Development of Galloyl Antioxidant for Dispersed and Bulk Oils through Incorporation of Branched Phytol Chain
Abstract
:1. Introduction
2. Results and Discussion
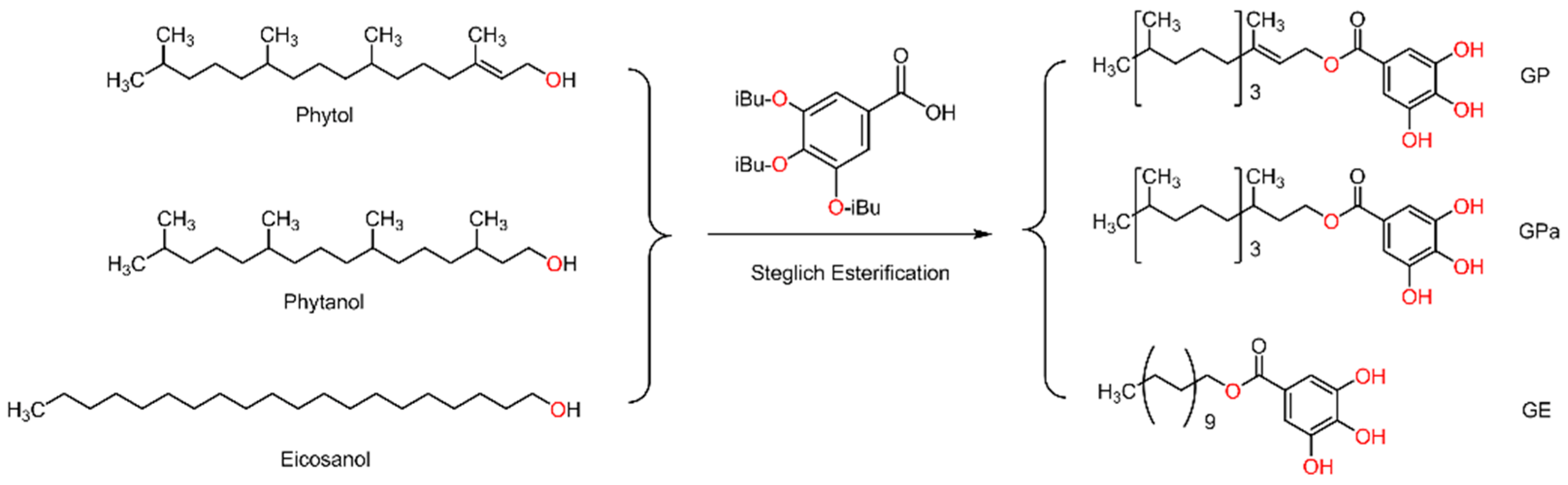
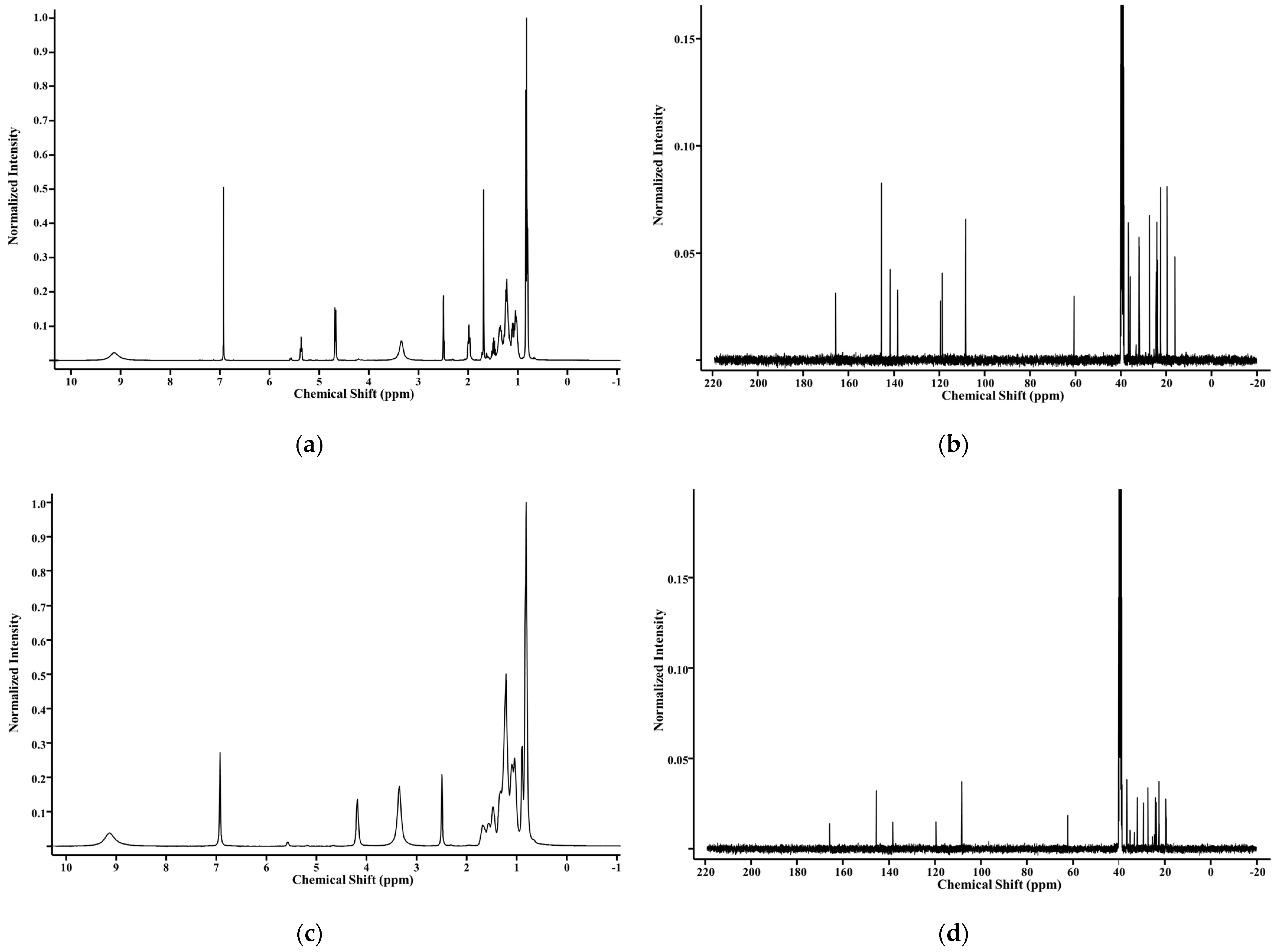
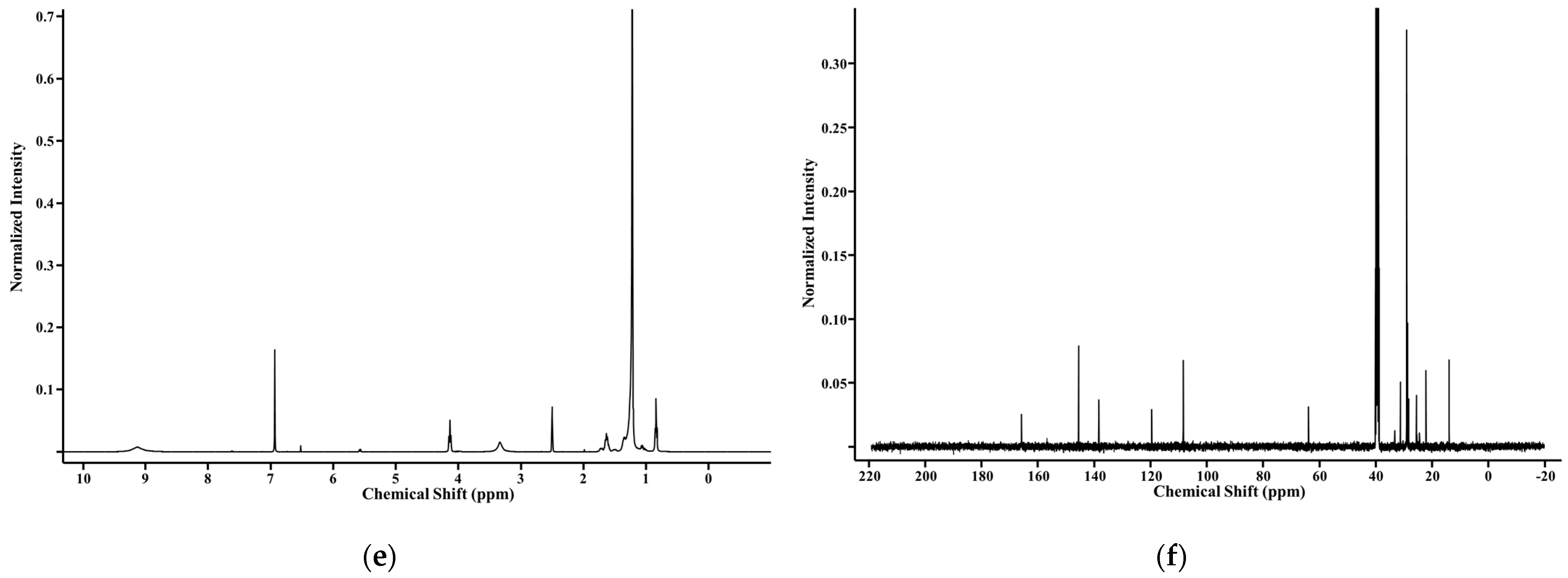
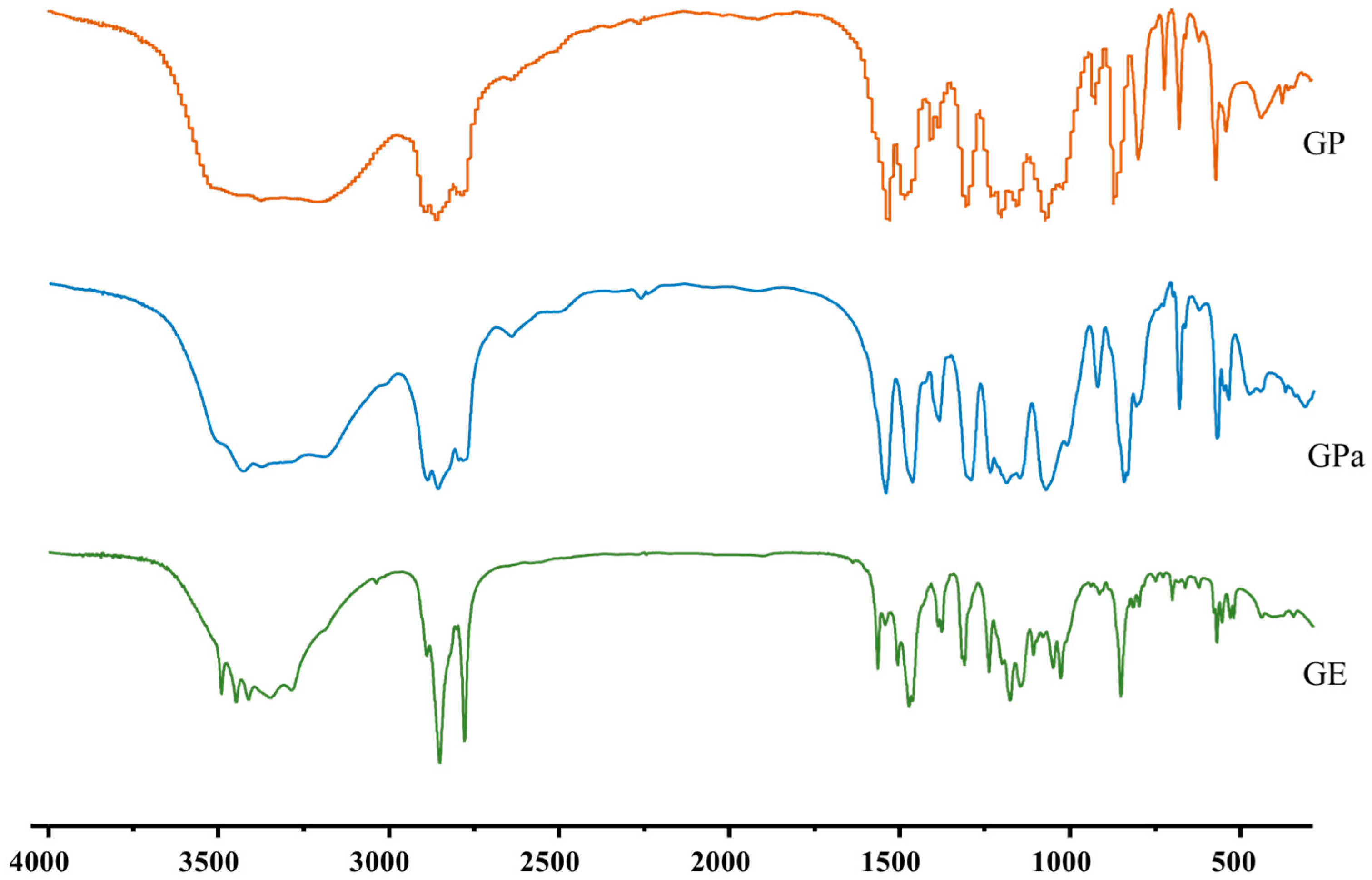
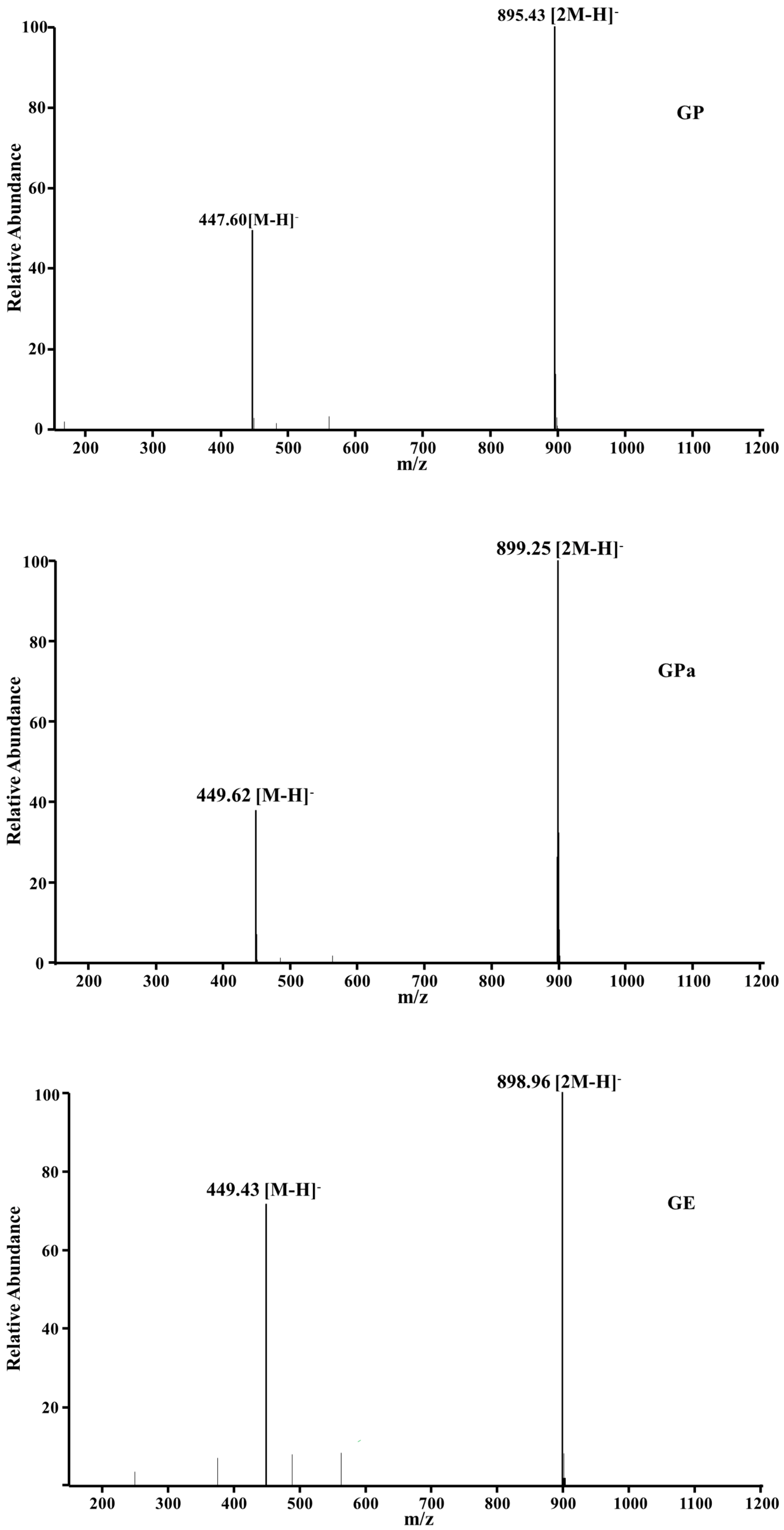
2.1. Synthesis and Structural Analysis of Galloyl Phytol (GP), Galloyl Phytanol (GPa), and Galloyl Eicosanol (GE)
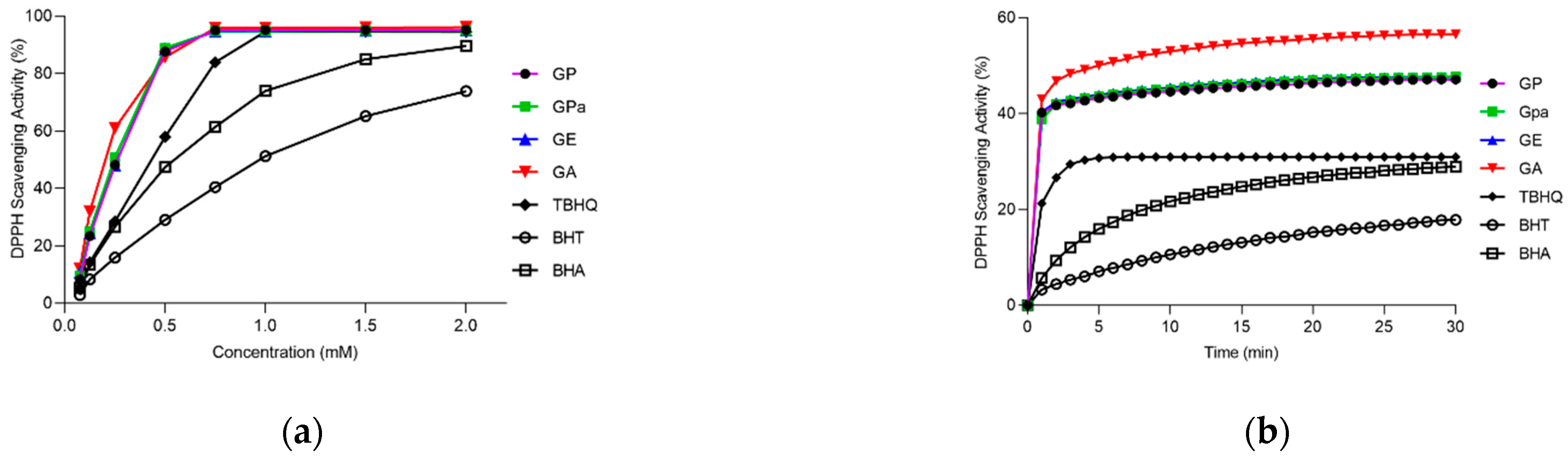
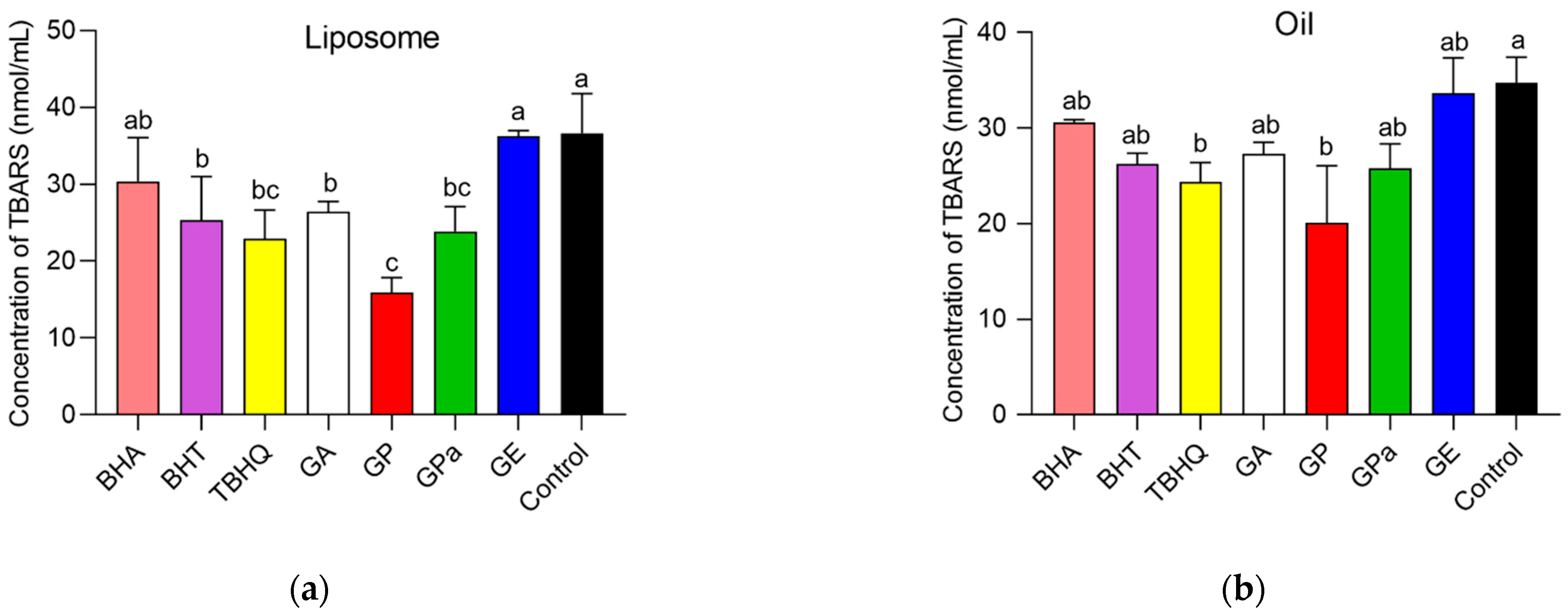
2.2. Antioxidant Activities of GP, GPa, and GE
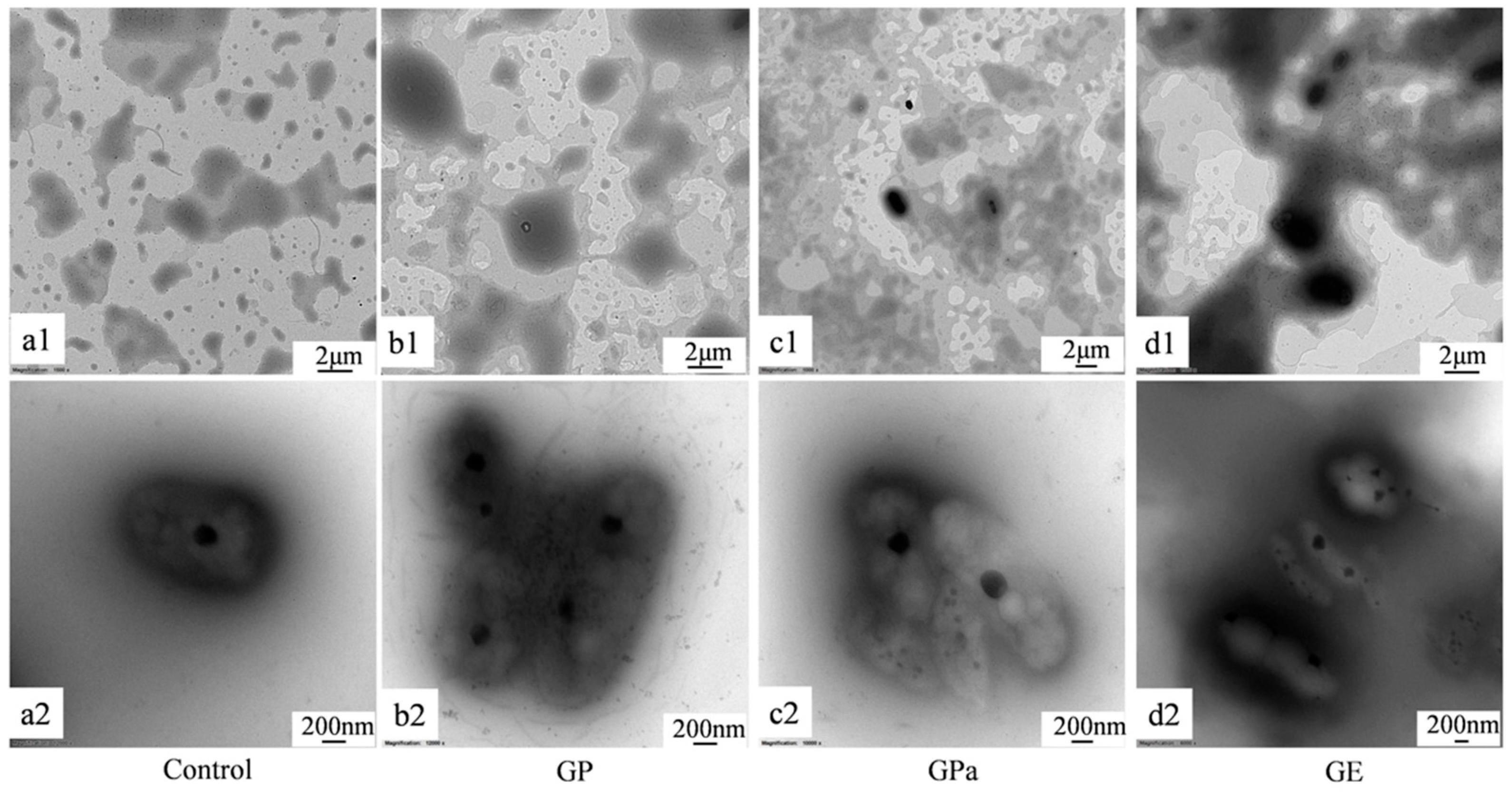
2.3. Antioxidant Activities of GP, GPa, and GE
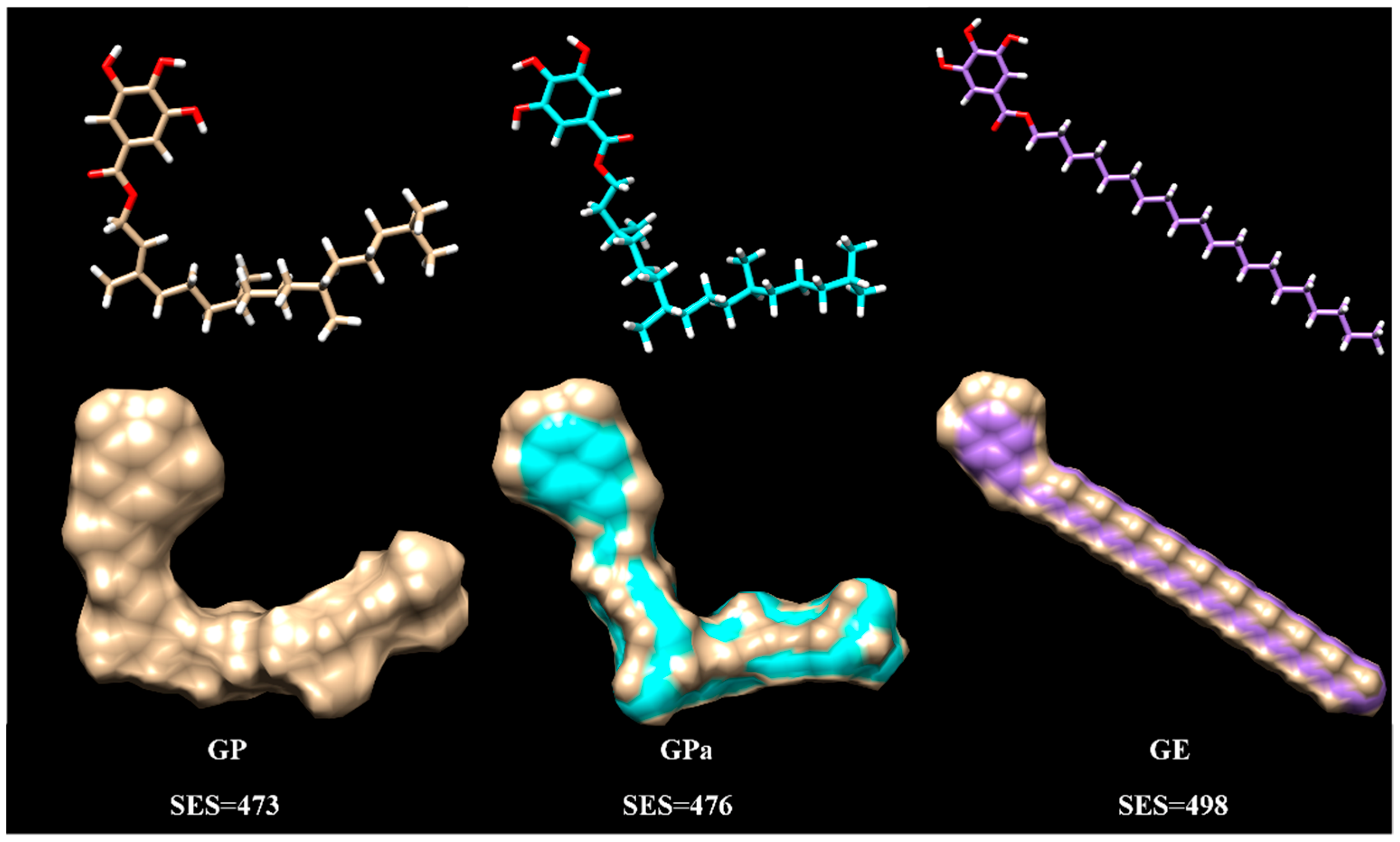
2.4. Rationalization of Antioxidant Activity Using Molecular Modeling
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Galloyl Phytol, Galloyl Eicosanol, and Galloyl Phytanol
3.3. Structural Determination
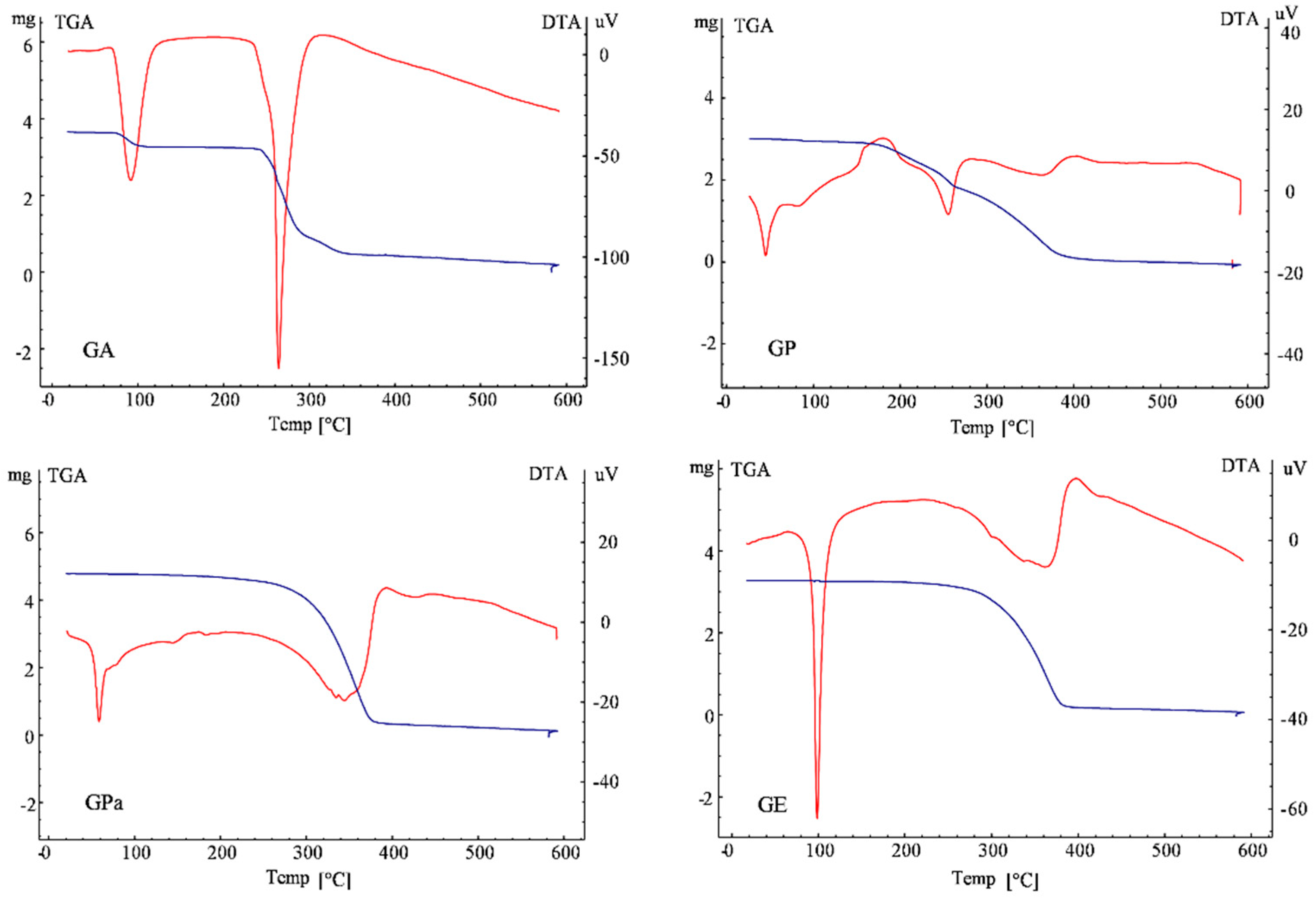
3.4. Thermal Gravimetry/Differential Thermal Analysis (TG/DTA) of Gallic Acid Esters
3.5. DPPH Scavenging Activity Assay
3.6. Inhibition of Lipid Oxidation in a Liposomal Model System
3.7. Inhibition of Lipid Oxidation in Oil
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini-Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Letizia, C.S.; Api, A.M. Fragrance material review on phytol. Food Chem. Toxicol. 2010, 48, S59–S63. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Vetter, W. Phytol and Phytyl Fatty Acid Esters: Occurrence, Concentrations, and Relevance. Eur. J. Lipid Sci. Technol. 2018, 120, 1700387. [Google Scholar] [CrossRef]
- Valentin, H.E.; Lincoln, K.; Moshiri, F.; Jensen, P.K.; Qi, Q.G.; Venkatesh, T.V.; Karunanandaa, B.; Baszis, S.R.; Norris, S.R.; Savidge, B.; et al. The Arabidopsis vitamin E pathway gene5-1 mutant reveals a critical role for phytol kinase in seed tocopherol biosynthesis. Plant Cell 2006, 18, 212–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- vom Dorp, K.; Holzl, G.; Plohmann, C.; Eisenhut, M.; Abraham, M.; Weber, A.P.M.; Hanson, A.D.; Dormann, P. Remobilization of Phytol from Chlorophyll Degradation Is Essential for Tocopherol Synthesis and Growth of Arabidopsis. Plant Cell 2015, 27, 2846–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, H.; Ohno, R.; Shibata, M.; Ikegami, I.; Onai, K.; Ohto, M.; Takamiya, K. Inactivation and deficiency of core proteins of photosystems I and II caused by genetical phylloquinone and plastoquinone deficiency but retained lamellar structure in a T-DNA mutant of Arabidopsis. Plant J. 2005, 41, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Yuan, Y.; Lu, D.F.; Du, B.W.; Xiong, L.; Shi, J.G.; Yang, L.J.; Liu, W.L.; Yuan, X.H.; Zhang, G.L.; et al. Two natural products, trans-phytol and (22E)-ergosta-6,9,22-triene-3 beta,5 alpha,8 alpha-triol, inhibit the biosynthesis of estrogen in human ovarian granulosa cells by aromatase (CYP19). Toxicol. Appl. Pharm. 2014, 279, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New Perspectives on the Efficacy of Gallic Acid in Cosmetics & Nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar] [PubMed]
- Weng, Y.P.; Hung, P.F.; Ku, W.Y.; Chang, C.Y.; Wu, B.H.; Wu, M.H.; Yao, J.Y.; Yang, J.R.; Lee, C.H. The inhibitory activity of gallic acid against DNA methylation: Application of gallic acid on epigenetic therapy of human cancers. Oncotarget 2018, 9, 361–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Z.; Li, Z.L.; Ma, Y.T.; Liu, Y.; Lin, C.C.; Li, S.M.; Zhan, J.F.; Ho, C.T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Guo, C.; Lu, T.; Ding, X.Y.; Zhao, M.T.; Zhang, M.; Liu, H.L.; Song, L.; Zhou, D.Y. Effects of gallic acid alkyl esters and their combinations with other antioxidants on oxidative stability of DHA algae oil. Food Res. Int. 2021, 143, 110280. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Wu, Z.X.; Xie, H.K.; Li, A.; Wang, Y.F.; Song, L.; Zhou, D.Y.; Wang, T. Improving the oxidative stability of flaxseed oil with composite antioxidants comprising gallic acid alkyl ester with appropriate chain length. LWT-Food Sci. Technol. 2021, 138, 110763. [Google Scholar] [CrossRef]
- Wang, P.F.; Liu, S.B. Development of a Gemini Interfacial Antioxidant for Oil in Water Emulsion with Gallic Acid and Dodecyl Gemini Chains. J. Agric. Food Chem. 2020, 68, 9953–9960. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Ye, K.; Shu, T.; Tang, X.W.; Wang, X.J.; Liu, S.B. Enhancement of Galloylation Efficacy of Stigmasterol and beta-Sitosterol Followed by Evaluation of Cholesterol-Reducing Activity. J. Agric. Food Chem. 2019, 67, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contrerasguzman, E.S.; Strong, F.C. Determination of Tocopherols (Vitamin-E) by Reduction of Cupric Ion. J. Assoc. Off. Anal. Chem. 1982, 65, 1215–1221. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera-A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Charsley, E.L.; Laye, P.G.; Palakollu, V.; Rooney, J.J.; Joseph, B. DSC studies on organic melting point temperature standards. Thermochim. Acta 2006, 446, 29–32. [Google Scholar] [CrossRef]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; De Amicis, C.V.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Sanchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Liu, S.B.; Hu, H.Y.; Li, C. Antioxidant activities of novel galloyl phytosterols evaluated by human erythrocytes with the aid of confocal microscopy imaging. J. Funct. Foods 2016, 22, 224–231. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT-Food Sci. Technol. 2006, 39, 191–198. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, H.; Yan, F.; Wang, J.; Liu, S. Development of Galloyl Antioxidant for Dispersed and Bulk Oils through Incorporation of Branched Phytol Chain. Molecules 2022, 27, 7301. https://doi.org/10.3390/molecules27217301
Wang S, Wang H, Yan F, Wang J, Liu S. Development of Galloyl Antioxidant for Dispersed and Bulk Oils through Incorporation of Branched Phytol Chain. Molecules. 2022; 27(21):7301. https://doi.org/10.3390/molecules27217301
Chicago/Turabian StyleWang, Shanshan, Hua Wang, Fujie Yan, Jie Wang, and Songbai Liu. 2022. "Development of Galloyl Antioxidant for Dispersed and Bulk Oils through Incorporation of Branched Phytol Chain" Molecules 27, no. 21: 7301. https://doi.org/10.3390/molecules27217301





