Tailoring the Electrical Energy Storage Capability of Dielectric Polymer Nanocomposites via Engineering of the Host–Guest Interface by Phosphonic Acids
Abstract
1. Introduction
2. Results
2.1. Characterization of the Modified BT
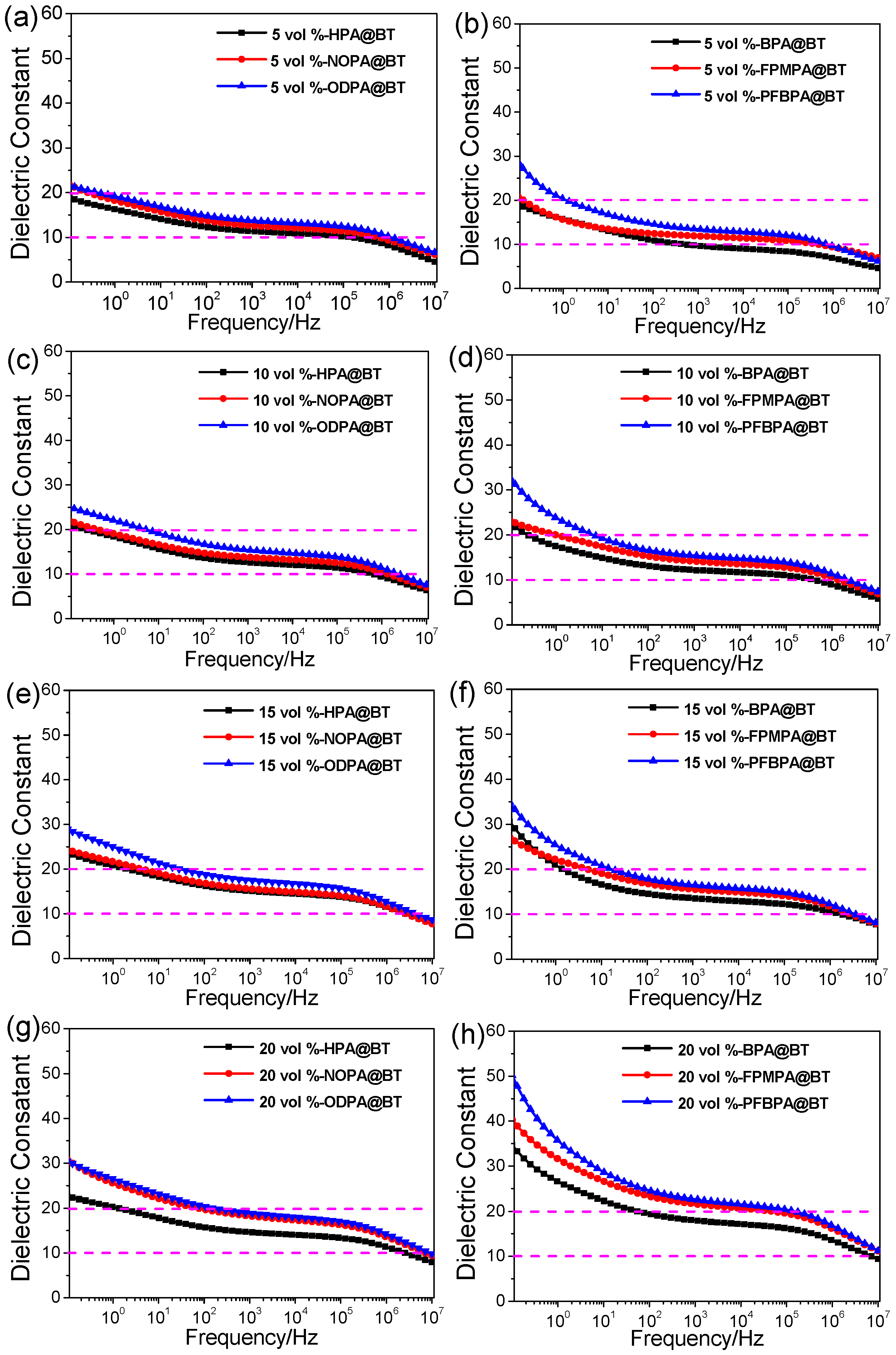
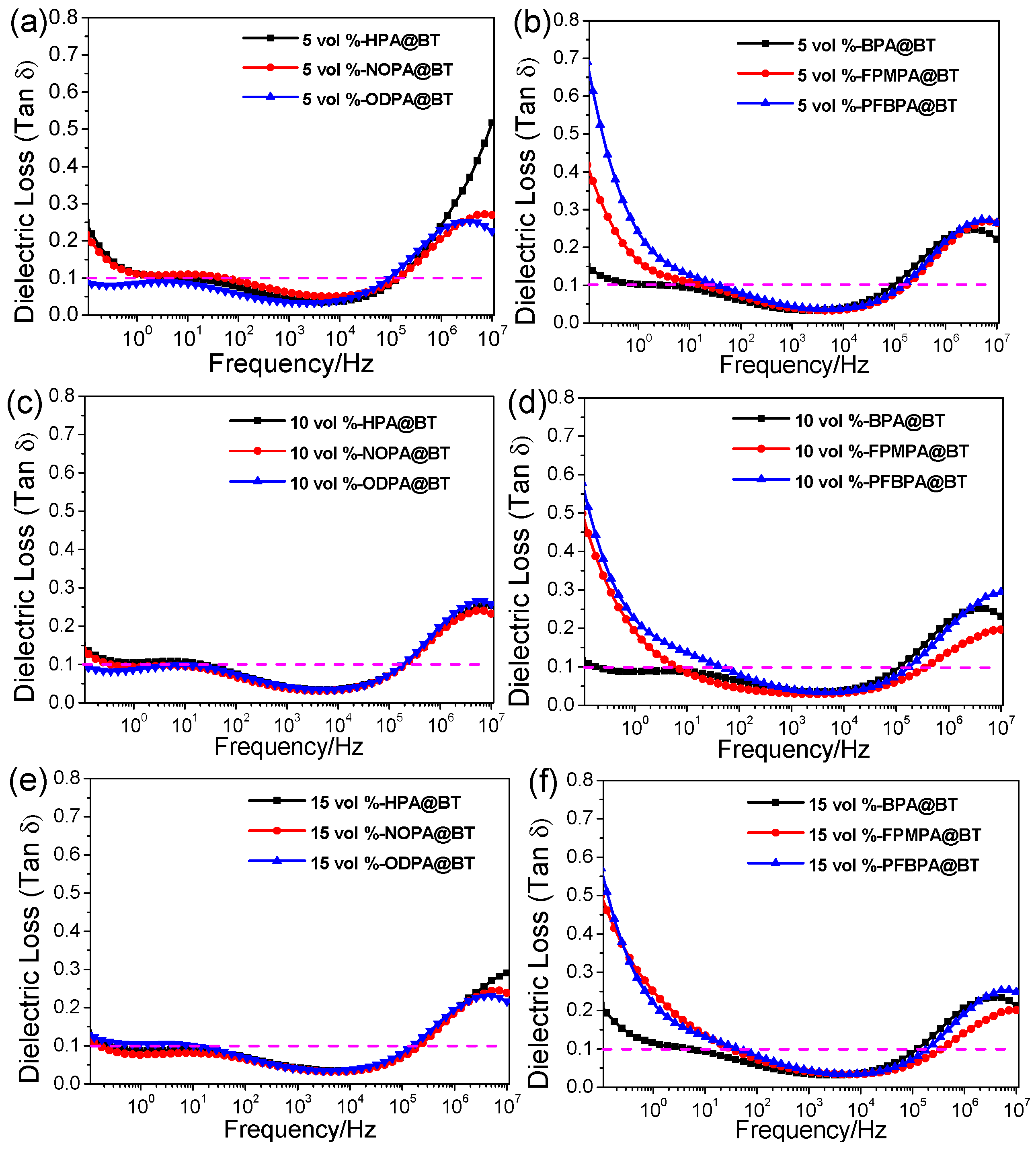
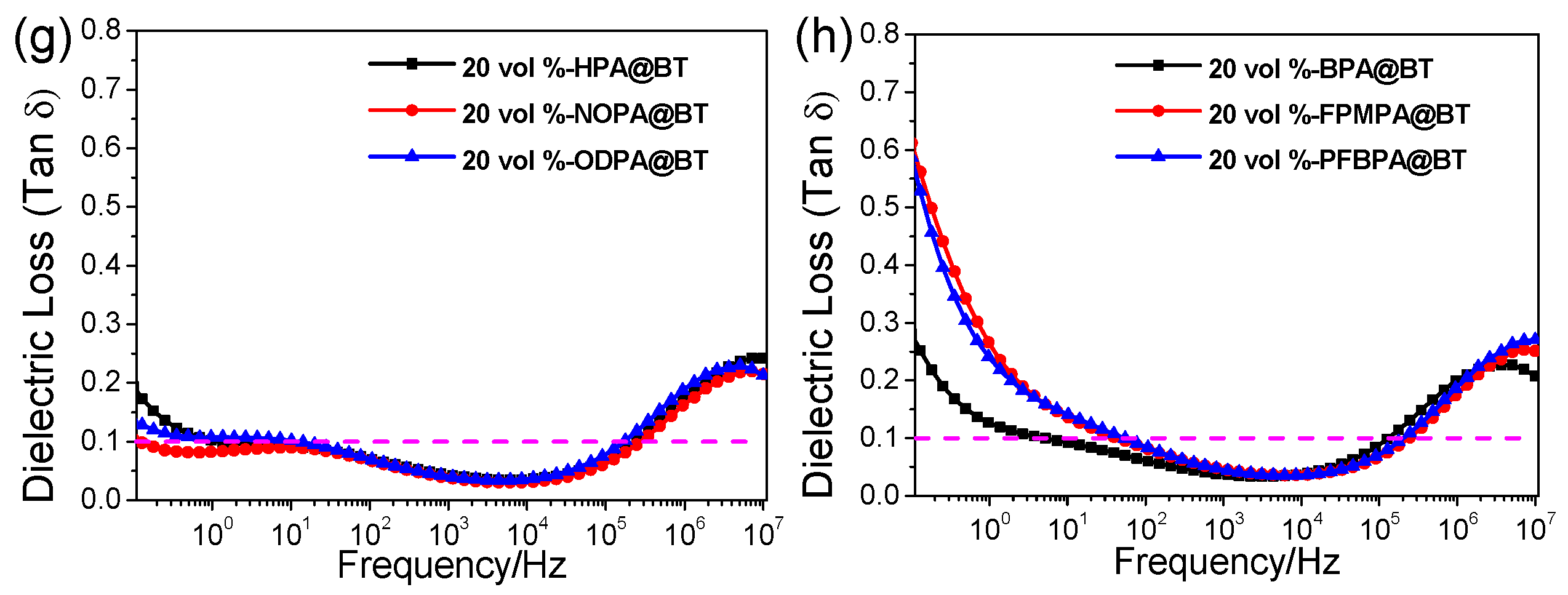
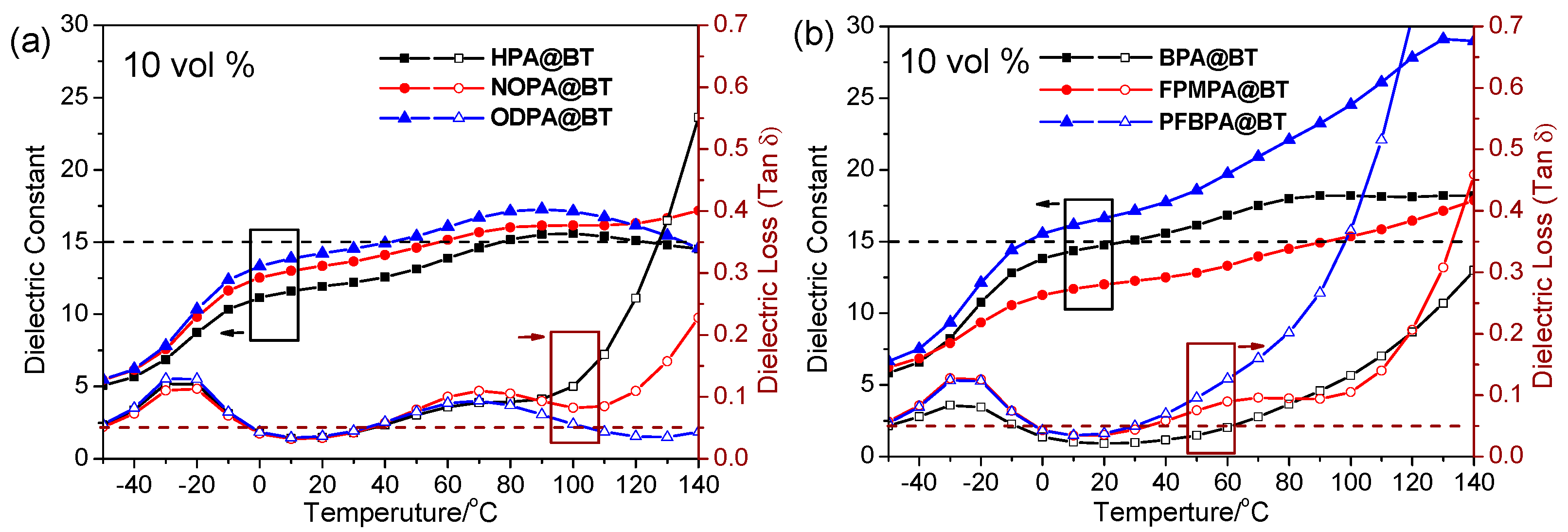
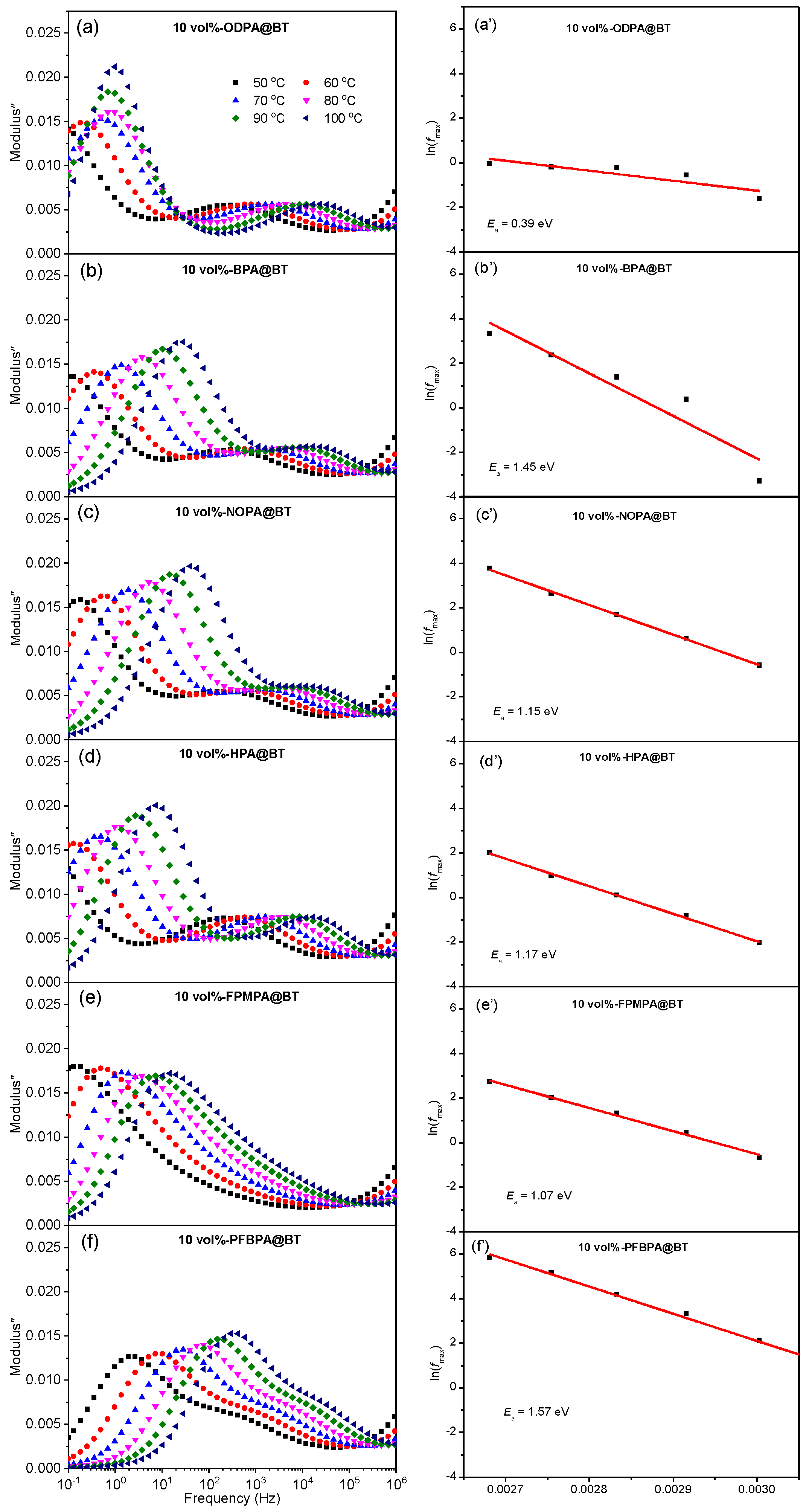
2.2. Dielectric Properties of Nanocomposite Films
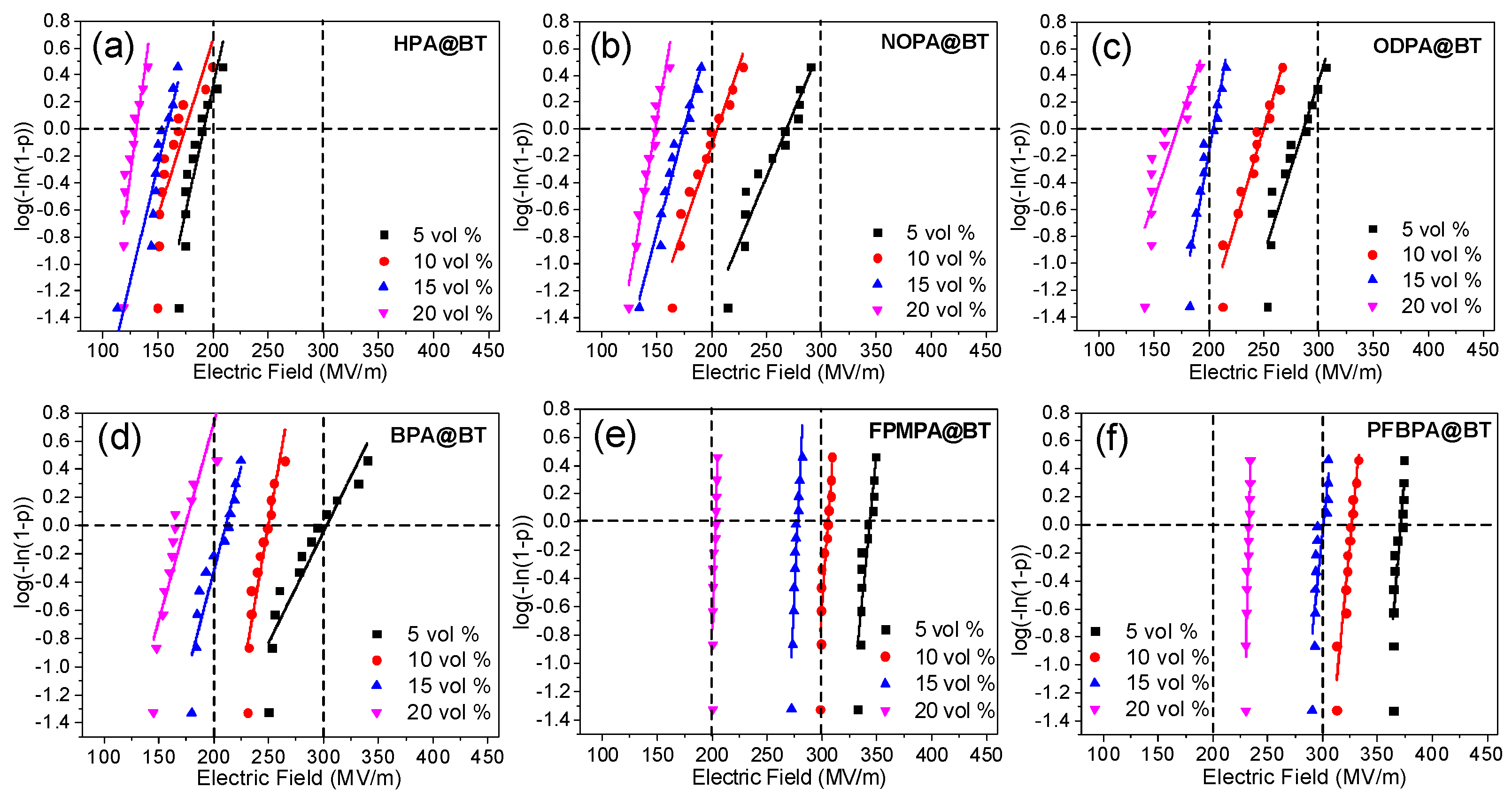
2.3. Breakdown Electric Field
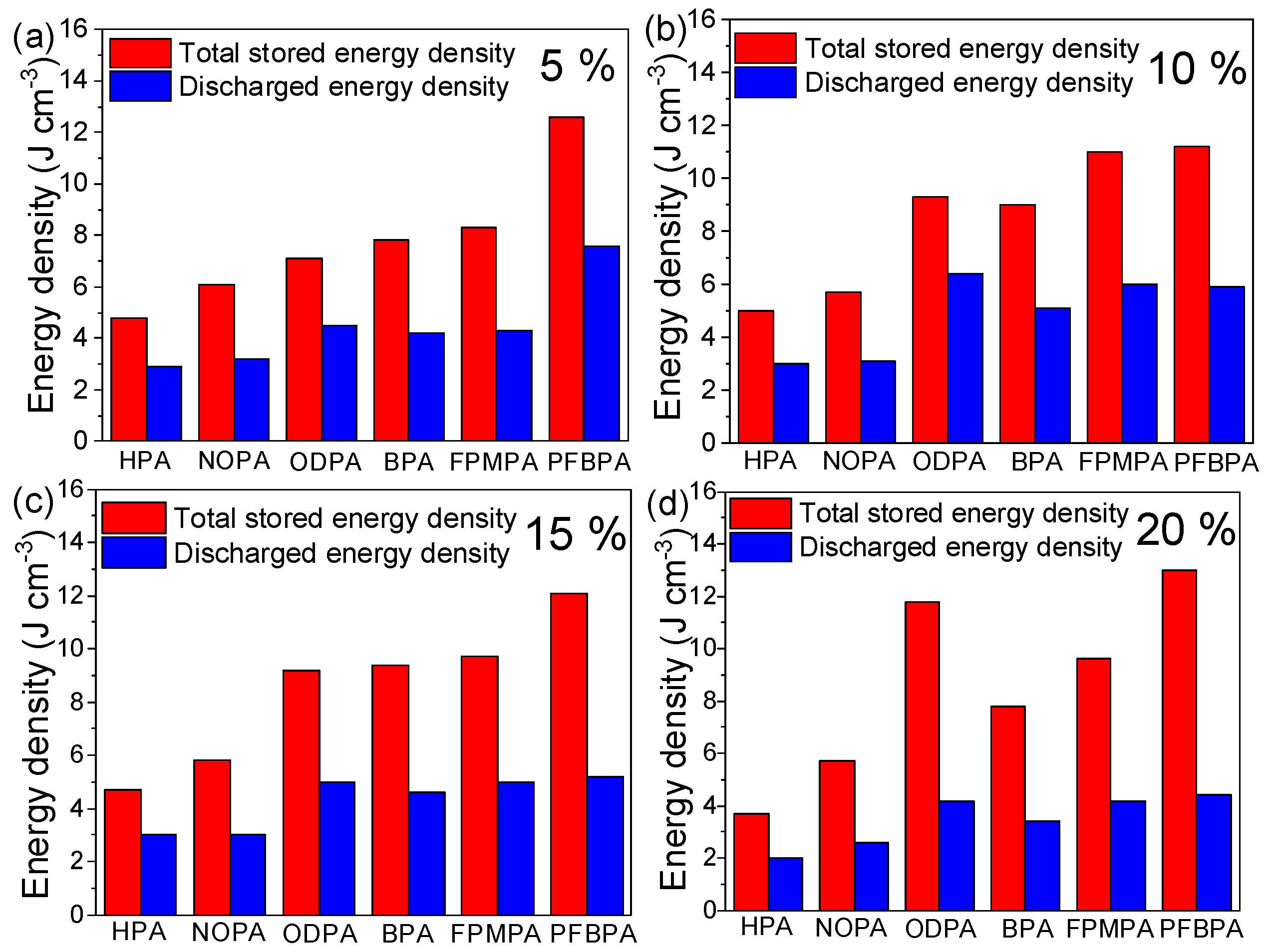
2.4. Electrical Energy Storage
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huang, X.; Sun, B.; Zhu, Y.; Li, S.; Jiang, P. High-k polymer nanocomposites with 1D filler for dielectric and energy storage applications. Prog. Mater. Sci. 2019, 100, 187–225. [Google Scholar] [CrossRef]
- Wu, C.; Deshmukh, A.A.; Li, Z.; Chen, L.; Alamri, A.; Wang, Y.; Ramprasad, R.; Sotzing, G.A.; Cao, Y. Flexible Temperature-Invariant Polymer Dielectrics with Large Bandgap. Adv. Mater. 2020, 32, e2000499. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W.; Ding, L. Dielectric/ultrathin metal/dielectric structured transparent conducting films for flexible electronics. Sci. Bull. 2020, 65, 1324–1326. [Google Scholar] [CrossRef]
- Yuan, C.; Zhou, Y.; Zhu, Y.; Liang, J.; Wang, S.; Peng, S.; Li, Y.; Cheng, S.; Yang, M.; Hu, J.; et al. Polymer/molecular semiconductor all-organic composites for high-temperature dielectric energy storage. Nat. Commun. 2020, 11, 3919. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P. Core-Shell Structured High-k Polymer Nanocomposites for Energy Storage and Dielectric Applications. Adv. Mater. 2015, 27, 546–554. [Google Scholar] [CrossRef]
- Zamperlin, N.; Bottacini, A.; Callone, E.; Pegoretti, A.; Fontana, M.; Dirè, S. Barium Titanate Functionalization with Organosilanes: Effect on Particle Compatibility and Permittivity in Nanocomposites. Molecules 2022, 27, 6499. [Google Scholar] [CrossRef]
- Guo, M.F.; Jiang, J.Y.; Shen, Z.H.; Lin, Y.H.; Nan, C.W.; Shen, Y. High-Energy-Density Ferroelectric Polymer Nanocomposites for Capacitive Energy Storage: Enhanced Breakdown Strength and Improved Discharge Efficiency. Mater Today 2019, 29, 49–67. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Huang, X.; Chen, J.; Li, Q.; He, J.; Jiang, P. High Energy Density Polymer Dielectrics Interlayered by Assembled Boron Nitride Nanosheets. Adv. Energy Mater. 2019, 9, 1901826. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, Z.; Li, Y.; Chai, B.; Chen, J.; Jiang, P.; Huang, X. High Conduction Band Inorganic Layers for Distinct Enhancement of Electrical Energy Storage in Polymer Nanocomposites. Nano-Micro Lett. 2022, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Gupta, R.K. Recent progress on ferroelectric polymer-based nanocomposites for high energy density capacitors: Synthesis, dielectric properties, and future aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar]
- Luo, H.; Zhou, X.; Ellingford, C.; Zhang, Y.; Chen, S.; Zhou, K.; Zhang, D.; Bowen, C.R.; Wan, C. Interface design for high energy density polymer nanocomposites. Chem. Soc. Rev. 2019, 48, 4424–4465. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khanchaitit, P.; Han, K.; Wang, Q. New Route Toward High-Energy-Density Nanocomposites Based on Chain-End Functionalized Ferroelectric Polymers. Chem. Mater. 2010, 22, 5350–5357. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Pradhan, N.; Karim, A.; Raghavan, D. Recent Advances in the Synthesis of Polymer-Grafted Low-K and High-K Nanoparticles for Dielectric and Electronic Applications. Molecules 2021, 26, 2942. [Google Scholar] [CrossRef]
- Badi, N.; Mekala, R.; Khasim, S.; Roy, A.; Ignatiev, A. Enhanced dielectric performance in PVDF/Al-Al2O3 core–shell nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 10593–10599. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, J.; Wang, L.; Yuan, Q.; Niu, Y.; Chen, J.; Wang, Q.; Wang, H. Compositional Tailoring Effect on Electric Field Distribution for Significantly Enhanced Breakdown Strength and Restrained Conductive Loss in Sandwich-Structured Ceramic/Polymer Nanocomposites. J. Mater. Chem. A 2017, 5, 4710–4718. [Google Scholar] [CrossRef]
- Kang, D.; Wang, G.; Huang, Y.; Jiang, P.; Huang, X. Decorating TiO2 Nanowires with BaTiO3 Nanoparticles: A New Approach Leading to Substantially Enhanced Energy Storage Capability of High k Polymer Nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Yuan, Q.; Niu, Y.; Chen, J.; Wang, Q.; Wang, H. Ultrahigh electric displacement and energy density in gradient layer-structured BaTiO3/PVDF nanocomposites with an interfacial barrier effect. J. Mater. Chem. A 2017, 5, 10849–10855. [Google Scholar] [CrossRef]
- Marwat, M.A.; Ma, W.; Fan, P.; Elahi, H.; Samart, C.; Nan, B.; Tan, H.; Salamon, D.; Ye, B.; Zhang, H. Ultrahigh energy density and thermal stability in sandwich-structured nanocomposites with dopamine@Ag@BaTiO3. Energy Storage Mater. 2020, 31, 492–504. [Google Scholar] [CrossRef]
- Li, J.; Claude, J.; Norena-Franco, L.; Seok, S.; Wang, Q. Electrical Energy Storage in Ferroelectric Polymer Nanocomposites Containing Surface-Functionalized BaTiO3 Nanoparticles. Chem. Mater. 2008, 20, 6304–6306. [Google Scholar] [CrossRef]
- Zhou, T.; Zha, J.W.; Cui, R.Y.; Fan, B.H.; Yuan, J.K.; Dang, Z.M. Improving Dielectric Properties of BaTiO3/Ferroelectric Polymer Composites by Employing Surface Hydroxylated BaTiO3 Nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 2184–2188. [Google Scholar] [CrossRef]
- Yu, K.; Niu, Y.; Xiang, F.; Zhou, Y.; Bai, Y.; Wang, H. Enhanced electric breakdown strength and high energy density of barium titanate filled polymer nanocomposites. J. Appl. Phys. 2013, 114, 174107. [Google Scholar] [CrossRef]
- Cho, S.; Paik, K. Relationships Between Suspension Formulations and the Properties of BaTiO3/Epoxy Composite Films for Integral Capacitors. In Proceedings of the 51st IEEE Electronic Components and Technology Conference, (ECTC), Orlando, FL, USA, 29 May–1 June 2001. [Google Scholar]
- Kim, P.; Jones, S.; Hotchkiss, P.; Haddock, J.; Kippelen, B.; Marder, S.; Perry, J. Phosphonic Acid-Modified Barium Titanate Polymer Nanocomposites with High Permittivity and Dielectric Strength. Adv. Mater. 2007, 19, 1001–1005. [Google Scholar] [CrossRef]
- Kim, P.; Doss, N.; Tillotson, J.; Hotchkiss, P.; Pan, M.; Marder, S.; Li, J.; Calame, J.; Perry, J. High Energy Density Nanocomposites Based on Surface-Modified BaTiO3 and a Ferroelectric Polymer. ACS Nano 2009, 3, 2581–2599. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, H.; Wang, Y.; Ratcliff, T.; Breneman, C.; Brinson, L.C.; Chen, W.; Schadler, L. Predicting the breakdown strength and lifetime of nanocomposites using a multiscale modeling approach. J. Appl. Phys. 2017, 122, 065101. [Google Scholar] [CrossRef]
- Routray, K.; Sanyal, D.; Behera, D. Dielectric, magnetic, ferroelectric, and Mossbauer properties of bismuth substituted nanosized cobalt ferrites through glycine nitrate synthesis method. J. Appl. Phys. 2017, 122, 224104. [Google Scholar] [CrossRef]
- Gao, W.; Dickinson, L.; Grozinger, C.; Morin, F.; Reven, L. Self-Assembled Monolayers of Alkyl phosphonic Acids on Metal Oxides. Langmuir 1996, 12, 6429–6435. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Zhang, Q.; Gu, L.; Hu, Y.; Du, J.; Lin, Y.; Nan, C. Ultrahigh Energy Density of Polymer Nanocomposites containing BaTiO3@TiO2 Nanofibers by Atomic-Scale Interface Engineering. Adv. Mater. 2015, 27, 819–824. [Google Scholar] [CrossRef]
- Aldas, M.; Boiteux, G.; Seytre, G.; Ghallabi, Z. Dielectric behaviour of BaTiO3/P(VDF-HFP) composite thin films prepared by solvent evaporation method. In Proceedings of the 2010 10th IEEE International Conference on Solid Dielectrics, 4–9 July 2010; Volume 978, pp. 1–4.
- Lin, Y.; Li, D.; Zhang, M.; Zhan, S.; Yang, Y.; Yang, H.; Yuan, Q. Excellent Energy-Storage Properties Achieved in BaTiO3-Based Lead-Free Relaxor Ferroelectric Ceramics via Domain Engineering on the Nanoscale. ACS Appl. Mater. Interfaces 2019, 11, 36824–36830. [Google Scholar] [CrossRef]
- Wang, G.; Huang, X.; Jiang, P. Tailoring Dielectric Properties and Energy Density of Ferroelectric Polymer Nanocomposites by High k Nanowires. ACS Appl. Mater. Interface 2015, 7, 18017–18027. [Google Scholar] [CrossRef]
- Chen, J.; Shen, Z.; Kang, Q.; Qian, X.; Li, S.; Jiang, P.; Huang, X. Chemical adsorption on 2D dielectric nanosheets for matrix free nanocomposites with ultrahigh electrical energy storage. Sci. Bull. 2022, 67, 609–618. [Google Scholar] [CrossRef]
- Siddabattuni, S.; Schuman, T.P.; Dogan, F. Dielectric Properties of Polymer-Particle Nanocomposites Influenced by Electronic Nature of Filler Surfaces. ACS Appl. Mater. Interfaces 2013, 5, 1917–1927. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xu, P.; Xu, X.; Kang, D.; Chen, J.; Li, Z.; Huang, X. Tailoring the Electrical Energy Storage Capability of Dielectric Polymer Nanocomposites via Engineering of the Host–Guest Interface by Phosphonic Acids. Molecules 2022, 27, 7225. https://doi.org/10.3390/molecules27217225
Wang S, Xu P, Xu X, Kang D, Chen J, Li Z, Huang X. Tailoring the Electrical Energy Storage Capability of Dielectric Polymer Nanocomposites via Engineering of the Host–Guest Interface by Phosphonic Acids. Molecules. 2022; 27(21):7225. https://doi.org/10.3390/molecules27217225
Chicago/Turabian StyleWang, Shaojing, Peng Xu, Xiangyi Xu, Da Kang, Jie Chen, Zhe Li, and Xingyi Huang. 2022. "Tailoring the Electrical Energy Storage Capability of Dielectric Polymer Nanocomposites via Engineering of the Host–Guest Interface by Phosphonic Acids" Molecules 27, no. 21: 7225. https://doi.org/10.3390/molecules27217225
APA StyleWang, S., Xu, P., Xu, X., Kang, D., Chen, J., Li, Z., & Huang, X. (2022). Tailoring the Electrical Energy Storage Capability of Dielectric Polymer Nanocomposites via Engineering of the Host–Guest Interface by Phosphonic Acids. Molecules, 27(21), 7225. https://doi.org/10.3390/molecules27217225






