Valorisation, Green Extraction Development, and Metabolomic Analysis of Wild Artichoke By-Product Using Pressurised Liquid Extraction UPLC–HRMS and Multivariate Data Analysis
Abstract
:1. Introduction
2. Results and Discussion
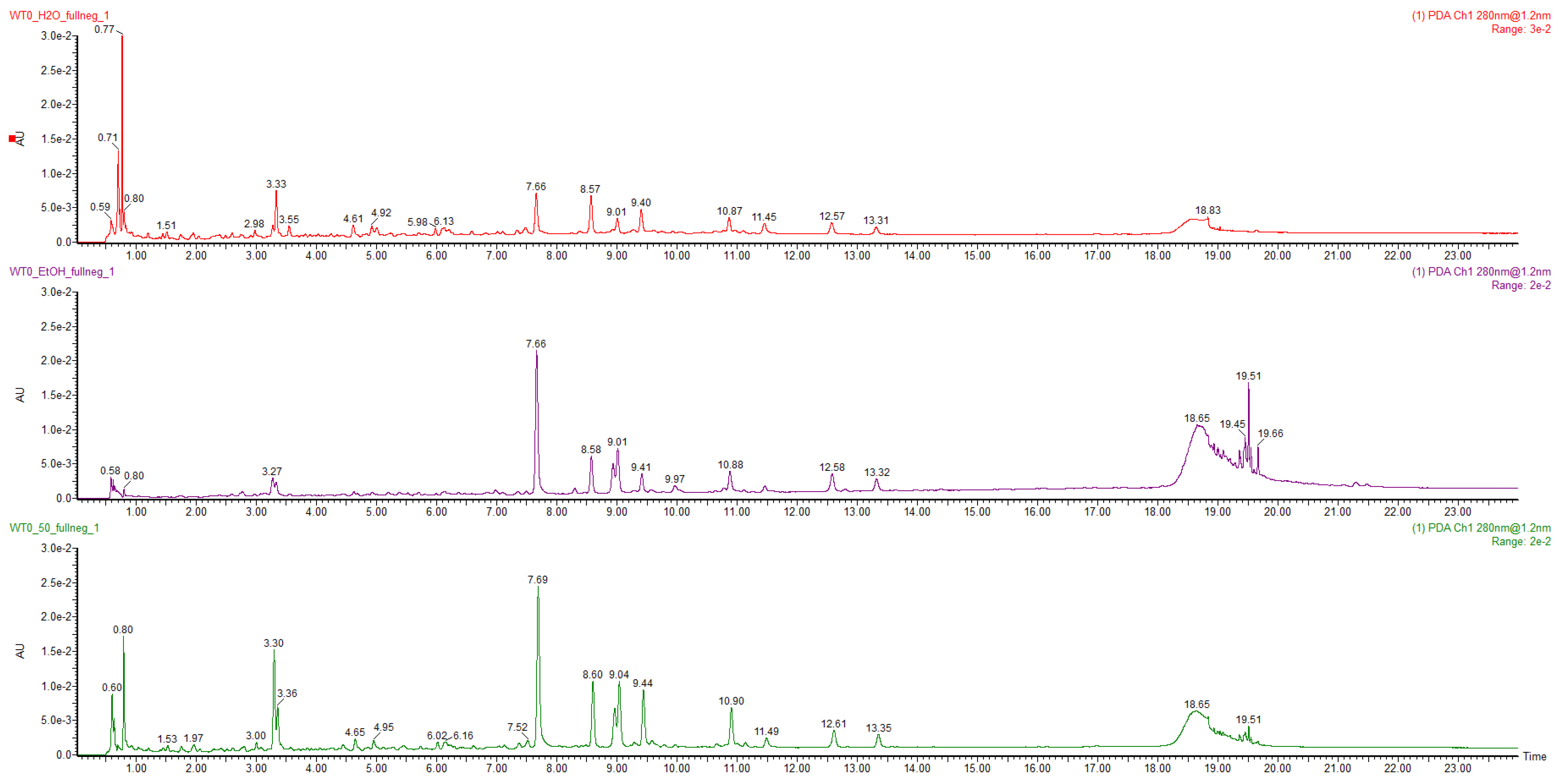
2.1. Characterisation of Ultrasound-Assisted Extraction by UPLC–HRMS Analysis
2.2. Optimisation of PLE Conditions
2.3. Multivariate Data Analysis
2.3.1. Supervised Principal Component Analysis (PCA) of C. cardunculus Extracts
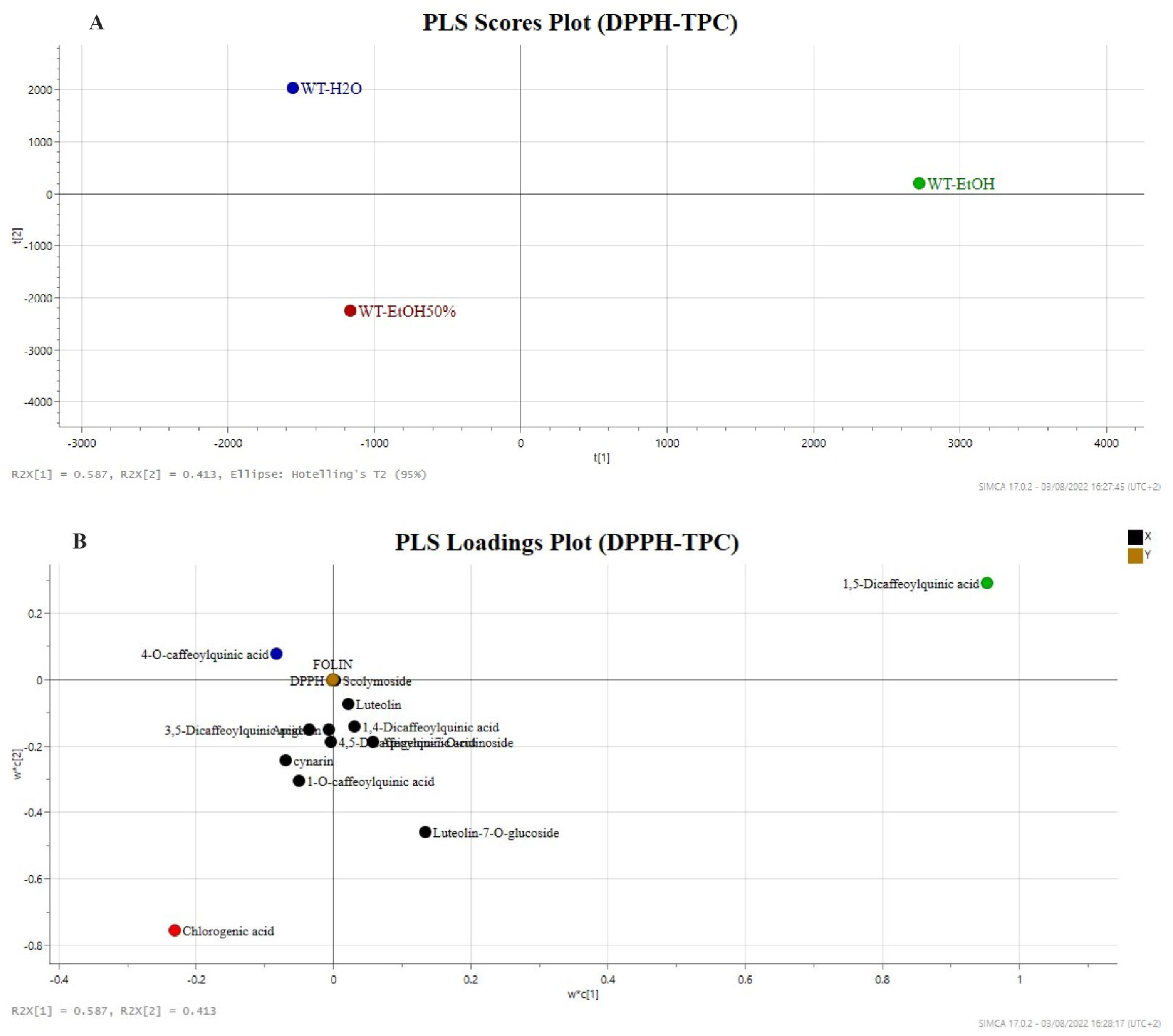
2.3.2. Partial Least Square (PLS) Regression Analysis with TPC and DPPH Tests
3. Materials and Methods
3.1. Chemicals
3.2. Plant Sample
3.3. Ultrasound-Assisted Extraction (USAE) and Pressurised Liquid Extraction (PLE)
3.4. Qualitative and Quantitative Analysis by UPLC-ESI/HRMS-UV
3.5. Determination of Total Phenol Content by Folins–Ciocalteu Assay
3.6. Determination of DPPH Radical Scavenging Activity
3.7. Statistical and Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mirabella, N.; Castellani, V.; Sala, S. Current Options for the Valorization of Food Manufacturing Waste: A Review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, Agronomical, Phytochemical, and Pharmacological Overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Mauro, R.; Portis, E.; Acquadro, A.; Lombardo, S.; Mauromicale, G.; Lanteri, S. Genetic Diversity of Globe Artichoke Landraces from Sicilian Small-Holdings: Implications for Evolution and Domestication of the Species. Conserv. Genet. 2009, 10, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Pignone, D.; Sonnante, G. Wild Artichokes of South Italy: Did the Story Begin Here? Genet. Resour. Crop Evol. 2004, 51, 577–580. [Google Scholar] [CrossRef]
- Portis, E.; Acquadro, A.; Comino, C.; Mauromicale, G.; Saba, E.; Lanteri, S. Genetic Structure of Island Populations of Wild Cardoon [Cynara cardunculus L. Var. sylvestris (Lamk) Fiori] Detected by AFLPs and SSRs. Plant Sci. 2005, 169, 199–210. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; De Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic Composition in Different Parts of Some Cultivars of Globe Artichoke (Cynara cardunculus L. Var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Pandino, G.; Courts, F.L.; Lombardo, S.; Mauromicale, G.; Williamson, G. Caffeoylquinic Acids and Flavonoids in the Immature Inflorescence of Globe Artichoke, Wild Cardoon, and Cultivated Cardoon. J. Agric. Food Chem. 2009, 58, 1026–1031. [Google Scholar] [CrossRef]
- Dabbou, S.; Dabbou, S.; Pandino, G.; Lombardo, S.; Mauromicale, G.; Chahdoura, H.; Gasco, L.; Helal, A.N. In Vitro Antioxidant Activities and Phenolic Content in Crop Residues of Tunisian Globe Artichoke. Sci. Hortic. 2015, 190, 128–136. [Google Scholar] [CrossRef]
- Zayed, A.; Serag, A.; Farag, M.A. Cynara cardunculus L.: Outgoing and Potential Trends of Phytochemical, Industrial, Nutritive and Medicinal Merits. J. Funct. Foods 2020, 69, 103937. [Google Scholar] [CrossRef]
- Cavini, S.; Guzzetti, L.; Givoia, F.; Regonesi, M.E.; Di Gennaro, P.; Magoni, C.; Campone, L.; Labra, M.; Bruni, I. Artichoke (Cynara cardunculus Var. scolymus L.) by-Products as a Source of Inulin: How to Valorise an Agricultural Supply Chain Extracting an Added-Value Compound. Nat. Prod. Res. 2022, 36, 2140–2144. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe Artichoke: A Functional Food and Source of Nutraceutical Ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Pagnotta, M.A. Artichoke: An Uncivilized Crop Rich of Diversity to Be Preserved and Valorized. Acta Hortic. 2016, 1147, 243–255. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palermo, M.; Colla, G.; Barbieri, G.; Fogliano, V. Polyphenol Metabolite Profile of Artichoke Is Modulated by Agronomical Practices and Cooking Method. J. Agric. Food Chem. 2013, 61, 7960–7968. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe Artichoke Leaves and Floral Stems as a Source of Bioactive Compounds. Ind. Crops Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Ruiz-Cano, D.; Pérez-Llamas, F.; Frutos, M.J.; Arnao, M.B.; Espinosa, C.; López-Jiménez, J.Á.; Castillo, J.; Zamora, S. Chemical and Functional Properties of the Different By-Products of Artichoke (Cynara scolymus L.) from Industrial Canning Processing. Food Chem. 2014, 160, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.T.C.; Eca, K.S.; Vieira, G.S.; Menegalli, F.C.; Martinez, J.; Hubinger, M.D. Prebiotic Oligosaccharides from Artichoke Industrial Waste: Evaluation of Different Extraction Methods. Ind. Crops Prod. 2015, 76, 141–148. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Ahmady, S.H.; Elian, F.S.; Wessjohann, L.A. Metabolomics Driven Analysis of Artichoke Leaf and Its Commercial Products via UHPLC–q-TOF-MS and Chemometrics. Phytochemistry 2013, 95, 177–187. [Google Scholar] [CrossRef]
- Órbenes, G.; Rodríguez-Seoane, P.; Torres, M.D.; Chamy, R.; Zúñiga, M.E.; Domínguez, H. Valorization of Artichoke Industrial By-Products Using Green Extraction Technologies: Formulation of Hydrogels in Combination with Paulownia Extracts. Molecules 2021, 26, 4386. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, Extraction Optimization and Technology Advancements of Artichoke Biowastes: Food and Non-Food Applications. LWT 2020, 132, 109883. [Google Scholar] [CrossRef]
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke Biorefinery: From Food to Advanced Technological Applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Mejri, F.; Baati, T.; Martins, A.; Selmi, S.; Luisa Serralheiro, M.; Falé, P.L.; Rauter, A.; Casabianca, H.; Hosni, K. Phytochemical Analysis and in Vitro and in Vivo Evaluation of Biological Activities of Artichoke (Cynara scolymus L.) Floral Stems: Towards the Valorization of Food by-Products. Food Chem. 2020, 333, 127506. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Castello, E.M.; Mayor, L.; Calvo-Ramirez, A.; Ruiz-Melero, R.; Rodriguez-Lopez, A.D. Response Surface Optimization of Inulin and Polyphenol Extraction from Artichoke (Cynara scolymus (L.)) Solid Wastes. Appl. Sci. 2022, 12, 7957. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food by-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef] [PubMed]
- Pagano, I.; del Pilar Sánchez-Camargo, A.; Mendiola, J.A.; Campone, L.; Cifuentes, A.; Rastrelli, L.; Ibañez, E. Selective Extraction of High-value Phenolic Compounds from Distillation Wastewater of Basil (Ocimum basilicum L.) by Pressurized Liquid Extraction. Electrophoresis 2018, 39, 1884–1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raccuia, S.A.; Cavallaro, V.; Melilli, M.G. Intraspecific Variability in Cynara cardunculus L. Var. sylvestris Lam. Sicilian Populations: Seed Germination under Salt and Moisture Stresses. J. Arid Environ. 2004, 56, 107–116. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic Acids and Flavonoids in Leaf and Floral Stem of Cultivated and Wild Cynara cardunculus L. Genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- d’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound Assisted Extraction of Polyphenols from Black Chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Bhebhe, M.; Füller, T.N.; Chipurura, B.; Muchuweti, M. Effect of Solvent Type on Total Phenolic Content and Free Radical Scavenging Activity of Black Tea and Herbal Infusions. Food Anal. Methods 2016, 9, 1060–1067. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Extensive Characterisation of Bioactive Phenolic Constituents from Globe Artichoke (Cynara scolymus L.) by HPLC–DAD-ESI-QTOF-MS. Food Chem. 2013, 141, 2269–2277. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Abass, K.; Hakkola, J.; Atawia, A.R.; Farag, M.A. The Wild Egyptian Artichoke as a Promising Functional Food for the Treatment of Hepatitis C Virus as Revealed via UPLC-MS and Clinical Trials. Food Funct. 2016, 7, 3006–3016. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Barreira, J.C.M.; Alves, M.J.; Barracosa, P.; Ferreira, I.C.F.R. Phenolic Profile and Bioactivity of Cardoon (Cynara cardunculus L.) Inflorescence Parts: Selecting the Best Genotype for Food Applications. Food Chem. 2018, 268, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, P.A.B.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Freire, C.S.R.; Rocha, S.M.; Duarte, M.F.; Silvestre, A.J.D. Phenolic Composition and Antioxidant Activity of Different Morphological Parts of Cynara cardunculus L. Var. altilis (DC). Ind. Crops Prod. 2014, 61, 460–471. [Google Scholar] [CrossRef]
- Tian, P.; Zhang, X.; Wang, H.; Li, P.; Liu, Y.; Li, S. Rapid Analysis of Components in Coptis Chinensis Franch by Ultra-Performance Liquid Chromatography with Quadrupole Time-of-Flight Mass Spectrometry. Pharmacogn. Mag. 2017, 13, 175. [Google Scholar]
- Jia, J.; Liu, M.; Wen, Q.; He, M.; Ouyang, H.; Chen, L.; Li, J.; Feng, Y.; Zhong, G.; Yang, S. Screening of Anti-Complement Active Ingredients from Eucommia ulmoides Oliv. Branches and Their Metabolism in Vivo Based on UHPLC-Q-TOF/MS/MS. J. Chromatogr. B 2019, 1124, 26–36. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; De La Ossa, E.M. Use of High Pressure Techniques to Produce Mangifera indica L. Leaf Extracts Enriched in Potent Antioxidant Phenolic Compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Piacente, S. Metabolite Profiling of Helichrysum italicum Derived Food Supplements by 1H-NMR-Based Metabolomics. Molecules 2021, 26, 6619. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Guzzetti, L.; Panzeri, D.; Ulaszewska, M.; Sacco, G.; Forcella, M.; Fusi, P.; Tommasi, N.; Fiorini, A.; Campone, L.; Labra, M. Assessment of Dietary Bioactive Phenolic Compounds and Agricultural Sustainability of an African Leafy Vegetable Corchorus olitorius L. Front. Nutr. 2021, 8, 383. [Google Scholar] [CrossRef]


| Extracts | Folins–Ciocalteu Assay [mgGAE/gDM] | DPPH Assay [µmolTrolox/gEXT] |
|---|---|---|
| H2O | 2.937 ± 0.054 | 0.159 ± 0.009 |
| EtOH | 2.499 ± 0.114 | 0.211 ± 0.003 |
| EtOH50% | 6.317 ± 0.161 | 0.246 ± 0.003 |
| Peak | Tr (Minutes) | [M−H]− (m/z) | Molecular Formula | Error (ppm) | MS/MS Fragments | Metabolite Identity | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 1,4 | 315.0716 | C13H15O9 | 0.1 | 152.0050; 108.0170 | protocatechuic acid-O-glycoside | [31] |
| 2 | 1,6 | 329.0784 | C14H17O9 | −0.5 | 167.1225 | vannilloyl-1-O-glycoside | Chemspider |
| 3 | 2,0 | 353.0718 | C7H12O6 | −0.2 | 191.0483 | 1-O-caffeoylquinic acid | Standard |
| 4 | 2,3 | 353.0718 | C25H24O12 | 0.3 | 191.0483 | chlorogenic acid | Standard |
| 5 | 3,4 | 353.0718 | C32H36O18 | −1.6 | 191.0483 | 4-O-caffeoylquinic acid | Standard |
| 6 | 4,2 | 471.1869 | C22H32O11 | 1.2 | 337.0681; 191.0483 | p-coumaric acid derivative | [32] |
| 7 | 5,4 | 515.1197 | C25H24O12 | 1.2 | 353.0718; 191.0483; 179.0281; 135.0378 | cynarin | Standard; [33,34] |
| 8 | 5,6 | 367.1025 | C17H20O9 | 1.3 | 191.0483; 173.0438 | 3-O-feruloylquinic acid | [31,35] |
| 9 | 6,0 | 681.2400 | C32H42O16 | 0.0 | 519.1870; 357.1329; 151.0378 | pinoresinol-O-glucopyranosyl-glycopiranoside | [36] |
| 10 | 6,7 | 535.1806 | C26H31O12 | −0.4 | 357.1358 | bidenilegnoside A | [31] |
| 11 | 7,5 | 593.1511 | C27H30O15 | −0.2 | 447.0939; 285.0398; 151.0037 | scolymoside | Standard |
| 12 | 7,8 | 447.0932 | C21H20O11 | −0.3 | 357.1332; 285.0398 | luteolina-7-O-glucoside | Standard |
| 13 | 8,6 | 515.1197 | C25H24O12 | 1.3 | 353.0858; 191.0561; 173.0391; 135.0378 | 1,4-dicaffeoylquinic acid | Standard |
| 14 | 8,7 | 519.1870 | C26H32O12 | −0.3 | 357.1332; 151.0378 | pinoresinol-4-O-glycoside | [31,36] |
| 15 | 8,7 | 515.1197 | C25H24O12 | 353.0858; 191.0561; 173.0391; 135.0378 | 1,5-dicaffeoylquinic acid | Standard | |
| 16 | 8,7 | 577.1570 | C27H30O12 | 1.2 | 269.0346 | apigenin-7-O-rutinoside | Standard; [31,34] |
| 17 | 9,1 | 515.1197 | C25H24O12 | 0.4 | 353.0718; 191.0483; 179.0281; 135.0378 | 3,5-dicaffeoylquinic acid | Standard |
| 18 | 9,2 | 489.1036 | C23H22O12 | −0.4 | 447.8031; 285.0398 | luteolin-7-O-(acetyl)-glycoside | [34] |
| 19 | 9,8 | 515.1197 | C25H24O12 | −2.0 | 353.0718; 191.0483; 179.0281; 173.0391; 161.0242; 135.0378 | 4,5-dicaffeoylquinic acid | Standard |
| 20 | 10,1 | 615.1359 | C29H27O15 | 0.7 | 353.0718; 335.1522; 191.0483; 179.0281; 173.0391, 161.0242 | dicaffeoyl-succinoylquinic acid | [34] |
| 21 | 10,5 | 561.1977 | C28H33O12 | −0.1 | 357.1332; 342.0462; 151.0037 | pinoresinol-acetilhexoside | [31] |
| 22 | 11,0 | 269.0346 | C15H9O5 | 0.2 | 117.0293 | apigenin | Standard |
| 23 | 11,6 | 285.0313 | C15H9O6 | 0.6 | 151.0037; 133.0218 | luteolin | Standard |
| EtOH | H2O | EtOH50% | |
|---|---|---|---|
| 1-O-caffeoylquinic acid | 11.45 ± 0.24 a | 14.35 ± 0.41 a | 13.73 ± 0.34 a |
| chlorogenic acid | 5.13 ± 0.09 a | 6.51 ± 0.12 a | 6.21 ± 0.16 a |
| 4-O-caffeoylquinic acid | 20.82 ± 0.34 a | 26.02 ± 0.35 a | 24.90 ± 0.61 a |
| cynarina | 79.98 ± 0.65 a | 103.15 ± 0.85 a | 98.16 ± 2.74 a |
| scolymoside | 5.11 ± 0.16 a | 6.24 ± 0.22 a | 6.00 ± 0.13 a |
| luteolin-7-O-glucoside | 11.99 ± 0.25 a | 15.21 ± 0.29 a | 14.51 ± 0.38 a |
| 1,4-dicaffeoylquinic acid | 7.68 ± 0.11 a | 9.19 ± 0.19 a | 8.87 ± 0.18 a |
| 1,5-dicaffeoylquinic acid | 3.42 ± 0.07 a | 4.20 ± 0.15 a | 4.03 ± 0.09 a |
| apigenin-7-O-rutinoside | 7.07 ± 0.23 a | 8.67 ± 0.38 a | 8.33 ± 0.19 a |
| 3,5-dicaffeoylquinic acid | 1.31 ± 0.03 a | 1.62 ± 0.10 a | 1.55 ± 0.04 a |
| 4,5-dicaffeoylquinic acid | 5.64 ± 0.31 a | 7.00 ± 0.06 a | 6.71 ± 0.16 a |
| luteolin | 3.91 ± 0.10 a | 4.97 ± 0.21 a | 4.74 ± 0.12 a |
| apigenin | 2.88 ± 0.18 a | 3.51 ± 0.13 a | 3.37 ± 0.07 a |
| total phenol content | 11.21 ± 0.70 b | 28.49 ± 1.53 b | 33.62 ± 1.73 b |
| radical scavenging activity | 36.86 ± 4.13 c | 105.54 ± 9.28 c | 144.37 ± 15.12 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagliari, S.; Cannavacciuolo, C.; Celano, R.; Carabetta, S.; Russo, M.; Labra, M.; Campone, L. Valorisation, Green Extraction Development, and Metabolomic Analysis of Wild Artichoke By-Product Using Pressurised Liquid Extraction UPLC–HRMS and Multivariate Data Analysis. Molecules 2022, 27, 7157. https://doi.org/10.3390/molecules27217157
Pagliari S, Cannavacciuolo C, Celano R, Carabetta S, Russo M, Labra M, Campone L. Valorisation, Green Extraction Development, and Metabolomic Analysis of Wild Artichoke By-Product Using Pressurised Liquid Extraction UPLC–HRMS and Multivariate Data Analysis. Molecules. 2022; 27(21):7157. https://doi.org/10.3390/molecules27217157
Chicago/Turabian StylePagliari, Stefania, Ciro Cannavacciuolo, Rita Celano, Sonia Carabetta, Mariateresa Russo, Massimo Labra, and Luca Campone. 2022. "Valorisation, Green Extraction Development, and Metabolomic Analysis of Wild Artichoke By-Product Using Pressurised Liquid Extraction UPLC–HRMS and Multivariate Data Analysis" Molecules 27, no. 21: 7157. https://doi.org/10.3390/molecules27217157






