Anticholinesterase Activity of Selected Medicinal Plants from Navarra Region of Spain and a Detailed Phytochemical Investigation of Origanum vulgare L. ssp. vulgare
Abstract
:1. Introduction
2. Results and Discussion
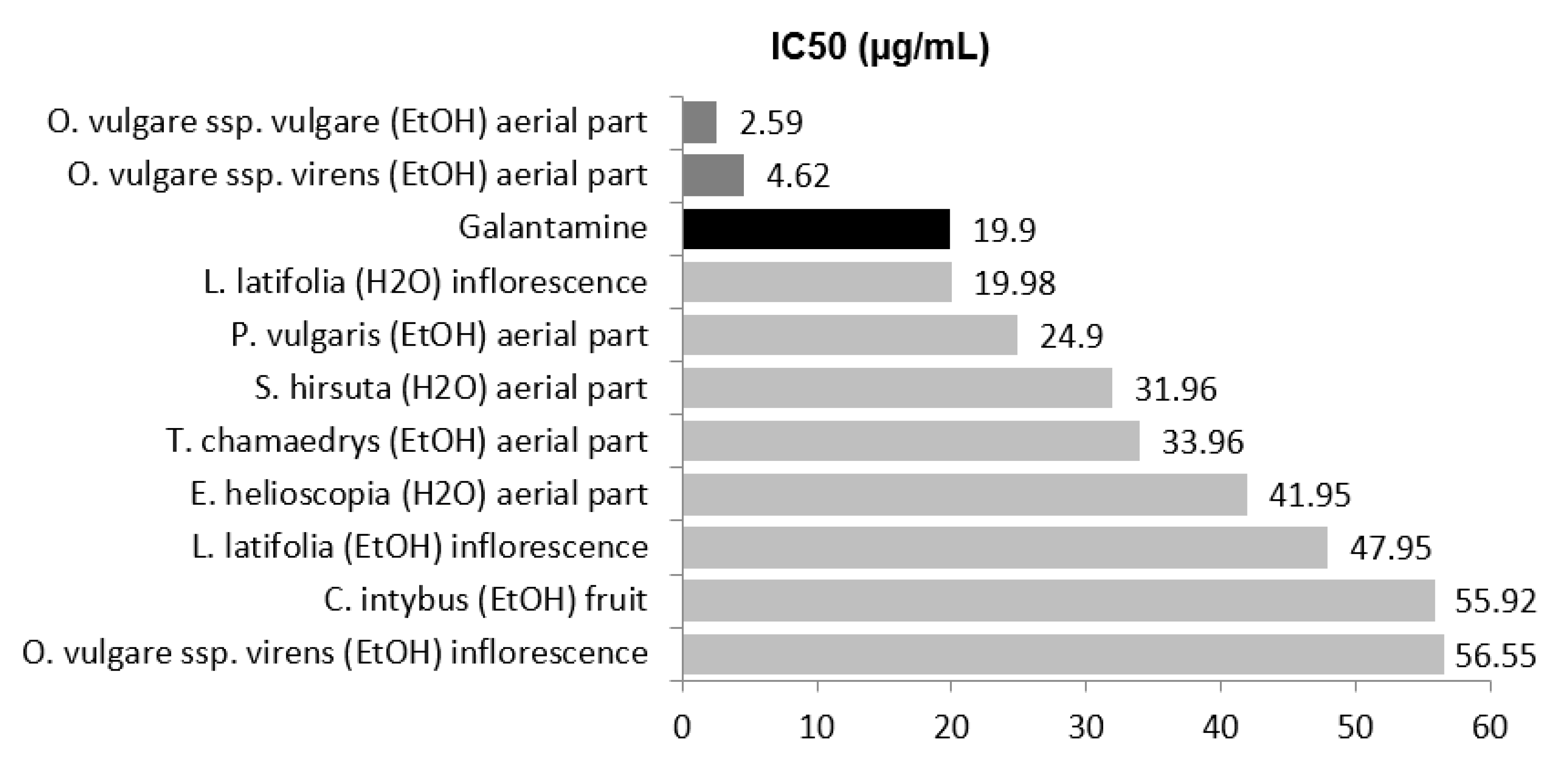
2.1. Antiacethylcholinesterase Activity
2.2. Chemical Characterization of Origanum vulgare ssp. vulgare Aerial Parts
2.2.1. Total Phenolic Compounds Determination
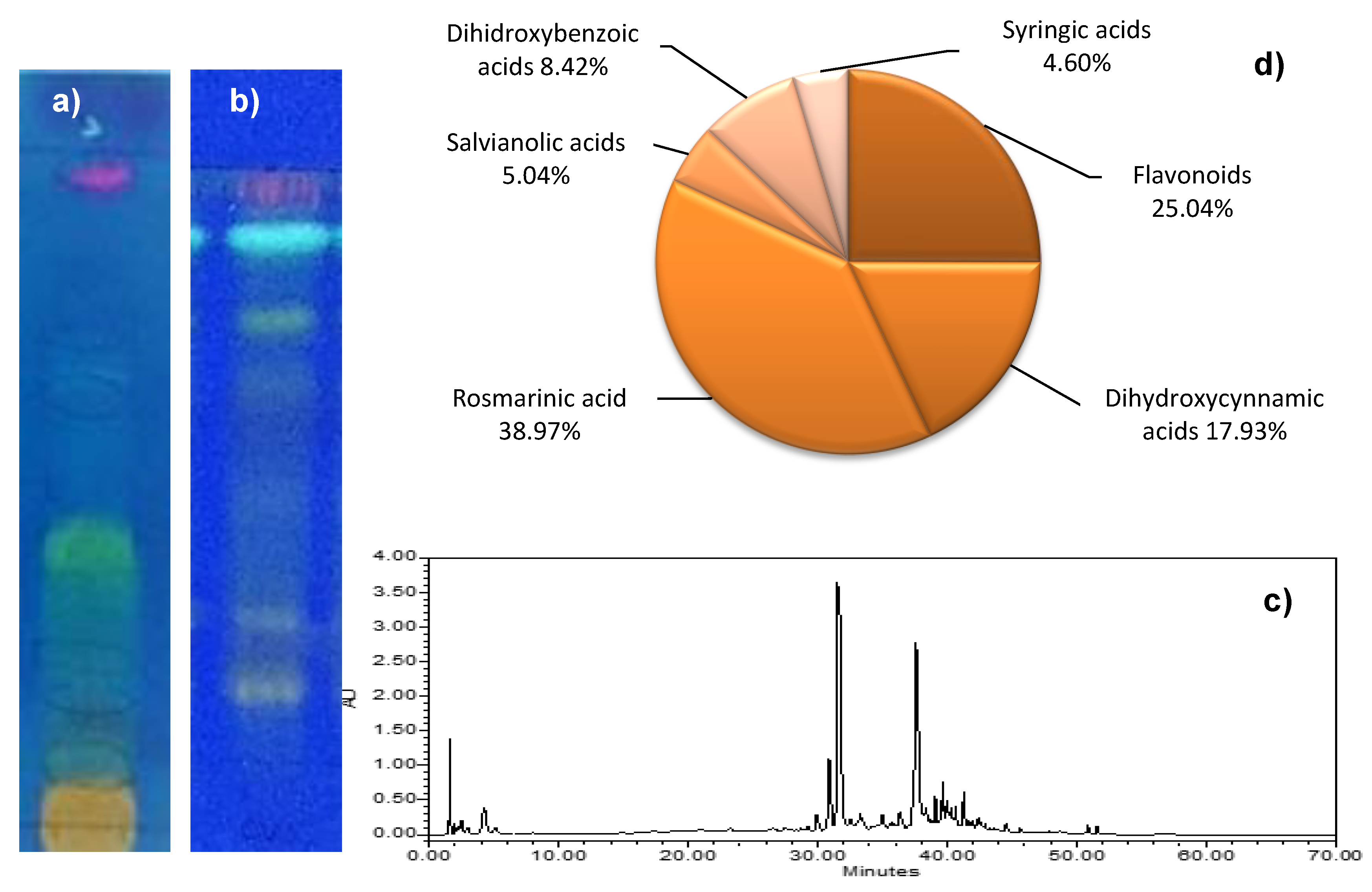
2.2.2. Identification and Quantification of Main Groups of Phenolic Compounds by TLC and HPLC-DAD
2.2.3. Identification of Main Compounds by LC-ESI-QTOF-MS
2.3. Antioxidant Activity
2.3.1. Antioxidant Activity In Vitro against DPPH Radical
2.3.2. Antioxidant Activity In Vitro against ABTS Radical
2.4. Chemical Composition—Biological Activity Relationship
3. Materials and Methods
3.1. Plant materials and extraction
3.2. Antiacetylcholinesterase Activity
3.3. Chemical Characterizationof Origanum vulgare ssp. vulgare Aerial Parts
3.3.1. Total Phenolic Compounds Determination
3.3.2. Identification and Quantification of Main Groups of Phenolic Compounds by TLC and HPLC-DAD
3.3.3. Identification of Main Compounds by LC-ESI-QTOF-MS
3.4. Antioxidant Activity
3.4.1. Antioxidant Activity In Vitro against DPPH Radical
3.4.2. Antioxidant Activity In Vitro against ABTS Radical
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Pueyo, I.; Calvo, M.I. Flavonoids as Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Gnanaraj, C.; Sekar, M.; Fuloria, S.; Swain, S.S.; Gan, S.H.; Chidambaram, K.; Rani, N.N.I.M.; Balan, T.; Stephenie, S.; Lum, P.T.; et al. In Silico Molecular Docking Analysis of Karanjin against Alzheimer’s and Parkinson’s Diseases as a Potential Natural Lead Molecule for New Drug Design, Development and Therapy. Molecules 2022, 27, 2834. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Calvo, M.I.; Cavero, R.Y. Medicinal plants used for neurological and mental disorders in Navarra and their validation from official sources. J. Ethnopharmacol. 2015, 169, 263–268. [Google Scholar] [CrossRef]
- Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef] [Green Version]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.M.; Cavero, R.Y.; Calvo, M.I. In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Food. Hum. Nutr. 2007, 62, 151–155. [Google Scholar] [CrossRef]
- López, V.; Akerreta, S.; Casasnova, E.; García-Mina, J.M.; Cavero, R.Y.; Calvo, M.I. Screening of Spanish medicinal plants for antioxidant and antifungal activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Uysal, S.; Senkardes, I.; Mollica, A.; Zengin, G.; Bulut, G.; Dogan, A.; Glamočlija, J.; Soković, M.; Lobine, D.; Mahomoodally, F.M. Biologically active compounds from two members of the Asteraceae family: Tragopogon dubius Scop. and Tussilago farfara L. J. Biomol. Struct. Dyn. 2019, 37, 3269–3281. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Kim, J.; Kang, J.; Koh, S.H.; Ahn, Y.J.; Kang, K.S.; Park, I.K. Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe). Pestic. Biochem. Phys. 2014, 13, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Fale, P.L.; Ascensao, L.; Serralheiro, M.L. Santolinaimpressa, a Portuguese endemic species: Inhibition of acetylcholinesterase, antioxidant activity and cell toxicity. Planta Med. 2014, 80, 1491. [Google Scholar] [CrossRef]
- Gomes, A.; Pimpão, R.C.; Fortalezas, S.; Figueira, I.; Miguel, C.; Ferreira, R.B.; Santos, C.N.; Aguiar, C.; Salgueiro, L.; Cavaleiro, C.; et al. Chemical characterization and bioactivity of phytochemicals from Iberian endemic Santolina semidentata and strategies for ex situ propagation. Ind. Crop. Prod. 2015, 74, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef] [Green Version]
- Truzzi, E.; Chaouch, M.A.; Rossi, G.; Tagliazucchi, L.; Bertelli, D.; Benvenuti, S. Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules 2022, 27, 1613. [Google Scholar] [CrossRef]
- Videira, R.; Castanheira, P.; Graos, M.; Salgueiro, L.; Faro, C.; Cavaleiro, C.A. Necrodane monoterpenoid from Lavandula luisieri essential oil as a cell-permeable inhibitor of BACE-1, the beta-secretase in Alzheimer’s disease. Flavour Frag. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Costa, P.; Gonalves, S.; Valentào, P.; Andrade, P.B.; Almeida, C.; Nogueira, J.M.F.; Romano, A. Metabolic profile and biological activities of Lavandula pedunculata subsp. lusitanica (Chaytor) Franco: Studies on the essential oil and polar extracts. Food Chem. 2013, 141, 2501–2506. [Google Scholar]
- Sebai, H.; Selmi, S.; Rtibi, K.; Gharbi, N.; Sakly, M. Protective Effect of Lavandula stoechas and Rosmarinus officinalis Essential Oils Against Reproductive Damage and Oxidative Stress in Alloxan-Induced Diabetic Rats. J. Med. Food 2015, 18, 241–249. [Google Scholar] [CrossRef]
- Costa, P.; Grevenstuk, T.; Rosa da Costa, A.M.; Gonçalves, S.; Romano, A. Antioxidant and anti-cholinesterase activities of Lavandula viridis L’Hér extracts after in vitro gastrointestinal digestion. Ind. Crop. Prod. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Al-Sarar, A.S.; Hussein, H.I.; Abobakr, Y.; Bayoumi, A.E.; Al-Otaibi, M.T. Fumigant toxicity and antiacetylcholinesterase activity of Saudi Mentha longifolia and Lavandula dentata species against Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Turk. Entomol. Derg. 2014, 38, 11–18. [Google Scholar]
- Stafford, G.I.; Pedersen, M.E.; van Staden, J.; Jäger, A.K. Review on plants with CNS-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 2008, 119, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.Y.; Fan, P.H.; Perez, R.G.; Lou, H.X. Neuroprotective effects of linarin through activation of the PI3K/Akt pathway in amyloid-beta-induced neuronal cell death. Bioorgan. Med. Chem. 2011, 19, 4021–4027. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Watanabe, H.; Kameoka, H.; Umemoto, K. Inhibition of Acetylcholinesterase Activity by Essential Oils of Mentha Species. J. Agr. Food Chem. 1998, 46, 3431–3434. [Google Scholar] [CrossRef]
- de Sousa Barros, A.; de Morais, S.M.; Ferreira, P.A.T.; Vieira, Í.G.P.; Craveiro, A.A.; dos Santos Fontenelle, R.O.; de Menezes, J.E.S.A.; da Silva, F.W.F.; de Sousa, H.A. Chemical composition and functional properties of essential oils from Mentha species. Ind. Crop. Prod. 2015, 76, 557–564. [Google Scholar] [CrossRef]
- Politeo, O.; Bektašević, M.; Carev, I.; Jurin, M.; Roje, M. Phytochemical Composition, Antioxidant Potential and Cholinesterase Inhibition Potential of Extracts from Mentha pulegium L. Chem. Biodivers. 2018, 15, e1800374. [Google Scholar] [CrossRef]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.M.; Araújo, M.E.M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, antioxidant, antimicrobial and enzyme inhibition activities of two Origanum vulgare subspecies (subsp. vulgare and subsp. hirtum) essential oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Park, S.Y. Terpenoids as Potential Anti-Alzheimer’s Disease Therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef] [Green Version]
- López, V.; Cascella, M.; Benelli, G.; Maggi, F.; Gómez-Rincón, C. Green drugs in the fight against Anisakis simplex-larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018, 117, 861–867. [Google Scholar] [CrossRef] [Green Version]
- López, V.; Pavela, R.; Gómez-Rincón, C.; Les, F.; Bartolucci, F.; Galiffa, V.; Petrelli, R.; Cappellacci, L.; Maggi, F.; Canale, A.; et al. Efficacy of Origanum syriacum Essential Oil against the Mosquito Vector Culex quinquefasciatus and the Gastrointestinal Parasite Anisakis simplex, with Insights on Acetylcholinesterase Inhibition. Molecules 2019, 24, 2563. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; Cindio, B.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, D.H.; Lee, I.K.; Jung, W.Y.; Park, D.H.; Kim, J.M.; Lee, K.R.; Lee, K.T.; Shin, C.Y.; Cheong, J.H.; et al. The ameliorating effect of the extract of the flower of Prunella vulgaris var. lilacina on drug-induced memory impairments in mice. Food Chem. Toxicol. 2010, 48, 1671–1676. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, J.; Yang, H.; Gao, J.; Chen, H.; Liu, C.; Gao, W. Prunella vulgaris L., an Edible and Medicinal Plant, Attenuates Scopolamine-Induced Memory Impairment in Rats. J. Agric. Food Chem. 2017, 65, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Bouchmaaa, N.; Aazza, S.; Gaamoussi, F.; Lyoussi, B. Antioxidant, anti-inflammatory and anti-acetylcholinesterase activities of eleven extracts of Moroccan plants. Fresen. Environ. Bull. 2014, 23, 1–14. [Google Scholar]
- Topcu, G.; Ertas, A.; Ozturk, M.; Dincel, D.; Kilic, T.; Halfon, B. Ent-kaurane diterpenoids isolated from Sideritis congesta. Phytochem. Lett. 2011, 4, 436–439. [Google Scholar] [CrossRef]
- Erdogan-Orhan, I.; Baki, E.; Şenol, S.; Yilmaz, G. Sage-called plant species sold in Turkey and their antioxidant activities. J. Serb. Chem. Soc. 2010, 75, 1491–1501. [Google Scholar] [CrossRef]
- Golfakhrabadi, F.; Yousefbeyk, F.; Mirnezami, T.; Khanavi, M.; Laghaei, P.; Hajimahmoodi, M. Antioxidant and Antiacetylcholinesterase Activity of Teucriumhyrcanicum. Pharmacog. Res. 2015, 7, S15–S19. [Google Scholar]
- Ahmad, B.; Mukarram Shah, S.M.; Khan, H.; Hassan Shah, S.M. Enzyme inhibition activities of Teucrium royleanum. J. Enzyme Inhib. Med. Chem. 2007, 22, 730–732. [Google Scholar] [CrossRef] [Green Version]
- Rabiei, Z.; Mokhtari, S.; Asgharzade, S.; Rahnama, S.; Rafieian-kopaei, M.; Gholami, M. Inhibitory effect of Thymus vulgaris extract on memory impairment induced by scopolamine in rat. Asian Pac. J. Trop. Biomed. 2015, 5, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Sezer Senol, F.; Orhan, I.E.; Ozgen, U.; Renda, G.; Bulut, G.; Guven, L.; Karaoglan, E.S.; Sevindik, H.G.; Skalicka-Wozniak, K.; Koca Caliskan, U.; et al. Memory-vitalizing effect of twenty-five medicinal and edible plants and their isolated compounds. S. Afr. J. Bot. 2016, 102, 102–109. [Google Scholar] [CrossRef]
- Singh, D.K.; Agarwal, R.A. Latex of Euphorbia antisyphlitica, a new potent molluscicide having antiacetylcholinesterase activity against the snail Lymnaea acuminata. Sci. Total Environ. 1987, 61, 211–215. [Google Scholar] [CrossRef]
- Pisano, M.B.; Cosentino, S.; Viale, S.; Spanò, D.; Corona, A.; Esposito, F.; Tramontano, E.; Montoro, P.; Tuberoso, C.I.; Medda, R.; et al. Biological Activities of Aerial Parts Extracts of Euphorbia characias. Biomed. Res. Int. 2016, 2016, 1538703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anuradha, H.; Srikumar, B.N.; Deepti, N.; Shankaranarayana Rao, B.S.; Lakshmana, M. Restoration of acetylcholinesterase activity by Euphorbia hirta in discrete brain regions of chronically stressed rats. Pharm. Biol. 2010, 48, 499–503. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, A. Piscicidal and anti-acetylcholinesterase activity of Euphorbia royleana stem bark extracts against freshwater common predatory fish Channa punctatus. Environ. Toxicol. Phar. 2004, 18, 47–53. [Google Scholar] [CrossRef]
- Bakry, F.A. Use of some plant extracts to control Biomphalaria alexandrina snails with emphasis on some biological effects. Pestic. Biochem. Phys. 2009, 95, 159–165. [Google Scholar] [CrossRef]
- Tiwari, S.; Singh, A. Biochemical stress response in freshwater fish Channa punctatus induced by aqueous extracts of Euphorbia tirucalli plant. Chemosphere 2006, 64, 36–42. [Google Scholar] [CrossRef]
- Wei, J.C.; Zhang, X.Y.; Gao, Y.N.; Wang, D.D.; He, X.L.; Gao, X.X.; Hu, G.S.; Wang, A.H.; JJia, J.M. Euphorfinoids E-L: Diterpenoids from the roots of Euphorbia fischeriana with acetylcholinesterase inhibitory activity. Phytochemistry 2021, 190, 112867. [Google Scholar] [CrossRef]
- Kim, D. Inhibitory effect of corynoline isolated from the aerial parts of Corydalis incisa on the acetylcholinesterase. Arch. Pharm. Res. 2002, 25, 817–819. [Google Scholar] [CrossRef]
- Gayoso, L.; Roxo, M.; Cavero, R.Y.; Calvo, M.I.; Ansorena, D.; Astiasarán, I.; Wink, M. Bioaccessibility and biological activity of Melissa officinalis, Lavandula latifolia and Origanum vulgare extracts: Influence of an in vitro gastrointestinal digestion. J. Funct. Foods 2018, 44, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Mahomoodally, M.F.; Zengin, G.; Aladag, M.O.; Ozparlak, H.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Aumeeruddy, M.Z. HPLC-MS/MS chemical characterization and biological properties of Origanum onites extracts: A recent insight. Int. J. Environ. Health Res. 2019, 29, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bladt, S. Plant Drug Analysis, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wang, C.N.; Shiao, Y.J.; Liu, T.Y.; Wang, W.Y. Benzolignanoid and polyphenols from Origanum vulgare. J. Chin. Chem. Soc. 2003, 50, 1079–1083. [Google Scholar] [CrossRef]
- Agiomyrgianaki, A.; Dais, P. Simultaneous determination of phenolic compounds and triterpenic acids in oregano growing wild in Greece by 31P NMR spectroscopy. Magn. Reson. Chem. 2012, 50, 739–748. [Google Scholar] [CrossRef]
- González, M.D.; Luis, C.M.; Lanzelotti, P.L. Polyphenolic profile of Origanum vulgare L. ssp. viridulum from Argentina. Fyt. Issn. 2014, 9457, 179–184. [Google Scholar]
- Koldaş, S.; Demirtas, I.; Ozen, T.; Demirci, M.A.; Behçet, L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [CrossRef]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and majoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Lin, L.Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compost. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ličina, B.Z.; Stefanović, O.D.; Vasić, S.M.; Radojević, I.D.; Dekić, M.S.; Čomić, L.R. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control 2013, 33, 498–504. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, A.C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- de Torre, M.P.; Vizmanos, J.L.; Cavero, R.Y.; Calvo, M.I. Improvement of antioxidant activity of oregano (Origanum vulgare L.) with an oral pharmaceutical form. Biomed. Pharmacother. 2020, 129, 110424. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, T.; Yan, W. Authentication of the Bilberry Extracts by an HPLC Fingerprint Method Combining Reference Standard Extracts. Molecules 2020, 25, 2514. [Google Scholar] [CrossRef]
- Jorge, T.F.; Mata, A.T.; António, C. Mass spectrometry as a quantitative tool in plant metabolomics. Philos. Trans. A. Math. Phys. Eng. Sci. 2016, 374, 20150370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamasaki, N.; Ishii, E.; Tominaga, K.; Tezuka, Y.; Nagaoka, T.; Kadota, S.; Kuroki, T.; Yano, I. Highly selective antibacterial activity of novel alkyl quinolone alkaloids from a Chinese herbal medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori in vitro. Microbiol. Immunol. 2000, 44, 9–15. [Google Scholar] [CrossRef]
- Lecomte, J.; López-Giraldo, L.J.; Laguerre, M.; Baréa, B.; Villeneuve, P. Synthesis, Characterization and Free Radical Scavenging Properties of Rosmarinic Acid Fatty Esters. J. Am. Oil Chem. Soc. 2010, 87, 615–620. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.S.; Calvo, M.I.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Agata, I.; Hatanp, T.; Okudaq, T.A. Tetrameric derivative of caffeic acid from Rabdosia japonica. Phytochemistry 1989, 28, 2447–2450. [Google Scholar] [CrossRef]
- Flegkas, A.; Milosević Ifantis, T.; Barda, C.; Samara, P.; Tsitsilonis, O.; Skaltsa, H. Antiproliferative Activity of (-)-Rabdosiin Isolated from Ocimum sanctum L. Medicines 2019, 6, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.H.; Chan, L.P.; Ding, H.Y.; So, E.C.; Lin, R.J.; Wang, H.M.; Chen, Y.G.; Chou, T.H. Free radical scavenging activity of 4-(3,4-Dihydroxybenzoyloxymethyl) phenyl-O-β-d-glucopyranoside from Origanum vulgare and its protection against oxidative damage. J. Agric. Food Chem. 2012, 60, 7690–7696. [Google Scholar] [CrossRef]
- Zengin, G.; Ferrante, C.; Orlando, G.; Zheleva-Dimitrova, D.; Gevrenova, R.; Recinella, L.; Chiavaroli, A.; Leone, S.; Brunetti, L.; Aumeeruddy, M.Z.; et al. Chemical profiling and pharmaco-toxicological activity of Origanum sipyleum extracts: Exploring for novel sources for potential therapeutic agents. J. Food Biochem. 2019, 43, 13003. [Google Scholar] [CrossRef]
- Li, F.; Tsona, N.T.; Li, J.; Du, L. Aqueous-phase oxidation of syringic acid emitted from biomass burning: Formation of light-absorbing compounds. Sci. Total Environ. 2021, 765, 144239. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic acid on antioxidant biomarkers and associated inflammatory markers in mice model of asthma. Drug Dev. Res. 2019, 80, 253–261. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. essential oil as a potential anti-acne topical nanoemulsion-in vitro and in vivo study. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2164. [Google Scholar] [CrossRef] [Green Version]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Angulo-Escalante, M.A.; León-Félix, J.; Heredia, J.B. Effect of in vitro digestion on the Total Antioxidant Capacity and Phenolic Content of 3 species of oregano (Hedeoma patens, Lippia graveolens, Lippia palmeri). J. Food Sci. 2017, 82, 2832–2839. [Google Scholar] [CrossRef]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical compositions and anti-skin-ageing activities of Origanum vulgare L. essential oil from tropical and mediterranean region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Wang, S.; Yu, Y.; Sun, S.; Zhang, Y.; Zhang, Y.; Yang, W.; Li, S.; Qiao, Y. Salvianolic Acid A, as a Novel ETA Receptor Antagonist, Shows Inhibitory Effects on Tumor in Vitro. Int. J. Mol. Sci. 2016, 17, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chishti, S.; Kaloo, Z.A.; Sultan, P. Medicinal importance of genus Origanum: A review. J. Pharmacogn. Phytotherapy 2013, 5, 170–177. [Google Scholar]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.L.; Guo, Y.S.; Wang, C.H.; Li, G.Q.; Xu, J.J.; Chung, H.Y.; Ye, W.C.; Li, Y.L.; Wang, G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef]
- Baâtour, O.; Kaddour, R.; Mahmoudi, H.; Tarchoun, I.; Bettaieb, I.; Nasri, N.; Mrah, S.; Hamdaoui, G.; Lachaâl, M.; Marzouk, B. Culture conditions and salt effects on essential oil composition of sweet marjoram (Origanum majorana) from Tunisia. Acta Pharm. 2012, 62, 251–261. [Google Scholar] [CrossRef] [Green Version]
- De Falco, E.; Roscigno, G.; Landolfi, S.; Scandolera, E.; Senatore, F. Growth, essential oil characterization, and antimicrobial activity of three wild biotypes of oregano under cultivation condition in Southern Italy. Ind. Crops Prod. 2014, 62, 242–249. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Viña, J.; Borras, C.; Abdelaziz, K.M.; Garcia–Valles, R.; Gomez-Cabrera, M.C. The Free Radical Theory of Aging Revisited The Cell Signaling Disruption Theory of Aging. Antioxid. Redox Signal. 2013, 19, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, S.; Khan, A. Antioxidants and diabetes. Indian J. Endocrinol. Metab. 2012, 16, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N.A.; Taitzoglou, I.A.; Botsoglou, E.; Lavrentiadou, S.N.; Kokoli, A.N.; Roubies, N. Effect of Long-Term Dietary Administration of Oregano on the Alleviation of Carbon Tetrachloride-Induced Oxidative Stress in Rats. J. Agric. Food Chem. 2008, 56, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Nur Alam, M.; Jahan Bristi, N.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- García-Herreros, C.; García-Iñiguez, M.; Astiasarán, I.; Ansorena, D. Antioxidant activity and phenolic content of water extracts of Borago officinalis L.: Influence of plant part and cooking procedure. Ital. J. Food Sci. 2010, 22, 156–164. [Google Scholar]
- Orhan, I.E.; Kucukboyaci, N.; Calis, I.; Cerón-Carrasco, H.P.; den Haan Alonso, H.; Peña-García, J.; Pérez-Sánchez, H. Acetylcholinesterase inhibitory assessment of isolated constituents from Salsola grandis Freitag, Vural & Adıgüzel and molecular modeling studies on N-acetyltryptophan. Phytochem. Lett. 2017, 20, 373–378. [Google Scholar]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids—Interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef]
- Szwajgier, D.; Baranowska-Wójcik, E.; Borowiec, K. Phenolic acids exert anticholinesterase and cognition-improving effects. Curr. Alzheimer Res. 2018, 15, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic Acids as Acetylcholinesterase Inhibitors: Calorimetric and Docking Simulation Studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Pueyo, I.; Calvo, M.I. Structure-activity relationships of acetylated flavone glycosides from Galeopsis ladanum L. (Lamiaceae). Food Chem. 2010, 120, 679–683. [Google Scholar] [CrossRef]
- Rhee, I.K.; Meent, M.V.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Carpinella, M.C.; Andrione, D.G.; Ruiz, G.; Palacios, S.M. Screening for acetylcholinesterase inhibitory activity in plant extracts from Argentina. Phytother. Res. 2010, 24, 259–263. [Google Scholar] [CrossRef]
- Guedes, L.; Reis, P.B.P.S.; Machuqueiro, M.; Ressaissi, A.; Pacheco, R.; Serralheiro, M.L. Bioactivities of Centaurium erythraea (Gentianaceae) Decoctions: Antioxidant activity, enzyme inhibition and docking studies. Molecules 2019, 24, 3795. [Google Scholar] [CrossRef]
| Ethanolic Extract | Aqueous Extract | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yield (w/w %) | Inhibitory Activity (%) | IC50 (μg/mL) | Yield (w/w %) | Inhibitory Activity (%) | IC50 (μg/mL) | ||||||
| 250 μg/mL | 125 μg/mL | 62.5 μg/mL | 250 μg/mL | 125 μg/mL | 62.5 μg/mL | ||||||
| Asteraceae | |||||||||||
| Achillea millefolium L. ssp. millefolium | Leaf | 8.39 | 56.96 ± 4.93 | 45.17 ± 6.10 | 38.70 ± 6.43 | 190.80 ± 8.02 | 10.7 | <10 | n.q. | n.q. | n.d. |
| Stem | 8.47 | <10 | n.q | n.q. | n.d. | 4.66 | 46.89 ± 4.29 | 32.57 ± 5.08 | 21.99 ± 5.83 | >250 | |
| Inflorescence | 11.99 | 60.78 ± 5.21 | 49.40 ± 6.14 | 39.00 ± 2.51 | 128.87 ± 2.57 | 9.73 | 38.95 ± 5.93 | 35.40 ± 6.03 | 32.16 ± 3.96 | >250 | |
| Anthemis arvensis L. ssp. arvensis | Aerial part | 20.09 | 53.80 ± 5.01 | 33.82 ± 6.80 | 29.24 ± 1.93 | 234.76 ± 5.54 | 10.26 | 67.17 ± 15.30 | 46.53 ± 4.79 | 36.73 ± 2.12 | 158.84 ± 1.58 |
| Anthemis cotula L. | Aerial part | 13.83 | 56.50 ± 5.96 | 49.62 ± 5.04 | 38.43 ± 5.61 | 156.84 ± 3.76 | 10.87 | <10 | n.q. | n.q. | n.d. |
| Cichorium intybus L. | Fruit | 9.68 | 66.57 ± 9.31 | 63.49 ± 1.06 | 61.24 ± 1.19 | 55.92 ± 4.75 | 14.82 | 60.08 ± 3.06 | 35.22 ± 4.79 | 30.82 ± 2.51 | 189.81 ± 2.57 |
| Helichrysum stoechas | Aerial part | 4.42 | <10 | n.q. | n.q. | n.d. | 8.0 | 49.57 ± 7.64 | 29.48 ± 4.48 | 25.52 ± 4.92 | >250 |
| Jasonia glutinosa | Inflorescence | 5.68 | 57.81 ± 6.99 | 48.60 ± 8.30 | 33.02 ± 7.15 | 239.76 ± 13.17 | 13.53 | 56.82 ± 1.90 | 56.10 ± 7.69 | 52.93 ± 4.55 | 60.83 ± 9.80 |
| Jasonia tuberosa | Aerial part | 17.17 | <10 | n.q. | n.q. | n.d. | 15.79 | 55.94 ± 4.25 | 30.35 ± 1.01 | 28.67 ± 5.63 | 219.10 ± 5.73 |
| Santolina chamaecyparesus L. ssp. squarrosa Nyman | Inflorescence | 13.15 | 75.82 ± 6.69 | 52.18 ± 6.00 | 24.20 ± 6.57 | 100.90 ± 5.74 | 8.43 | <10 | n.q. | n.q. | n.d. |
| Sylibum marianum (L.) Gaertner | Inflorescence | 5.39 | 49.79 ± 2.44 | 22.43 ± 8.35 | 16.45 ± 0.42 | >250 | 6.87 | <10 | n.q. | n.q. | n.d. |
| Tanacetum parthenium (L.) Schultz | Stem | 10.25 | 40.83 ± 6.77 | 39.69 ± 5.02 | 14.71 ± 2.14 | >300 | 8.21 | 53.46 ± 5.22 | 38.86 ± 4.02 | 40.96 ± 8.15 | 193.80 ± 4.25 |
| Leaf | 15.61 | 67.57 ± 1.25 | 59.66 ± 19.73 | 48.53 ± 2.24 | 92.80 ± 4.29 | 10.95 | 49.80 ± 1.32 | 45.60 ± 1.19 | 28.61 ± 1.35 | >250 | |
| Inflorescence | 8.07 | 69.74 ± 1.02 | 59.46 ± 1.18 | 44.77 ± 8.61 | 116.95 ± 3.56 | 8.8 | 25.78 ± 2.06 | n.q. | n.q. | >300 | |
| Tussilago farfara L. | Leaf | 5.68 | 70.76 ± 8.87 | 44.61 ± 6.98 | 31.65 ± 3.06 | 160.83 ± 4.25 | 10 | 80.25 ± 13.78 | 37.47 ± 7.13 | 34.95 ± 1.18 | 178.92 ± 8.71 |
| Crassulaceae | |||||||||||
| Hylotelephium maximum | Aerial part | 20.94 | <10 | n.q. | n.q. | n.d. | 4.35 | 44.85 ± 4.70 | 238.76 ± 1.68 | ||
| Equisetaceae | |||||||||||
| Equisetum arvense L. | Sterile stem | 20.62 | 84.72 ± 9.20 | 60.95 ± 6.67 | 50.53 ± 3.80 | 62.16 ± 2.35 | 17.65 | 62.59 ± 9.02 | 59.85 ± 1.99 | 42.73 ± 1.56 | 155.61 ± 7.52 |
| Equisetum telmateia L | Sterile stem | 13.06 | 84.79 ± 1.97 | 74.61 ± 6.14 | 48.21 ± 1.33 | 63.93 ± 1.88 | 15.52 | 42.90 ± 6.41 | 25.18 ± 6.62 | 19.49 ± 5.71 | >300 |
| Euphorbiaceae | |||||||||||
| Euphorbia characias | Aerial part | 17.29 | <10 | n.q. | n.q. | n.d. | 8.93 | 44.25 ± 5.01 | 32.03 ± 7.09 | 27.14 ± 6.72 | >300 |
| Euphorbia helioscopia | Aerial part | 16.26 | <10 | n.q. | n.q. | n.d. | 6.06 | 78.74 ± 4.15 | 68.74 ± 4.15 | 56.24 ± 5.01 | 41.95 ± 0.69 |
| Lamiaceae | |||||||||||
| Calamintha sylavatica Bromf ssp. ascendens (Jordan) P.W.Ball | Aerial part | 12.27 | 64.58 ± 3.39 | 51.835.17 | 27.13 ± 2.20 | 67.90 ± 2.57 | 9.03 | 68.64 ± 2.06 | 28.58 ± 3.26 | 27.15 ± 3.53 | 157.84 ± 3.06 |
| Lavandula latifolia Medicus | Inflorescence | 13.38 | 98.73 ± 5.00 | 91.73 ± 9.67 | 66.13 ± 6.52 | 47.95 ± 0.59 | 8.76 | 91.25 ± 13.14 | 59.13 ± 1.90 | 72.18 ± 8.27 | 19.98 ± 0.49 |
| Stem and leaf | 12.65 | 99.79 ± 4.00 | 98.27 ± 1.97 | 23.32 ± 2.59 | 70.92 ± 0.20 | 3.55 | 96.32 ± 2.71 | 63.07 ± 5.53 | 33.75 ± 1.70 | 71.92 ± 2.47 | |
| Melissa officinalis L. ssp. officinalis | Aerial part | 6.79 | 61.81 ± 1.52 | 58.55 ± 16.81 | 48.99 ± 3.32 | 84.91 ± 2.16 | 11.82 | 59.40 ± 1.12 | 45.52 ± 3.58 | 23.81 ± 3.77 | 171.82 ± 4.25 |
| Mentha aquatica L. | Aerial part | 14.40 | <10 | n.q. | n.q. | n.d. | 9.89 | 61.81 ± 1.25 | 55.00 ± 0.70 | 29.47 ± 3.56 | 118.87 ± 7.32 |
| Mentha longifolia (L.) Hudson | Aerial part | 9.45 | 77.98 ± 2.18 | 44.47 ± 2.40 | 30.18 ± 1.78 | 130.86 ± 7.22 | 6.44 | 90.45 ± 5.35 | 64.74 ± 1.00 | 44.08 ± 5.27 | 62.93 ± 1.68 |
| Mentha pullegium L. | Aerial part | 10.84 | <10 | n.q. | n.q. | n.d. | 5.88 | 46.29 ± 1.40 | 38.91 ± 4.95 | 30.79 ± 1.34 | 226.7 ±10.98 |
| Mentha suaveolens Ehrh. | Aerial part | 10.34 | 62.29 ± 8.91 | 47.37 ± 5.81 | 34.01 ± 8.04 | 217.78 ± 11.48 | 8.61 | 57.74 ± 5.60 | 55.73 ± 7.08 | 47.91 ± 2.85 | 113.88 ± 3.96 |
| Origanum vulgare L. spp. virens Bonnier and Layens | Inflorescence | 8.50 | 61.89 ± 4.11 | 59.38 ± 2.10 | 56.25 ± 3.92 | 56.55 ± 0.62 | 6.54 | 62.91 ± 8.22 | 50.76 ± 2.15 | 43.54 ± 1.37 | 120.85 ± 1.88 |
| Aerial part | 14.0 | 91.75 ± 1.38 | 80.21 ± 2.88 | 64.77 ± 5.35 | 4.62 ± 0.01 | 8.38 | 32.50 ± 3.30 | 18.07 ± 7.34 | 16.38 ± 1.09 | >250 | |
| Origanum vulgare L. spp. vulgare | Aerial part | 8.13 | 95.61 ± 2.02 | 91.50 ± 3.01 | 75.45 ± 2.92 | 2.59 ± 0.01 | 13.91 | 52.55 ± 7.86 | 44.35 ± 9.51 | 32.65 ± 1.03 | 175.82 ± 5.84 |
| Phlomis herba-venti L. | Aerial part | 13.54 | 72.47 ± 6.34 | 47.82 ± 5.14 | 48.57 ± 6.52 | 189.1 ± 2.67 | 6.16 | 62.84 ± 6.20 | 47.66 ± 4.52 | 42.84 ± 7.91 | 190.90 ± 4.15 |
| Phlomis lychnitis L. | Inflorescence | 14.52 | 53.63 ± 13.75 | 30.21 ± 2.90 | 39.33 ± 9.94 | 248.73 ± 5.64 | 6.22 | <10 | n.q. | n.q. | n.d. |
| Stem and leaf | 7.90 | 55.26 ± 5.04 | 28.27 ± 4.63 | 21.65 ± 4.53 | 247.75 ± 6.13 | 4.79 | <10 | n.q. | n.q. | n.d. | |
| Prunella vulgaris L. | Aerial part | 6.15 | 78.86 ± 7.39 | 68.78 ± 1.86 | 63.05 ± 3.11 | 24.97 ± 1.48 | 15.7 | <10 | n.q. | n.q. | n.d. |
| Salvia pratensis L. | Aerial part | 9.99 | 41.17 ± 6.50 | 37.20 ± 5.14 | 21.86 ± 2.26 | >300 | 13.95 | <10 | n.q. | n.q. | n.d. |
| Sideritis hirsuta | Aerial part | 13.44 | <10 | n.q. | n.q. | n.d. | 11.32 | 93.27 ± 3.85 | 87.40 ± 9.28 | 74.68 ± 3.67 | 31.96 ± 0.39 |
| Sideritis hyssopifolia ssp. guillonii | Aerial part | 3.44 | <10 | n.q. | n.q. | n.d. | 4.83 | 47.45 ± 6.64 | 30.94 ± 1.06 | 28.33 ± 6.44 | >250 |
| Teucrium chamaedrys | Aerial part | 11.01 | 98.09 ± 1.10 | 94.58 ± 11.84 | 69.98 ± 1.49 | 33.96 ± 0.19 | 7.45 | 98.96 ± 9.85 | 59.00 ± 8.80 | 41.05 ± 1.01 | 97.90 ± 1.88 |
| Thymus praecox Opiz ssp. polytrichus | Aerial part | 10.17 | 97.81 ± 10.9 | 60.16 ± 4.26 | 60.79 ± 5.39 | 71.92 ± 3.07 | 11.08 | 51.03 ± 1.16 | 33.05 ± 7.95 | 25.79 ± 3.04 | >250 |
| Thymus vulgaris L. ssp. vulgaris | Aerial part | 5.09 | 82.48 ± 9.05 | 68.62 ± 8.91 | 45.47 ± 2.43 | 79.92 ± 3.36 | 9.64 | <10 | n.q. | n.q. | n.d. |
| Lytraceae | |||||||||||
| Lythrum salicaria L. | Aerial part | 20.04 | <10 | n.q. | n.q. | n.d. | 4.07 | 98.50 ± 13.50 | 74.28 ± 6.04 | 48.62 ± 9.31 | 69.93 ± 1.48 |
| Papaveraceae | |||||||||||
| Papaver rhoeas L. | Capsule/petal | 29.65 | 99.78 ± 7.57 | 56.02 ± 9.88 | 14.80 ± 1.57 | 76.92 ± 8.51 | 15.13 | 52.64 ± 5.31 | 42.64 ± 8.77 | 36.42 ± 1.40 | 150.84 ± 9.70 |
| Primulaceae | |||||||||||
| Anagallis arvensis L. | Aerial part | 14.06 | 63.10 ± 1.08 | 57.92 ± 4.57 | 51.42 ± 5.56 | 57.94 ± 3.36 | 13.04 | 80.02 ± 1.25 | 58.98 ± 9.41 | 38.08 ± 8.33 | 102.89 ± 2.97 |
| Verbenaceae | |||||||||||
| Verbena officinalis L. | Aerial part | 10.94 | 58.04 ± 7.63 | 43.20 ± 3.17 | 28.40 ± 5.13 | 166.83 ± 1.78 | 8.80 | 58.05 ± 1.61 | 39.96 ± 1.24 | 33.54 ± 1.85 | 140.85 ± 6.73 |
| Galantamine | 91.33 ± 1.31 | 88.38 ± 2.23 | 74.26 ± 6.20 | 19.9 ± 4.80 | |||||||
| Compound | Rt (min) | λmax (nm) | [M–H]– (m/z) | Fragment Ions (m/z) | Tentative Identification | Molecular Formula |
|---|---|---|---|---|---|---|
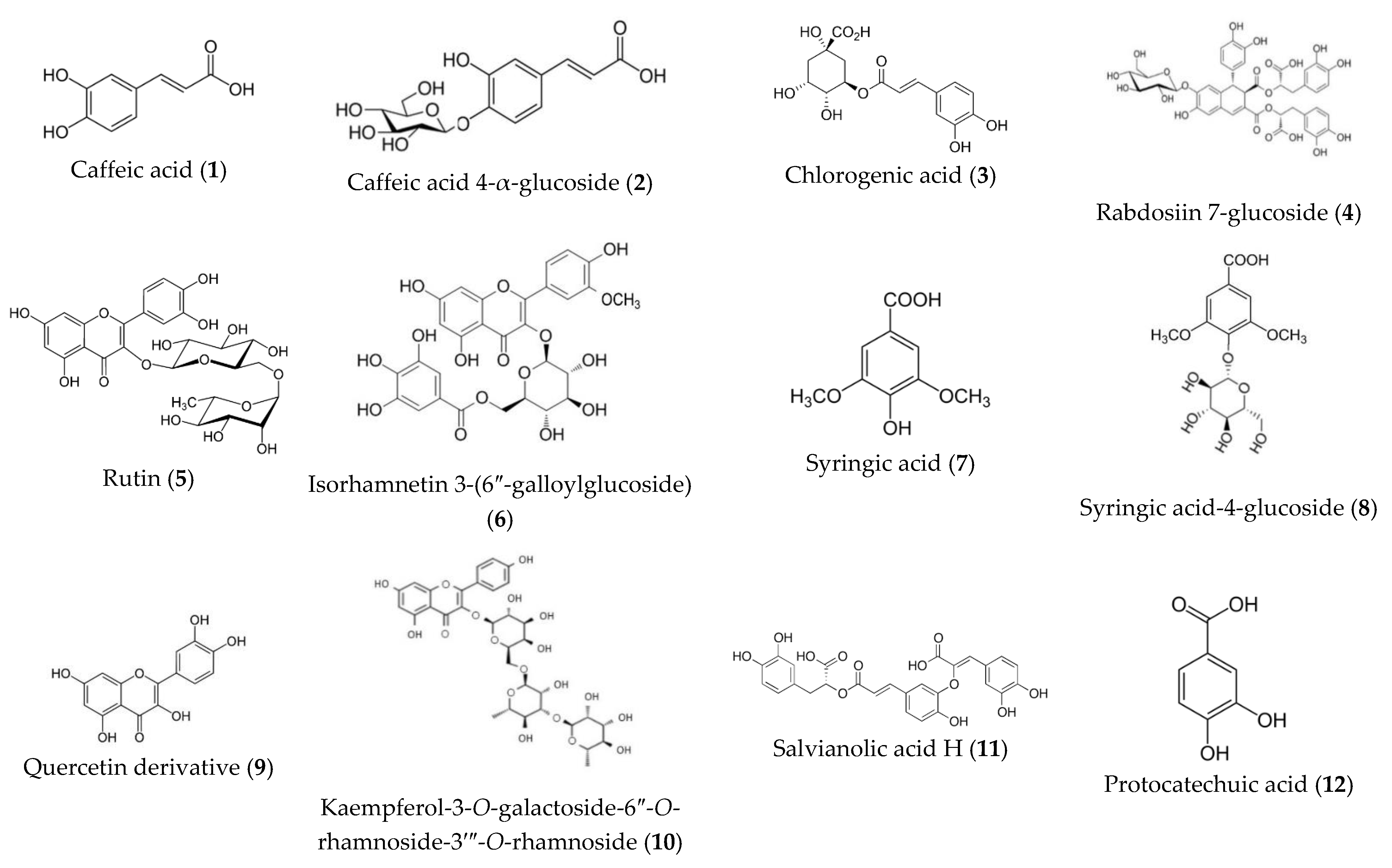
| 1 | 1.1 | 296 sh, 324 | 179.05 | 135.04, 89.03 | Caffeic acid (3,4-Dihydroxycinnamic acid) | C9H8O4 |
| 2 | 0.9 | 287 sh, 331 | 341.07 | 179.03, 149.04, 96.95 | Caffeic acid 4-α-glucoside | C15H18O9 |
| 3 | 1.3 | 287 sh, 329 | 353.10 | 191.01, 179.03 | Chlorogenic acid | C16H18O9 |
| 4 | 1.4 | 287 sh, 329 | 879.05 | 717, 1-, 179.05, 96.95 | Caffeic acid tetramer glucoside (Rabdosiin 7-O-β-glucoside) | C42H40O21 |
| 5 | 2.4 | 254.6 348.5 | 609.17 | 463.3, 301.80 | Rutin | C27H30O16 |
| 6 | 2.5 | 253, 290 sh, 370 | 629.13 | 477,03, 315.06, 96.95 | Isorhamnetin 3-(6″-galloylglucoside) | C29H26O16 |
| 7 | 3.4 | 220.5 sh, 278.3 | 197.03 | – | Syringic acid | C9H10O5 |
| 8 | 3.7 | 213.4 sh, 280.7 | 359.08 | 197.04 | Syringic acid-4-β-glucoside | C15H20O10 |
| 9 | 5.6 | 269, 290 sh, 355 | 387.15 | 301.80 | Quercetin oxalate | C17H8O11 |
| 10 | 5.8 | 266, 346 | 739.05 | 659.07, 593.3, 447.01, 285.03 | Kaempferol-3-O-galactoside-6″-O-rhamnoside-3′″-O-rhamnoside | C33H40O19 |
| 11 | 6.3 | 289.0, 323.1 sh | 537.09 | 493.11, 358.06, 295.06, 253.04, 185.02, 179.04, 135.04 | Salvianolic acid H or Salvianolic I | C27H22O12 |
| 12 | 6.5 | 217.0, 261.7, 294.9 | 153.01 | 109.02 | 3,4-Dihydroxybenzoic acid (Protocatechuic acid) | C7H6O4 |
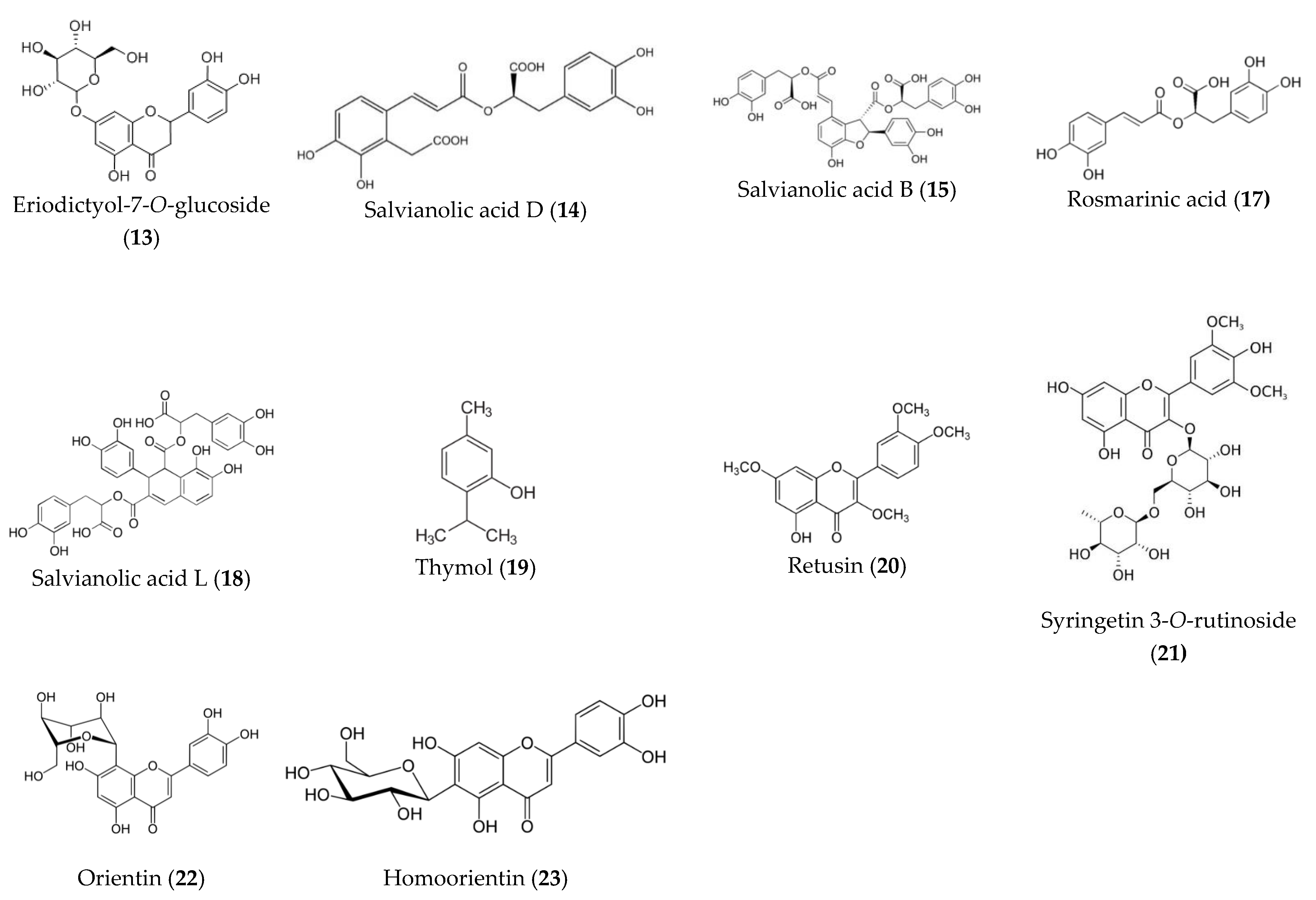
| 13 | 6.6 | 285, 325 | 449.19 | 377.04, 287.05 153.01 | Eriodictyol-7-O-glucoside | C21H22O11 |
| 14 | 6.8 | 289.0, 323.1 sh | 717.12 | 553.08, 519.09, 419.21, 358.06, 339.05, 321.04, 295.06, 179.04 | Salvianolic acid D | C36H30O16 |
| 15 | 7.1 | 289.0, 323.1 sh | 717.12 | 519.09, 421.1, 358.06, 339.05, 321.04, 179.04 | Salvianolic acid B | C36H30O16 |
| 16 | 7.3 | 289.0, 323.1 sh | 987.22 | 451.11, 179.04 | Caffeic acid hexamer | C52H44O20 |
| 17 | 7.4 | 329.1 | 359.06 | 197,1, 179.05, 161.3, 135.04, 133.03, 123.04 | Rosmarinic acid | C18H16O8 |
| 18 | 7.6 | 289.0, 323.1 sh | 717.12 | 553.08, 519.09, 419.21, 358.06, 339.05, 321.04, 185.02, 179.04 | Salvianolic acid L | C36H30O16 |
| 19 | 7.8 | 254,4 | 149.1- | - | Thymol | C10H14O |
| 20 | 7.9 | 350, 268 | 357.06 | 357.09, 342.12, 327.07, 312.02, 297.02 | Retusin | C19H18O7 |
| 21 | 8.0 | 254.6 348.5 | 653.14 | 507.4, 345.07, 330.1, 315.2, 96.95 | Syringetin 3-O-rutinoside | C29H34O17 |
| 22 | 9.2 | 254, 267 | 447.05 | 357.78, 327.21, 285.4 | Orientin (Luteolin 8-C-glucoside) | C21H20O11 |
| 23 | 9.4 | 254, 267 | 447.09 | 357.78, 327.22, 285.4 | Homoorientin (Luteolin 6-C-glucoside) | C21H20O11 |
| Time (min) | ||||||
|---|---|---|---|---|---|---|
| [Extract] (µg/mL) | 15 | 30 | 45 | 60 | 75 | 90 |
| 125 | 105.29 ± 0.65 | 105.21 ± 0.64 | 105.35 ± 0.59 | 105.51 ± 0.71 | 105.49 ± 0.64 | 105.71 ± 0.67 |
| 62.5 | 104.89 ± 0.77 | 105.14 ± 0.59 | 105.16 ± 0.45 | 105.41 ± 0.71 | 105.28 ± 0.65 | 105.43 ± 0.67 |
| 31.25 | 102.98 ± 1.83 | 104.36 ± 0.85 | 104.71 ± 0.73 | 105.21 ± 0.89 | 105.28 ± 0.68 | 105.50 ± 0.64 |
| 15.62 | 90.40 ± 8.90 | 100.97 ± 6.86 | 103.29 ± 7.71 | 105.23 ± 6.09 | 106.00 ± 6.18 | 106.37 ± 5.29 |
| 7.81 | 71.11 ± 1.38 | 67.81 ± 6.37 | 72.48 ± 7.36 | 77.24± 6.10 | 78.11 ± 8.20 | 80.10 ± 8.31 |
| 3.91 | 50.46 ± 1.81 | 51.00 ± 2.27 | 51.02 ± 1.31 | 50.90 ± 1.26 | 49.53 ± 1.18 | 50.19 ± 1.41 |
| 1.95 | 36.66 ± 0.94 | 36.27 ± 0.76 | 38.89± 1.23 | 38.91 ± 1.45 | 41.78 ± 2.17 | 35.37 ± 1.68 |
| 0.98 | 19.45 ± 0.87 | 22.52 ± 2.46 | 21.71± 1.18 | 29.01 ± 0.80 | 29.26± 0.88 | 28.00 ± 1.32 |
| IC50 (µg/mL) | 4.05 ± 0.22 b | 3.82 ± 0.27 b | 3.58 ± 0.38 b | 3.22 ± 0.19 a | 3.15 ± 0.34 a | 3.28 ± 0.29 a |
| AAI | 4.94 ± 0.09 b | 5.23 ± 0.07 b | 5.59 ± 0.05 b | 6.21 ± 0.10 a | 6.35 ± 0.06 a | 6.10 ± 0.07 a |
| DPPH-AAI | ABTS | TPC | FL | DHBA | DHCA | SRA | SALVA | RA | |
|---|---|---|---|---|---|---|---|---|---|
| AChE-IC50 | −0.8649 | −0.9487 | −0.5984 | −0.9563 | −0.9247 | −0.7667 | −0.9864 | −0.8806 | −0.7693 |
| DPPH-AAI | 0.9378 | 0.8145 | 0.9141 | 0.9324 | 0.9011 | 0.9409 | 0.9208 | 0.9022 | |
| ABTS | 0.8210 | 0.9878 | 0.9762 | 0.9304 | 0.9976 | 0.9923 | 0.9318 | ||
| TPC | 0.7220 | 0.9253 | 0.9732 | 0.8584 | 0.7439 | 0.9722 | |||
| FL | 0.9304 | 0.8618 | 0.9747 | 0.9995 | 0.8639 | ||||
| DHBA | 0.9877 | 0.9888 | 0.9417 | 0.9884 | |||||
| DHCA | 0.9534 | 0.8777 | 1.0000 | ||||||
| SRA | 0.9814 | 0.9546 | |||||||
| SALVA | 0.8796 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Torre, M.P.; Cavero, R.Y.; Calvo, M.I. Anticholinesterase Activity of Selected Medicinal Plants from Navarra Region of Spain and a Detailed Phytochemical Investigation of Origanum vulgare L. ssp. vulgare. Molecules 2022, 27, 7100. https://doi.org/10.3390/molecules27207100
de Torre MP, Cavero RY, Calvo MI. Anticholinesterase Activity of Selected Medicinal Plants from Navarra Region of Spain and a Detailed Phytochemical Investigation of Origanum vulgare L. ssp. vulgare. Molecules. 2022; 27(20):7100. https://doi.org/10.3390/molecules27207100
Chicago/Turabian Stylede Torre, María Pilar, Rita Yolanda Cavero, and María Isabel Calvo. 2022. "Anticholinesterase Activity of Selected Medicinal Plants from Navarra Region of Spain and a Detailed Phytochemical Investigation of Origanum vulgare L. ssp. vulgare" Molecules 27, no. 20: 7100. https://doi.org/10.3390/molecules27207100
APA Stylede Torre, M. P., Cavero, R. Y., & Calvo, M. I. (2022). Anticholinesterase Activity of Selected Medicinal Plants from Navarra Region of Spain and a Detailed Phytochemical Investigation of Origanum vulgare L. ssp. vulgare. Molecules, 27(20), 7100. https://doi.org/10.3390/molecules27207100






