Research Progress of Antioxidant Nanomaterials for Acute Pancreatitis
Abstract
:1. Introduction
2. The Pathogenesis and Current Treatment Status of AP
2.1. Oxidative Stress Response
2.2. Oxidative Stress and AP
2.3. Antioxidant Drugs for AP
3. Advantages of Nanomaterials in the Treatment of AP
4. Antioxidant Nanomaterials for AP
4.1. Nano-Drug Delivery Systems Loaded with Antioxidant Drugs
4.1.1. Liposome Drug Delivery Systems
4.1.2. Dendritic Macromolecular Drug Delivery Systems
4.1.3. Micellar Drug Delivery Systems
4.1.4. Polymeric Drug Delivery Systems
4.2. Antioxidant Nanomedicines
4.2.1. Nanomedicine Particles
4.2.2. Nanozymes
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boxhoorn, L.; Voermans, R.P.; Bouwense, S.A.; Bruno, M.J.; Verdonk, R.C.; Boermeester, M.A.; van Santvoort, H.C.; Besselink, M.G. Acute pancreatitis. Lancet 2020, 396, 726–734. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Zheng, Y.W.; Bao, S.; Zhang, H.; Chen, R.; Yao, Q.; Kou, L. Drug discovery and formulation development for acute pancreatitis. Drug Deliv. 2020, 27, 1562–1580. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kim, H. Oxidative stress and inflammatory signaling in cerulein pancreatitis. World J. Gastroenterol. 2014, 20, 17324–17329. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J.; Pereda, J.; Arduini, A.; Sandoval, J.; Sabater, L.; Aparisi, L.; López-Rodas, G.; Sastre, J. Cross-talk between oxidative stress and pro-inflammatory cytokines in acute pancreatitis: A key role for protein phosphatases. Curr. Pharm. Des. 2009, 15, 3027–3042. [Google Scholar] [CrossRef]
- Hardman, J.; Shields, C.; Schofield, D.; McMahon, R.; Redmond, H.P.; Siriwardena, A.K. Intravenous antioxidant modulation of end-organ damage in L-arginine-induced experimental acute pancreatitis. Pancreatology 2005, 5, 380–386. [Google Scholar] [CrossRef]
- Kambhampati, S.; Park, W.; Habtezion, A. Pharmacologic therapy for acute pancreatitis. World J. Gastroenterol. 2014, 20, 16868–16880. [Google Scholar] [CrossRef]
- Perez, S.; Pereda, J.; Sabater, L.; Sastre, J. Redox signaling in acute pancreatitis. Redox Biol. 2015, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Wu, H.; Wang, H.B.; Zaldivar-Silva, D.; Aguero, L.; Liu, Y.F.; Zhang, Z.R.; Yin, Y.C.; Qiu, B.W.; Zhao, J.L.; et al. An injectable anti-microbial and adhesive hydrogel for the effective noncompressible visceral hemostasis and wound repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112422. [Google Scholar] [CrossRef]
- Xie, M.; Liu, X.; Wang, S. Degradation of methylene blue through Fenton-like reaction catalyzed by MoS2-doped sodium alginate/Fe hydrogel. Colloids Surf. B Biointerfaces 2022, 214, 112443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, C.; Zhang, Z.; Zhao, J.; Yuan, Y.; Wang, S. Oxidation triggered formation of polydopamine-modified carboxymethyl cellulose hydrogel for anti-recurrence of tumor. Colloids Surf. B Biointerfaces 2021, 207, 112025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, J.; Chen, Y.; Zheng, Y.; Li, J.; Zhao, J.; Zhang, J.; Liu, Y.; Liu, X.; Wang, S. MoS2-ALG-Fe/GOx hydrogel with Fenton catalytic activity for combined cancer photothermal, starvation, and chemodynamic therapy. Colloids Surf. B Biointerfaces 2020, 195, 111243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, G.; Yu, Y.; Zhang, Y.; Li, X.; Wang, S. Design of Biocompatible Chitosan/Polyaniline/Laponite Hydrogel with Photothermal Conversion Capability. Biomolecules 2022, 12, 1089. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000, 3, 234–258. [Google Scholar]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiang, H.; Li, J.; Wang, S.; Feng, T.; Cai, H.; Liu, Z.; Yuan, J.; Zhang, W.; Zhang, J.; Zhang, Z. Distribution of systemically administered nanoparticles during acute pancreatitis: Effects of particle size and disease severity. Pharmazie 2021, 76, 180–188. [Google Scholar] [CrossRef]
- Hashimoto, D.; Ohmuraya, M.; Hirota, M.; Yamamoto, A.; Suyama, K.; Ida, S.; Okumura, Y.; Takahashi, E.; Kido, H.; Araki, K.; et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J. Cell Biol. 2008, 181, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Pandol, S.J.; Saluja, A.K.; Imrie, C.W.; Banks, P.A. Acute pancreatitis: Bench to the bedside. Gastroenterology 2007, 132, 1127–1151. [Google Scholar] [CrossRef]
- Rammal, H.; Bouayed, J.; Soulimani, R. A direct relationship between aggressive behavior in the resident/intruder test and cell oxidative status in adult male mice. Eur. J. Pharmacol. 2010, 627, 173–176. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants--Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial metabolism of reactive oxygen species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef] [Green Version]
- Holzerová, E.; Prokisch, H. Mitochondria: Much ado about nothing? How dangerous is reactive oxygen species production? Int. J. Biochem. Cell Biol. 2015, 63, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Herst, P.M.; Tan, A.S.; Scarlett, D.J.; Berridge, M.V. Cell surface oxygen consumption by mitochondrial gene knockout cells. Biochim. Biophys. Acta BBA Bioenerg. 2004, 1656, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher-Wellman, K.; Bell, H.K.; Bloomer, R.J. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid. Med. Cell Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Feissner, R.F.; Skalska, J.; Gaum, W.E.; Sheu, S.S. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front. Biosci. 2009, 14, 1197–1218. [Google Scholar] [CrossRef] [Green Version]
- Criddle, D.N.; Gerasimenko, J.V.; Baumgartner, H.K.; Jaffar, M.; Voronina, S.; Sutton, R.; Petersen, O.H.; Gerasimenko, O.V. Calcium signalling and pancreatic cell death: Apoptosis or necrosis? Cell Death Differ. 2007, 14, 1285–1294. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Burgos, M.A.; Granados, M.P.; González, A.; Rosado, J.A.; Yago, M.D.; Salido, G.M.; Martínez-Victoria, E.; Mañas, M.; Pariente, J.A. Involvement of ryanodine-operated channels in tert-butylhydroperoxide-evoked Ca2+ mobilisation in pancreatic acinar cells. J. Exp. Biol. 2006, 209, 2156–2164. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.; Edwards, S.W. Stimulation of primed neutrophils by soluble immune complexes: Priming leads to enhanced intracellular Ca2+ elevations, activation of phospholipase D, and activation of the NADPH oxidase. Biochem. Biophys. Res. Commun. 1998, 247, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Eu, J.P.; Sun, J.; Xu, L.; Stamler, J.S.; Meissner, G. The skeletal muscle calcium release channel: Coupled O2 sensor and NO signaling functions. Cell 2000, 102, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Meissner, G. Regulation of mammalian ryanodine receptors. Front. Biosci. 2002, 7, d2072–d2080. [Google Scholar] [CrossRef]
- Sun, J.; Xu, L.; Eu, J.P.; Stamler, J.S.; Meissner, G. Classes of thiols that influence the activity of the skeletal muscle calcium release channel. J. Biol. Chem. 2001, 276, 15625–15630. [Google Scholar] [CrossRef] [Green Version]
- Scherer, N.M.; Deamer, D.W. Oxidative stress impairs the function of sarcoplasmic reticulum by oxidation of sulfhydryl groups in the Ca2+-ATPase. Arch. Biochem. Biophys. 1986, 246, 589–601. [Google Scholar] [CrossRef]
- Baggaley, E.M.; Elliott, A.C.; Bruce, J.I. Oxidant-induced inhibition of the plasma membrane Ca2+-ATPase in pancreatic acinar cells: Role of the mitochondria. Am. J. Physiol. Cell Physiol. 2008, 295, C1247–C1260. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.Y.; Xu, J.; Jensen, M.D.; Yu, S.; Wood, W.G.; González, F.A.; Simonyi, A.; Sun, A.Y.; Weisman, G.A. Phospholipase A2 in astrocytes: Responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol. Neurobiol. 2005, 31, 27–41. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Stafforini, D.M. Modulation of oxidative stress, inflammation, and atherosclerosis by lipoprotein-associated phospholipase A2. J. Lipid Res. 2012, 53, 1767–1782. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Dong, C.G.; Wang, G.; Jiang, H.C.; Meng, Q.H.; Li, J.; Liu, J.; Wu, L.F. Analysis of fatal risk factors for severe acute pancreatitis: A report of 141 cases. Chin. J. Surg. 2007, 45, 1619–1622. [Google Scholar] [PubMed]
- Yoon, S.O.; Yun, C.H.; Chung, A.S. Dose effect of oxidative stress on signal transduction in aging. Mech. Ageing Dev. 2002, 123, 1597–1604. [Google Scholar] [CrossRef]
- Dabrowski, A.; Boguslowicz, C.; Dabrowska, M.; Tribillo, I.; Gabryelewicz, A. Reactive oxygen species activate mitogen-activated protein kinases in pancreatic acinar cells. Pancreas 2000, 21, 376–384. [Google Scholar] [CrossRef]
- Zhou, Z.G.; Chen, Y.D. Influencing factors of pancreatic microcirculatory impairment in acute panceatitis. World J. Gastroenterol. 2002, 8, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.A.; Sugden, P.H.; Clerk, A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: Association with changes in mitochondrial membrane potential. Circ. Res. 1999, 85, 940–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T.; Enokido, Y.; Aoshima, H.; Uchiyama, Y.; Hatanaka, H. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J. Neurosci. Res. 1997, 50, 413–420. [Google Scholar] [CrossRef]
- Siriwardena, A.K.; Mason, J.M.; Balachandra, S.; Bagul, A.; Galloway, S.; Formela, L.; Hardman, J.G.; Jamdar, S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut 2007, 56, 1439–1444. [Google Scholar] [CrossRef] [Green Version]
- Buyukberber, M.; Savaş, M.C.; Bagci, C.; Koruk, M.; Gulsen, M.T.; Tutar, E.; Bilgic, T.; Ceylan, N.O. Therapeutic effect of caffeic acid phenethyl ester on cerulein-induced acute pancreatitis. World J. Gastroenterol. 2009, 15, 5181. [Google Scholar] [CrossRef] [PubMed]
- Lawinski, M.; Sledzinski, Z.; Kubasik-Juraniec, J.; Spodnik, J.H.; Wozniak, M.; Boguslawski, W. Does resveratrol prevent free radical-induced acute pancreatitis? Pancreas 2005, 31, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Al-Malki, A.L. Suppression of acute pancreatitis by L-lysine in mice. BMC Complement. Altern. Med. 2015, 15, 193. [Google Scholar] [CrossRef] [Green Version]
- Ramudo, L.; Manso, M.A.; Vicente, S.; De Dios, I. Pro- and anti-inflammatory response of acinar cells during acute pancreatitis. Effect of N-acetyl cysteine. Cytokine 2005, 32, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Sevillano, S.; De la Mano, A.M.; De Dios, I.; Ramudo, L.; Manso, M.A. Major pathological mechanisms of acute pancreatitis are prevented by N-acetylcysteine. Digestion 2003, 68, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Wu, A.R.; Shen, Y.Z. Effects of N-acetylcysteine on mRNA expression of monocyte chemotactic protein and macrophage inflammatory protein 2 in acute necrotizing pancreatitis: Experiment with rats. Chin. Med. J. 2008, 88, 711–715. [Google Scholar]
- Barlas, A.; Cevik, H.; Arbak, S.; Bangir, D.; Sener, G.; Yeğen, C.; Yeğen, B.C. Melatonin protects against pancreaticobiliary inflammation and associated remote organ injury in rats: Role of neutrophils. J. Pineal. Res. 2004, 37, 267–275. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, J.C.; Ng, C.J.; Chiu, D.F.; Chen, M.F. Melatonin reduces pancreatic prostaglandins production and protects against caerulein-induced pancreatitis in rats. J. Pineal. Res. 2006, 40, 34–39. [Google Scholar] [CrossRef]
- Sheu, S.S.; Nauduri, D.; Anders, M.W. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2006, 1762, 256–265. [Google Scholar] [CrossRef] [Green Version]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirtürk, N.; Bilensoy, E. Nanocarriers targeting the diseases of the pancreas. Eur. J. Pharm. Biopharm. 2022, 170, 10–23. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, e1901223. [Google Scholar] [CrossRef]
- Kou, L.; Sun, R.; Jiang, X.; Lin, X.; Huang, H.; Bao, S.; Zhang, Y.; Li, C.; Chen, R.; Yao, Q. Tumor Microenvironment-Responsive, Multistaged Liposome Induces Apoptosis and Ferroptosis by Amplifying Oxidative Stress for Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 30031–30043. [Google Scholar] [CrossRef]
- Bami, M.S.; Raeisi Estabragh, M.A.; Khazaeli, P.; Ohadi, M.; Dehghannoudeh, G. pH-responsive drug delivery systems as intelligent carriers for targeted drug therapy: Brief history, properties, synthesis, mechanism and application. J. Drug Deliv. Sci. Technol. 2022, 70, 102987. [Google Scholar] [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-Responsive ‘Smart’ Drug Delivery and Tumor Targeting. Trends Pharmacol. Sci. 2018, 39, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Mao, H.; Guo, M.; Li, Y.; Zhu, A.; Yang, H.; He, H.; Shen, J.; Zhou, L.; Jiang, Z.; et al. Highly efficient hierarchical micelles integrating photothermal therapy and singlet oxygen-synergized chemotherapy for cancer eradication. Theranostics 2014, 4, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Xiao, Y.; Li, W.; Yang, Q.; Tan, L.; Jia, Y.; Qu, Y.; Qian, Z. Photosensitizer Micelles Together with IDO Inhibitor Enhance Cancer Photothermal Therapy and Immunotherapy. Adv. Sci. 2018, 5, 1700891. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Safinya, C.R.; Ewert, K.K. Materials chemistry: Liposomes derived from molecular vases. Nature 2012, 489, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W. Therapeutic Potential of Heme Oxygenase-1 and Carbon Monoxide in Acute Organ Injury, Critical Illness, and Inflammatory Disorders. Antioxidants 2020, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Cottin, Y.; Zeller, M.; Vergely, C. Carbon monoxide: Mechanisms of action and potential clinical implications. Pharmacol. Ther. 2013, 137, 133–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, S.; Taguchi, K.; Sakai, H.; Yamasaki, K.; Watanabe, H.; Otagiri, M.; Maruyama, T. Carbon monoxide-bound hemoglobin vesicles ameliorate multiorgan injuries induced by severe acute pancreatitis in mice by their anti-inflammatory and antioxidant properties. Int. J. Nanomed. 2016, 11, 5611–5620. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, K.; Nagao, S.; Maeda, H.; Yanagisawa, H.; Sakai, H.; Yamasaki, K.; Wakayama, T.; Watanabe, H.; Otagiri, M.; Maruyama, T. Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity. Drug Deliv. 2018, 25, 1266–1274. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.; Wei, Y.; Wu, Q.; Zhou, K.; Liu, T.; Zhang, Y.; Jiang, N.; Xiao, W.; Chen, J.; Liu, Q.; et al. Liposomes for effective drug delivery to the ocular posterior chamber. J. Nanobiotechnol. 2019, 17, 64. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, A.; Jaafari, M.R.; Ramezanian, N.; Gholami, L.; Malaekeh-Nikouei, B. BR2 and CyLoP1 enhance in-vivo SN38 delivery using pegylated PAMAM dendrimers. Int. J. Pharm. 2019, 564, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Wang, Y.; Hao, T.; Wang, C.Y.; Wang, Q.Y.; Jiang, X.X. Efficient GSH delivery using PAMAM-GSH into MPP-induced PC12 cellular model for Parkinson’s disease. Regen. Biomater. 2016, 3, 299–307. [Google Scholar] [CrossRef] [Green Version]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournié, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimisation of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex-vivo activation of human monocytes. Chemistry 2008, 14, 4836–4850. [Google Scholar] [CrossRef]
- Marchand, P.; Griffe, L.; Poupot, M.; Turrin, C.O.; Bacquet, G.; Fournié, J.J.; Majoral, J.P.; Poupot, R.; Caminade, A.M. Dendrimers ended by non-symmetrical azadiphosphonate groups: Synthesis and immunological properties. Bioorg. Med. Chem. Lett. 2009, 19, 3963–3966. [Google Scholar] [CrossRef]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef]
- Chauhan, A.S.; Diwan, P.V.; Jain, N.K.; Tomalia, D.A. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules 2009, 10, 1195–1202. [Google Scholar] [CrossRef]
- Tang, Y.; Han, Y.; Liu, L.; Shen, W.; Zhang, H.; Wang, Y.; Cui, X.; Wang, Y.; Liu, G.; Qi, R. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromolecules 2015, 16, 174–182. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, E.F.; Rifaai, R.A.; Abdel-Latif, R.G. Empagliflozin attenuates transient cerebral ischemia/reperfusion injury in hyperglycemic rats via repressing oxidative-inflammatory-apoptotic pathway. Fundam. Clin. Pharmacol. 2020, 34, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, F.; Lu, Q.; Li, X.; Ren, D.; Zhang, J.; Han, Y.; Xiang, Y.K.; Li, J. Empagliflozin Ameliorates Obesity-Related Cardiac Dysfunction by Regulating Sestrin2-Mediated AMPK-mTOR Signaling and Redox Homeostasis in High-Fat Diet-Induced Obese Mice. Diabetes 2020, 69, 1292–1305. [Google Scholar] [CrossRef]
- Ashrafi Jigheh, Z.; Ghorbani Haghjo, A.; Argani, H.; Roshangar, L.; Rashtchizadeh, N.; Sanajou, D.; Nazari Soltan Ahmad, S.; Rashedi, J.; Dastmalchi, S.; Mesgari Abbasi, M. Empagliflozin alleviates renal inflammation and oxidative stress in streptozotocin-induced diabetic rats partly by repressing HMGB1-TLR4 receptor axis. Iran. J. Basic. Med. Sci. 2019, 22, 384–390. [Google Scholar] [CrossRef]
- Li, Q.; Cao, Q.; Yuan, Z.; Wang, M.; Chen, P.; Wu, X. A novel self-nanomicellizing system of empagliflozin for oral treatment of acute pancreatitis: An experimental study. Nanomedicine 2022, 42, 102534. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2019, 7, 489. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Li, Y.; Xie, M.B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Anchi, P.; Khurana, A.; Swain, D.; Samanthula, G.; Godugu, C. Sustained-Release Curcumin Microparticles for Effective Prophylactic Treatment of Exocrine Dysfunction of Pancreas: A Preclinical Study on Cerulein-Induced Acute Pancreatitis. J. Pharm. Sci. 2018, 107, 2869–2882. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Jiang, X.; Zhai, Y.Y.; Luo, L.Z.; Xu, H.L.; Xiao, J.; Kou, L.; Zhao, Y.Z. Protective effects and mechanisms of bilirubin nanomedicine against acute pancreatitis. J. Control. Release 2020, 322, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, S.; Lee, D.Y.; Yu, B.; Miao, W.; Jon, S. Multistimuli-Responsive Bilirubin Nanoparticles for Anticancer Therapy. Angew. Chem. Int. Ed. Engl. 2016, 55, 10676–10680. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland, M.G., 3rd; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid-bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Brannon, E.R.; Guevara, M.V.; Pacifici, N.J.; Lee, J.K.; Lewis, J.S.; Eniola-Adefeso, O. Polymeric particle-based therapies for acute inflammatory diseases. Nat. Rev. Mater. 2022, 7, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.H.; AbouSamra, M.M.; Awad, G.E.A.; Mansy, S.S. Promising bioadhesive ofloxacin-loaded polymeric nanoparticles for the treatment of ocular inflammation: Formulation and in vivo evaluation. Drug Deliv. Transl. Res. 2021, 11, 1943–1957. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ding, Y.; Fan, B.; Wang, Y.; Mao, Z.; Wang, W.; Wu, J. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J. Control. Release 2020, 321, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, S.; Ding, Q.; Fan, Q.; Dai, Y.; Guo, S.; Ye, Y.; Li, C.; Zhou, M. Recent advances in cell membrane-camouflaged nanoparticles for inflammation therapy. Drug Deliv. 2021, 28, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Timur, S.S.; Kuralay, F.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Current status of micro/nanomotors in drug delivery. J. Drug Target. 2021, 29, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Rahim, M.A.; Jan, N.; Shah, H.; Rawas-Qalaji, M.; Khan, S.; Sohail, M.; Thu, H.E.; Ramli, N.A.; Sarfraz, R.M.; et al. Cell membrane cloaked nanomedicines for bio-imaging and immunotherapy of cancer: Improved pharmacokinetics, cell internalization and anticancer efficacy. J. Control. Release 2021, 335, 130–157. [Google Scholar] [CrossRef]
- Ju, S.M.; Youn, G.S.; Cho, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates cytokine toxicity and pro-inflammatory immune responses by suppressing NF-κB activation in RINm5F beta cells. BMB Rep. 2015, 48, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yuan, Y.; Zhang, Y.; He, Q.; Xu, R.; Ge, F.; Wu, C. Celastrol inhibits IL-1β-induced inflammation in orbital fibroblasts through the suppression of NF-κB activity. Mol. Med. Rep. 2016, 14, 2799–2806. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Lee, M.H.; Nam, D.H.; Song, H.K.; Kang, Y.S.; Lee, J.E.; Kim, H.W.; Cha, J.J.; Hyun, Y.Y.; Han, S.Y.; et al. Celastrol, an NF-κB inhibitor, improves insulin resistance and attenuates renal injury in db/db mice. PLoS ONE 2013, 8, e62068. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Cao, X.; Tu, H.; Zhang, Z.R.; Deng, L. Inflammation-Targeted Delivery of Celastrol via Neutrophil Membrane-Coated Nanoparticles in the Management of Acute Pancreatitis. Mol. Pharm. 2019, 16, 1397–1405. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Arbabi, E.; Rostami, F. Coating of ferulic acid-loaded silk fibroin nanoparticles with neutrophil membranes: A promising strategy against the acute pancreatitis. Life Sci. 2021, 270, 119128. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, O.A.R.; Moawed, F.S.M.; Ahmed, E.S.A.; Ibrahim, Z.A. Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165904. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Liu, D.; Yan, X.; Wang, F.; Niu, G.; Yang, M.; Chen, X. Biomimetic RNA-silencing nanocomplexes: Overcoming multidrug resistance in cancer cells. Angew. Chem. Int. Ed. Engl. 2014, 53, 1997–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ran, X.; Lin, Y.; Ren, J.; Qu, X. Self-assembly of an organic-inorganic hybrid nanoflower as an efficient biomimetic catalyst for self-activated tandem reactions. Chem. Commun. 2015, 51, 4386–4389. [Google Scholar] [CrossRef]
- Xue, S.; Schlosburg, J.E.; Janda, K.D. A New Strategy for Smoking Cessation: Characterization of a Bacterial Enzyme for the Degradation of Nicotine. J. Am. Chem. Soc. 2015, 137, 10136–10139. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Polypyrrole nanoparticles as promising enzyme mimics for sensitive hydrogen peroxide detection. Chem. Commun. 2014, 50, 3030–3032. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Zhao, X.; Wu, J.; Muhammad, F.; Lin, S.; He, J.; Zhou, L.; Zhang, C.; Deng, Y.; et al. Surface-Enhanced Raman Scattering Active Gold Nanoparticles with Enzyme-Mimicking Activities for Measuring Glucose and Lactate in Living Tissues. ACS Nano 2017, 11, 5558–5566. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chemistry 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, J.; Gao, W.; Chen, J.; Hu, B.; Cai, X.; Zheng, Y. Prussian blue nanozyme-mediated nanoscavenger ameliorates acute pancreatitis via inhibiting TLRs/NF-kappaB signaling pathway. Theranostics 2021, 11, 3213–3228. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, J.; Yang, X.; Pan, C.; Chen, W.; Wang, M.; Yu, P.; Mao, L.; Li, Y. A single-atom Fe-N(4) catalytic site mimicking bifunctional antioxidative enzymes for oxidative stress cytoprotection. Chem. Commun. 2018, 55, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Gao, W.; Cai, X.; Xu, J.; Zou, D.; Li, Z.; Hu, B.; Zheng, Y. Nanozyme-mediated catalytic nanotherapy for inflammatory bowel disease. Theranostics 2019, 9, 2843–2855. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Zhang, L.; Shen, H.; Wu, H.; Zhao, J.; Wang, S.; Hu, L. Biodegradable MoSe2-polyvinylpyrrolidone nanoparticles with multi-enzyme activity for ameliorating acute pancreatitis. J. Nanobiotechnol. 2022, 20, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xie, P.; Wu, H.; Zhao, J.; Wang, S. 2D MoSe2@PVP nanosheets with multi-enzyme activity alleviate the acute pancreatitis via scavenging the reactive oxygen and nitrogen species. Chem. Eng. J. 2022, 446, 136792. [Google Scholar] [CrossRef]
- Khurana, A.; Anchi, P.; Allawadhi, P.; Kumar, V.; Sayed, N.; Packirisamy, G.; Godugu, C. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation. Nanomedicine 2019, 18, 54–65. [Google Scholar] [CrossRef]
- Khurana, A.; Anchi, P.; Allawadhi, P.; Kumar, V.; Sayed, N.; Packirisamy, G.; Godugu, C. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine 2019, 14, 1805–1825. [Google Scholar] [CrossRef]
- Abdel-Hakeem, E.A.; Abdel-Hamid, H.A.; Abdel Hafez, S.M.N. The possible protective effect of Nano-Selenium on the endocrine and exocrine pancreatic functions in a rat model of acute pancreatitis. J. Trace Elem. Med. Biol. 2020, 60, 126480. [Google Scholar] [CrossRef]
- Fan, J.J.; Mei, Q.X.; Deng, G.Y.; Huang, Z.H.; Fu, Y.; Hu, J.H.; Huang, C.L.; Lu, Y.Y.; Lu, L.G.; Wang, X.P.; et al. Porous SiO(2)-coated ultrasmall selenium particles nanospheres attenuate cerulein-induce acute pancreatitis in mice by downregulating oxidative stress. J. Dig. Dis. 2021, 22, 363–372. [Google Scholar] [CrossRef]

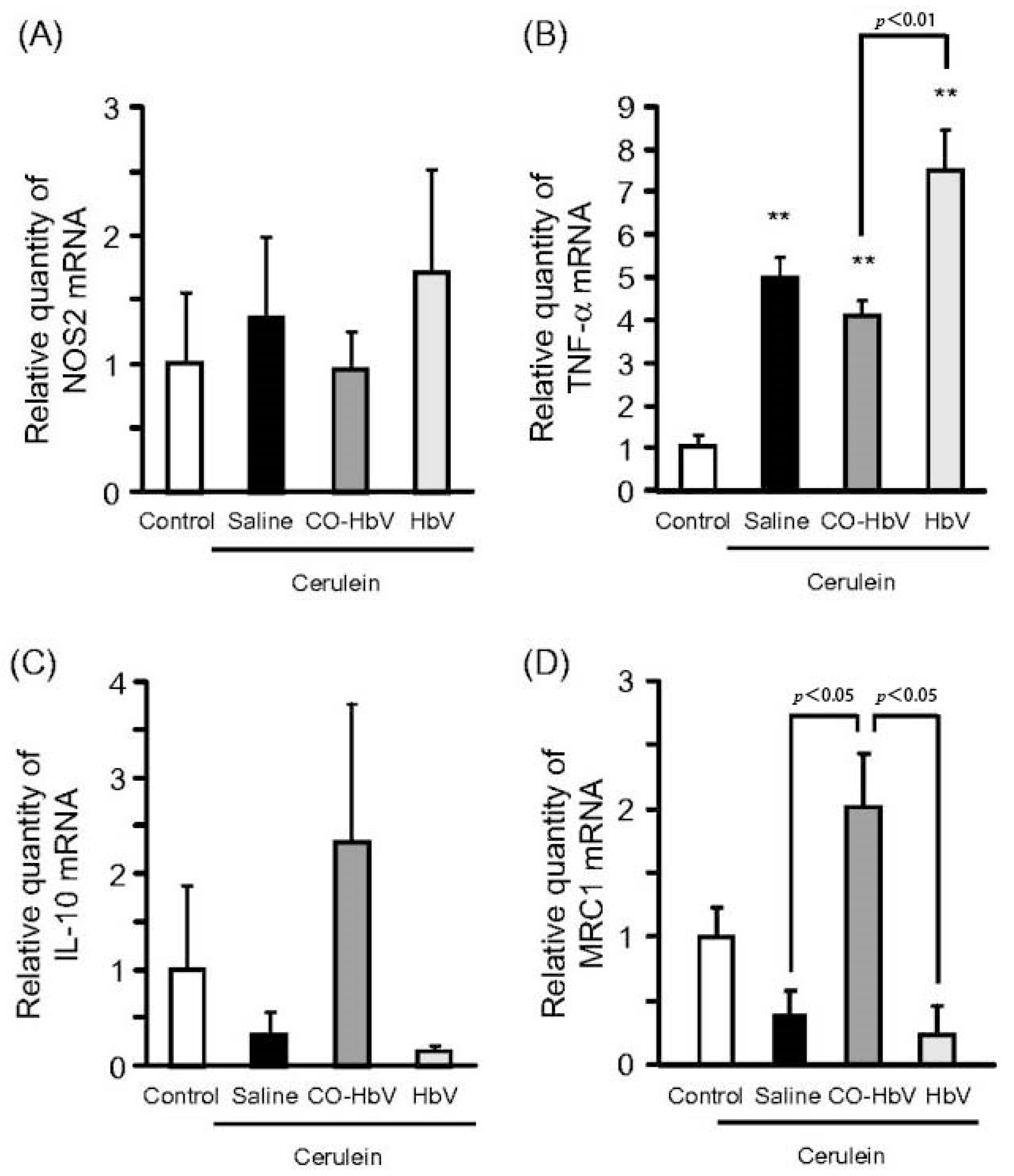
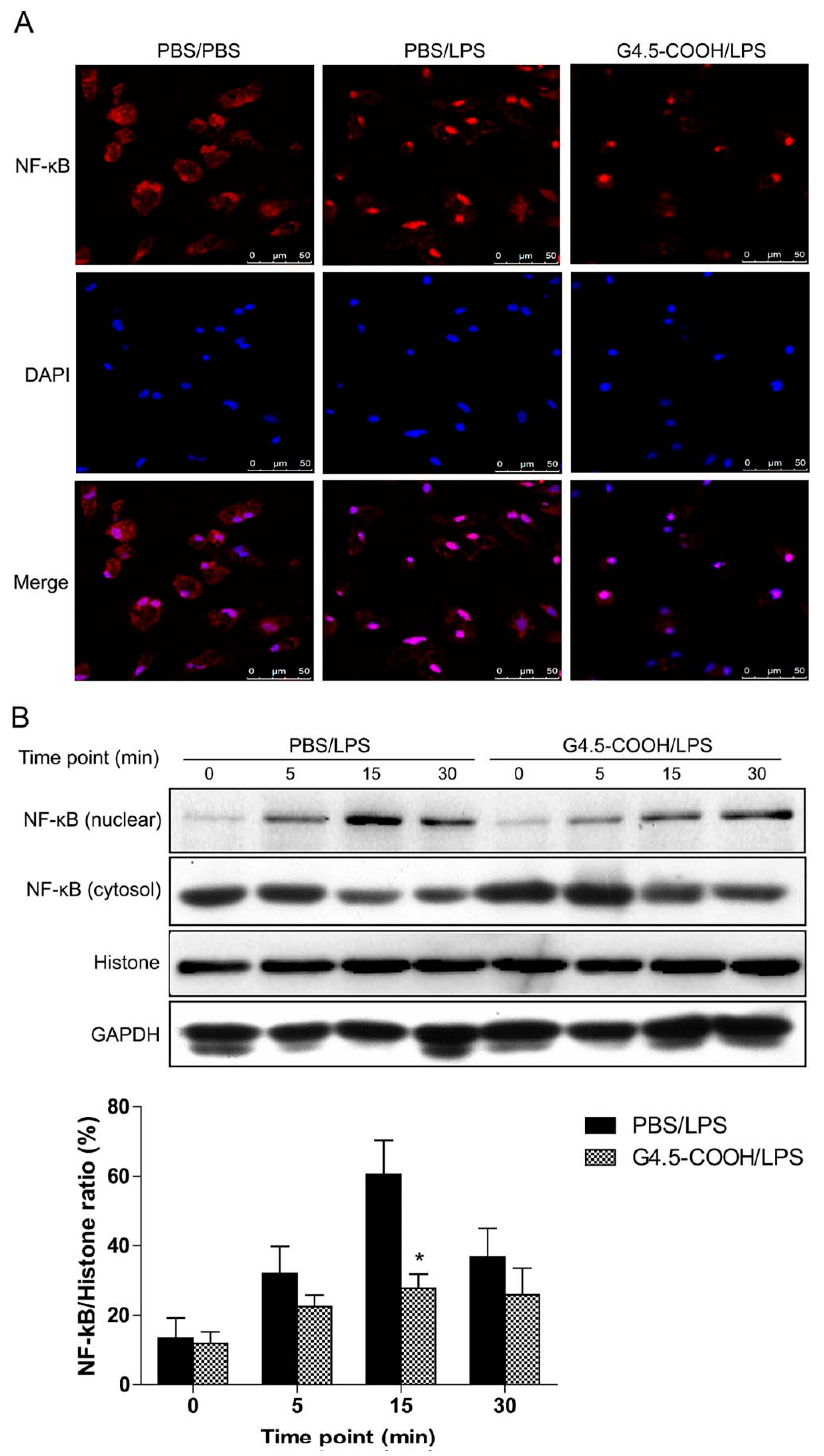
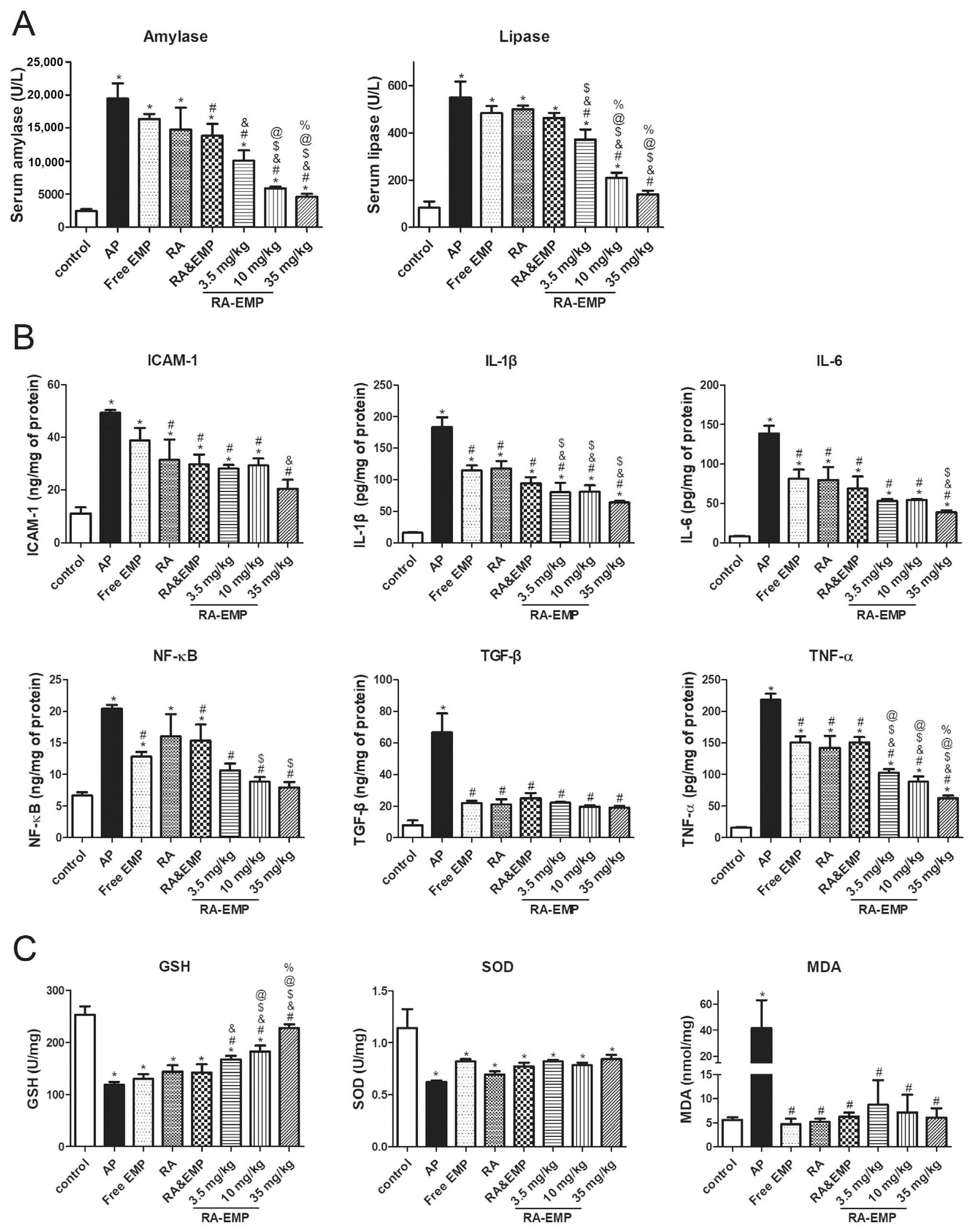
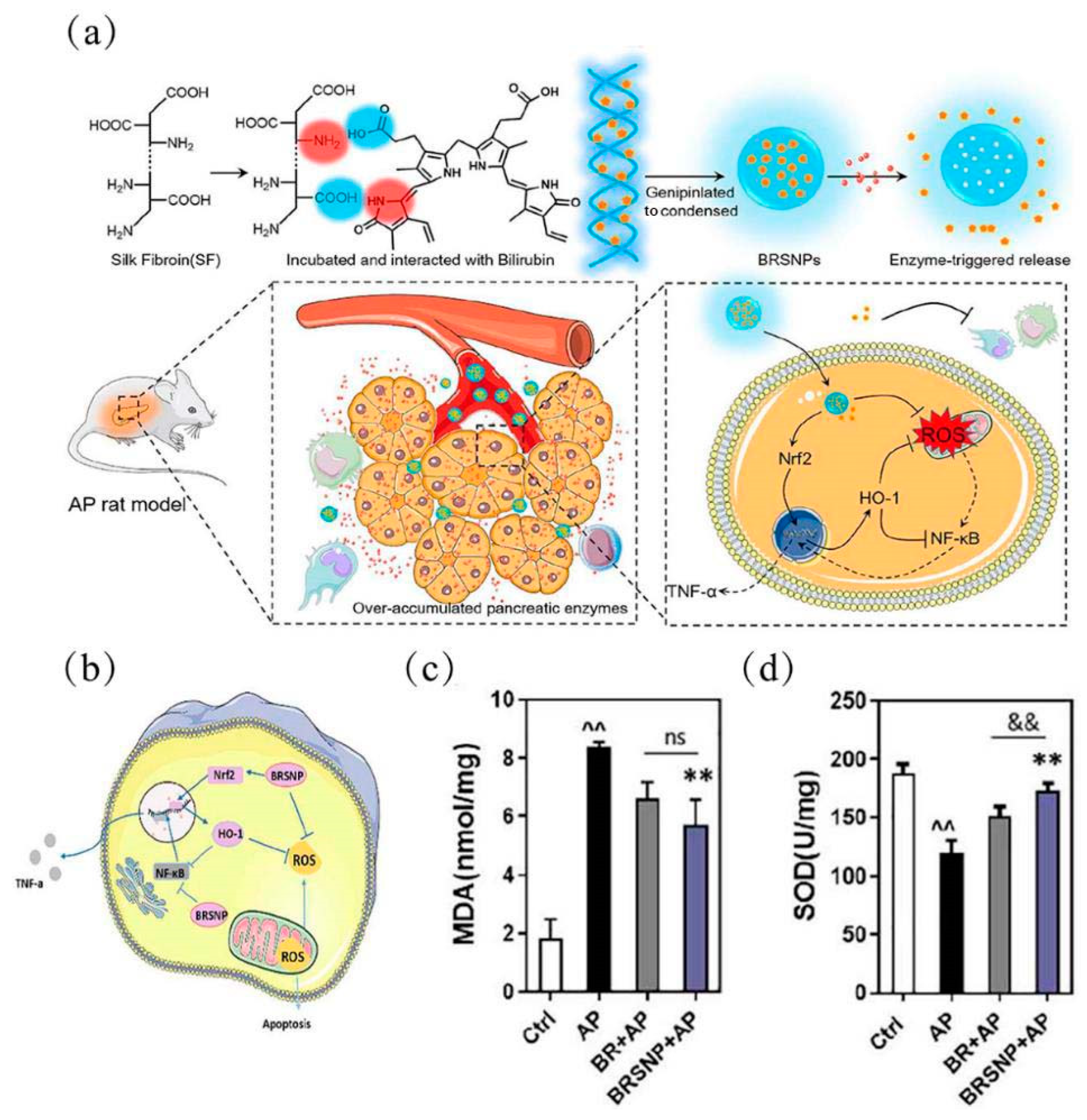
| Nanocarrier | Drug | Characteristics | Ref. |
|---|---|---|---|
| liposomes | Carbon monoxide (CO) | A by-product of inducible heme oxygenase-1, which effectively regulates inflammation and oxidative stress | [69] |
| liposomes | Carbon monoxide (CO) | It has powerful cytoprotective effects and multiple biological functions, such as antioxidant and anti-apoptotic effects, while inhibiting the expression of pro-inflammatory cytokines and increasing the production of anti-inflammatory cytokines | [70] |
| Dendrimers | G4.5-COOH; G5-OH | Multiple biological activities, high drug loading rate, high biocompatibility, low cytotoxicity and hemolysis, low protein interactions | [78] |
| Micellar | Empagliflozin (EMP) | Anti-oxidative stress and anti-inflammatory effects involve modulation associated with high mobility group box 1 signaling | [83] |
| Polymeric (PLGA) | Curcumin (CUR) | A polyphenol derivative from the traditional Indian plant turmeric (Curcuma longa), Cur enhances NF-κB pathway, c-Jun N-terminal ki-nases, and other related signaling pathways exert their anti-inflammatory, anti-oxidative stress | [87] |
| Polymeric (SF) | Bilirubin | Endogenous antioxidant compounds derived from the catabolism of heme, and low levels of bilirubin in tissues can adequately scavenge ROS and reduce intracellular oxidative stress | [88] |
| Polymeric (PEG-PLGA) | Celastrol (CLT) | A compound isolated from the roots of the thunder god vine with a variety of activities including NF-κB inhibition, anti-inflammatory, antioxidant, and anti-cancer | [100] |
| Polymeric (SF) | Ferulic acid (FA) | A multifunctional bioflavonoid that is more effective in scavenging free radicals, stimulating cytoprotective enzymes or the immune system | [101] |
| Types and Sizes of Nanomaterials | Animal Model | Effects and Cellular Mechanisms | Ref. |
|---|---|---|---|
| liposomes (CO-HbV) (280 nm) | CDE (choline-deficient ethionine supplemented); mice | Reduction of TNF-α and IL-1β, neutrophil infiltration, MPO, and NO2-Tyr (anti-oxidative stress; anti-inflammatory) | [69] |
| liposomes (CO-HbV) (280 nm) | Caerulein; mice | Transformation of macrophage phenotype, reduction of HMGB1, MPO, and NO2-Tyr levels, and reduction of neutrophil infiltration (anti-oxidative stress; anti-inflammatory) | [70] |
| Dendrimers (G4.5-COOH; G5-OH) (5 nm) | Caerulein; mice | Inhibits nuclear translocation of NF-κB and reduces the expression of inflammatory factors such as IL-1β and IL-6 and infiltration of macrophages (anti-inflammatory) | [78] |
| Micellar (RA-EMP) (4.703 ± 0.114 nm) | L-Arginine; mice | Decrease the expression of GSH, SOD, IL-6, IL-1β, and TGF-β in serum and increase the expression of MDA (anti-oxidative stress; anti-inflammatory) | [83] |
| Polymeric (CuMPs) (14.34 ± 5.98 µm) | Caerulein; mice | Increases GSH and Nrf-2, decreases serum amylase and lipase and levels of inflammatory factors such as IL-1β (anti-oxidative stress; anti- nitrosative stress; anti-inflammatory) | [87] |
| Polymeric (BRSNPs) (268 ± 6 nm) | L-Arginine; rat | Inhibition of NF-κB pathway, activation of Nrf2/HO-1 pathway, reduction of pro-inflammatory cytokines, and inflammatory cell infiltration (anti-oxidative stress; anti-inflammatory) | [88] |
| Polymeric (PEG-PLGA/CLT) (150 nm) | Injection of 3% sodium taurine into the pancreatic duct after aseptic dissection; rat | Reduces serum amylase and MPO levels inhibit the NF-κB pathway and reduce expression of inflammatory cytokines (anti-oxidative stress; anti-inflammatory) | [100] |
| Polymeric (FA-SF-NPs) (186 nm) | Biliopancreatic duct ligation; rats | Decreased expression levels of SOD, GPx, GSSH/GSSG, IL-β, and TNF-a and increased expression levels of MDA (anti-oxidative stress; anti-inflammatory) | [101] |
| Particles (CA-NPs) (50–90 nm) | L-Arginine and gamma radiation; rats | Downregulation of NLRP3, NF-κB, and ASK1/MAPK signaling pathways; decreased caspase-3 expression levels (anti-oxidative stress; anti-inflammatory) | [102] |
| Nanoenzymes (PBzyme) (60 nm) | Caerulein; mice | Reduced MDA levels, increased SOD and GSH levels, and inhibited TLRs/NF-κB signaling pathway (anti-oxidative stress; anti-inflammatory) | [111] |
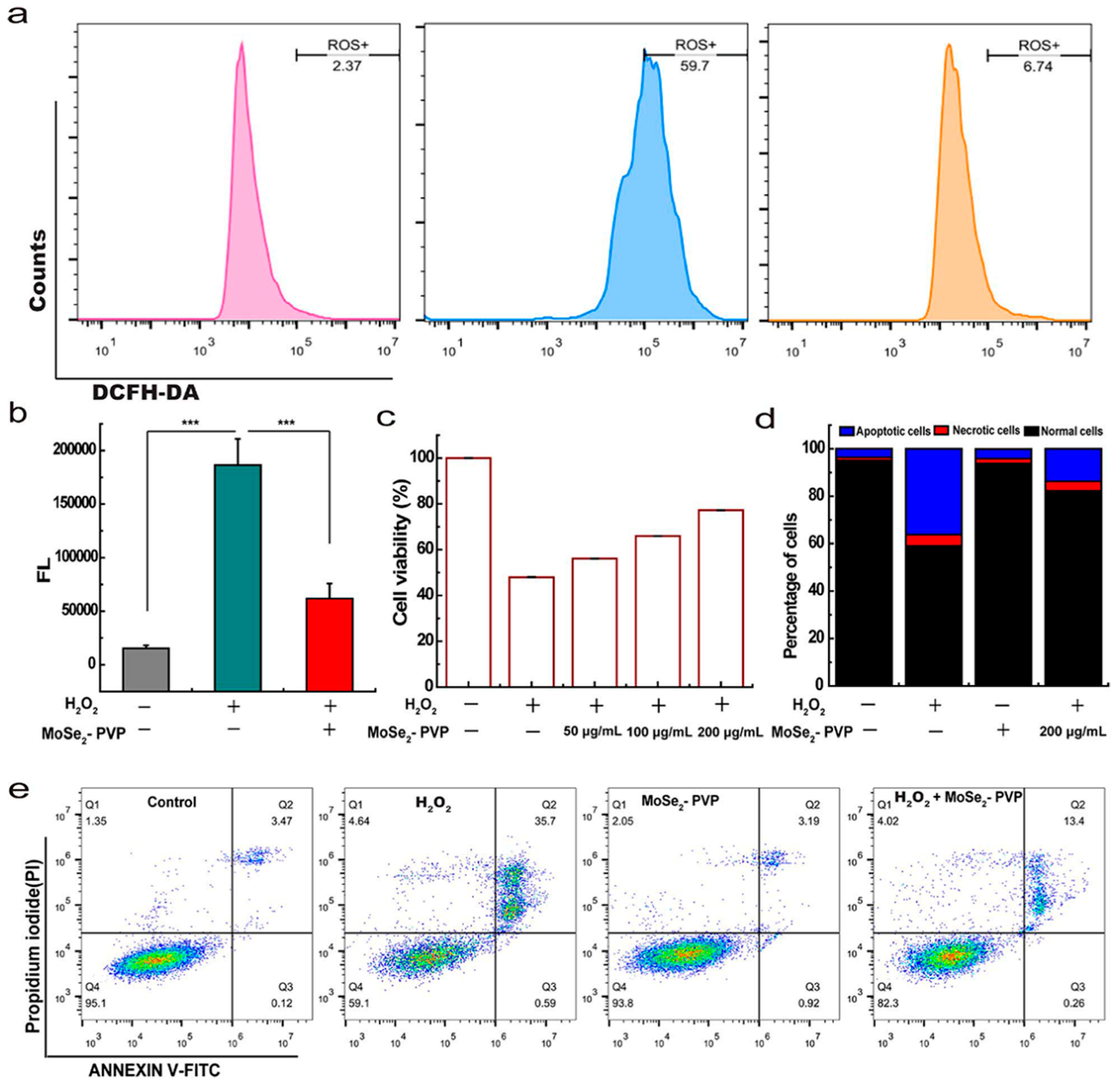
| Nanoenzymes (MoSe2-PVP NPs) (119.39 ± 13.94 nm) | Caerulein; mice | Mimics the activity of CAT, SOD, POD, and GPx eliminates multiple ROS, reduces serum amylase levels and inflammatory factors (anti-oxidative stress; anti-inflammatory) | [115] |
| Nanoenzymes (MoSe2@PVP NSs) (86.278 ± 11.82 nm) | Caerulein; mice | Mimics the activity of CAT, SOD, POD, and GPx eliminates ROS and RNS, reduces serum amylase levels, IL-6, IL-1β, and TGF-β levels (anti-oxidative stress; anti-inflammatory) | [116] |
| Inorganic (NYs) (159.2 ± 7.5 nm) | Caerulein; mice | Mimics CAT and SOD activity, inhibits inflammatory cell infiltration, regulates Nrf2/NF-κB pathway, and restores mitochondrial and endoplasmic reticulum homeostasis (anti-oxidative stress; anti-inflammatory) | [117] |
| Inorganic (NC) (82 ± 5.4 nm) | Caerulein; mice | mimicking CAT and SOD activities, decreasing the levels of p65-NF-κB, Hsp27, and Hsp70, and upregulating the expression of Nrf2, SOD1, and NQO1 (anti-oxidative stress; anti-inflammatory) | [118] |
| Inorganic (nano-Se) (20–60 nm) | L-Arginine; rat | Inhibition of NF-kB pathway and reduction of serum MDA, IL-1β, IL-6, and TNF-a expression levels (anti-oxidative stress; anti-inflammatory) | [119] |
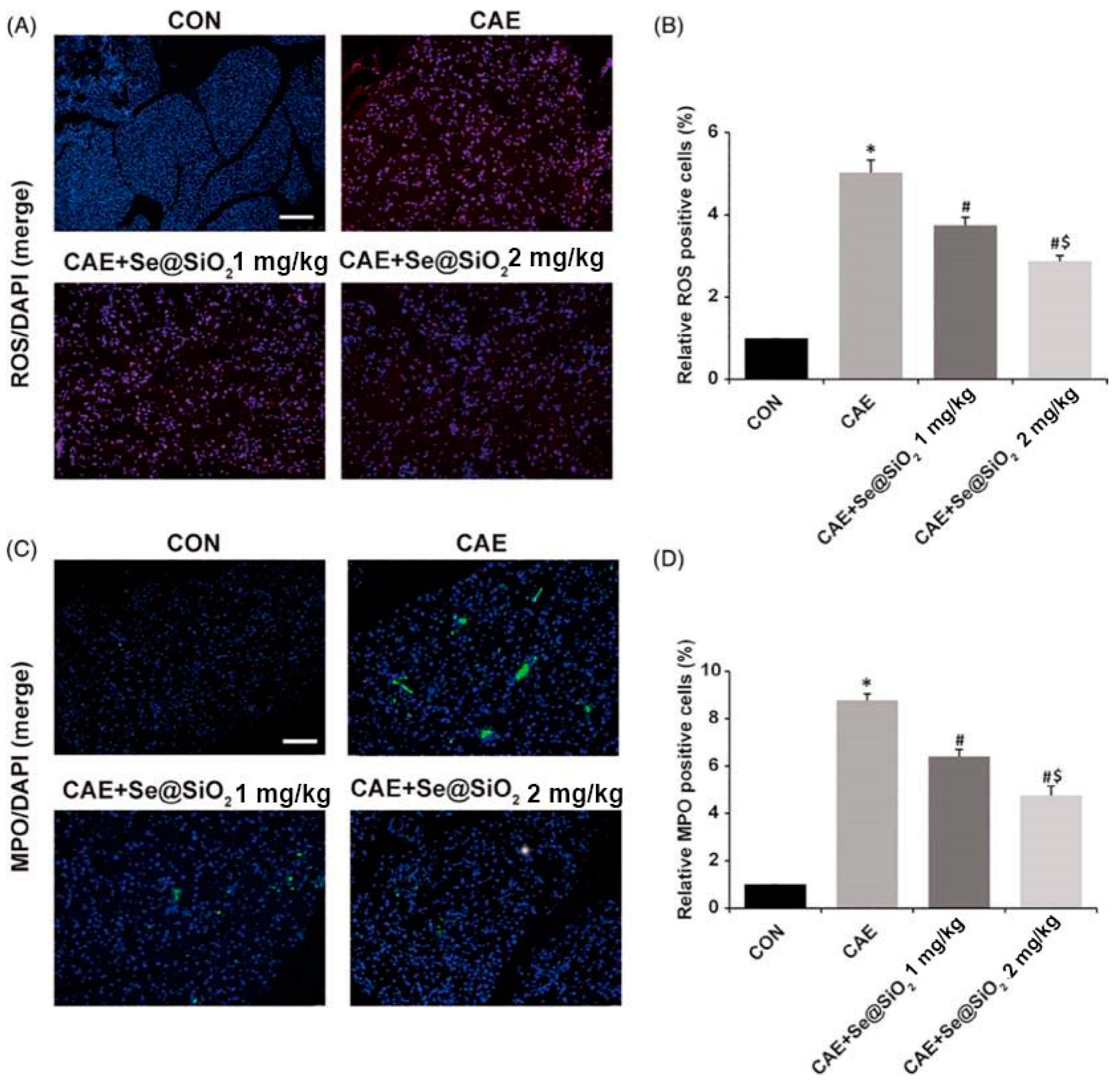
| Inorganic (Se@SiO2) (50 nm) | Caerulein; mice | Targeting TLR4/Myd88/p-p65 and NQO1/Nrf2/HO-1 pathways to reduce ROS, MPO, and MDA levels and increase GSH and SOD levels (anti-oxidative stress; anti-inflammatory) | [120] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Zhao, J.; Wang, S.; Hu, L. Research Progress of Antioxidant Nanomaterials for Acute Pancreatitis. Molecules 2022, 27, 7238. https://doi.org/10.3390/molecules27217238
Zheng X, Zhao J, Wang S, Hu L. Research Progress of Antioxidant Nanomaterials for Acute Pancreatitis. Molecules. 2022; 27(21):7238. https://doi.org/10.3390/molecules27217238
Chicago/Turabian StyleZheng, Xiaoyi, Jiulong Zhao, Shige Wang, and Lianghao Hu. 2022. "Research Progress of Antioxidant Nanomaterials for Acute Pancreatitis" Molecules 27, no. 21: 7238. https://doi.org/10.3390/molecules27217238







