Experimental and Theoretical Studies of the Optical Properties of the Schiff Bases and Their Materials Obtained from o-Phenylenediamine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ligand Synthesis and Characterization
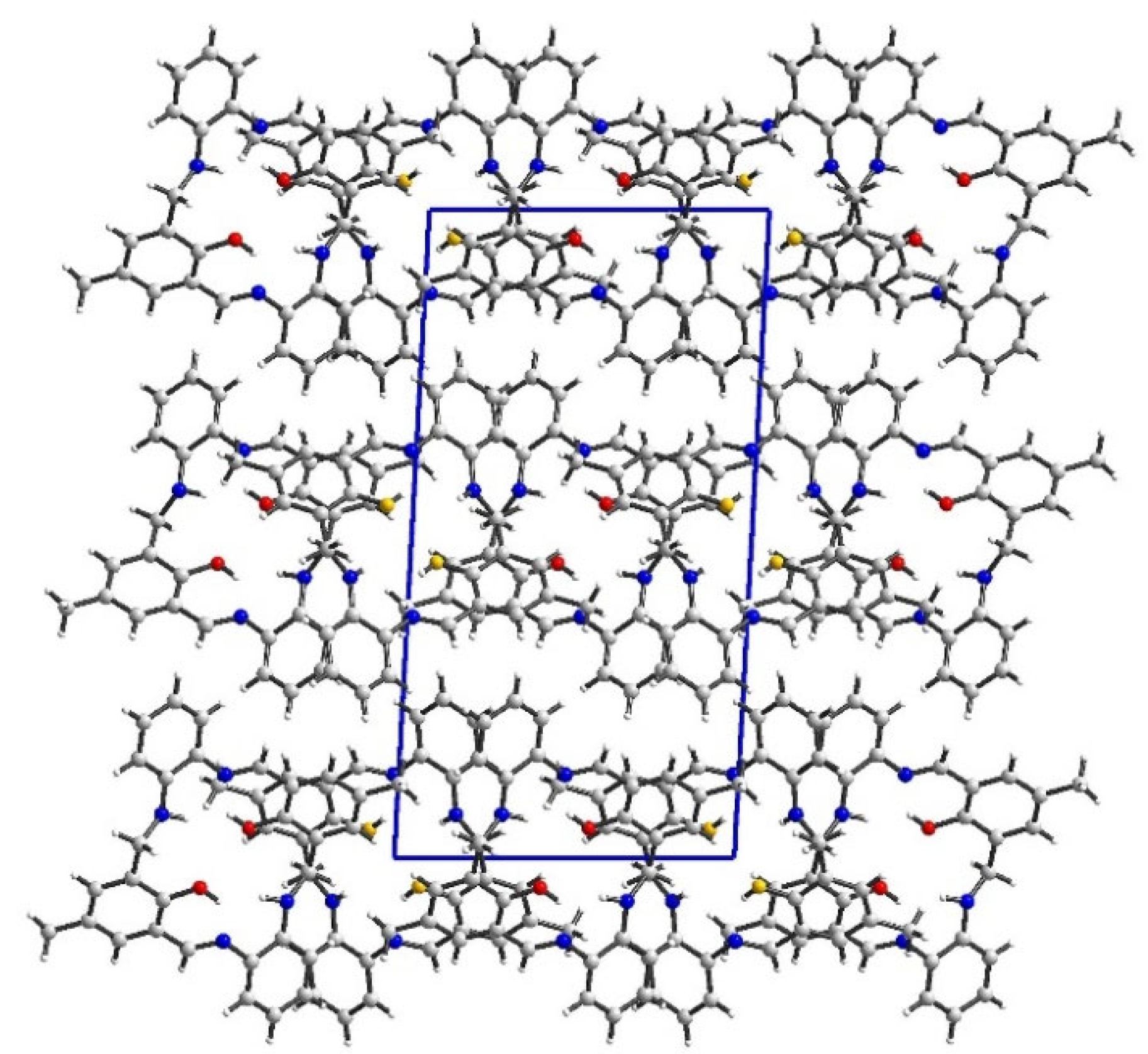
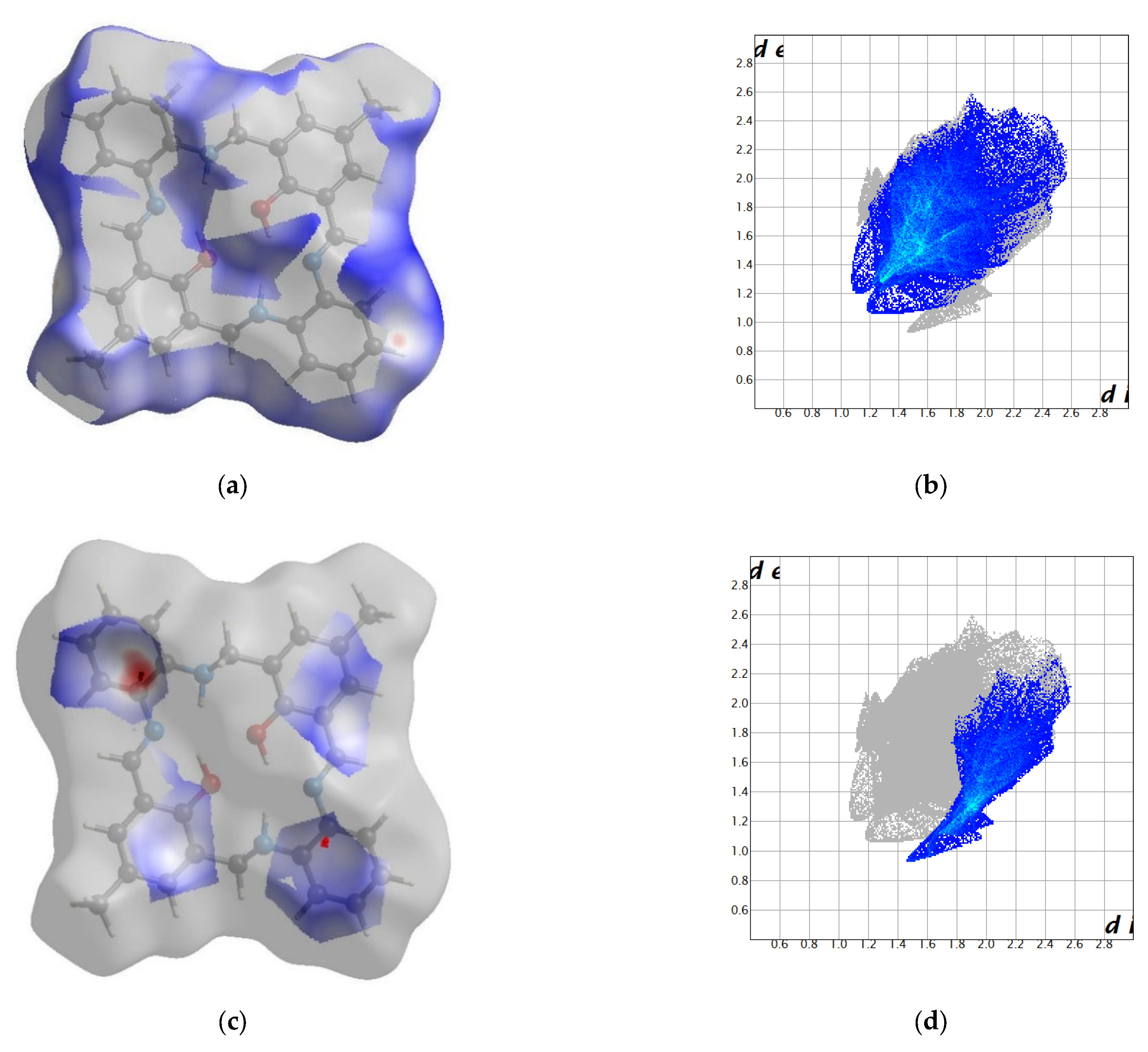
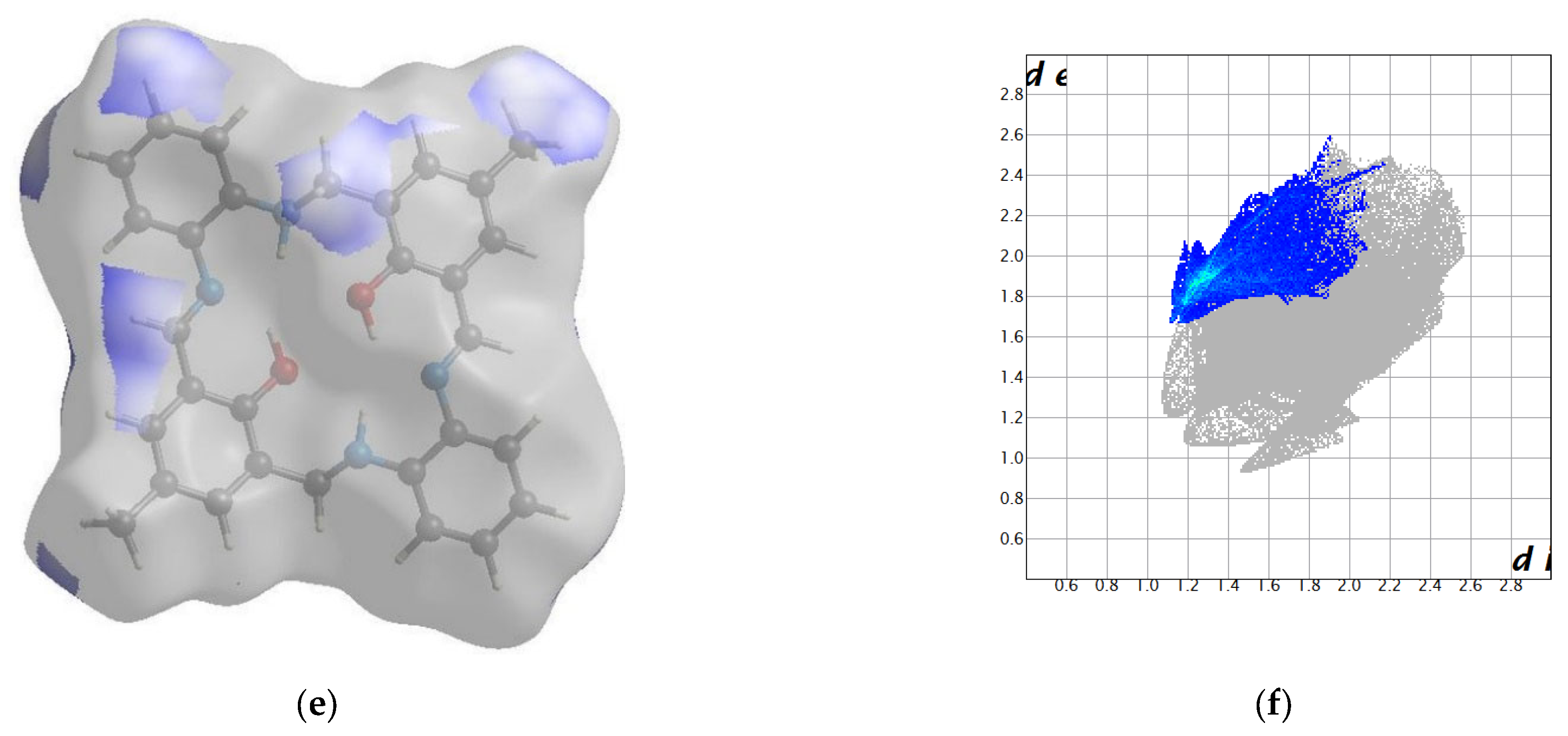
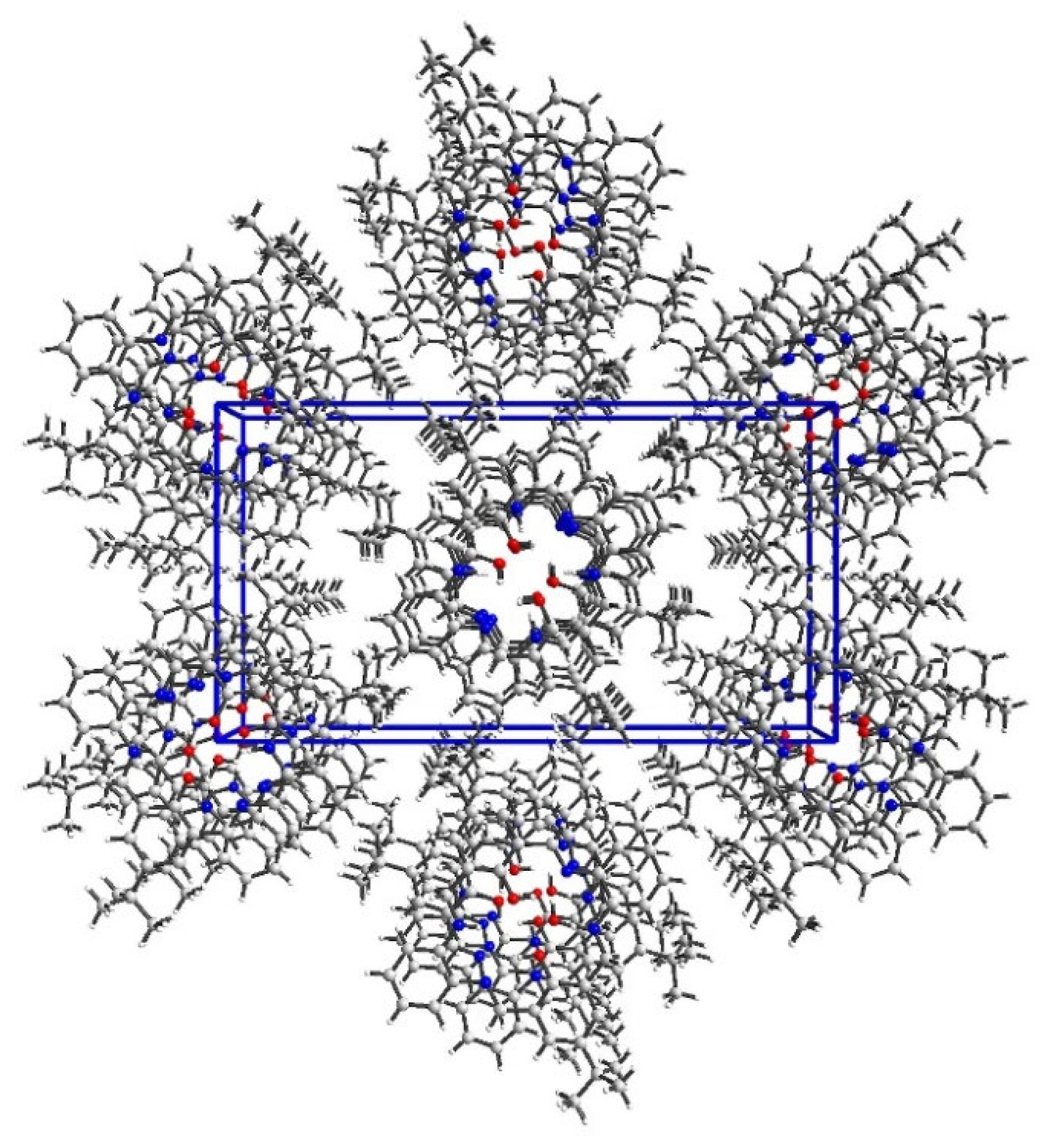
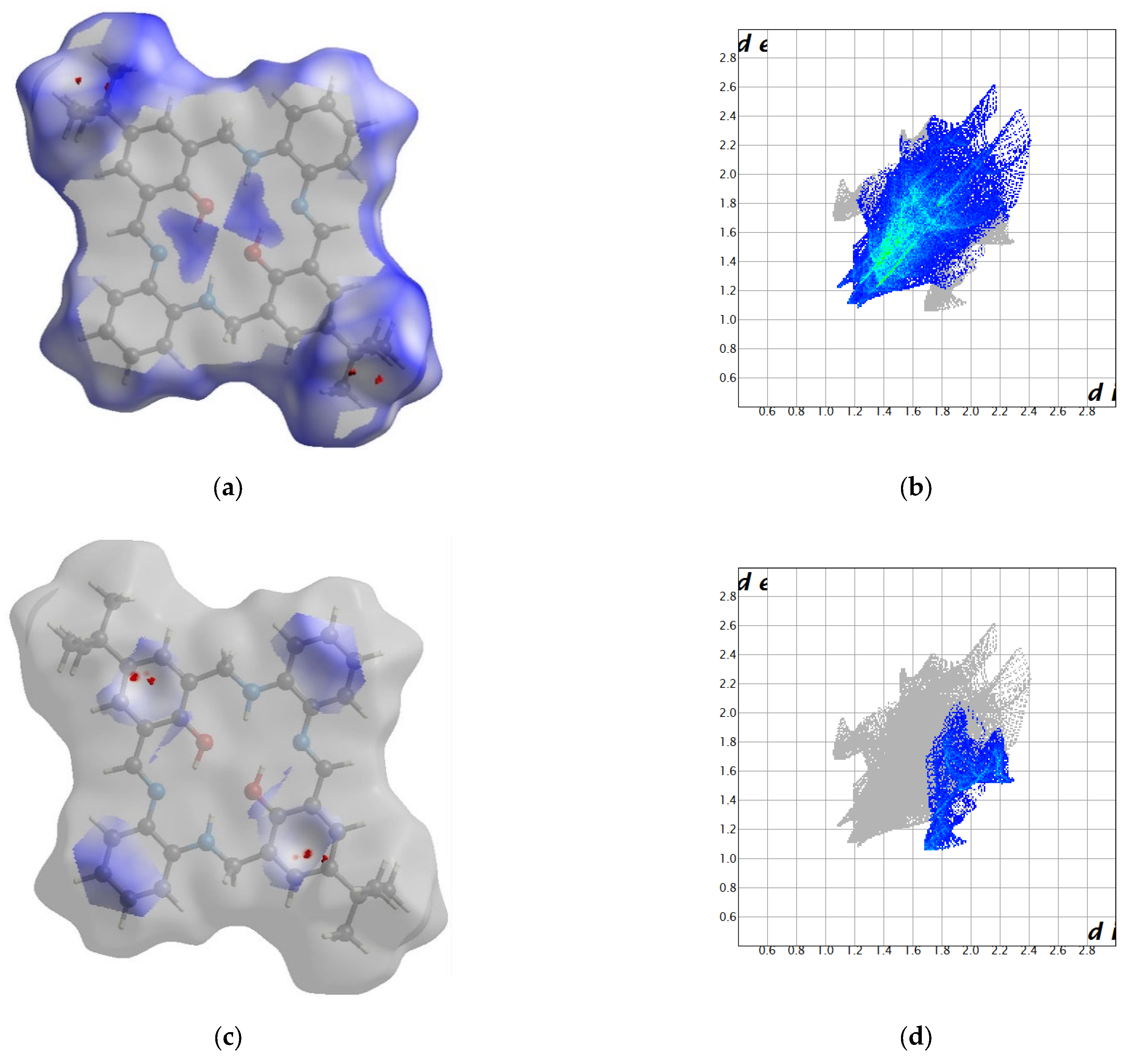
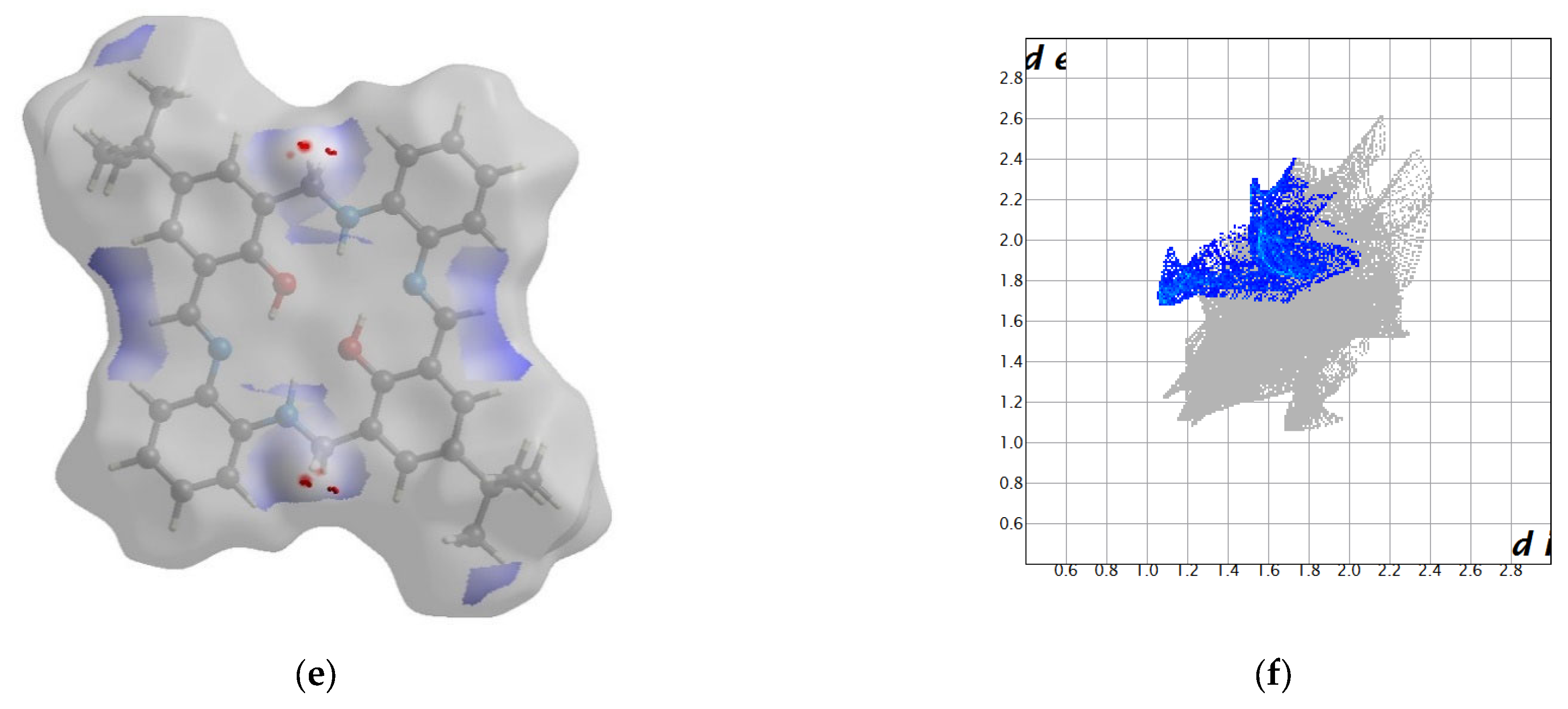
2.2. Crystal Structure Description—Hirshfeld Analysis
2.2.1. L1
2.2.2. L2
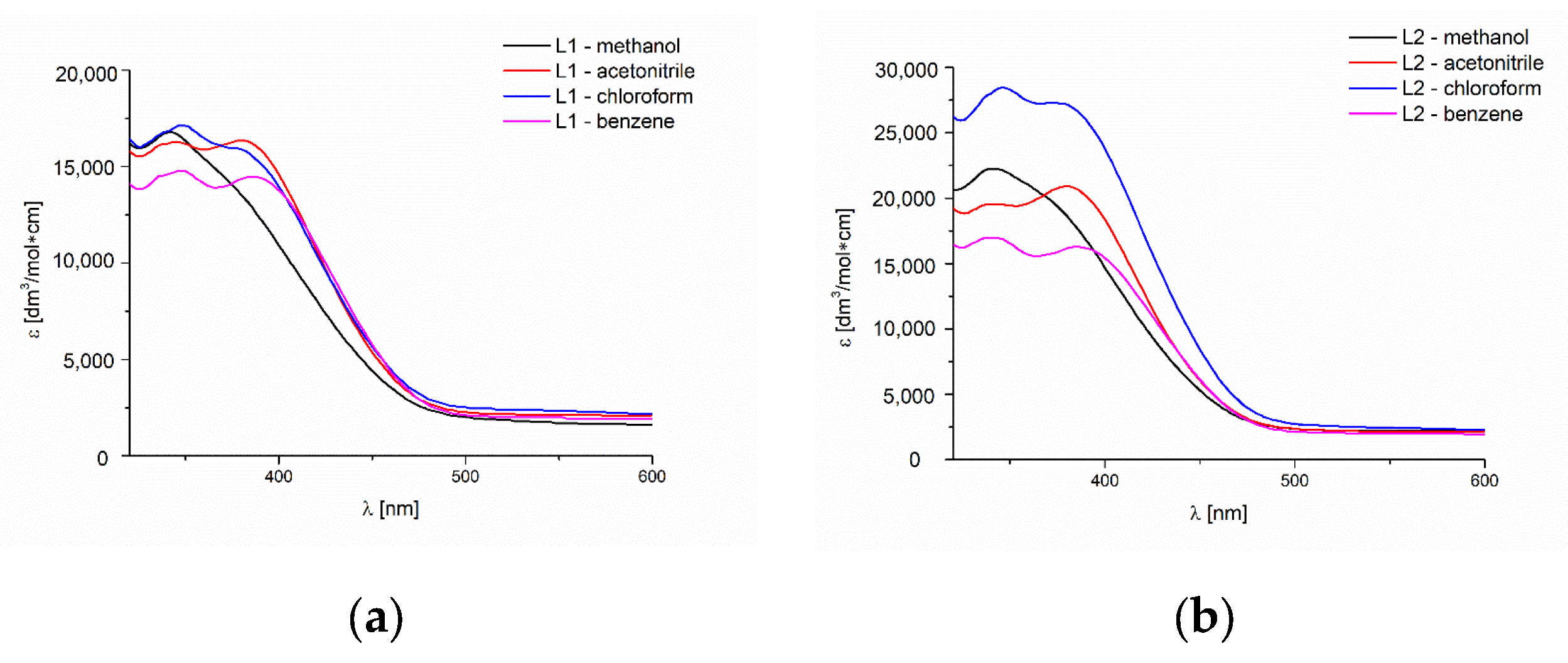
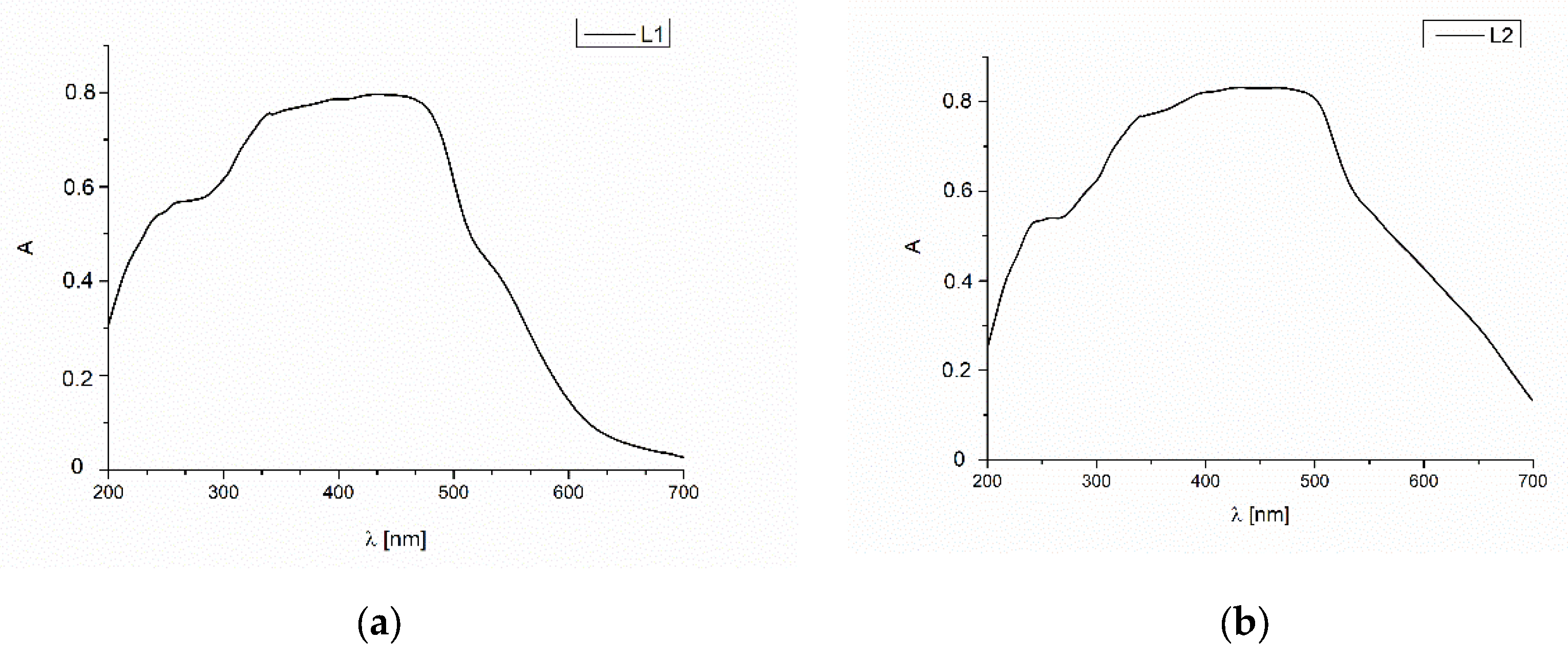
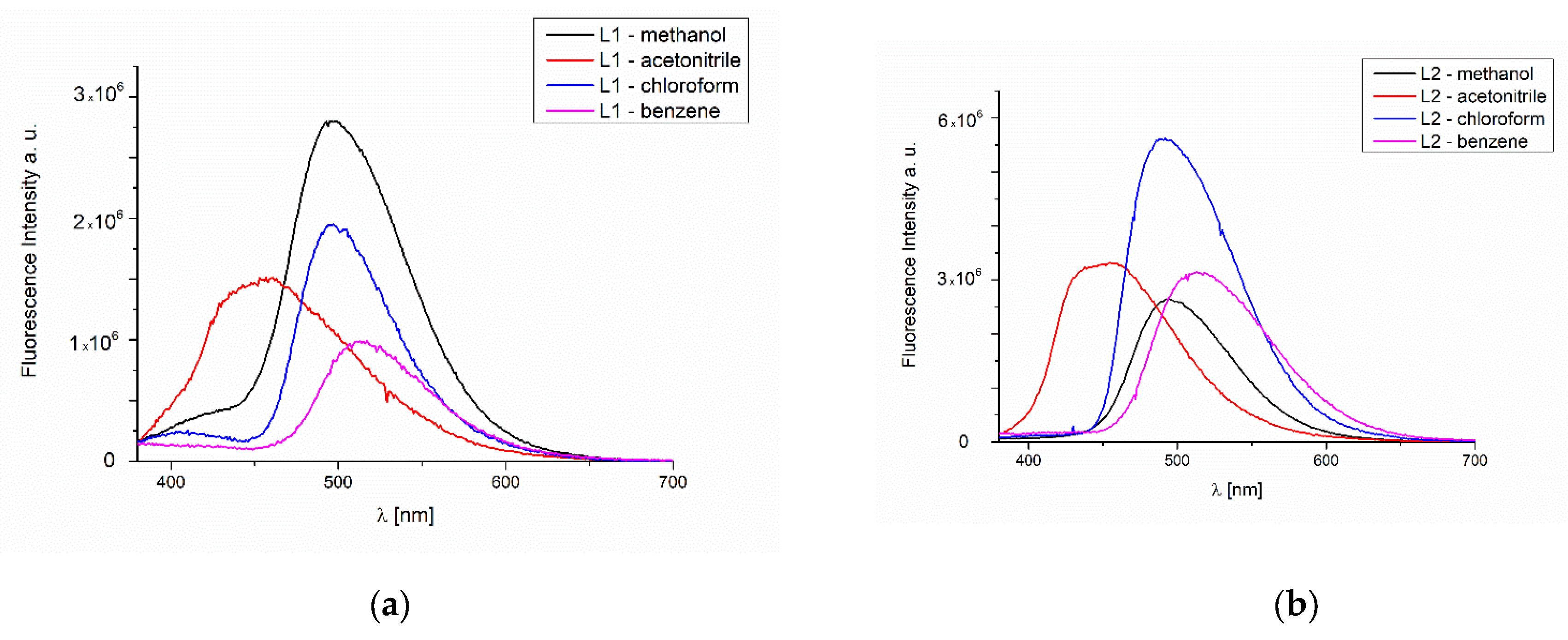
2.3. UV-Vis and Fluorescence Spectroscopy
2.4. Thin Materials of Macrocyclic Ligands
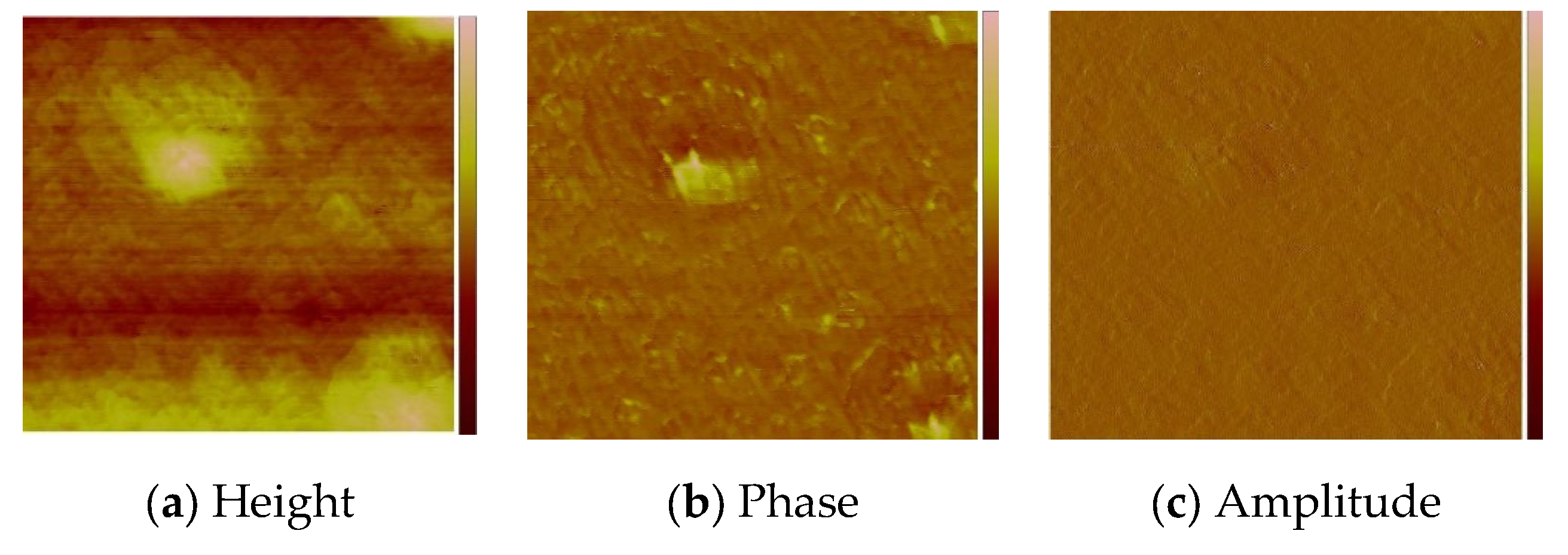
2.4.1. Thin Layers Obtained by Spin Coating
2.4.2. Thin Layers Obtained by Thermal Vapor Method
2.4.3. Spectroscopic Ellipsometry Results of the Thin Materials
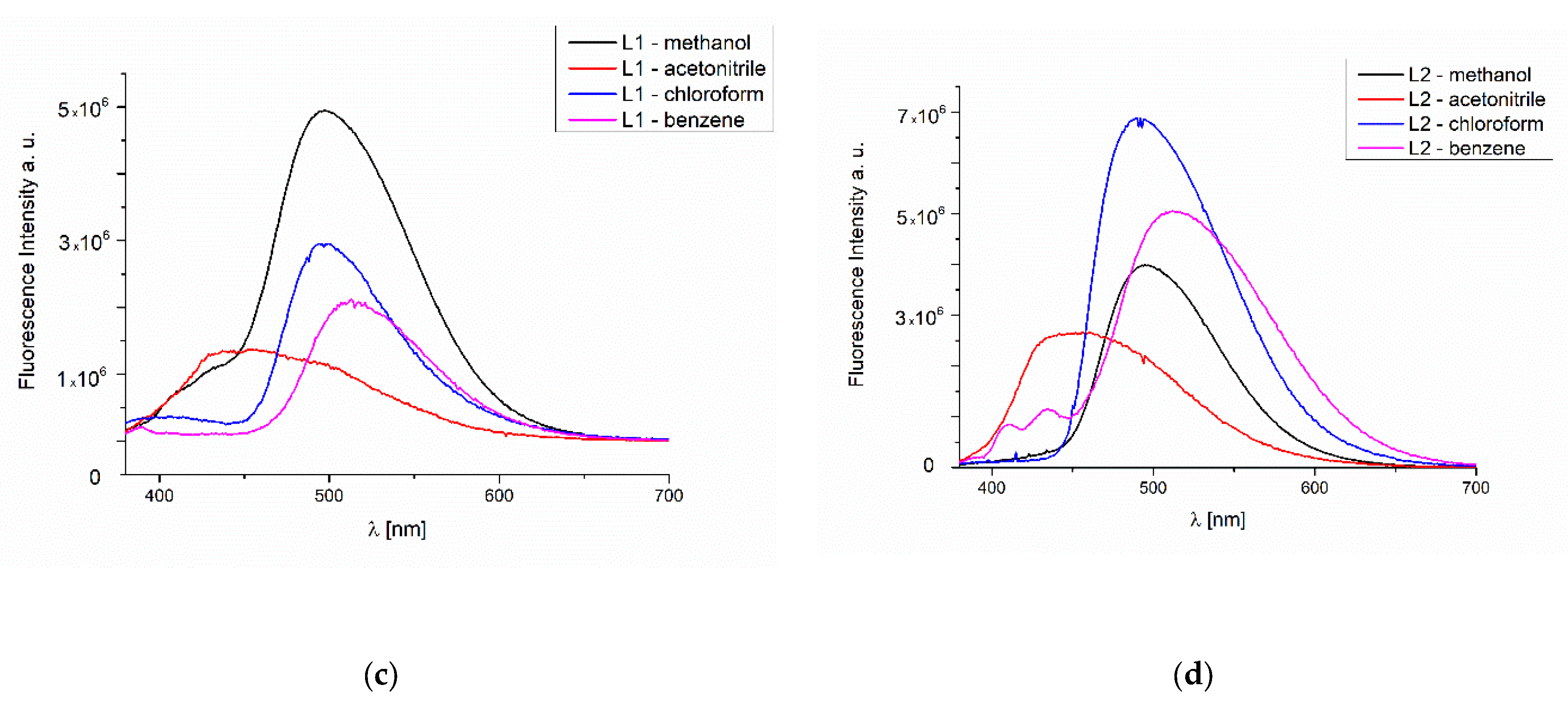
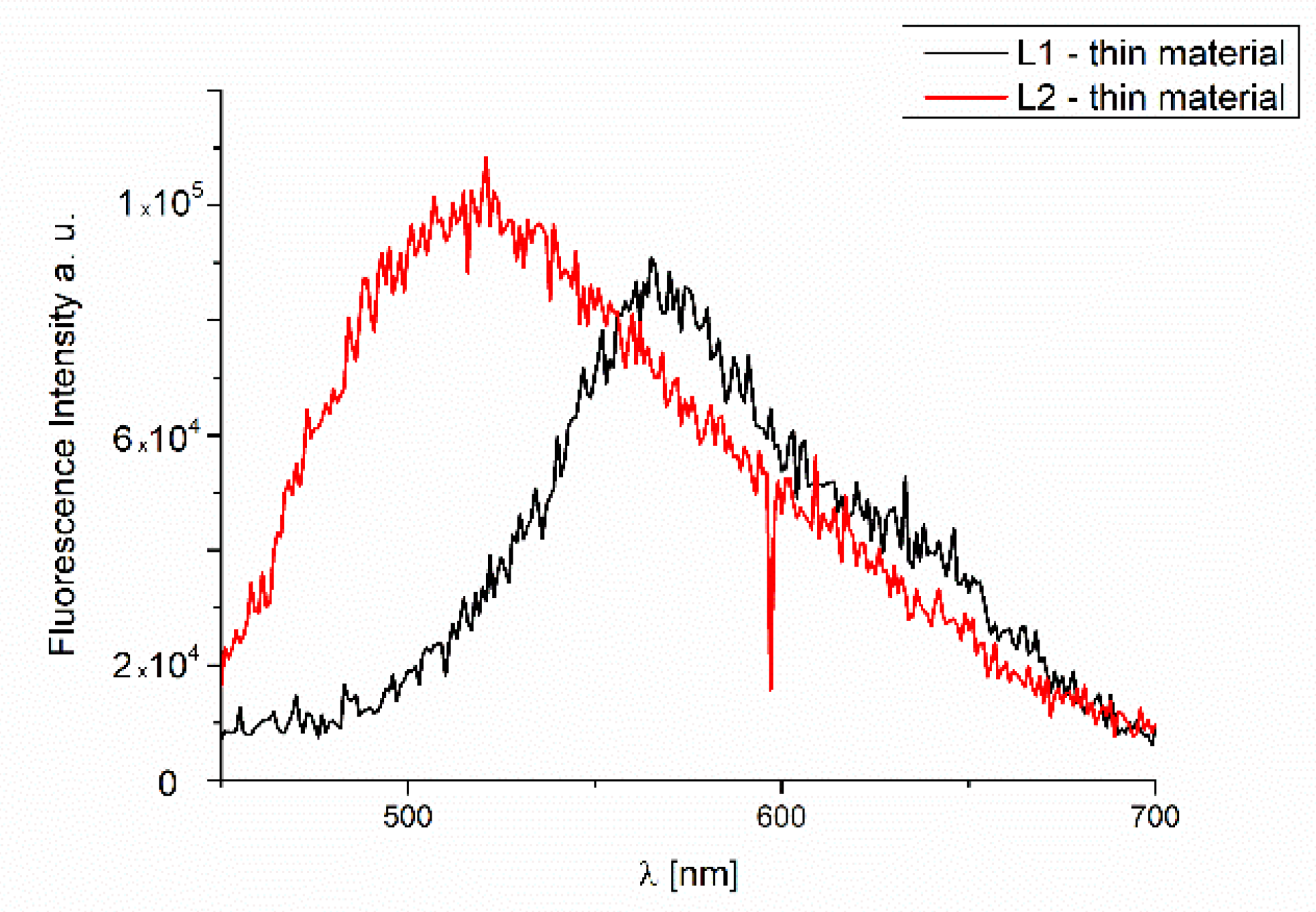
2.4.4. Fluorescence of Thin Materials
3. Materials and Methods
3.1. Methods and Instrumentation
3.2. Crystal Structure Determination
3.3. Computational Details
3.4. Thin Materials
3.4.1. Spin Coating
3.4.2. Thermal Vapor Deposition
3.5. Spectroscopic Ellipsometry
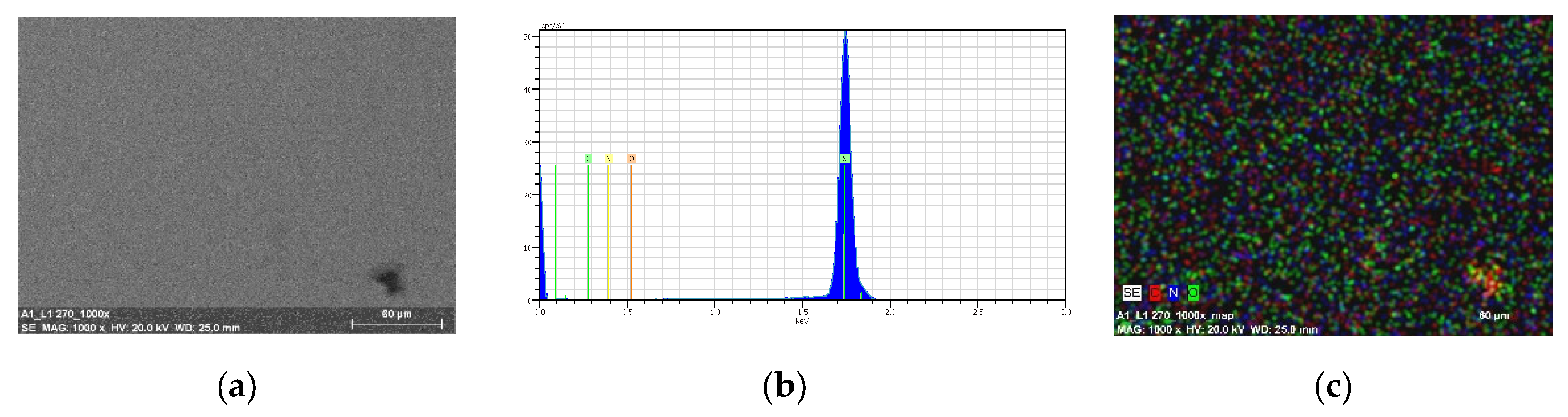
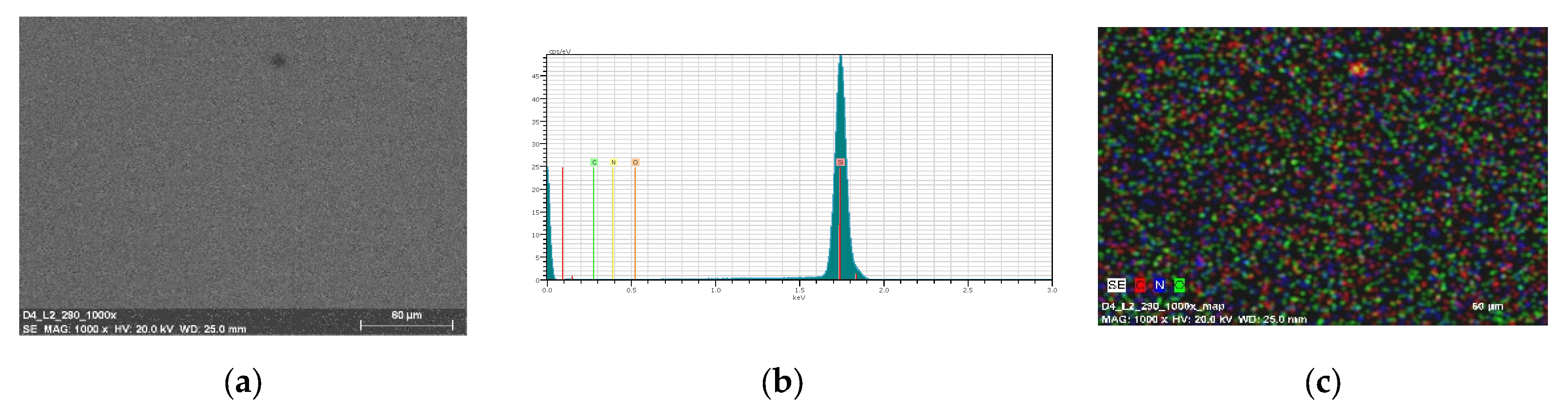
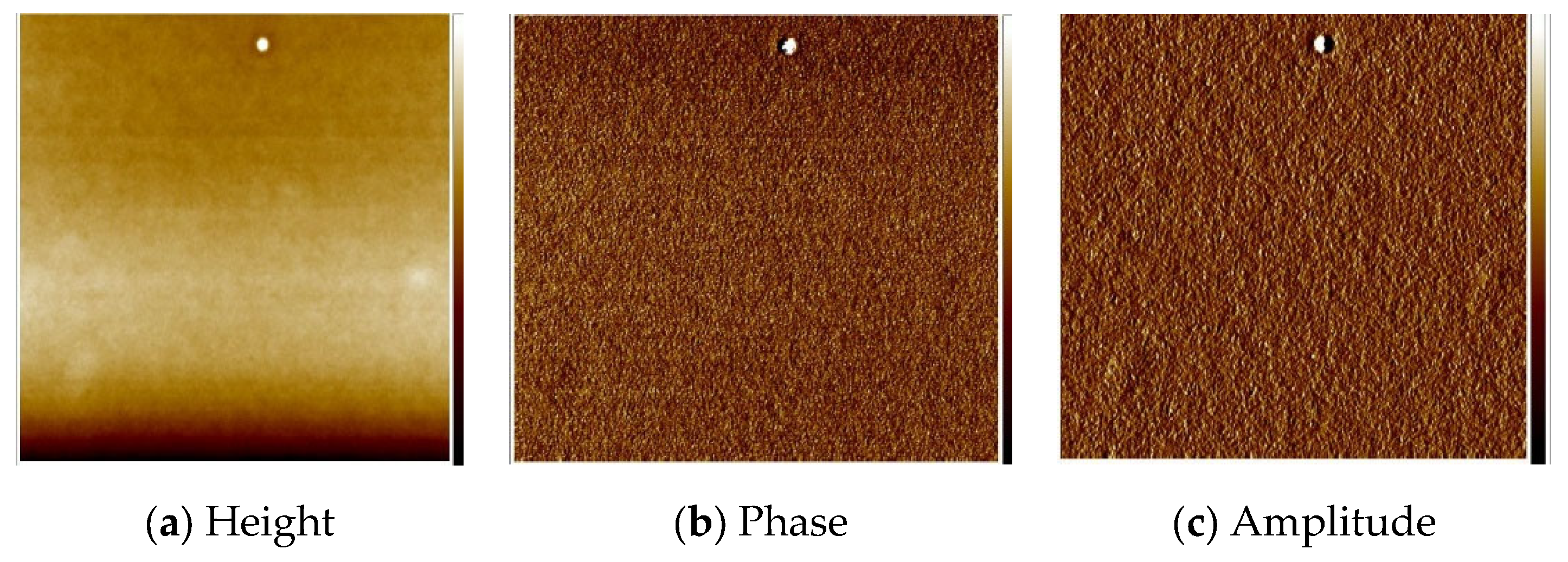
3.6. Morphology and Composition of the Materials
3.7. Synthesis of Compounds
3.7.1. L1
3.7.2. L2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, D.; Kumar, K.; Sharma, C. Antimicrobial active macrocyclic complexes of Cr(III), Mn(III) and Fe(III) with their spectroscopic approach. Eur. J. Med. Chem. 2009, 44, 3299–3304. [Google Scholar] [CrossRef]
- Rajakkani, P.; Alagarraj, A.; Thangavelu, S.A.G. Tetraaza macrocyclic Schiff base metal complexes bearing pendant groups: Synthesis, characterization and bioactivity studies. Inorg. Chem. Commun. 2021, 134, 108989. [Google Scholar] [CrossRef]
- Shalini, A.S.; Amaladasan, M.; Prasannabalaji, N.; Revathi, J.; Muralitharan, G. Synthesis, characterization and antimicrobial studies on 13-membered-N6-macrocyclic transition metal complexes containing trimethoprim. Arab. J. Chem. 2019, 12, 1176–1185. [Google Scholar] [CrossRef] [Green Version]
- Urbani, M.; Torres, T. A Constrained and “Inverted” [3 + 3] Salphen Macrocycle with an ortho-Phenylethynyl Substitution Pattern. Chem.-Eur. J. 2019, 26, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Feng, G.-F.; Hu, Y.; Chang, F.-F.; Huang, W. Two Types of Anion-Induced Reconstruction of Schiff-Base Macrocyclic Zinc Complexes: Ring-Contraction and Self-Assembly of a Molecular Box. Inorg. Chem. 2015, 55, 16–21. [Google Scholar] [CrossRef]
- Zheng, H.-W.; Yang, D.-D.; Liang, Q.-F.; Zheng, X.-J. Multi-stimuli-responsive Zn(II)-Schiff base complexes adjusted by rotatable aromatic rings. Dalton Trans. 2021, 50, 16803–16809. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Z.; Yang, J.; Yi, L.; Liao, L.; Xiao, X. Development of a method for the detection of Cu2+ in the environment and live cells using a synthesized spider web-like fluorescent probe. Biosens. Bioelectron. 2021, 182, 113174. [Google Scholar] [CrossRef]
- Mayhugh, J.T.; Niklas, J.E.; Forbes, M.G.; Gorden, J.D.; Gorden, A.E.V. Pyrrophens: Pyrrole-Based Hexadentate Ligands Tailor-Made for Uranyl (UO22+) Coordination and Molecular Recognition. Inorg. Chem. 2020, 59, 9560–9568. [Google Scholar] [CrossRef]
- Lindeboom, W.; Fraser, D.A.X.; Durr, C.B.; Williams, C.K. Heterodinuclear Zn(II), Mg(II) or Co(III) with Na(I) Catalysts for Carbon Dioxide and Cyclohexene Oxide Ring Opening Copolymerizations. Chem.-Eur. J. 2021, 27, 12224–12231. [Google Scholar] [CrossRef]
- Chinnaraja, E.; Arunachalam, R.; Suresh, E.; Sen, S.K.; Natarajan, R.; Subramanian, P.S. Binuclear Double-Stranded Helicates and Their Catalytic Applications in Desymmetrization of Mesodiols. Inorg. Chem. 2019, 58, 4465–4479. [Google Scholar] [CrossRef] [PubMed]
- Borisova, N.E.; Reshetova, M.D.; Ustynyuk, Y.A. Metal-Free Methods in the Synthesis of Macrocyclic Schiff Bases. Chem. Rev. 2006, 107, 46–79. [Google Scholar] [CrossRef] [PubMed]
- Gennarini, F.; David, R.; López, I.; Le Mest, Y.; Réglier, M.; Belle, C.; Thibon-Pourret, A.; Jamet, H.; Le Poul, N. Influence of Asymmetry on the Redox Properties of Phenoxo- and Hydroxo-Bridged Dicopper Complexes: Spectroelectrochemical and Theoretical Studies. Inorg. Chem. 2017, 56, 7707–7719. [Google Scholar] [CrossRef] [PubMed]
- Roznyatovsky, V.V.; Borisova, N.E.; Reshetova, M.D.; Ustynyuk, Y.A.; Aleksandrov, G.G.; Eremenko, I.L.; Moiseev, I.I. Dinuclear and polynuclear transition metal complexes with macrocyclic ligands. 6. New dinuclear copper(II) complexes with macrocyclic Schiff bases derived from 4-tert-butyl-2,6-diformylphenol. Bull. Acad. Sci. USSR Div. Chem. Sci. 2004, 53, 1208–1217. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.-R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 381, 94–118. [Google Scholar] [CrossRef]
- Sheoran, M.; Bhar, K.; Khan, T.A.; Sharma, A.K.; Naik, S.G. Phosphatase activity and DNA binding studies of dinuclear phenoxo-bridged zinc (II) complexes with an N, N, O-donor ligand and halide ions in a rare cis-configuration. Polyhedron 2017, 129, 82–91. [Google Scholar] [CrossRef]
- Wang, K.; Chen, K.; Prior, T.J.; Feng, X.; Redshaw, C. Pd-Immobilized Schiff Base Double-Layer Macrocycle: Synthesis, Structures, Peroxidase Mimic Activity, and Antibacterial Performance. ACS Appl. Mater. Interfaces 2021, 14, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Gregoliński, J.; Ślepokura, K.; Kłak, J.; Witwicki, M. Multinuclear Ni(II) and Cu(II) complexes of a meso 6 + 6 macrocyclic amine derived from trans-1,2-diaminocyclopentane and 2,6-diformylpyridine. Dalton Trans. 2022, 51, 9735–9747. [Google Scholar] [CrossRef]
- Vigato, P.; Tamburini, S.; Bertolo, L. The development of compartmental macrocyclic Schiff bases and related polyamine derivatives. Co-ord. Chem. Rev. 2007, 251, 1311–1492. [Google Scholar] [CrossRef]
- Chang, F.-F.; Zhang, K.; Huang, W. Schiff-base macrocyclic ZnII complexes based upon flexible pendant-armed extended dialdehydes. Dalton Trans. 2018, 48, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Mandal, L.; Majumder, S.; Mohanta, S. Syntheses, crystal structures and steady state and time-resolved fluorescence properties of a PET based macrocycle and its dinuclear ZnII/CdII/HgII complexes. Dalton Trans. 2016, 45, 17365–17381. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Adhikary, J.; Chakraborty, P.; Chakraborty, T.; Das, D. Macrocyclization of N,N′-propylenebis(3-formyl-5-tert-butylsalicylaldimine): A ratiometric fluorescence chemodosimeter for ZnII. RSC Adv. 2016, 6, 98620–98631. [Google Scholar] [CrossRef]
- Chakraborty, T.; Mukherjee, S.; Parveen, R.; Chandra, A.; Samanta, D.; Das, D. A combined experimental and theoretical rationalization of an unusual zinc(II)-mediated conversion of 18-membered Schiff-base macrocycles to 18-membered imine–amine macrocycles with imidazolidine side rings: An investigation of their bio-relevant catalytic activities. New J. Chem. 2021, 45, 2550–2562. [Google Scholar] [CrossRef]
- Wang, K.; Chen, K.; Bian, T.; Chao, Y.; Yamato, T.; Xing, F.; Prior, T.J.; Redshaw, C. Emission and theoretical studies of Schiff-base [2+2] macrocycles derived from 2,2′-oxydianiline and zinc complexes thereof. Dyes Pigments 2021, 190, 109300. [Google Scholar] [CrossRef]
- Ullmann, S.; Schnorr, R.; Handke, M.; Laube, C.; Abel, B.; Matysik, J.; Findeisen, M.; Rüger, R.; Heine, T.; Kersting, B. Zn2+ -Ion Sensing by Fluorescent Schiff Base Calix [4]arene Macrocycles. Chem.-Eur. J. 2017, 23, 3824–3827. [Google Scholar] [CrossRef] [PubMed]
- Malthus, S.J.; Cameron, S.A.; Brooker, S. Improved Access to 1,8-Diformyl-carbazoles Leads to Metal-Free Carbazole-Based [2 + 2] Schiff Base Macrocycles with Strong Turn-On Fluorescence Sensing of Zinc(II) Ions. Inorg. Chem. 2018, 57, 2480–2488. [Google Scholar] [CrossRef]
- Chang, F.-F.; Li, W.-Q.; Feng, F.-D.; Huang, W. Construction and Photoluminescent Properties of Schiff-Base Macrocyclic Mono-/Di-/Trinuclear ZnII Complexes with the Common 2-Ethylthiophene Pendant Arm. Inorg. Chem. 2019, 58, 7812–7821. [Google Scholar] [CrossRef] [PubMed]
- Ustynyuk, Y.A.; Borisova, N.; Nosova, V.M.; Reshetova, M.D.; Talismanov, S.S.; Nefedov, S.E.; Aleksandrov, G.A.; Eremenko, I.; Moiseev, I.I. Binuclear and polynuclear transition metal complexes with macrocyclic ligands. 2. New macrocyclic Schiff"s base in the reaction of 4-tert-butyl-2,6-diformylphenol with 1,2-diaminobenzene. Synthesis and structural, spectroscopic, and theoretical study. Bull. Acad. Sci. USSR Div. Chem. Sci. 2002, 51, 488–498. [Google Scholar] [CrossRef]
- Chinna Ayya Swamy, P.; Solel, E.; Reany, O.; Keinan, E. Synthetic Evolution of the Multifarene Cavity from Planar Predecessors. Chem.-Eur. J. 2018, 24, 15319–15328. [Google Scholar] [CrossRef] [PubMed]
- Aguiari, A.; Bullita, E.; Casellato, U.; Guerriero, P.; Tamburini, S.; Vigato, P. Macrocyclic and macroacyclic compartmental Schiff bases: Synthesis, characterization, X-ray structure and interaction with metal ions. Inorganica Chim. Acta 1992, 202, 157–171. [Google Scholar] [CrossRef]
- Barwiolek, M.; Jankowska, D.; Chorobinski, M.; Kaczmarek-Kędziera, A.; Łakomska, I.; Wojtulewski, S.; Muzioł, T.M. New dinuclear zinc(ii) complexes with Schiff bases obtained from o-phenylenediamine and their application as fluorescent materials in spin coating deposition. RSC Adv. 2021, 11, 24515–24525. [Google Scholar] [CrossRef]
- Berhanu, A.L.; Gaurav; Mohiuddin, I.; Malik, A.K.; Aulakh, J.S.; Kumar, V.; Kim, K.-H. A review of the applications of Schiff bases as optical chemical sensors. TrAC Trends Anal. Chem. 2019, 116, 74–91. [Google Scholar] [CrossRef]
- Alorabi, A.Q.; Abdelbaset, M.; Zabin, S.A. Colorimetric Detection of Multiple Metal Ions Using Schiff Base 1-(2-Thiophenylimino)-4-(N-dimethyl)benzene. Chemosensors 2019, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Chowdhury, T.; Ghosh, A.; Das, A.K.; Das, D. Effect of O-substitution in imidazole based Zn(II) dual fluorescent probes in the light of arsenate detection in potable water: A combined experimental and theoretical approach. Dalton Trans. 2022, 51, 7174–7187. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Maity, S.; Halder, S.; Dutta, B.; Jana, S.; Jana, K.; Sinha, C. Idiosyncatic recognition of Zn2+ and CN− using pyrazolyl-hydroxy-coumarin scaffold and live cell imaging: Depiction of luminescent Zn(II)-metallocryptand. Dalton Trans. 2022, 51, 3198–3212. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Wen, G.; Wang, J.; Yao, H.; Zhao, Y.; Lin, Z.; Ling, Q. A Λ-shaped donor–π–acceptor–π–donor molecule with AIEE and CIEE activity and sequential logic gate behaviour. J. Mater. Chem. C 2015, 3, 7267–7271. [Google Scholar] [CrossRef]
- Faure, M.D.M.; Lessard, B.H. Layer-by-layer fabrication of organic photovoltaic devices: Material selection and processing conditions. J. Mater. Chem. C 2020, 9, 14–40. [Google Scholar] [CrossRef]
- Rani, P.; Kiran; Chahal, S.; Priyanka; Kataria, R.; Kumar, P.; Kumar, S.; Sindhu, J. Unravelling the thermodynamics and binding interactions of bovine serum albumin (BSA) with thiazole based carbohydrazide: Multi-spectroscopic, DFT and molecular dynamics approach. J. Mol. Struct. 2022, 127, 133939. [Google Scholar] [CrossRef]
- Lalhruaizela; Marak, B.N.; Hazarika, B.; Pandey, S.K.; Kataria, R.; Singh, V.P. Study of self-assembly features in 4H-pyrans: Synthesis, Hirshfeld surface, and energy framework analysis. J. Mol. Struct. 2022, 1265, 133361. [Google Scholar] [CrossRef]
- Kitajgorodskij, A.I. Molecular Crystals and Molecules; Academic Press: New York, NY, USA, 1973; pp. 18–21. [Google Scholar]
- Barwiolek, M.; Wojtczak, A.; Kozakiewicz, A.; Babinska, M.; Tafelska-Kaczmarek, A.; Larsen, E.; Szlyk, E. The synthesis, characterization and fluorescence properties of new benzimidazole derivatives. J. Lumin. 2019, 211, 88–95. [Google Scholar] [CrossRef]
- Su, Q.; Wu, Q.-L.; Li, G.-H.; Liu, X.-M.; Mu, Y. Bis-salicylaldiminato zinc complexes: Syntheses, characterization and luminescent properties. Polyhedron 2007, 26, 5053–5060. [Google Scholar] [CrossRef]
- Woollam, J.A. Guide to Using WVASE32®; Wextech Systems Inc.: New York, NY, USA, 2010. [Google Scholar]
- Fujiwara, H. Spectroscopic Ellipsometry. Principles and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum: New York, NY, USA, 1974; ISBN 978-1-4615-8707-1. [Google Scholar]
- Gordillo, N.; Gonzalez-Arrabal, R.; Martin-Gonzalez, M.; Olivares, J.; Rivera, A.; Briones, F.; Agulló-López, F.; Boerma, D. DC triode sputtering deposition and characterization of N-rich copper nitride thin films: Role of chemical composition. J. Cryst. Growth 2008, 310, 4362–4367. [Google Scholar] [CrossRef]
- CrysAlis CCD. CrysAlis Red and CrysAlis CCD; Oxford Diffraction, Ltd.: Abingdon, UK, 2000. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barwiolek, M.; Jankowska, D.; Kaczmarek-Kędziera, A.; Wojtulewski, S.; Skowroński, L.; Rerek, T.; Popielarski, P.; Muziol, T.M. Experimental and Theoretical Studies of the Optical Properties of the Schiff Bases and Their Materials Obtained from o-Phenylenediamine. Molecules 2022, 27, 7396. https://doi.org/10.3390/molecules27217396
Barwiolek M, Jankowska D, Kaczmarek-Kędziera A, Wojtulewski S, Skowroński L, Rerek T, Popielarski P, Muziol TM. Experimental and Theoretical Studies of the Optical Properties of the Schiff Bases and Their Materials Obtained from o-Phenylenediamine. Molecules. 2022; 27(21):7396. https://doi.org/10.3390/molecules27217396
Chicago/Turabian StyleBarwiolek, Magdalena, Dominika Jankowska, Anna Kaczmarek-Kędziera, Slawomir Wojtulewski, Lukasz Skowroński, Tomasz Rerek, Paweł Popielarski, and Tadeusz M. Muziol. 2022. "Experimental and Theoretical Studies of the Optical Properties of the Schiff Bases and Their Materials Obtained from o-Phenylenediamine" Molecules 27, no. 21: 7396. https://doi.org/10.3390/molecules27217396
APA StyleBarwiolek, M., Jankowska, D., Kaczmarek-Kędziera, A., Wojtulewski, S., Skowroński, L., Rerek, T., Popielarski, P., & Muziol, T. M. (2022). Experimental and Theoretical Studies of the Optical Properties of the Schiff Bases and Their Materials Obtained from o-Phenylenediamine. Molecules, 27(21), 7396. https://doi.org/10.3390/molecules27217396







