.07 |
| 29. | cold plasma (10 min; He/N2) and 30 mg/L potassium metabisulfite | 3.44 ± 0.03 a | 9.44 ± 0.06 c | 38.94 ± 0.06 bc | 16.28 ± 0.06 c | 42.21 ± 0.07 c | 22.69 ± 0.07 c | 4.49 ± 0.06 |
| 30. | 100 mg/L potassium metabisulfite | 3.47 ± 0.01 abc | 16.52 ± 0.12 o | 47.60 ± 0.09 s | 26.94 ± 0.03 s | 54.70 ± 0.05 r | 29.51 ± 0.07 s | 10.98 ± 0.05 |
| Sample Number | Preservation Methods | TPC [mg/L] | TAC [mg/L] | DPPH [% Inhibition] | ABTS [% Inhibition] | FRAP [mM TE/L] |
|---|---|---|---|---|---|---|
| BEFORE STORAGE | ||||||
| 1. | no preservation | 2442.75 ± 12.30 mn | 690.92 ± 3.00 lmno | 72.22 ± 0.87 hij | 95.24 ± 1.73 a | 10.35 ± 0.53 fghijk |
| 2. | cold plasma (2 min *; He/O2 **) | 2300.25 ± 10.15 j | 647.92 ± 3.64 ijk | 67.82 ± 1.27 h | 95.13 ± 2.54 a | 10.16 ± 0.59 fghij |
| 3. | cold plasma (5 min; He/O2) | 2497.03 ± 11.26 pq | 607.00 ± 17.82 h | 74.14 ± 1.46 jk | 95.39 ± 0.95 a | 10.72 ± 0.11 hijkl |
| 4. | cold plasma (10 min; He/O2) | 2449.53 ± 4.02 n | 634.56 ± 3.08 hi | 72.22 ± 0.88 hij | 94.81 ± 1.40 a | 9.81 ± 0.19 defghi |
| 5. | cold plasma (2 min; He/N2) | 2483.46 ± 5.26 op | 693.84 ± 4.98 mno | 70.50 ± 1.09 hij | 95.22 ± 2.33 a | 12.06 ± 0.46 lm |
| 6. | cold plasma (5 min; He/N2) | 2388.46 ± 3.59 k | 642.35 ± 10.51 ij | 72.61 ± 4.31 ijk | 94.26 ± 1.68 a | 8.24 ± 0.27 abcd |
| 7. | cold plasma (10 min; He/N2) | 2517.39 ± 4.07 q | 649.17 ± 3.01 ijk | 72.03 ± 0.99 hij | 95.39 ± 1.56 a | 11.64 ± 0.61 jklm |
| 8. | 30 mg/L potassium metabisulfite | 2422.39 ± 3.03 lm | 702.19 ± 8.22 nop | 73.75 ± 1.84 ijk | 95.27 ± 1.12 a | 11.42 ± 0.65 jklm |
| 9. | cold plasma (2 min; He/O2) and 30 mg/L potassium metabisulfite | 2381.68 ± 4.80 k | 658.35 ± 5.74 ijk | 72.61 ± 2.52 ijk | 95.42 ± 0.62 a | 11.45 ± 0.43 jklm |
| 10. | cold plasma (5 min; He/O2) and 30 mg/L potassium metabisulfite | 2483.46 ± 5.71 op | 670.46 ± 5.75 jklm | 69.73 ± 1.51 hij | 95.30 ± 1.25 a | 10.17 ± 0.24 fghij |
| 11. | cold plasma (10 min; He/O2) and 30 mg/L potassium metabisulfite | 2463.10 ± 8.96 no | 662.67 ± 22.00 ijkl | 72.22 ± 2.35 hij | 95.04 ± 1.03 a | 9.81 ± 0.25 defghi |
| 12. | cold plasma (2 min; He/N2) and 30 mg/L potassium metabisulfite | 2578.46 ± 9.04 s | 673.80 ± 6.31 klmn | 70.11 ± 0.97 hij | 95.51 ± 0.93 a | 12.09 ± 0.76 lm |
| 13. | cold plasma (5 min; He/N2) and 30 mg/L potassium metabisulfite | 2415.61 ± 4.34 l | 658.56 ± 14.13 ijk | 69.16 ± 1.08 hi | 95.24 ± 0.37 a | 11.45 ± 0.69 jklm |
| 14. | cold plasma (10 min; He/N2) and 30 mg/L potassium metabisulfite | 2551.31 ± 3.46 r | 707.20 ± 5.21 op | 73.95 ± 0.95 jk | 95.42 ± 1.59 a | 11.86 ± 0.77 klm |
| 15. | 100 mg/L potassium metabisulfite | 2598.81 ± 5.18 s | 730.16 ± 14.61 p | 77.31 ± 1.34 k | 95.56 ± 1.55 a | 12.41 ± 0.59 m |
| AFTER STORAGE | ||||||
| 16. | no preservation | 1954.20 ± 5.37 i | 571.10 ± 1.67 g | 57.21 ± 0.89 g | 94.80 ± 0.93 a | 11.32 ± 0.61 ijklm |
| 17. | cold plasma (2 min; He/O2) | 1750.64 ± 5.38 e | 485.10 ± 7.23 cd | 46.66 ± 0.65 ef | 93.36 ± 0.56 a | 8.17 ± 0.14 abc |
| 18. | cold plasma (5 min; He/O2) | 1791.35 ± 4.18 f | 476.47 ± 12.11 cd | 36.13 ± 0.57 d | 93.86 ± 1.16 a | 8.15 ± 0.46 abc |
| 19. | cold plasma (10 min; He/O2) | 1587.79 ± 6.28 a | 425.40 ± 3.26 a | 23.81 ± 0.34 a | 92.54 ± 0.51 a | 7.16 ± 0.17 a |
| 20. | cold plasma (2 min; He/N2) | 1832.06 ± 7.03 g | 436.95 ± 11.12 ab | 31.44 ± 1.70 cd | 94.14 ± 1.88 a | 10.41 ± 0.56 ghijk |
| 21. | cold plasma (5 min; He/N2) | 1944.20 ± 5.09 i | 529.91 ± 15.49 ef | 50.18 ± 1.30 f | 94.20 ± 1.44 a | 11.46 ± 0.77 jklm |
| 22. | cold plasma (10 min; He/N2) | 1791.35 ± 7.91 f | 436.12 ± 4.89 ab | 46.07 ± 1.03 ef | 93.20 ± 0.88 a | 9.05 ± 0.30 bcdefg |
| 23. | 30 mg/L potassium metabisulfite | 1832.06 ± 5.95 g | 543.83 ± 9.96 fg | 31.99 ± 0.67 cd | 94.51 ± 0.73 a | 10.07 ± 0.42 efghij |
| 24. | cold plasma (2 min; He/O2) and 30 mg/L potassium metabisulfite | 1750.64 ± 8.50 e | 467.57 ± 10.85 c | 30.85 ± 0.25 bc | 94.20 ± 1.44 a | 9.73 ± 0.16 cdefghi |
| 25. | cold plasma (5 min; He/O2) and 30 mg/L potassium metabisulfite | 1628.50 ± 7.48 b | 437.23 ± 5.03 ab | 26.16 ± 0.71 ab | 93.46 ± 1.74 a | 7.82 ± 0.16 ab |
| 26. | cold plasma (10 min; He/O2) and 30 mg/L potassium metabisulfite | 1709.92 ± 4.34 d | 433.54 ± 2.55 ab | 32.02 ± 0.98 cd | 93.30 ± 1.66 a | 8.52 ± 0.36 abcde |
| 27. | cold plasma (2 min; He/N2) and 30 mg/L potassium metabisulfite | 1669.21 ± 5.93 c | 458.38 ± 7.07 bc | 35.51 ± 0.52 cd | 93.17 ± 1.84 a | 9.12 ± 0.42 bcdefg |
| 28. | cold plasma (5 min; He/N2) and 30 mg/L potassium metabisulfite | 1709.92 ± 2.37 d | 482.32 ± 6.27 cd | 43.72 ± 0.90 e | 93.70 ± 0.66 a | 9.19 ± 0.60 bcdefgh |
| 29. | cold plasma (10 min; He/N2) and 30 mg/L potassium metabisulfite | 1832.06 ± 6.93 g | 501.24 ± 4.12 de | 44.90 ± 1.65 e | 94.20 ± 1.45 a | 8.81 ± 0.31 bcdef |
| 30. | 100 mg/L potassium metabisulfite | 1913.49 ± 11.40 h | 553.57 ± 9.45 fg | 50.18 ± 2.61 f | 94.72 ± 0.56 a | 10.31 ± 0.57 fghijk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedźwiedź, I.; Simeonov, V.; Waśko, A.; Polak-Berecka, M. Comparison of the Effect of Cold Plasma with Conventional Preservation Methods on Red Wine Quality Using Chemometrics Analysis. Molecules 2022, 27, 7048. https://doi.org/10.3390/molecules27207048
Niedźwiedź I, Simeonov V, Waśko A, Polak-Berecka M. Comparison of the Effect of Cold Plasma with Conventional Preservation Methods on Red Wine Quality Using Chemometrics Analysis. Molecules. 2022; 27(20):7048. https://doi.org/10.3390/molecules27207048
Chicago/Turabian StyleNiedźwiedź, Iwona, Vasil Simeonov, Adam Waśko, and Magdalena Polak-Berecka. 2022. "Comparison of the Effect of Cold Plasma with Conventional Preservation Methods on Red Wine Quality Using Chemometrics Analysis" Molecules 27, no. 20: 7048. https://doi.org/10.3390/molecules27207048






