Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells
Abstract
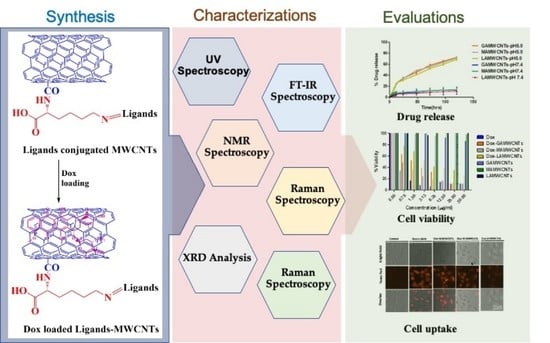
:1. Introduction
2. Results and Discussion
2.1. UV Spectroscopy
2.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3. NMR Spectroscopy
2.4. Raman Spectroscopy
2.5. X-ray Diffraction (XRD) Analysis
2.6. Particle Size Distribution and Zeta Potential Studies
2.7. Determination of Surface Morphology Using Field Emission Scanning Electron Microscope (FE-SEM)
2.8. Drug Loading
2.9. In Vitro Drug Release
2.10. In Vitro Cytotoxicity Assay
2.11. Cellular Uptake Analysis
3. Materials and Methods
3.1. General Materials
3.2. Cell Culture Reagents
3.3. Methods
3.3.1. Acylation of MWCNTs
3.3.2. Lysinated MWCNTs (LyMWCNTs)
3.3.3. Carbohydrate Conjugated with LyMWCNTs
3.4. Characterization and Evaluation of the Functionalized MWCNTs
3.4.1. UV Spectroscopy
3.4.2. Fourier Transform Infrared (FT-IR) Spectroscopy
3.4.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.4.4. Raman Spectroscopy
3.4.5. X-ray Diffraction (XRD) Analysis
3.4.6. Particle Size Distribution and Zeta Potential Studies
3.4.7. Surface Morphology Using Field Emission Scanning Electron Microscope (FE-SEM)
3.4.8. Drug Loading
3.4.9. Assessment of In Vitro Drug Release
3.4.10. Cell Cultures
3.4.11. In Vitro Cell Cytotoxicity
3.4.12. Cellular Uptake Analysis
3.4.13. Statical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wilson, B.E.; Jacob, S.; Yap, M.L.; Ferlay, J.; Bray, F.; Barton, M.B. Estimates of global chemotherapy demands and corresponding physician workforce requirements for 2018 and 2040: A population-based study. Lancet Oncol. 2019, 20, 769–780. [Google Scholar] [CrossRef]
- Ozgen, P.S.O.; Atasoy, S.; Kurt, B.Z.; Durmus, Z.; Yigit, G.; Dag, A. Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy. J. Mater. Chem. B 2020, 8, 3123–3137. [Google Scholar] [CrossRef] [PubMed]
- Dizaji, B.F.; Farboudi, A.; Rahbar, A.; Azarbaijan, M.H.; Asgary, M.R. The role of single-and multi-walled carbon nanotube in breast cancer treatment. Ther. Deliv. 2020, 11, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, P.Y.; Hettiarachchi, S.D.; Zhou, Y.; Ouhtit, A.; Seven, E.S.; Oztan, C.Y.; Celik, E.; Leblanc, R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2019, 1871, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Wahab, R.; Ahmad, J.; Farshori, N.N.; Musarrat, J.; Al-Khedhairy, A.A. Evaluation of cytotoxic responses of raw and functionalized multi-walled carbon nanotubes in human breast cancer (MCF-7) cells. Vacuum 2017, 146, 578–585. [Google Scholar] [CrossRef]
- Neupane, R.; Boddu, S.H.; Abou-Dahech, M.S.; Bachu, R.D.; Terrero, D.; Babu, R.J.; Tiwari, A.K. Transdermal delivery of chemotherapeutics: Strategies, requirements, and opportunities. Pharmaceutics 2021, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Kumar, A.; Bhatkar, D.; Sharma, N.K. Molecular avenues in targeted doxorubicin cancer therapy. Future Oncol. 2020, 16, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Battogtokh, G.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-targeting anticancer agent conjugates and nanocarrier systems for cancer treatment. Front. Pharmacol. 2018, 9, 922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistone, A.; Iannazzo, D.; Ansari, S.; Milone, C.; Salamò, M.; Galvagno, S.; Cirmi, S.; Navarra, M. Tunable doxorubicin release from polymer-gated multiwalled carbon nanotubes. Int. J. Pharm. 2016, 515, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Chudoba, D.; Łudzik, K.; Jażdżewska, M.; Wołoszczuk, S. Kinetic and equilibrium studies of doxorubicin adsorption onto carbon nanotubes. Int. J. Mol. Sci. 2020, 21, 8230. [Google Scholar] [CrossRef]
- Cao, X.; Tao, L.; Wen, S.; Hou, W.; Shi, X. Hyaluronic acid-modified multiwalled carbon nanotubes for targeted delivery of doxorubicin into cancer cells. Carbohydr. Res. 2015, 405, 70–77. [Google Scholar] [CrossRef]
- Hoa, N.T.; Siggelc, M.; Camachoa, K.V.; Bhaskarac, R.M.; Hicksa, M.J.; Yaoa, Y.C.; Zhanga, Y.; Jurgen, K.; Hummerc, G.; Noy, A. Membrane fusion and drug delivery with carbon nanotube porins. Biophys. Comput. Biol. 2021, 118, e2016974118. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.K.; Thotakura, N.; Kumar, R.; Kumar, P.; Singh, B.; Chitkara, D.; Raza, K. Chitosan-modified PLGA polymeric nanocarriers with better delivery potential for tamoxifen. Int. J. Biol. Macromol. 2016, 93, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Kumar, D.; Kiran, C.; Kumar, M.; Guru, S.K.; Kumar, P.; Arora, S.; Sharma, G.; Bhushan, S.; Katare, O. Conjugation of docetaxel with multiwalled carbon nanotubes and codelivery with piperine: Implications on pharmacokinetic profile and anticancer activity. Mol. Pharm. 2016, 13, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhang, Q.; Wang, S.; Bharate, P.; Varela-Aramburu, S.; Lu, M.; Seeberger, P.H.; Yin, J. Tumour-targeted drug delivery with mannose-functionalized nanoparticles self-assembled from amphiphilic β-cyclodextrins. Chem.–A Eur. J. 2016, 22, 15216–15221. [Google Scholar] [CrossRef] [PubMed]
- Chadar, R.; Afzal, O.; Alqahtani, S.M.; Kesharwani, P. Carbon nanotubes as an emerging nanocarrier for the delivery of doxorubicin for improved chemotherapy. Colloids Surf. B Biointerfaces 2021, 208, 112044. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ramazani, A. Anticancer DOX delivery system based on CNTs: Functionalization, targeting and novel technologies. J. Control. Release 2020, 327, 198–224. [Google Scholar] [CrossRef]
- Grosso, R.; de-Paz, M.-V. Thiolated-Polymer-Based Nanoparticles as an Avant-Garde Approach for Anticancer Therapies—Reviewing Thiomers from Chitosan and Hyaluronic Acid. Pharmaceutics 2021, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Singh, A.; Sharma, P.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- de Menezes, B.R.C.; Rodrigues, K.F.; da Silva Fonseca, B.C.; Ribas, R.G.; do Amaral Montanheiro, T.L.; Thim, G.P. Recent advances in the use of carbon nanotubes as smart biomaterials. J. Mater. Chem. B 2019, 7, 1343–1360. [Google Scholar] [CrossRef]
- Singhai, N.J.; Maheshwari, R.; Jain, N.K.; Ramteke, S. Chondroitin sulphate and α-tocopheryl succinate tethered multiwalled carbon nanotubes for dual-action therapy of triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 102080. [Google Scholar] [CrossRef]
- Singhai, N.J.; Maheshwari, R.; Ramteke, S. CD44 receptor targeted ‘smart’multi-walled carbon nanotubes for synergistic therapy of triple-negative breast cancer. Colloid Interface Sci. Commun. 2020, 35, 100235. [Google Scholar] [CrossRef]
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surf. B Biointerfaces 2017, 154, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Calavia, P.G.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangia-Makker, P.; Conklin, J.; Hogan, V.; Raz, A. Carbohydrate-binding proteins in cancer, and their ligands as therapeutic agents. Trends Mol. Med. 2002, 8, 187–192. [Google Scholar] [CrossRef]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. A review of glycosylated carriers for drug delivery. Biomaterials 2012, 33, 4166–4186. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Huang, H. Sugar ligand-mediated drug delivery. Future Med. Chem. 2020, 12, 161–171. [Google Scholar] [CrossRef]
- Garg, N.K.; Singh, B.; Kushwah, V.; Tyagi, R.K.; Sharma, R.; Jain, S.; Katare, O.P. The ligand (s) anchored lipobrid nanoconstruct mediated delivery of methotrexate: An effective approach in breast cancer therapeutics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2043–2060. [Google Scholar] [CrossRef]
- Garg, N.K.; Singh, B.; Jain, A.; Nirbhavane, P.; Sharma, R.; Tyagi, R.K.; Kushwah, V.; Jain, S.; Katare, O.P. Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeutics. Colloids Surf. B Biointerfaces 2016, 146, 114–126. [Google Scholar] [CrossRef]
- Qi, X.; Rui, Y.; Fan, Y.; Chen, H.; Ma, N.; Wu, Z. Galactosylated chitosan-grafted multiwall carbon nanotubes for pH-dependent sustained release and hepatic tumor-targeted delivery of doxorubicin in vivo. Colloids Surf. B Biointerfaces 2015, 133, 314–322. [Google Scholar] [CrossRef]
- Joshi, D.C.; Saxena, S.; Jayakannan, M. Development of l-lysine based biodegradable polyurethanes and their dual-responsive amphiphilic nanocarriers for drug delivery to cancer cells. ACS Appl. Polym. Mater. 2019, 1, 1866–1880. [Google Scholar] [CrossRef]
- Boddu, S.H.; Bhargav, P.; Karla, P.K.; Jacob, S.; Adatiya, M.D.; Dhameliya, T.M.; Ranch, K.M.; Tiwari, A.K. Polyamide/Poly (Amino Acid) Polymers for Drug Delivery. J. Funct. Biomater. 2021, 12, 58. [Google Scholar] [CrossRef]
- Dhankhar, R.; Gupta, V.; Kumar, S.; Kapoor, R.K.; Gulati, P. Microbial enzymes for deprivation of amino acid metabolism in malignant cells: Biological strategy for cancer treatment. Appl. Microbiol. Biotechnol. 2020, 104, 2857–2869. [Google Scholar] [CrossRef]
- Uttekar, P.S.; Lakade, S.H.; Beldar, V.K.; Harde, M.T. Facile synthesis of multi-walled carbon nanotube via folic acid grafted nanoparticle for precise delivery of doxorubicin. IET Nanobiotechnol. 2019, 13, 688–696. [Google Scholar] [CrossRef]
- Jain, A.K.; Dubey, V.; Mehra, N.K.; Lodhi, N.; Nahar, M.; Mishra, D.K.; Jain, N.K. Carbohydrate-conjugated multiwalled carbon nanotubes: Development and characterization. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Zardini, H.Z.; Shanbedi, M.; Maghrebi, M.; Baniadam, M.; Tolueinia, B. Efficient method for functionalization of carbon nanotubes by lysine and improved antimicrobial activity and water-dispersion. Mater. Lett. 2012, 72, 153–156. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Amiri, A.; Shanbedi, M.; Maghrebi, M.; Baniadam, M. Enhanced antibacterial activity of amino acids-functionalized multi walled carbon nanotubes by a simple method. Colloids Surf. B Biointerfaces 2012, 92, 196–202. [Google Scholar] [CrossRef]
- Selvakannan, P.; Mandal, S.; Phadtare, S.; Pasricha, R.; Sastry, M. Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible. Langmuir 2003, 19, 3545–3549. [Google Scholar] [CrossRef]
- Guo, C.; Holland, G.P.; Yarger, J.L. Lysine-Capped Silica Nanoparticles: A Solid-State NMR Spectroscopy Study. Mater. Res. Soc. 2017, 1, 2261–2266. [Google Scholar] [CrossRef]
- Zeini, D.; Glover, J.C.; Knudsen, K.D.; Nyström, B. Influence of Lysine and TRITC Conjugation on the Size and Structure of Dextran Nanoconjugates with Potential for Biomolecule Delivery to Neurons. ACS Appl. Bio Mater. 2021, 4, 6832–6842. [Google Scholar] [CrossRef]
- Chen, X.; Lai, H.; Xiao, C.; Tian, H.; Chen, X.; Tao, Y.; Wang, X. New bio-renewable polyester with rich side amino groups from L-lysine via controlled ring-opening polymerization. Polym. Chem. R. Soc. Chem. 2014, 5, 6495–6502. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Morariu, S.; Tartau-Mititelu, L. Development of biocompatible glycodynameric hydrogels joining two natural motifs by dynamic constitutional chemistry. Carbohydr. Polym. 2017, 170, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Iftime, M.-M.; Morariu, S.; Marin, L. Salicyl-imine-chitosan hydrogels: Supramolecular architecturing as a crosslinking method toward multifunctional hydrogels. Carbohydr. Polym. 2017, 165, 39–50. [Google Scholar] [CrossRef]
- Muda, M.; Ramli, M.; Isa, S.M.; Halin, D.; Talip, L.; Mazelan, N.; Anhar, N.; Danial, N. Structural and Morphological Investigation for Water-Processed Graphene Oxide/Single-Walled Carbon Nanotubes Hybrids; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012030. [Google Scholar]
- Delhaes, P.; Couzi, M.; Trinquecoste, M.; Dentzer, J.; Hamidou, H.; Vix-Guterl, C. A comparison between Raman spectroscopy and surface characterizations of multiwall carbon nanotubes. Carbon 2006, 44, 3005–3013. [Google Scholar] [CrossRef]
- Haroun, A.; Gospodinova, Z.; Krasteva, N. Amino Acid Functionalization of Multi-Walled Carbon Nanotubes for Enhanced Apatite Formation and Biocompatibility. Nano Biomed. Eng. 2021, 13, 380–393. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Improved solubilization of multiwalled carbon nanotubes (MWCNTs) in water by surface functionalization with d-glucose and d-fructose: Properties comparison of functionalized MWCNTs/alanine-based poly (amide–imide) nanocomposites. High Perform. Polym. 2016, 28, 936–944. [Google Scholar] [CrossRef]
- Mallakpour, S. Fructose functionalized MWCNT as a filler for starch nanocomposites: Fabrication and characterizations. Prog. Org. Coat. 2018, 114, 244–249. [Google Scholar] [CrossRef]
- Zan, W.; Zhaozan, F.; Bengt, S. A Comparative Study on Thermal Conductivity and Rheology Properties of Alumina and Multi-Walled Nanotube Nanofluids. Front. Heat Mass Transf. 2014, 18, 1–10. [Google Scholar]
- Ruhung, W.; Lohray, R.; Chow, E.; Gangupantula, P.; Loren, S.R.D. Selective Uptake of Carboxylated Multi-Walled Carbon Nanotubes by Class A Type 1 Scavenger Receptors and Impaired Phagocytosis in Alveolar Macrophages. Nanomaterials 2020, 10, 2417. [Google Scholar]
- De Sousa, M.; Martinez, D.S.T.; Alves, O.L. Alternative mannosylation method for nanomaterials: Application to oxidized debris-free multiwalled carbon nanotubes. J. Nanopart. Res. 2016, 18, 143. [Google Scholar] [CrossRef]
- Murugesan, R.; Haldorai, Y.; Sibi, L.; Sureshkumar, R. Ibrutinib conjugated surface-functionalized multiwalled carbon nanotubes and its biopolymer composites for targeting prostate carcinoma. J. Mater. Sci. 2021, 56, 18684–18696. [Google Scholar] [CrossRef]
- Kumar, N.A.; Bund, A.; Cho, B.G.; Lim, K.T.; Jeong, Y.T. Novel amino-acid-based polymer/multi-walled carbon nanotube bio-nanocomposites: Highly water dispersible carbon nanotubes decorated with gold nanoparticles. Nanotechnology 2009, 20, 225608. [Google Scholar] [CrossRef]
- Sharma, P.; Jain, K.; Jain, N.; Mehra, N.K. Ex vivo and in vivo performance of anti-cancer drug loaded carbon nanotubes. J. Drug Deliv. Sci. Technol. 2017, 41, 134–143. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, R.; Hu, Y.; Sun, R.; Song, T.; Shi, X.; Yin, S. Stacking of doxorubicin on folic acid-targeted multiwalled carbon nanotubes for in vivo chemotherapy of tumors. Drug Deliv. 2018, 25, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Sutoo, S.; Maeda, T.; Suzuki, A.; Kato, Y. Adaptation to chronic acidic extracellular pH elicits a sustained increase in lung cancer cell invasion and metastasis. Clin. Exp. Metastasis 2020, 37, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Dang, G.; Zhou, H.; Jing, J.; Chen, C. Efficient direct water dispersion of multi-walled carbon nanotubes by functionalization with lysine. Mater. Lett. 2007, 61, 5285–5287. [Google Scholar] [CrossRef]
- Mulvey, J.J.; Feinberg, E.N.; Alidori, S.; McDevitt, M.R.; Heller, D.A.; Scheinberg, D.A. Synthesis, pharmacokinetics, and biological use of lysine-modified single-walled carbon nanotubes. Int. J. Nanomed. 2014, 9, 4245. [Google Scholar]
- Pastorin, G.; Wu, W.; Wieckowski, S.; Briand, J.-P.; Kostarelos, K.; Prato, M.; Bianco, A. Double functionalisation of carbon nanotubes for multimodal drug delivery. Chem. Commun. 2006, 11, 1182–1184. [Google Scholar] [CrossRef]
- Pruthi, J.; Mehra, N.K.; Jain, N.K. Macrophages targeting of amphotericin B through mannosylated multiwalled carbon nanotubes. J. Drug Target. 2012, 20, 593–604. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: Optimization of length and PEGylation degree. Colloids Surf. B Biointerfaces 2018, 168, 43–49. [Google Scholar] [CrossRef]
- Wu, H.; Shi, H.; Zhang, H.; Wang, X.; Yang, Y.; Yu, C.; Hao, C.; Du, J.; Hu, H.; Yang, S. Prostate stem cell antigen antibody-conjugated multiwalled carbon nanotubes for targeted ultrasound imaging and drug delivery. Biomaterials 2014, 35, 5369–5380. [Google Scholar] [CrossRef]
- Neupane, R.; Malla, S.; Abou-Dahech, M.S.; Balaji, S.; Kumari, S.; Waiker, D.K.; Moorthy, N.; Trivedi, P.; Ashby, C.R.; Karthikeyan, C. Antiproliferative Efficacy of N-(3-chloro-4-fluorophenyl)-6, 7-dimethoxyquinazolin-4-amine, DW-8, in Colon Cancer Cells Is Mediated by Intrinsic Apoptosis. Molecules 2021, 26, 4417. [Google Scholar] [CrossRef]












| S. No. | Formulations | Peak Position (cm−1) | Interpretation |
|---|---|---|---|
| 1 | COOHMWCNTs | 3423 | O-H stretching of carboxylic acid |
| 2358 | C-H stretching vibration | ||
| 1701 | C=O stretching of carboxylic acid | ||
| 2 | COClMWCNTs | 2357 | C-H stretching vibration |
| 801 | C-Cl stretching of an acylated group | ||
| 3 | LyMWCNTs | 3440 | N-H and O-H stretching of an amine and carboxylic group |
| 2359 | C-H stretching vibration | ||
| 1677 | C=O stretching of an amide bond | ||
| 1403 | -C-N stretch vibration | ||
| 4 | GAMWCNTs | 3433 | O-H stretching of a free hydroxylic group |
| 1625 | -C=N- stretch of an imine bond | ||
| 1401 | -C-N- stretching vibration | ||
| 5 | MAMWCNTs | 3428 | O-H stretching of a free hydroxylic group |
| 1623 | -C=N- stretch of imine bond | ||
| 1407 | -C-N- stretching vibration | ||
| 6 | LAMWCNTs | 3430 | O-H stretching of free a hydroxylic group |
| 1629 | -C=N- stretch of an imine bond | ||
| 1414 | -C-N- stretching vibration |
| S. No. | Formulations | Raman Peak Position (cm−1) | ID/IG Ratio | ||
|---|---|---|---|---|---|
| D-Band | G-Band | 2D-Band | |||
| 1 | COOHMWCNTs | 1359 | 1579 | 2709 | 0.82 |
| 2 | COClMWCNTs | 1350 | 1573 | 2702 | 0.90 |
| 3 | LyMWCNTs | 1356 | 1576 | 2709 | 0.94 |
| 4 | GAMWCNTs | 1358 | 1576 | 2702 | 0.78 |
| 5 | MAMWCNTs | 1357 | 1575 | 2707 | 0.75 |
| 6 | LAMWCNTs | 1354 | 1567 | 2702 | 0.77 |
| Formulations | Particle Size (nm) ± SD | PDI ± SD | Zeta Potential (mV) ± SD |
|---|---|---|---|
| COOHMWCNTs | 112 ± 1.08 | 0.31 ± 0.03 | −14.4 ± 1.06 |
| LyMWCNTs | 128 ± 1.25 | 0.25 ± 0.05 | 12.8 ± 1.32 |
| Dox-GAMWCNTs | 171 ± 0.95 | 0.21 ± 0.01 | 19.7 ± 1.09 |
| Dox-MAMWCNTs | 204 ± 0.50 | 0.23 ± 0.03 | 16.6 ± 1.41 |
| Dox-LAMWCNTs | 157 ± 1.06 | 0.24 ± 0.04 | 15.9 ± 1.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, C.K.; Neupane, R.; Karthikeyan, C.; Ashby, C.R., Jr.; Babu, R.J.; Boddu, S.H.S.; Tiwari, A.K.; Moorthy, N.S.H.N. Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells. Molecules 2022, 27, 7461. https://doi.org/10.3390/molecules27217461
Thakur CK, Neupane R, Karthikeyan C, Ashby CR Jr., Babu RJ, Boddu SHS, Tiwari AK, Moorthy NSHN. Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells. Molecules. 2022; 27(21):7461. https://doi.org/10.3390/molecules27217461
Chicago/Turabian StyleThakur, Chanchal Kiran, Rabin Neupane, Chandrabose Karthikeyan, Charles R. Ashby, Jr., R. Jayachandra Babu, Sai H. S. Boddu, Amit K. Tiwari, and Narayana Subbiah Hari Narayana Moorthy. 2022. "Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells" Molecules 27, no. 21: 7461. https://doi.org/10.3390/molecules27217461
APA StyleThakur, C. K., Neupane, R., Karthikeyan, C., Ashby, C. R., Jr., Babu, R. J., Boddu, S. H. S., Tiwari, A. K., & Moorthy, N. S. H. N. (2022). Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells. Molecules, 27(21), 7461. https://doi.org/10.3390/molecules27217461