Abstract
Magnolia champaca (L.) Baill. ex Pierre of family Magnoliaceae, is a perennial tree with aromatic, ethnobotanical, and medicinal uses. The M. champaca leaf is reported to have a myriad of therapeutic activities, however, there are limited reports available on the chemical composition of the leaf essential oil of M. champaca. The present study explored the variation in the yield and chemical composition of leaf essential oil isolated from 52 accessions of M. champaca. Through hydrodistillation, essential oil yield was obtained, varied in the range of 0.06 ± 0.003% and 0.31 ± 0.015% (v/w) on a fresh weight basis. GC-MS analysis identified a total of 65 phytoconstituents accounting for 90.23 to 98.90% of the total oil. Sesquiterpene hydrocarbons (52.83 to 65.63%) constituted the major fraction followed by sesquiterpene alcohols (14.71 to 22.45%). The essential oils were found to be rich in β-elemene (6.64 to 38.80%), γ-muurolene (4.63 to 22.50%), and β-caryophyllene (1.10 to 20.74%). Chemometrics analyses such as PCA, PLS-DA, sPLS-DA, and cluster analyses such as hierarchical clustering, i.e., dendrogram and partitional clustering, i.e., K-means classified the essential oils of M. champaca populations into three different chemotypes: chemotype I (β-elemene), chemotype II (γ-muurolene) and chemotype III (β-caryophyllene). The chemical polymorphism analyzed in the studied populations would facilitate the selection of chemotypes with specific compounds. The chemotypes identified in the M. champaca populations could be developed as promising bio-resources for conservation and pharmaceutical application and further improvement of the taxa.
1. Introduction
Essential oils are volatile organic compounds with a strong aroma. These are produced by aromatic plants as secondary metabolites to protect against microbes, fungi, viruses, and insects. The essential oils attract pollinators to disperse pollens and seeds and also repel insignificant others [1]. Essential oils (EOs) possess various bioactivities, i.e., antimicrobial, anti-inflammatory, antioxidant, etc., which make them a valuable commercial product in the pharmaceutical, cosmetic, food, and beverages industries [2,3]. As evidenced by literature survey, there is growing research interest in essential oils of various aromatic plants and their bioactive components [4,5,6]. An essential oil’s biological activity might result from the presence of only one active ingredient or the synergistic interactions of numerous different molecules [5]. Numerous researches have demonstrated the connection between essential oil variations and their bioactivities [2,7]. The yield and quality of essential oils are greatly influenced by genetic makeup, agronomic practices, plant age, climate, soil type, and composition [8,9,10]. Edaphic and abiotic factors affect the biosynthetic pathways of the volatile compounds, thereby leading to the development of chemotypes in a single species [11]. Furthermore, understanding the diversity of EOs would also make it easier to market certain chemotypes for use in food or phytopharmaceutical utilizations as well as for breeding better genotypes [3,12]. Therefore, a qualitative and quantitative analysis of EOs collected from different geographical regions would necessitate developing a promising bioresource for industrial purposes.
Magnolia champaca (L.) Baill. ex Pierre (syn. Michelia champaca L.), commonly known as Champak/Swarna Champa/Golden Champa, is a tall and magnificent evergreen tree belonging to the family Magnoliaceae. It is well known for its soothing and beautiful aromatic flowers, which help in relaxation. Geographically, Magnolia champaca is distributed throughout tropical and sub-tropical regions of Asia covering India, Nepal, China, Indonesia, Myanmar, Vietnam, Sri Lanka, Malaysia, and Thailand [13]. In India, wild populations of Champak are mainly found in the sub-Himalayan zone, south India, western ghat, and Assam. Traditionally, several parts of the plant are utilized in the treatment of various diseases such as inflammation, eye disorders, leprosy, cephalalgia, cough, gout, fever, colic, and antidote for scorpion and snake venoms, etc. [14]. The plant is reported to have several substantial pharmacological activities such as anti-diuretic, memory-enhancing, anti-diabetic, anti-inflammatory, antioxidant, antitumor, anti-microbial, and wound-healing properties [13,14].
M. champaca leaves are simple, alternate, and spiral, with a lamina of about 10–25 cm, lanceolate to elliptic-lanceolate, acuminate apex, acute base, the margin is slightly undulate, glabrous, strongly and reticulately nerved [15]. The leaf extract of M. champaca possesses anti-fertility, antibacterial, anti-inflammatory, antioxidant, analgesic, cytotoxic, antiulcerogenic, pro-cognitive, and helmintholytic activities [14,16,17,18,19,20,21]. Additionally, the leaves in an acidic medium inhibit mild steel corrosion [22]. Further, Champak leaf juice mixed with coconut oil is used for hair cleaning and removing lice and dandruff [23]. In addition, the market value of the dry leaf powder is $50 (for 100 leaves) and the essential oil is $13 (for 100 mL) (Sajee Sales; Shiva exports, India).
In spite of having enormous potential, few reports are available on phytoconstituent analysis of the leaf essential oil of M. champaca [24,25]. The available reports show that the M. champaca leaf essential oil is rich in sesquiterpenoids such as β-elemene, β-caryophyllene, α-humulene, β-selinene, and α-selinene, etc. [25]. However, to date, there is no report available regarding the variations in yield and phytochemical content of M. champaca leaf essential oil, necessitating the comparative assessment of essential oil among different populations.
Thus, the present research was conducted to explore the intraspecific variations in the phytochemical composition of M. champaca leaf essential oil collected from different geographic regions of Odisha, India. In order to find out the relationship among the accessions of Magnolia champaca, different chemometric analyses such as agglomerative hierarchical clustering (HCA), principal component analysis (PCA), partial least squares projection of latent structures (PLS), and partial least squares regression discriminant analysis (PLS-DA) were performed based on the essential oil compositions of the accessions. The results thus obtained were used to determine the Magnolia champaca chemotypes.
2. Results and Discussion
2.1. Essential Oil Yield
The hydrodistilled leaf essential oils of Magnolia champaca accessions were pale yellow in color with a strong odor. The essential oils from 52 accessions of M. champaca leaves were extracted, and the mean essential oil yield was determined using one-way ANOVA, followed by Tukey’s HSD test with a 95% confidence interval. A noteworthy difference in EO yield (%) on a fresh weight basis ranging from 0.06% to 0.31% (v/w) was observed among the populations of M. champaca (Table 1). The EO yield of accession MCL27 (0.31% v/w), collected from the Khordha district, was found to be the highest, followed by accessions MCL9 (0.29% v/w) and MCL32 (0.26% v/w) of Ganjam and Khordha district respectively. Similarly, the lowest essential oil yield was found in the accessions MCL3 and MCL4 (0.06% v/w) which were collected from the Bhadrak district. The essential oil yield of M. champaca in the present study was higher than that of the previous report [24], which reported an EO yield of 0.04% in fresh leaves collected from Brazil in different seasons. Further, the resulting variations in the essential oil yield might be due to seasonal variation, geographical location, genetic and environmental factors, etc., as reported in other species [2,3,4]. Our finding showing intraspecific variation in essential oil yield in M. champaca is in agreement with reports available in other species such as Croton gratissimus [26], Hypericum gaitii [3], Hedychium coronarium [27] and Curcuma longa [10], etc.

Table 1.
Geographical characteristics and essential oil yield of collected M. champaca accessions.
2.2. Essential Oil Composition
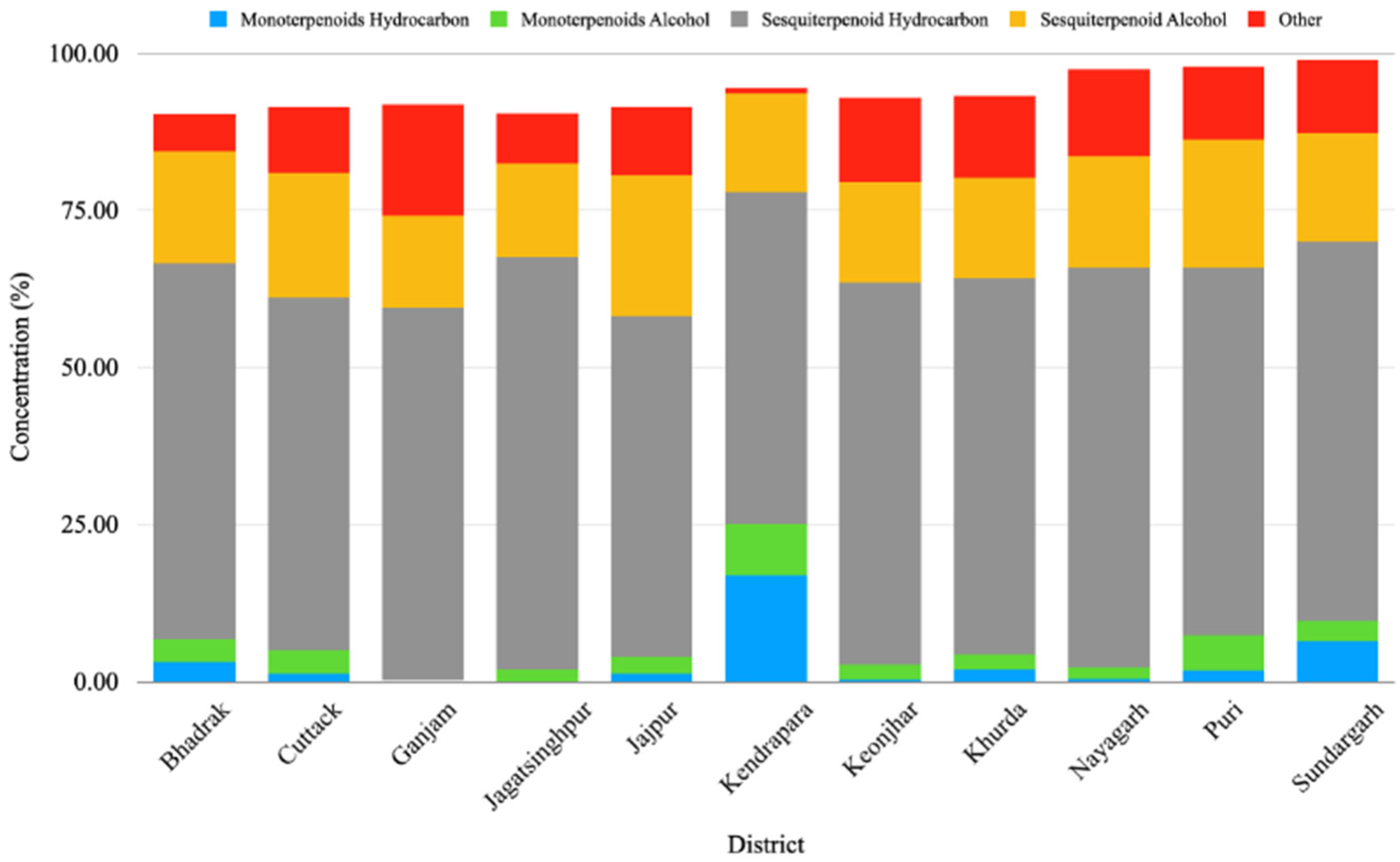
The GC-MS analysis of the leaf EO of different M. champaca populations collected from eleven districts of Odisha showed wide heterogeneity in chemical constituents. The study revealed a total of 65 compounds through GC-MS analysis, showcasing 90.23 to 98.90% of the total oil composition. The compounds detected through the Elite-5 MS column are listed based on their elution order (Table 2). In all accessions, sesquiterpenes (68.58 to 81.44%) were found to be the major chemical class followed by monoterpenes (0.8 to 25.04%). To be specific, EOs were dominated by mostly sesquiterpene hydrocarbons (52.83 to 65.63%) followed by sesquiterpene alcohol (14.71 to 22.45%) (Table 2, Figure 1). The present result is also consistent with the previous literature, where the leaf essential oil of M. champaca collected from Brazil was rich in sesquiterpene hydrocarbons followed by oxygenated sesquiterpenes [24]. In all the populations, the major constituents were β-elemene, γ-muurolene, β-caryophyllene, germacrene B, n-hexadecanol, α-humulene, viridiflorene, bicyclogermacrene, methyl linoleate, linalool, etc. (Table 3). β-elemene (0.23 to 38.76%) (Table 3) was found to be the major constituent of the accessions collected from Jagatsinghpur, Puri, Cuttack, Khordha, Nayagarh, and Ganjam districts (Table 2). γ-muurolene (0.31 to 22.48%) (Table 3) was found to be the predominant constituent of the accessions collected from the Sundargarh and Keonjhar districts (Table 2). Likewise, β-caryophyllene (0.03 to 20.70%) (Table 3) was the major constituent of the accessions of the Jajpur, Bhadrak, and Kendrapara districts (Table 2). The occurrence of three different types of major constituents in different accessions might be due to the difference in geographical location, genetic and environmental factors prevailing at different germplasm collection sites. The phytoconstituents identified herewith have already been reported in the leaf essential oil of different Magnolia species [24,25,28,29,30].

Table 2.
Relative contents of each constituent in Magnolia champaca leaf essential oil from Bhadrak (N = 4), Cuttack (N = 3), Ganjam (N = 3), Jajpur (N = 3), Jagatsinghpur (N = 3), Kendrapara (N = 3), Keonjhar (N = 3), Khordha (N = 11), Nayagarh (N = 4), Puri (N = 4), Sundargarh (N = 11).
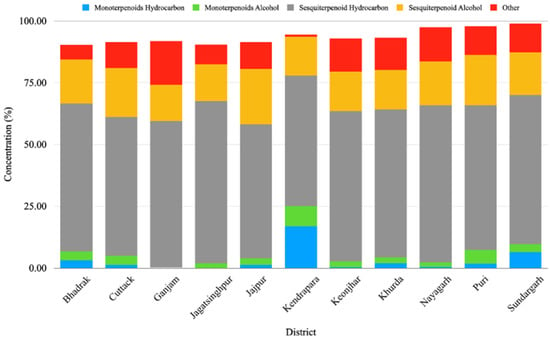
Figure 1.
Relative percentages of chemical constituents’ classes in the essential oil of Magnolia champaca from different regions of Odisha, India.

Table 3.
Concentration (min and max) of top 10 constituents of Magnolia champaca leaf essential oil as identified by GC-MS.
Although several phytoconstituents were restricted to one or a small number of populations, the majority were found in varying concentrations in different accessions (Table 2). For example, α-fenchene (0.22–0.79%) was observed only in populations of Kendrapara and Sundargarh districts. Similarly, α-pinene (0.15–0.58%), myrcene (0.17–0.6%), and (E)-β-ocimene (0.27–3.82%) were detected in populations collected from Khordha, Kendrapara and Sundargarh district, however, they were completely absent in the other groups. Our finding showing intraspecific variation in essential oil composition in M. champaca is in agreement with reports available in other species such as Croton gratissimus [26], Myristica fragrans [32], Hypericum gaitii [3], Hedychium coronarium [27] and Curcuma longa [10], etc. The variation in phytoconstituent levels might be due to the place of occurrence of the plant populations, soil characteristics, and predominant environmental factors, of which rainfall and temperature are important parameters [2,3]. As reported [33], the distribution of essential oil chemotypes is related to abiotic factors (temperature, moisture, topography, and edaphic factors) of the region which act on genes of the terpenoid biosynthetic pathways and contribute to the development of diverse essential oil profiles. The chemical diversity observed among M. champaca populations resulting from bioclimatic and geographical variables necessitates designing suitable conservation and sustainable utilization strategies, by considering these aspects. Furthermore, all populations representing different chemotypes need to be conserved utilizing different conservation approaches [3,34].
2.3. Multivariate Analysis of phytoconstituents
Chemometrics analyses such as PCA, PLS-DA, and sPLS-DA were performed to determine the chemotypes and cluster analyses such as hierarchical clustering, i.e., dendrogram and partitional clustering, i.e., K-means to understand the associations among studied populations of M. champaca concerning their EOs’ composition and contents.
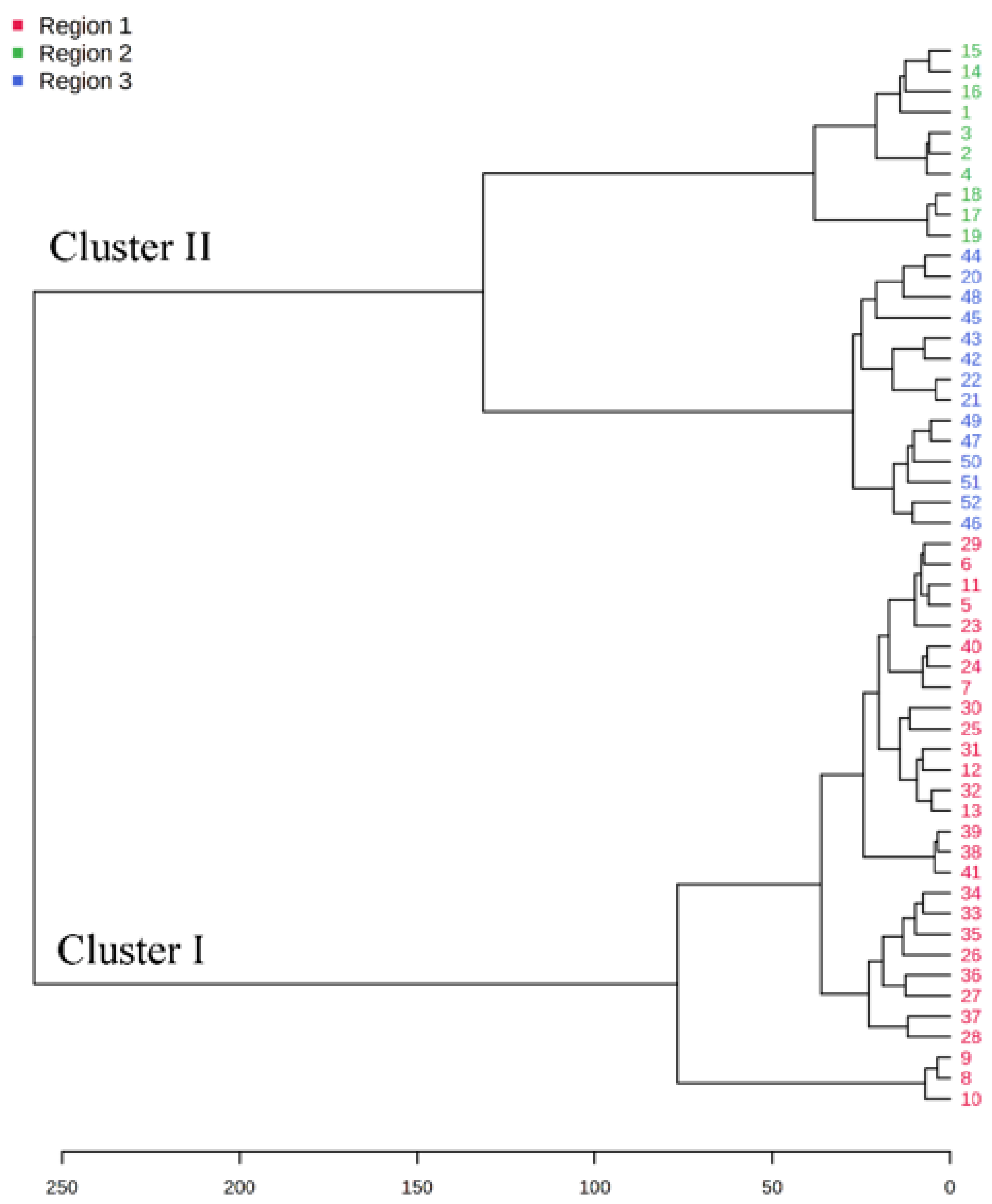
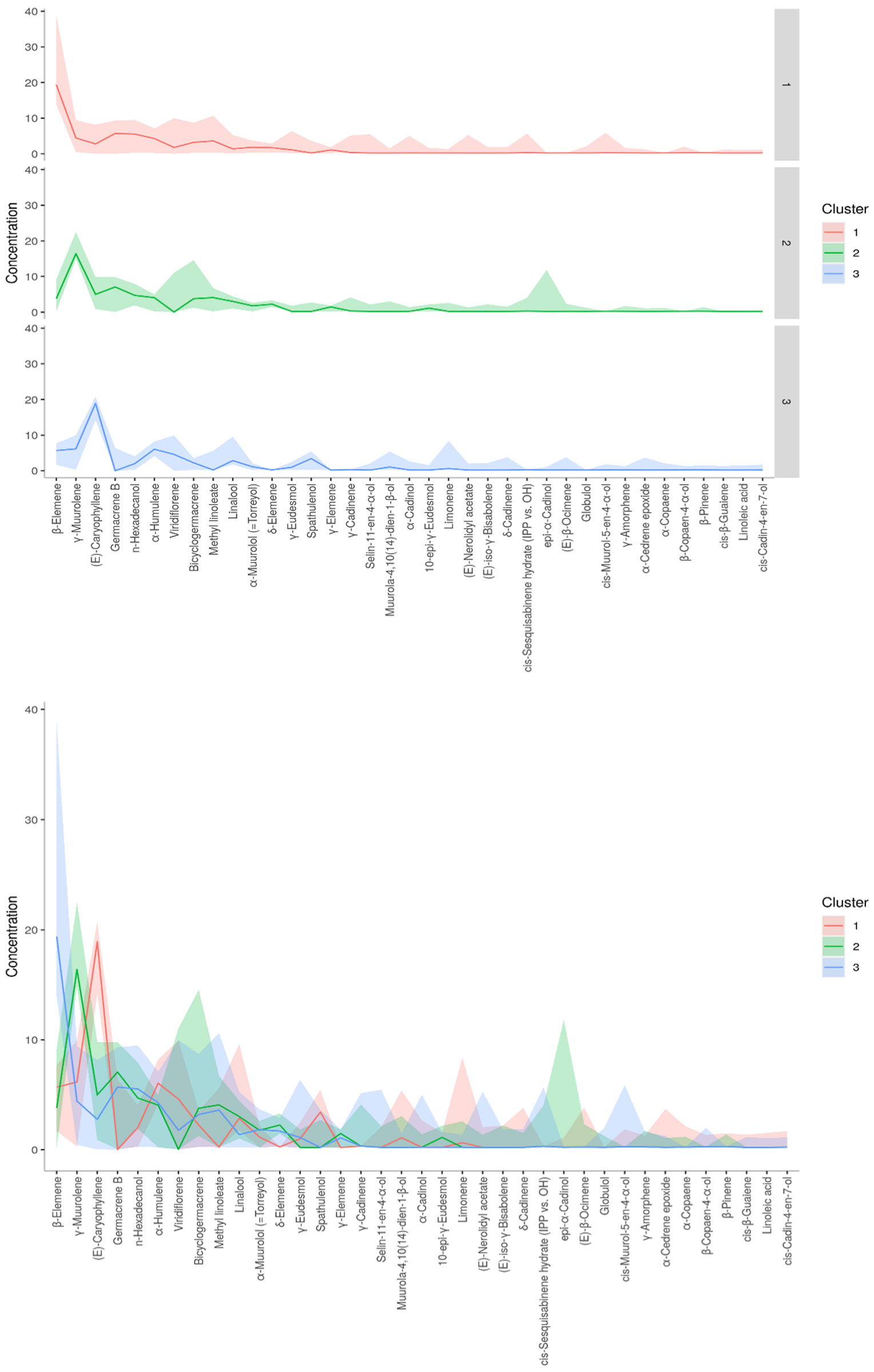
A dendrogram was constructed based on the Ward linkage-clustering algorithm method using Euclidean distance measures between groups. Concerning the constructed dendrogram, the studied populations were separated into two major clusters, i.e., cluster I and cluster II (Figure 2). Cluster I included the populations collected from Jagatsinghpur, Cuttack, Puri, Khordha, Nayagarh, and Ganjam districts and is characterized by a high content of β-elemene (14.28–38.80%). Cluster II was further subdivided into two subclusters (cluster IIA and IIB) representing two distinct chemotypes. The subcluster IIA included the populations collected from Sundargarh and Keonjhar districts and is characterized by a high content of γ-muurolene (14.92–22.50%) whereas the subcluster IIB included the populations collected from Jajpur, Bhadrak and Kendrapara district and was rich in β-caryophyllene (14.22–20.74%). The variations in the composition of the essential oils in plant species and their principal compounds are possibly because of the changes in abiotic factors such as moisture, temperature and topography which regulate the terpene biosynthesis pathway. This alteration in the biosynthetic pathway can lead to the occurrence of new chemotypes in a plant species [2,35]. Also, the variations in the constituents of the EOs might be connected with microclimatic criteria such as temperature, precipitation or phenological state, which are found to be different in the collection site of M. champaca and are possible explanation to alter the oil phytochemical compositions [24,36,37]. Further K-means cluster analysis was prepared by taking the components of EO (variable indices) on the X-axis and their relative intensities on the Y-axis (Figure 3). Different colors represent different clusters and their concentrations. The lines are the median intensities of corresponding clusters. Cluster 1 (red), cluster 2 (green), and cluster 3 (blue) represent the accessions rich in β-elemene, γ-muurolene, and β-caryophyllene, respectively.
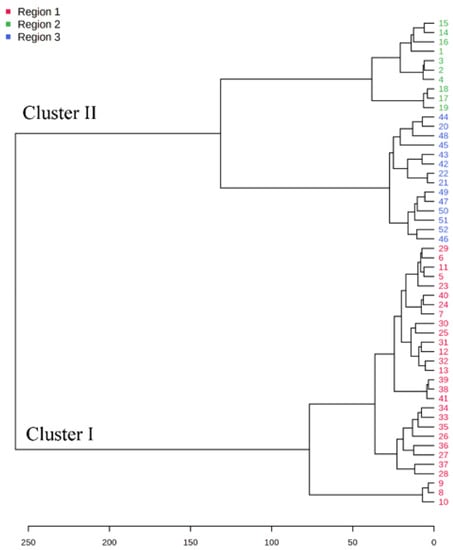
Figure 2.
Dendrogram illustrating cluster analysis (distance measure and clustering algorithm were. done using Euclidean and Ward). M. champaca accessions are indicated by names according to Table 1.
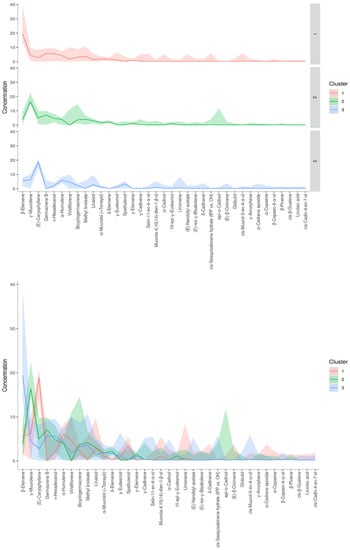
Figure 3.
K-means cluster analysis. The X-axis represents phytoconstituents and the Y-axis represents its relative intensities. The median intensities of corresponding clusters are shown in different colored lines.
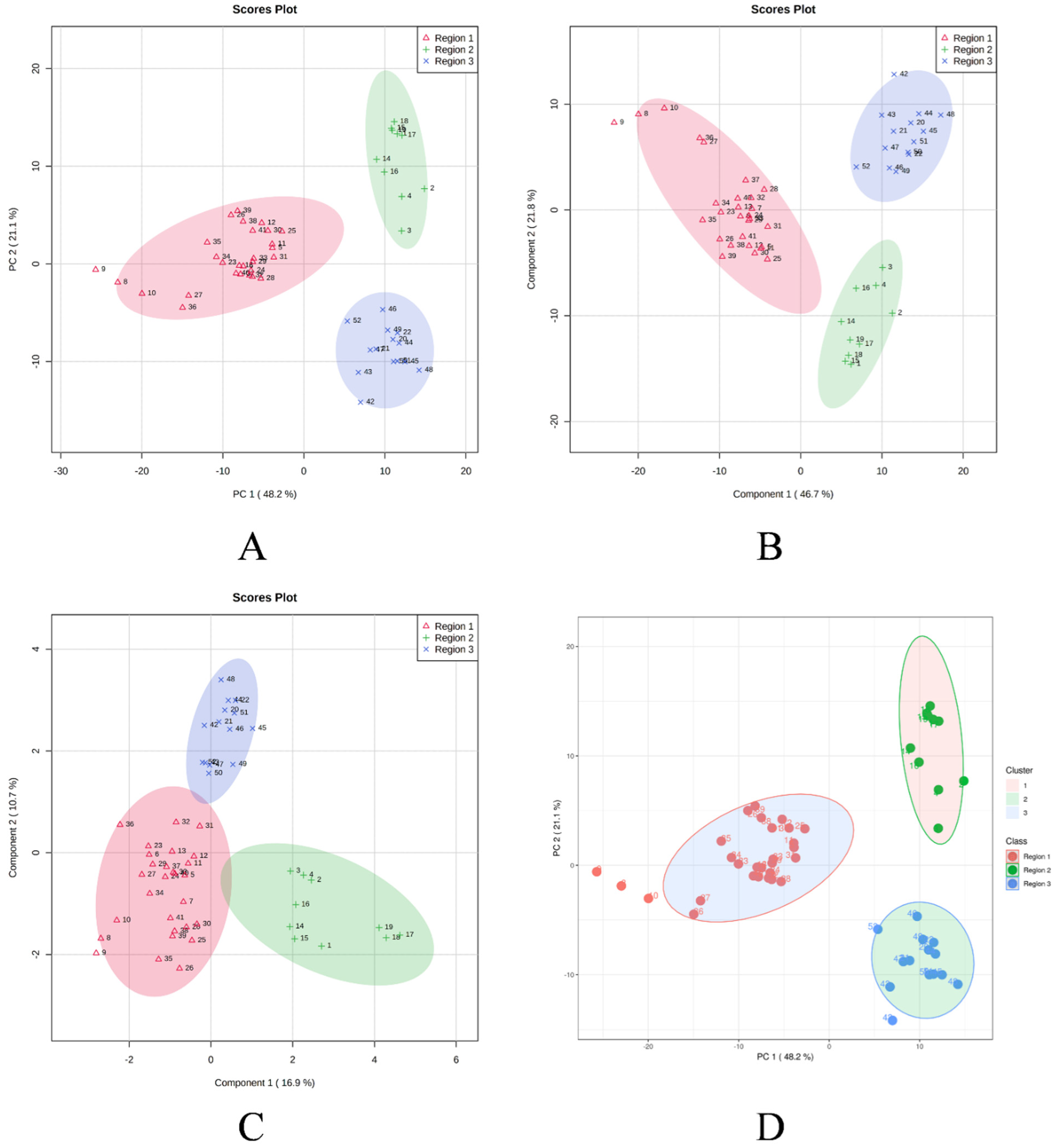
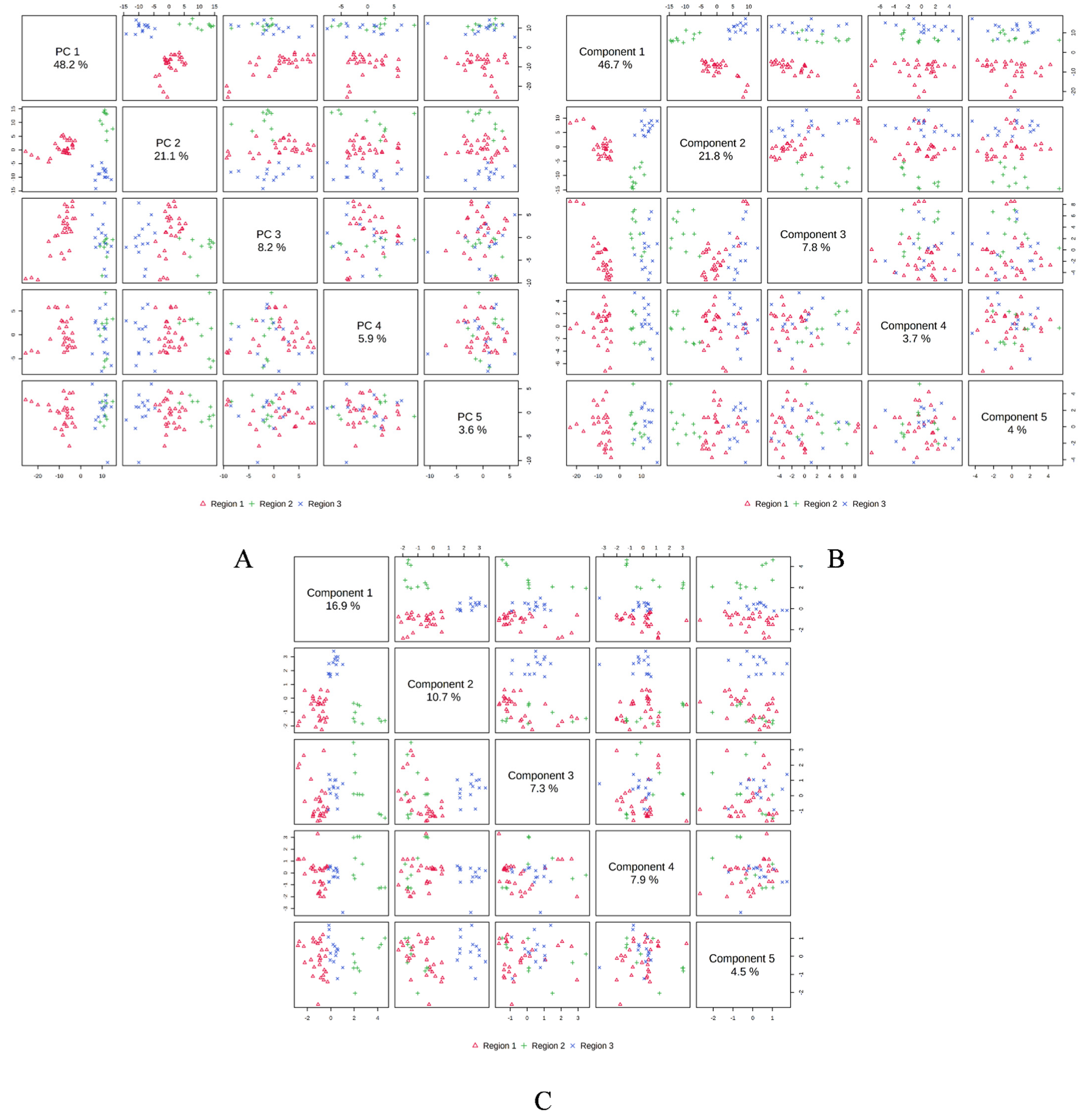
In order to determine the linkages between the M. champaca populations, a PCA analysis was performed based on the composition of the essential oils. PCA divided the populations into three groups, confirming the dendrogram’s clustering structure. PCA revealed a total of five principal components (PC) explaining approximately 86.90% of the overall variance. In the score plot, the two principal components (PC1 and PC2) that account for the maximum variation of 69.30% (amongst the populations under study) are shown. (Figure 4). Similarly, score plots generated by PLS-DA, sPLS-DA and K-means clustering show similar distinctions within PCs. Cumulatively pairwise score plots generated through PCA, PLS-DA, and sPLS-DA show a similar trend in the distribution of populations according to their variance (Figure 5).
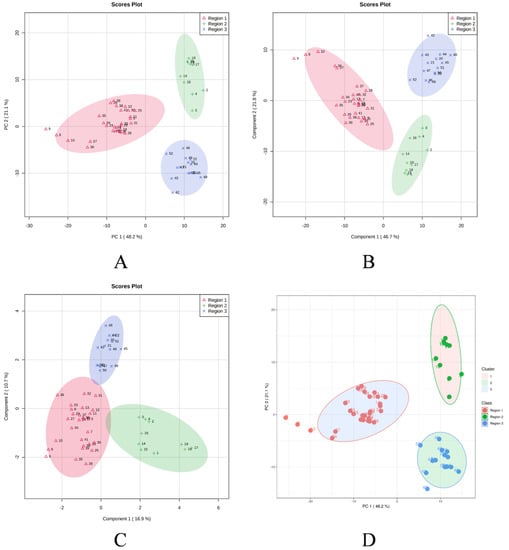
Figure 4.
Score plot generated between the selected PCs with their variances. (A) PCA; (B) PLS-DA; (C) sPLS-DA; (D) K-means clustering.
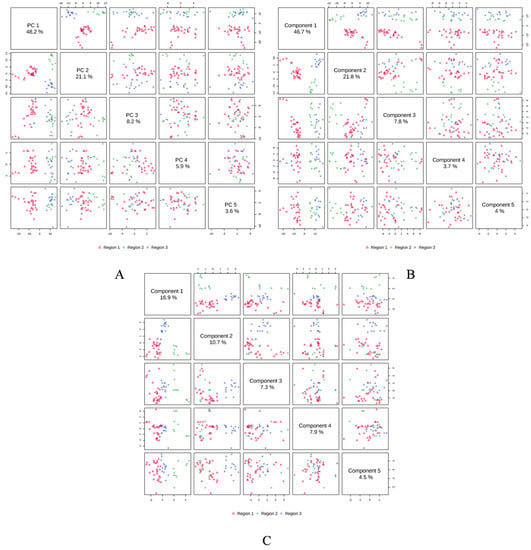
Figure 5.
Pairwise score plots between the 5 selected components with their variances shown in the corresponding diagonal cell. (A) PCA; (B) PLSDA; (C) sPLS-DA.
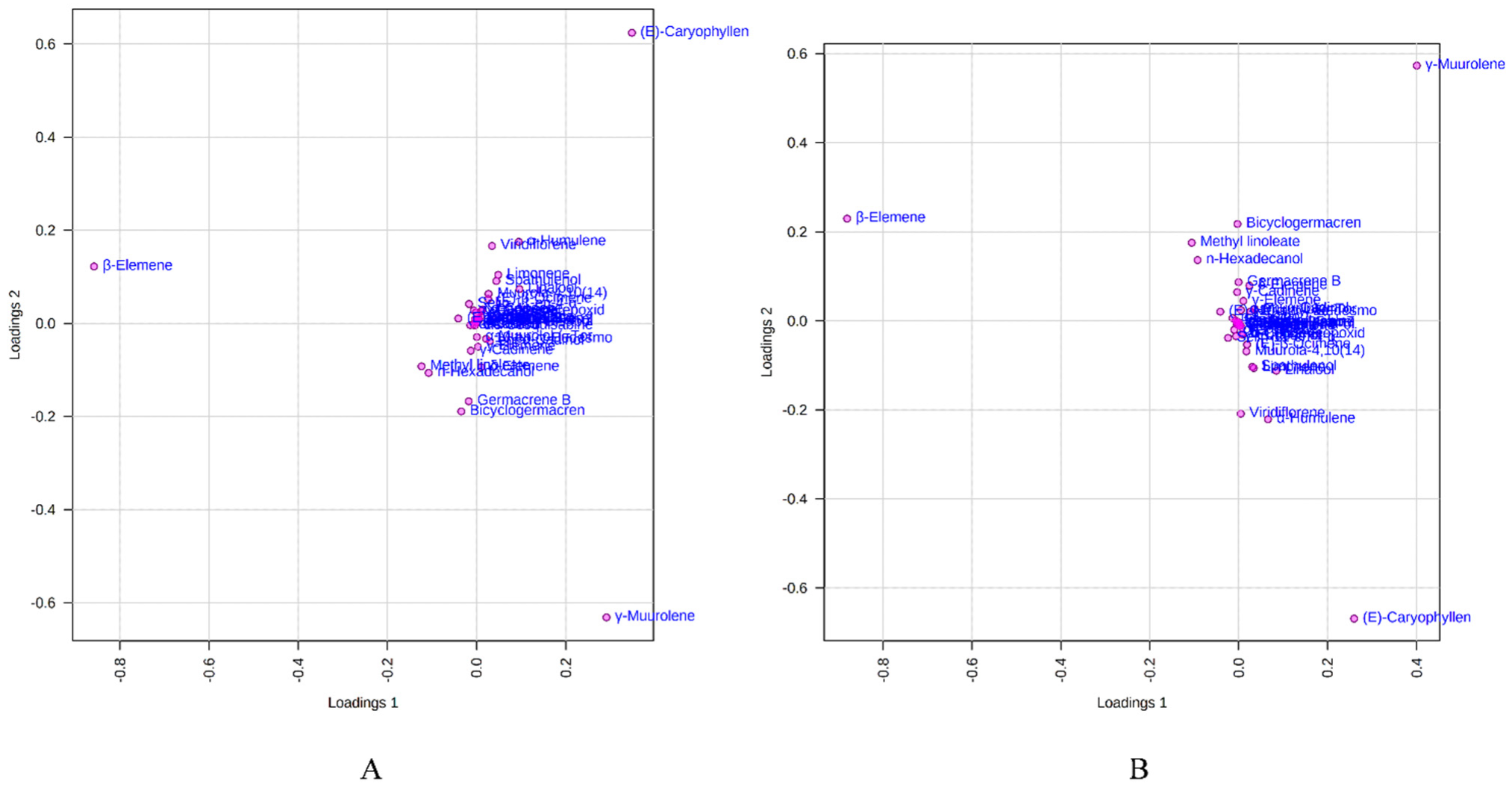
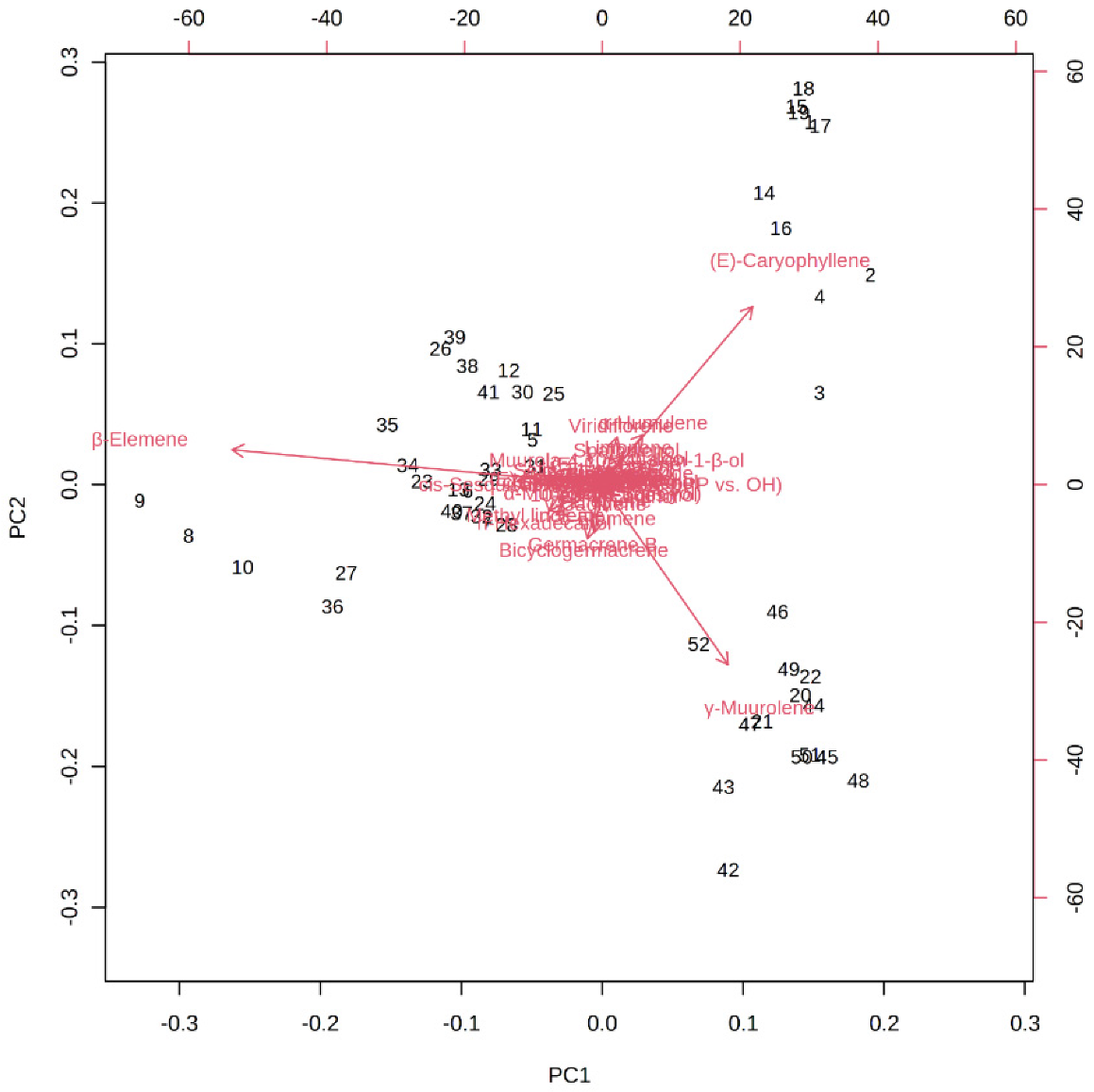
Figure 6 depicts the loading plot of PC1 and PC2 illustrating the contribution of significant EO elements (>1%) in the M. champaca populations. The first principal component (PC1) accounts for up to 48.20% variation and had a positive correlation with γ-muurolene and β-caryophyllene (>0.20) but had a negative correlation with β-elemene (>−0.80). Whereas the second principal component (PC2) could explain 21.10% of the total variation and showed a negative correlation with γ-muurolene (>−0.60) and a positive relationship with β-elemene (>0.10) and β-caryophyllene (>0.60). Further biplot (Figure 7) was generated from the loading plots (Figure 6) by following the centering and normalization scaling method. Here the biplot was generated between two principal components, i.e., PC1 and PC2 which once again proved the three chemotypes with major constituents β-elemene, γ-muurolene and β-caryophyllene.
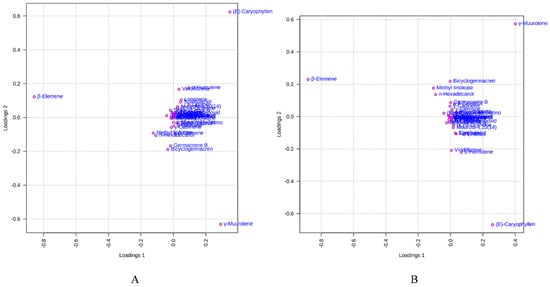
Figure 6.
Loadings plot between the selected PCs (PC1 vs. PC2) showing the involvement of major essential oil components (>1%) in the grouping of M. champaca accessions. (A) PCA; (B) PLS-DA.
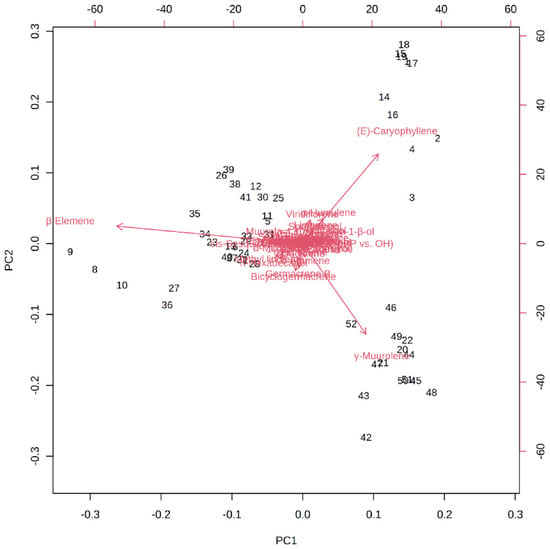
Figure 7.
PCA biplot between the selected PCs (PC1 vs. PC2) showing the involvement of major essential oil components (>1%) in the grouping of M. champaca accessions. M. champaca accessions are indicated by names according to Table 1.
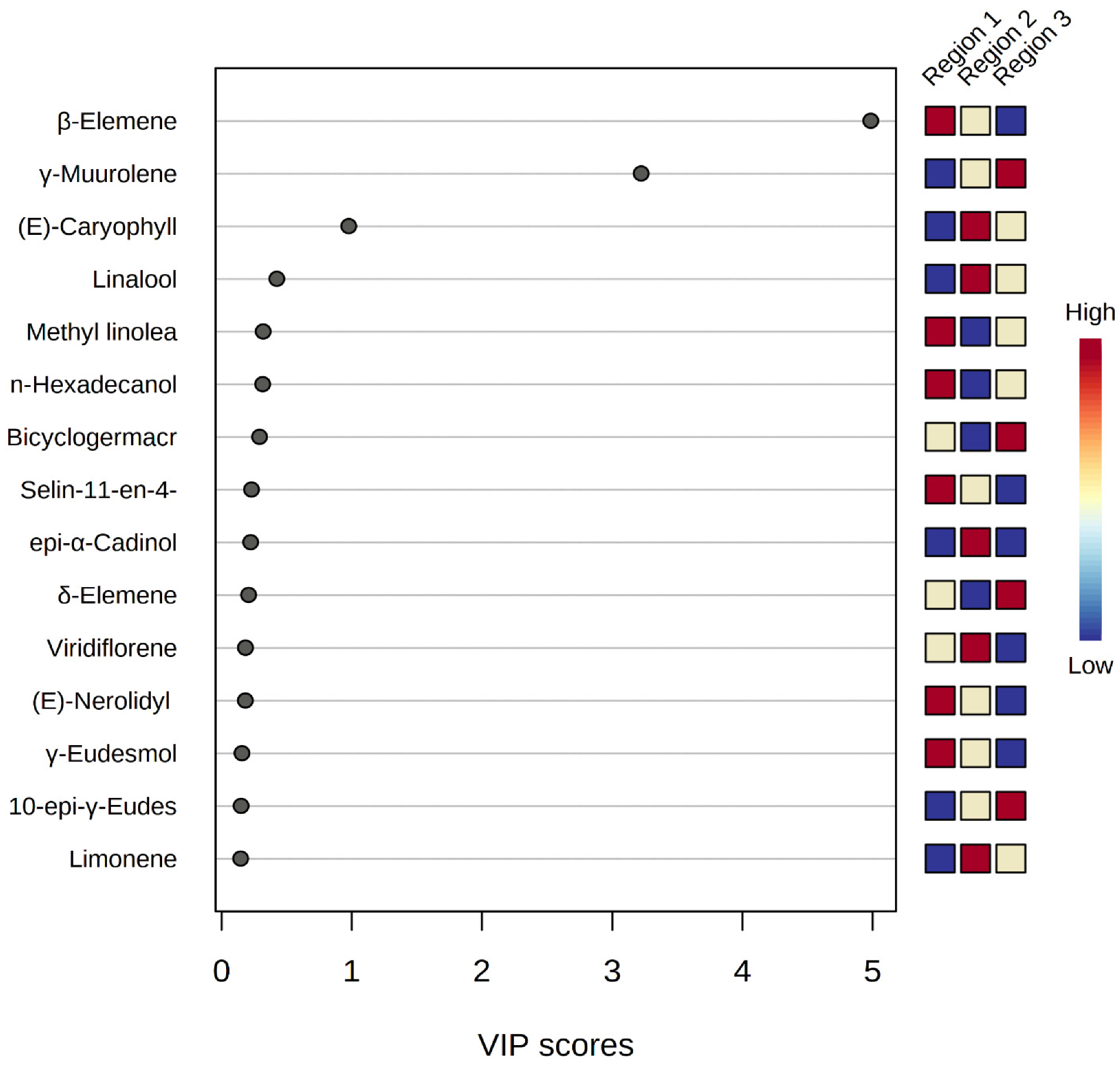
The resulting VIP score plot (Figure 8) represented the strength of each peak distinguishing M. champaca samples from different geographical origins. Populations with Variable Importance for the Projection (VIP) > 1 have more influence on population discrimination. The colored boxes against the plot indicate relative concentrations of the corresponding metabolite in each region. The phytoconstituent β-elemene, leading with a VIP score of > 5, is found to be dominant in the accessions of Jagatsinghpur, Cuttack, Puri, Khordha, Nayagarh and Ganjam districts, followed by γ-muurolene, with a score of >3, is found to be highest in the accessions of Sundargarh and Keonjhar district. Furthermore, β-caryophyllene, with a score of >1, is found to be the leading compound of M. champaca populations collected from the Jajpur, Bhadrak and Kendrapara districts.
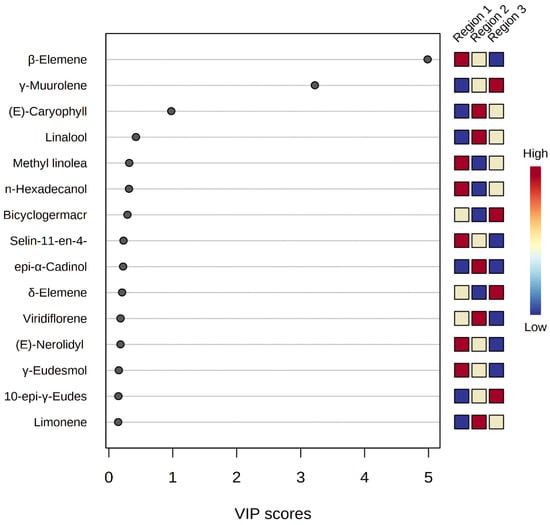
Figure 8.
PLS-DA-VIP score plot showing important features of the major constituents of M. champaca leaf essential oil. The colored boxes on the right indicate the relative concentrations of the corresponding metabolite in each group under study.
Due to the diverse geographic origin of M. champaca accessions, the variances in the phyto-constituents are quite normal. Geographical differences expose the species to a variety of exogenous factor-related stresses that result in the development of a variety of secondary metabolites for their defense [38]. There are reports on other species where the variations in phytoconstituents of different eco-regions were explored by showing the effect of different genetic, climatic and edaphic factors on the variation of essential oil yield and its quality [3,26,27,32]. For the first time, the chemical diversity of leaf essential oil of M. champaca has been studied in association with different chemometric approaches such as clustering analysis, PCA, PLS-DA, and sPLS-DA. The chemometric approach has also been effectively used to analyze the correlation and variation in essential oil composition of other species [3,26,27,32].
3. Materials and Methods
3.1. Plant Material
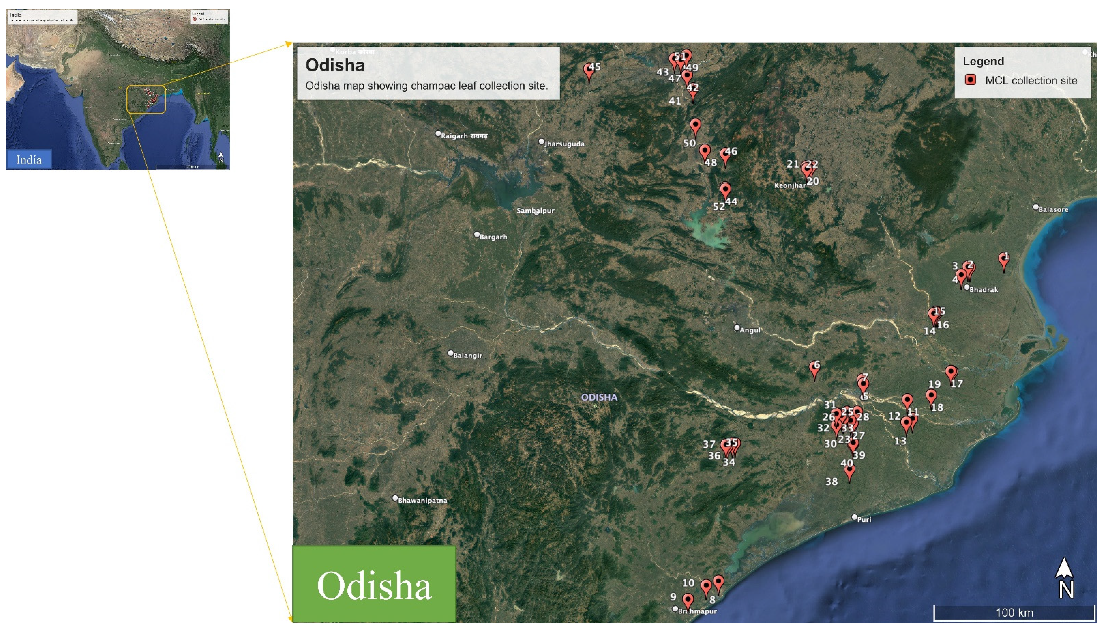
Plant leaves of M. champaca were collected from different regions of Odisha in the months of February–August 2022 (Figure 9). The botanical identification of the species was done with the help of the literature and authenticated by Prof. Pratap Chandra Panda, Taxonomist, Centre for Biotechnology, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India. The herbarium specimens were prepared and deposited at the Herbarium of the Centre for Biotechnology, as a voucher. The geocoordinates of the areas of occurrence of plant accessions were logged by the GPS and the details are provided in Table 1.
Figure 9.
Map showing different regions of sample collection from Odisha, India.
3.2. Extraction of Essential Oils
For the isolation of the essential oil, fresh mature leaves measuring 150 g from each accession were cleaned and finely chopped before being subjected to hydrodistillation utilizing a Clevenger-type apparatus for 5 h. EO isolation from each accession was done in three replications to confirm the yield reproducibility. The EO was collected and dried over anhydrous sodium sulfate (Na2SO4). The EO, was stored in a glass vial at 4 ℃ for further GC-MS analysis. The EO’s yield was calculated following the equation:
% Yield of oil (v/w) = [volume of oil (in mL)/weight of fresh sample (in g)] × 100
3.3. GC-MS Analysis of Isolated EOs
For the analysis of the EOs of M. champaca, a gas chromatograph (Clarus 580, PerkinElmer, Waltham, MA, USA) attached to an SQ8S mass spectrometric detector was utilized where 99.99% pure helium was taken as mobile phase (flow rate = 1 mL/min). The EO obtained above was analyzed by injecting 0.1 μL of neat EO into the GC-MS. Separation was carried out on an Elite-5 MS column (30 m in length × 0.25 mm I.D., film thickness 0.25 μm). The oven temperature in the GC-MS was programmed as follows: 60 °C for 0 min, heated at 3 ℃/min to 220 ℃ with 7 min hold, and the total run time was set for 60.33 min. The source and transfer interface temperatures were set at 150 °C and 250 °C respectively. Scanning was performed over a mass scan range of 50–600 m/z with electron ionization mode at an ionization voltage of 70 eV. Turbo mass TM software 6.1 was utilized to obtain the mass spectra and ion chromatogram.
Identification of each constituent was performed based on the RI (Retention Index) determined by co-injecting a homologous series of straight chain n-alkanes (C8–C20) run under similar experimental conditions and by comparing calculated RI values with the literature values [31]. The identification of constituents was further confirmed by matching their mass spectra with the inbuilt NIST MS library (NIST 08). Quantification of the essential oil constituents was performed based on relative area percentages.
3.4. Statistical Analysis
To compare the statistical differences in EO yield (%) and quality amongst M. champaca populations, one-way ANOVA followed by the Tukey HSD test at a 95% confidence level was performed using the statistical software Minitab 17. To investigate the similarity and relationship among M. champaca populations based on EO chemical constituents, chemometrics analyses such as Principal Component Analysis (PCA), Partial Least Squares—Discriminant Analysis (PLS-DA), and Sparse Partial Least Squares—Discriminant Analysis (sPLS-DA), and cluster analyses such as hierarchical clustering, i.e., dendrogram and partitional clustering, i.e., K-means clustering was used. The chemometric analysis such as PCA, PLS-DA, sPLS-DA, dendrogram, and K-means clustering was performed using the MetaboAnalyst 5.0, a comprehensive web-based metabolomics analysis tool (https://www.metaboanalyst.ca/ accessed on 21 September 2022). For the chemometrics analysis phytoconstituents with a peak area >1% in at least a single population were chosen as variables. Euclidean distance was used to measure the dissimilarity between populations, and Ward’s variance-minimizing method was performed for hierarchical clustering [39,40].
4. Conclusions
This is the first report on the chemical diversity analysis of the leaf essential oil of Magnolia champaca. This research revealed significant quantitative and qualitative variations in the chemical profile of the M. champaca leaf essential oils from eleven districts of Odisha. Chemometrics techniques could effectively classify the accessions into three different chemotypes rich with β-elemene, β-caryophyllene and γ-muurolene. The result demonstrated that the chemical composition and yield of the essential oil of M. champaca might be influenced by the geographical origin of populations and environmental factors. The chemical polymorphism analyzed in the studied populations would facilitate the selection of chemotypes with specific compounds. The chemotypes identified in the Magnolia champaca populations could be developed as promising bio-resources for conservation, pharmaceutical application, and further improvement of the taxa.
Author Contributions
Conceptualization, S.N. and A.S.; methodology, C.S. and B.B.C.; software, C.S. and B.D.; validation, C.S., A.S. and A.R.; formal analysis, C.S. and S.J.; investigation, C.S., S.J. and B.B.C.; Resources, S.N., A.R. and A.S.; data curation, C.S. and B.B.C.; writing-original draft preparation, C.S.; writing-review and editing, A.S., S.N. and A.R.; supervision, A.S., P.C.P. and S.N.; project administration, P.C.P., A.S. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We are grateful to Manoj Ranjan Nayak and S.C. Si for their constant support and encouragement in and out of the centre.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crops Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Kamila, P.K.; Ray, A.; Jena, S.; Sahoo, A.; Kar, S.K.; Nayak, S.; Panda, P.C. Intraspecific variability in yield and chemical composition of essential oil of the endemic species Hypericum gaitii from different natural habitats of Eastern India. Plant Biosyst. 2021, in press. [CrossRef]
- Behura, S.; Sahoo, A.; Singh, S.; Jena, S.; Kar, B.; Nayak, S. Variation in essential oil yield and volatile constituents of Curcuma aromatica rhizome from different regions of eastern and southern India. J. Essent. Oil Bear. Plants 2021, 24, 1248–1255. [Google Scholar] [CrossRef]
- Sahoo, A.; Jena, S.; Ray, A.; Dash, K.T.; Nayak, S.; Panda, P.C. Chemical constituent analysis and antioxidant activity of leaf essential oil of Curcuma xanthorrhiza. J. Essent. Oil Bear. Plants 2021, 24, 736–744. [Google Scholar] [CrossRef]
- Sahoo, A.; Dash, B.; Jena, S.; Ray, A.; Panda, P.C.; Nayak, S. Phytochemical composition of flower essential oil of Plumeria alba grown in India. J. Essent. Oil Bear. Plants 2021, 24, 671–676. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Yao, X.H.; Duan, M.H.; Wei, F.Y.; Wu, G.H.; Li, L. Variation of essential oil content and antioxidant activity of Lonicera species in different sites of China. Ind. Crops Prod. 2015, 77, 772–779. [Google Scholar] [CrossRef]
- Masotti, V.; Juteau, F.; Bessière, J.M.; Viano, J. Seasonal and phenological variations of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. Stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef]
- Sahoo, A.; Kar, B.; Jena, S.; Dash, B.; Ray, A.; Sahoo, S.; Nayak, S. Qualitative and quantitative evaluation of rhizome essential oil of eight different cultivars of Curcuma longa L. (Turmeric). J. Essent. Oil Bear. Plants 2019, 22, 239–247. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Ebadi, A.; Maggi, F.; Fattahi, R.; Yazdani, D.; Jafari, M. Chemical characterization of the essential oil compositions from Iranian populations of Hypericum perforatum L. Ind. Crops Prod. 2015, 76, 565–573. [Google Scholar] [CrossRef]
- Aghbash, B.N.; Dehghan, G.; Movafeghi, A.; Talebpour, A.H.; Pouresmaeil, M.; Maggi, F.; Nojadeh, M.S. Chemical compositions and biological activity of essential oils from four populations of Satureja macrantha C. A. Mey. J. Essent. Oil Bear. Plants 2021, 33, 133–142. [Google Scholar] [CrossRef]
- Sinha, R.; Varma, R. Michelia champaca L. (swarna champa): A review. Int. J. Enhanc. Res. Sci. Technol. Eng. 2016, 5, 78–83. [Google Scholar]
- Taprial, S.; Kashyap, D.; Mehta, V.; Kumar, S.; Kumar, D. Antifertility effect of hydroalcoholic leaves extract of Michelia champaca L.: An ethnomedicine used by Bhatra women in Chhattisgarh state of India. J. Ethnopharmacol. 2013, 147, 671–675. [Google Scholar] [CrossRef]
- Ramyashree, C.; Hemalatha, K. Ethnomedicinal profile on magnolia species (Magnoliaceae): A review. Int. J. Herb. Med. 2020, 8, 39–46. [Google Scholar]
- Khan, M.R.; Kihara, M.; Omoloso, A.D. Antimicrobial activity of Michelia champaca. Fitoterapia 2002, 73, 744–748. [Google Scholar] [CrossRef]
- Gupta, S.; Mehla, K.; Chauhan, D.; Nair, A. Anti-inflammatory activity of leaves of Michelia champaca investigated on acute inflammation induced rats. Lat. Am. J. Pharm. 2011, 30, 819–822. [Google Scholar]
- Hossain, M.M.; Jahangir, R.; Hasan, S.R.; Akter, R.; Ahmed, T.; Islam, M.I.; Faruque, A. Antioxidant, analgesic and cytotoxic activity of Michelia champaca Linn. Leaf. Stamford J. Pharm. Sci. 2009, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Mullaicharam, A.R.; Kumar, M.S. Effect of Michelia champaca Linn on pylorous ligated rats. J. Appl. Pharm. Sci. 2011, 1, 60–64. [Google Scholar]
- Ahmad, H.; Saxena, V.; Mishra, A.; Gupta, R.; Saraf, S.A. Procognitive effects of hexane extracts of Michelia champaca leaves in normal and memory deficit mice. Pharmacogn. Commun. 2011, 1, 30–36. [Google Scholar] [CrossRef]
- Dama, G.; Bidkar, J.; Deore, S.; Jori, M.; Joshi, P. Helmintholytic activity of the methanolic and aqueous extracts of leaves of Michelia champaca. Res. J. Pharmacol. Pharmacodyn. 2011, 3, 25–26. [Google Scholar]
- Kumar, S.A.; Sankar, A.; Kumar, S.R.; Vijayan, M. Magnolia champaca plant leaves extract for the inhibition of mild steel corrosion in acidic medium. Asian J. Res. Chem. 2013, 6, 1023–1026. [Google Scholar]
- Mandal, U.; Mallick, S.K.; Mahalik, G. Ethnomedicinal plants used for the treatment and healing of skin diseases in Odisha, India: A review. Shodh Sanchar Bull. 2020, 10, 100–108. [Google Scholar]
- Lago, J.H.G.; Fávero, O.A.; Romoff, P. Chemical composition and seasonal variation of the volatile oils from leaves of Michelia champaca L., Magnoliaceae. Rev. Bras. Farmacogn. 2009, 19, 880–882. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Y.; Xia, B.; He, F.; Pan, H. Components and antibacterial functions of volatile organic compounds from leaves and flowers of Michelia champaka. J. Northeast For. Univ. 2012, 40, 71–74. [Google Scholar]
- Moremi, M.P.; Kamatou, G.P.; Viljoen, A.M.; Tankeu, S.Y. Croton gratissimus-essential oil composition and chemometric analysis of an ethnomedicinally important tree from South Africa. S. Afr. J. Bot. 2021, 138, 141–147. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Haldar, T.; Sahoo, A.; Kar, B.; Patnaik, J.; Ghosh, B.; Panda, P.C.; Mahapatra, N.; Nayak, S. Population genetic structure and diversity analysis in Hedychium coronarium populations using morphological, phytochemical and molecular markers. Ind. Crops Prod. 2019, 132, 118–133. [Google Scholar] [CrossRef]
- Dai, D.N.; Thang, T.D.; Ogunwande, I.A. Essential oil composition of four Magnoliaceae species cultivated in Vietnam. J. Herbs Spices Med. Plants 2016, 22, 279–287. [Google Scholar] [CrossRef]
- Ha, C.T.T.; Thai, T.H.; Thanh, T.X.; Thuy, D.T.T.; Tra, N.T.; Ha, N.T.T. Chemical composition and antimicrobial activity of the essential oils from stems and leaves of Michelia alba DC growing in Vietnam. Acad. J. Biol. 2018, 40, 96–105. [Google Scholar]
- Ha, C.T.T.; Thai, T.H.; Thuy, D.T.T.; Ky, N.D.; Tam, H.M. Chemical composition and antimicrobial activity of the essential oil from twigs and leaves of Magnolia macclurei (Dandy) Figlar from Ha Giang Province, Vietnam. Acad. J. Biol. 2020, 42, 41–49. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Component by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; pp. 1–811. [Google Scholar]
- Dupuy, N.; Molinet, J.; Mehl, F.; Nanlohy, F.; Le Dréau, Y.; Kister, J. Chemometric analysis of mid infrared and gas chromatography data of Indonesian nutmeg essential oils. Ind. Crops Prod. 2013, 43, 596–601. [Google Scholar] [CrossRef]
- Croteau, R.; Gershenzon, J. Genetic Control of Monoterpene Biosynthesis in Mints (Mentha: Lamiaceae). In Genetic Engineering of Plant Secondary Metabolism, 1st ed.; Ellis, B.E., Kuroki, G.W., Stafford, H.A., Eds.; Springer: Boston, MA, USA, 1994; Volume 28, pp. 193–229. [Google Scholar]
- Al-Sagheer, N.A. Magnolia champaca (L.) Baill. Ex Pierre (Magnoliaceae): A first report and a new record in the Arabian Peninsula (Yemen). J. Saudi Soc. Agric. Sci. 2021, 20, 243–247. [Google Scholar] [CrossRef]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. Et Reut., a North African endemic Species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Fávero, O.A.; Romoff, P. Microclimatic factors and phenology influences in the chemical composition of the essential oils from Pittosporum undulatum Vent. Leaves. J. Braz. Chem. Soc. 2006, 17, 1334–1338. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Ward, J.H., Jr.; Hook, M.E. Application of an hierarchical grouping procedure to a problem of grouping profiles. Educ. Psychol. Meas. 1963, 23, 69–81. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).