The Chemical Composition and Antimitotic, Antioxidant, Antibacterial and Cytotoxic Properties of the Defensive Gland Extract of the Beetle, Luprops tristis Fabricius
Abstract
:1. Introduction
2. Results
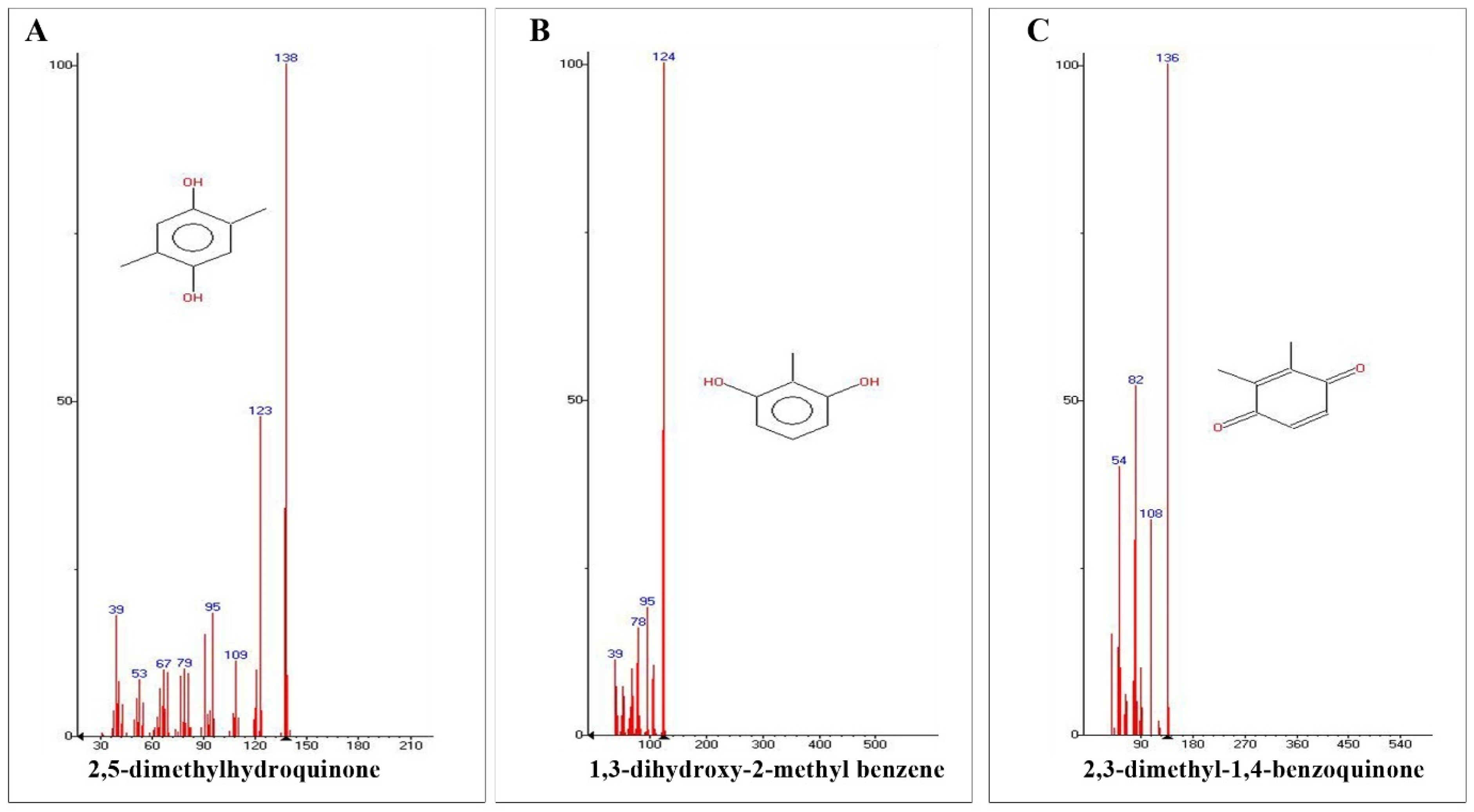
2.1. GC-HRMS Analysis
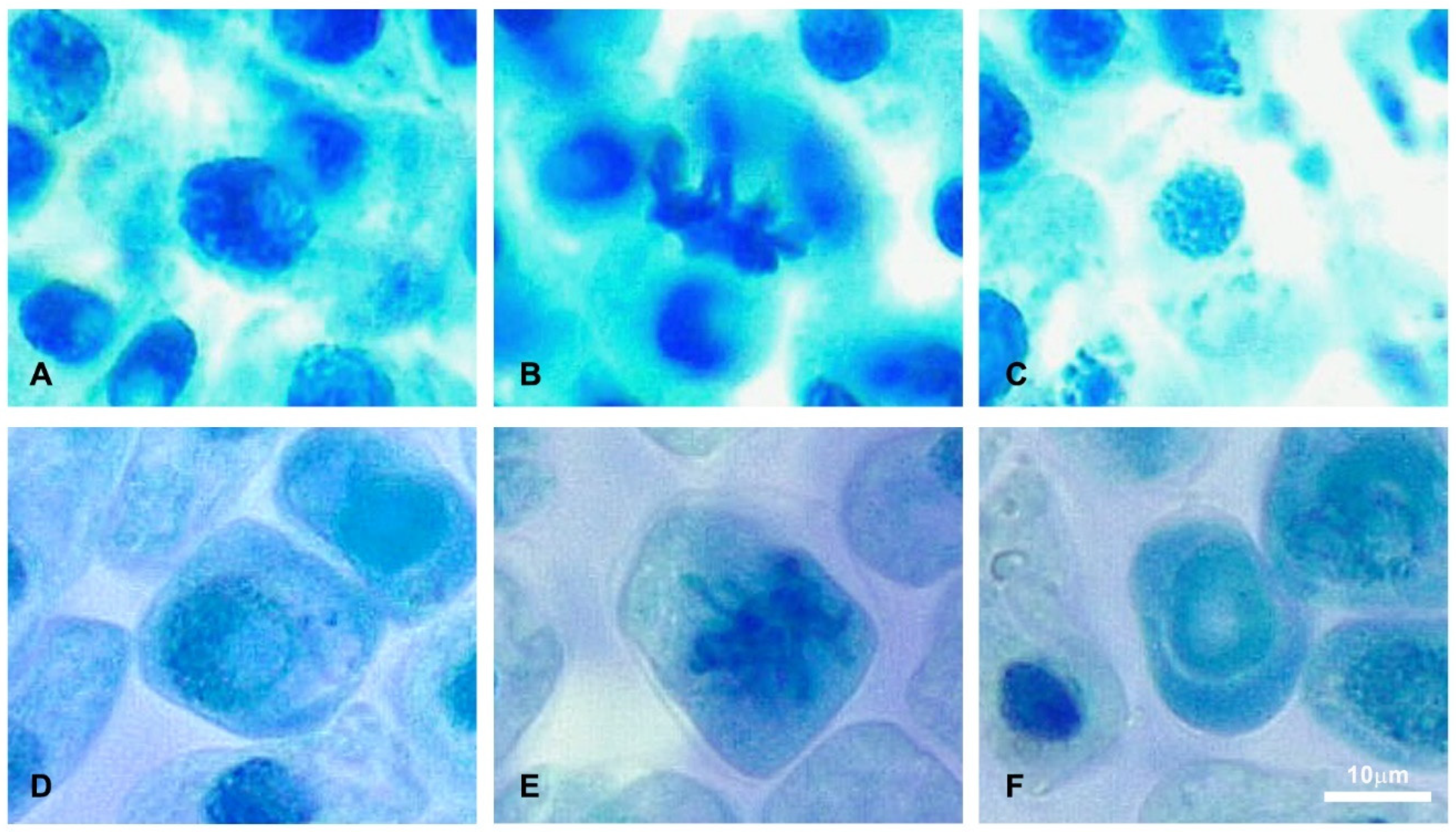
2.2. Antimitotic Activity
2.3. Antioxidant Activity
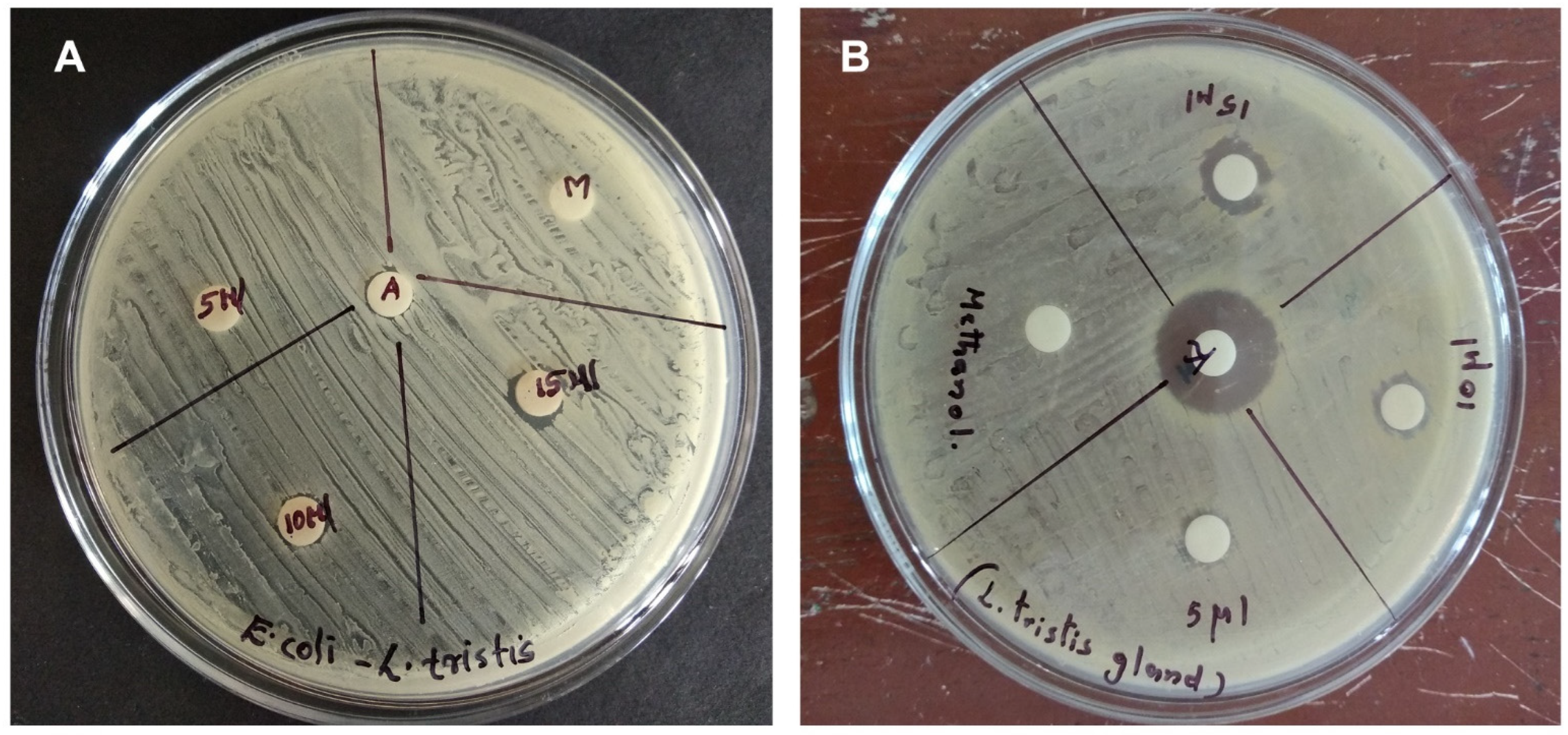
2.4. Antibacterial Activity
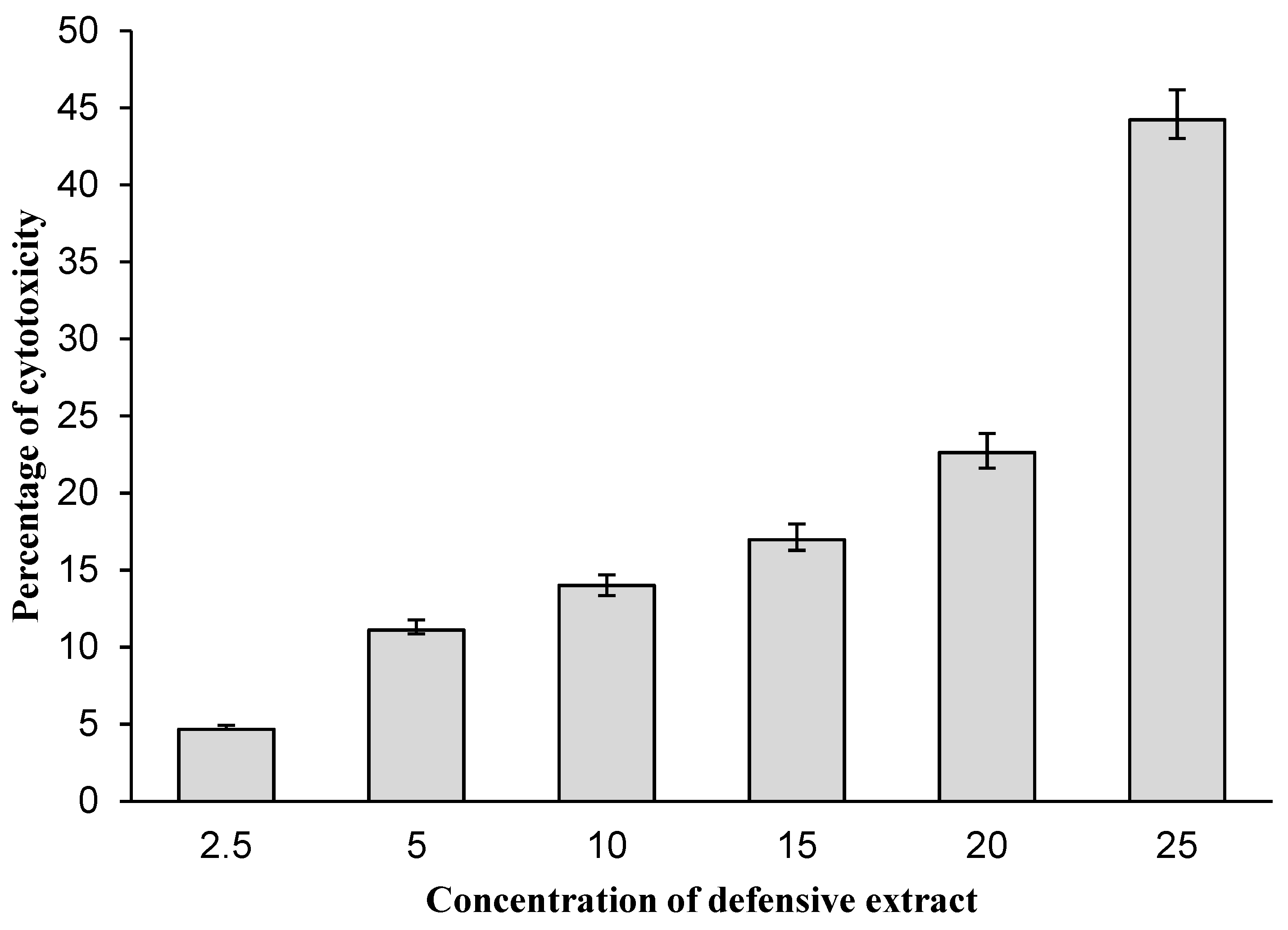
2.5. Cytotoxicity Assay
3. Discussion
4. Materials and Methods
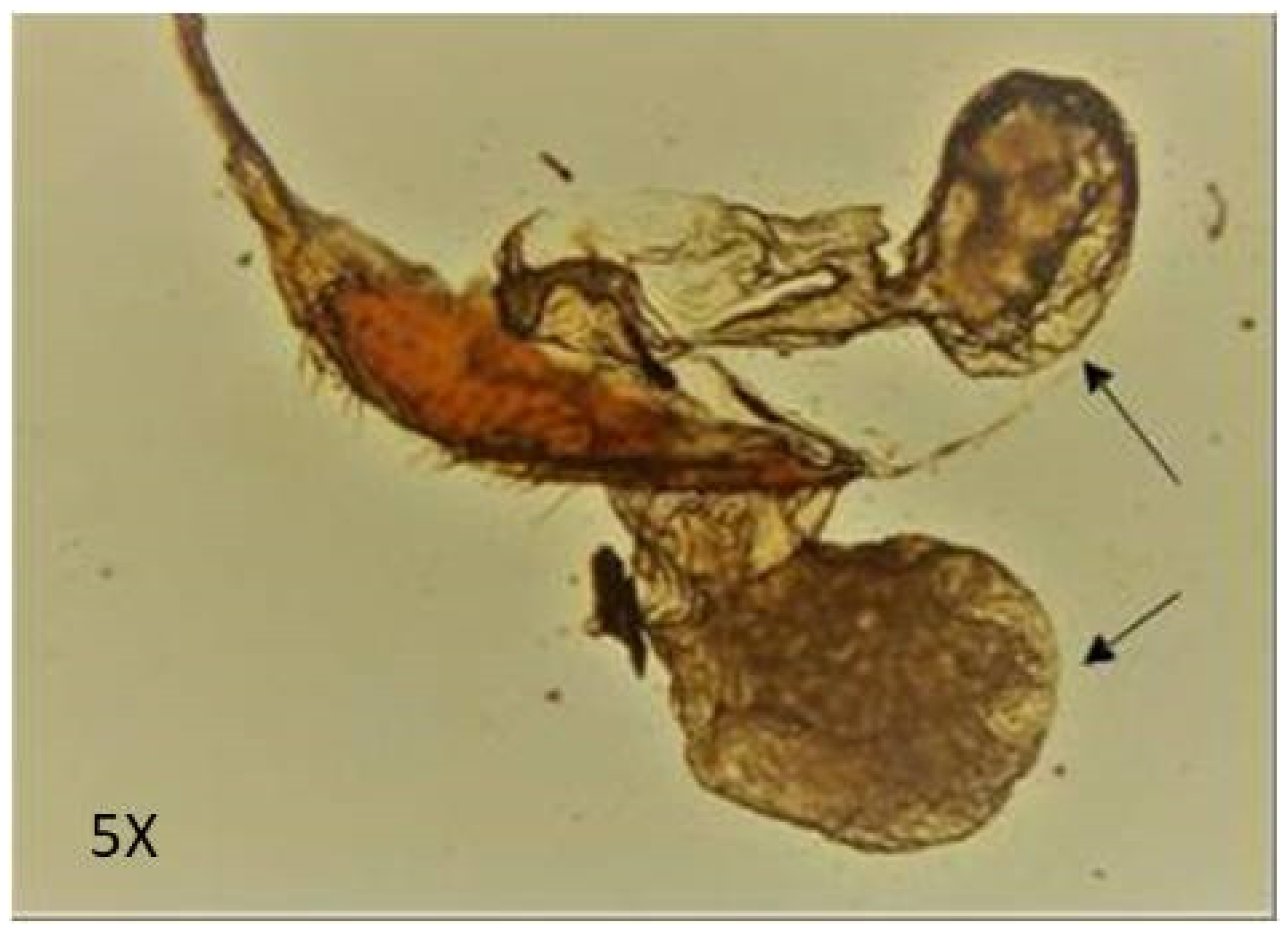
4.1. Collection of Experimental Organism
4.2. Collection of Defensive Secretion from the Gland
4.3. GC-HRMS Analysis
4.4. Antimitotic Activity
4.5. In Vitro Antioxidant Activity Analysis
4.5.1. DPPH Radical Scavenging Assay
4.5.2. ABTS Radical Scavenging Assay
4.5.3. Hydrogen Peroxide Scavenging Assay
4.5.4. Ferric Reducing Antioxidant Power
4.6. Antibacterial Activity
4.7. Anticancer Activity
4.8. Statistical Analysis and Data Representation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tschinkel, W.R. A Comparative Study of the Chemical Defensive System of Tenebrionid Beetles III. Morphology of the Glands. J. Morphol. 1975, 145, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Tschinkel, W.R. A Comparative Study of the Chemical Defensive System of Tenebrionid Beetles: Chemistry of the Secretions. J. Insect Physiol. 1975, 21, 753–783. [Google Scholar] [CrossRef]
- Tschinkel, W.R. A Comparative Study of the Chemical Defensive System of Tenebrionid Beetles. Defensive Behavior and Ancillary Features1,2. Ann. Entomol. Soc. Am. 1975, 68, 439–453. [Google Scholar] [CrossRef]
- Kanehisa, K. Comparative Study of the Abdominal Defensive Systems in Tenebrionid Beetles. Ber. Ohara Inst. Für Landwirtsch. Biol. Okayama Univ. 1978, 17, 47–55. [Google Scholar]
- Zvereva, E.L.; Kozlov, M.V. The Costs and Effectiveness of Chemical Defenses in Herbivorous Insects: A Meta-Analysis. Ecol. Monogr. 2016, 86, 107–124. [Google Scholar] [CrossRef]
- Brown, W.V.; Doyen, J.T.; Moore, B.P.; Lawrence, J.F. Chemical Composition and Taxonomic Significance of Defensive Secretions of Some Australian Tenebrionidae (Coleoptera). Aust. J. Entomol. 1992, 31, 79–89. [Google Scholar] [CrossRef]
- Bao, T.; Zhang, X.; Walczyńska, K.S.; Wang, B.; Rust, J. Earliest Mordellid-like Beetles from the Jurassic of Kazakhstan and China (Coleoptera: Tenebrionoidea). Proc. Geol. Assoc. 2019, 130, 247–256. [Google Scholar] [CrossRef]
- Abhitha, P.; Vinod, K.V.; Sabu, T.K. Defensive Glands in the Adult and Larval Stages of the Darkling Beetle, Luprops Tristis. J. Insect Sci. 2010, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Blum, M.S.; Crewe, R.M.; Pasteels, J.M. Defensive Secretion of Lomechusa strumosa, a Myrmecophilous Beetle1,2. Ann. Entomol. Soc. Am. 1971, 64, 975–976. [Google Scholar] [CrossRef]
- Markarian, H.; Florentine, G.J.; Pratt, J.J. Quinone Production of Some Species of Tribolium. J. Insect Physiol. 1978, 24, 785–790. [Google Scholar] [CrossRef]
- Gross, J.; Podsiadlowski, L.; Hilker, M. Antimicrobial activity of exocrine glandular secretion of chrysomela larvae. J. Chem. Ecol. 2002, 28, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Peschke, K.; Eisner, T. Defensive secretion of the tenebrionid beetle, Blaps mucronata: Physical and chemical determinants of effectiveness. J. Comp. Physiol. A 1987, 161, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Lečić, S.; Ćurčić, S.; Vujisić, L.; Ćurčić, B.; Curcic, N.; Nikolić, Z.; Anđelković, B.; Milosavljević, S.; Tešević, V.; Makarov, S. Defensive secretions in three ground-beetle species (Insecta: Coleoptera: Carabidae). Ann. Zool. Fenn. 2014, 51, 285–300. [Google Scholar] [CrossRef]
- Nenadić, M.; Soković, M.; Calhelha, R.C.; Ferreira, I.C.F.R.; Ćirić, A.; Vesović, N.; Ćurčić, S. Inhibition of tumour and non-tumour cell proliferation by pygidial gland secretions of four ground beetle species (coleoptera: Carabidae). Biologia 2018, 73, 787–792. [Google Scholar] [CrossRef]
- Fukushima, J.; Kuwahara, Y.; Yamada, A.; Suzuki, T. New Non-Cyclic Homo-Diterpene from the Sting Glands of Bracon hebetor Say (Hymenoptera: Braconidae). Agric. Biol. Chem. 1990, 54, 809–810. [Google Scholar] [CrossRef] [Green Version]
- Solazzo, G.; Seidelmann, K.; Moritz, R.F.A.; Settele, J. Tetracosane on the Cuticle of the Parasitic Butterfly Phengaris (Maculinea) nausithous Triggers the First Contact in the Adoption Process by Myrmica rubra Foragers. Physiol. Entomol. 2015, 40, 10–17. [Google Scholar] [CrossRef]
- Dettner, K.; Schwinger, G.; Wunderle, P. Sticky Secretion from Two Pairs of Defensive Glands of Rove Beetle Deleaster dichrous (Grav.) (Coleoptera: Staphylinidae): Gland Morphology, Chemical Constituents, Defensive Functions, and Chemotaxonomy. J. Chem. Ecol. 1985, 11, 859–883. [Google Scholar] [CrossRef]
- Pfeiffer, L.; Ruther, J.; Hofferberth, J.; Stökl, J. Interference of Chemical Defence and Sexual Communication can Shape the Evolution of Chemical Signals. Sci. Rep. 2018, 8, 321. [Google Scholar] [CrossRef] [Green Version]
- Surender, P.; Mogili, T.; Thirupathi, D.; Janaiah, C.; Vidyavati. Effect of scent components on somatic cells of Allium sativum L. Curr. Sci. 1987, 56, 964–967. [Google Scholar]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Yang, Q. Antioxidant Activity and Phenolic Compounds of Holotrichia parallela Motschulsky Extracts. Food Chem. 2012, 134, 1885–1891. [Google Scholar] [CrossRef]
- Tang, Y.; Debnath, T.; Choi, E.-J.; Kim, Y.W.; Ryu, J.P.; Jang, S.; Chung, S.U.; Choi, Y.-J.; Kim, E.-K. Changes in the Amino Acid Profiles and Free Radical Scavenging Activities of Tenebrio molitor Larvae Following Enzymatic Hydrolysis. PLoS ONE 2018, 13, e0196218. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.; Gu, F.; Wu, C.; Ma, Z.; Zhou, J.; Yang, P. Influences of Nano-TiO2 on the Chloroplast Aging of Spinach under Light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar] [CrossRef]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beheshti, A.; Norouzi, P.; Ganjali, M.R. A simple and robust model for predicting the reduction potential of quinones family; electrophilicity index effect. Int. J. Electrochem. Sci. 2012, 7, 11. [Google Scholar]
- Stanković, S.; Dimkić, I.; Vujisić, L.; Pavković-Lučić, S.; Jovanović, Z.; Stević, T.; Sofrenić, I.; Mitić, B.; Tomić, V. Chemical Defence in a Millipede: Evaluation and Characterization of Antimicrobial Activity of the Defensive Secretion from Pachyiulus hungaricus (Karsch, 1881) (Diplopoda, Julida, Julidae). PLoS ONE 2016, 11, e0167249. [Google Scholar] [CrossRef] [Green Version]
- Hoback, W.W.; Bishop, A.A.; Kroemer, J.; Scalzitti, J.; Shaffer, J.J. Differences among antimicrobial properties of carrion beetle secretions reflect phylogeny and ecology. J. Chem. Ecol. 2004, 30, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Villaverde, M.L.; Girotti, J.R.; Güerci, A.; Juárez, M.P.; de Bravo, M.G. Cytotoxic and Genotoxic Effects of Defence Secretion of Ulomoides dermestoides on A549 Cells. J. Ethnopharmacol. 2011, 136, 204–209. [Google Scholar] [CrossRef]
- Honda, K. GC-MS and 13C-NMR Studies on the Biosynthesis of Terpenoid Defensive Secretions by the Larvae of Papilionid Butterflies (Luehdorfia and Papilio). Insect Biochem. 1990, 20, 245–250. [Google Scholar] [CrossRef]
- Anusmitha, K.M.; Aruna, M.; Job, J.T.; Narayanankutty, A.; Pb, B.; Rajagopal, R.; Alfarhan, A.; Barcelo, D. Phytochemical Analysis, Antioxidant, Anti-Inflammatory, Anti-Genotoxic, and Anticancer Activities of Different Ocimum Plant Extracts Prepared by Ultrasound-Assisted Method. Physiol. Mol. Plant Pathol. 2022, 117, 101746. [Google Scholar] [CrossRef]
- Malayil, D.; Jose, B.; Narayanankutty, A.; Ramesh, V.; Rajagopal, R.; Alfarhan, A. Phytochemical Profiling of Azima tetracantha Lam. Leaf Methanol Extract and Elucidation of Its Potential as a Chain-Breaking Antioxidant, Anti-Inflammatory and Anti-Proliferative Agent. Saudi J. Biol. Sci. 2021, 28, 6040–6044. [Google Scholar] [CrossRef]
- Malayil, D.; House, N.C.; Puthenparambil, D.; Job, J.T.; Narayanankutty, A. Borassus flabellifer Haustorium Extract Prevents Pro-Oxidant Mediated Cell Death and LPS-Induced Inflammation. Drug Chem. Toxicol. 2022, 45, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Es, B. Antibacterial Potential of Luprops tristis–The Nuisance Rubber Plantation Pest from Western Ghats of India. Int. J. Agric. Innov. Res. 2014, 3, 1–6. [Google Scholar]
- Ahn, M.Y.; Ryu, K.S.; Lee, Y.W.; Kim, Y.S. Cytotoxicity and L-Amino Acid Oxidase Activity of Crude Insect Drugs. Arch. Pharm. Res. 2000, 23, 477–481. [Google Scholar] [CrossRef] [PubMed]
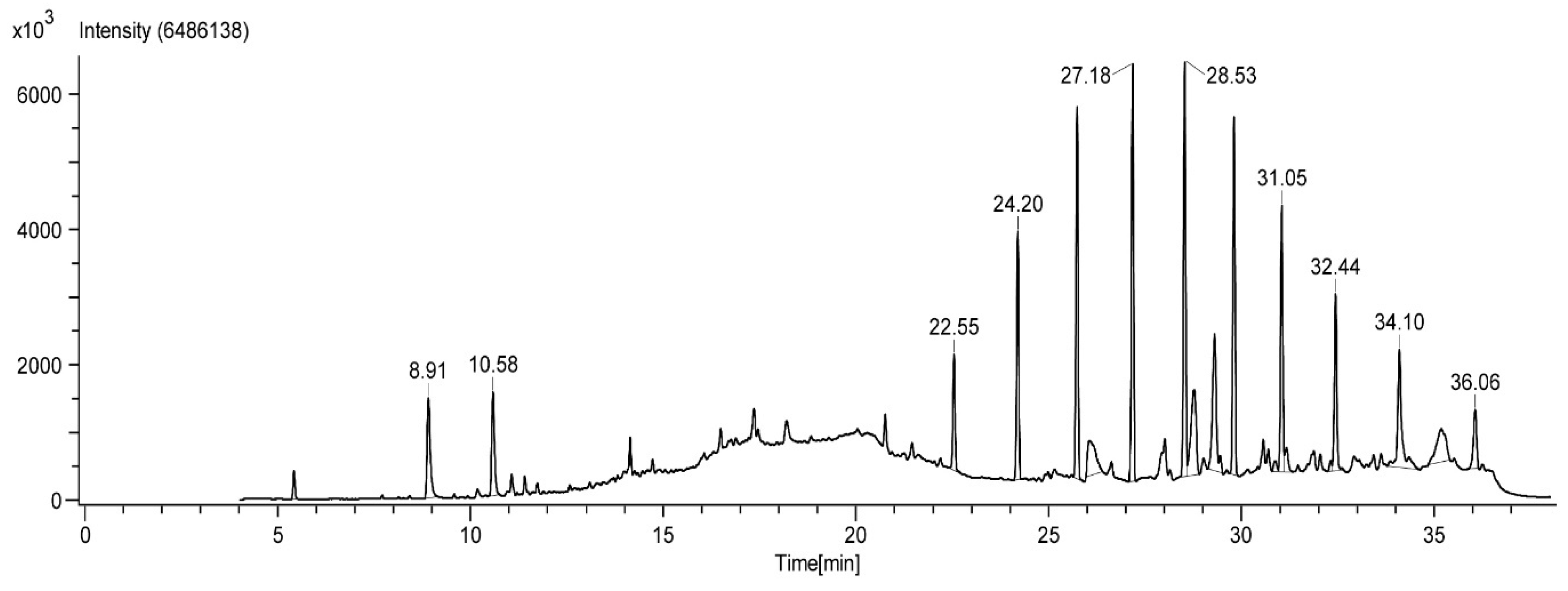
| Sl. No | Compound Name | Molecular Formula | Retention Time (min) |
|---|---|---|---|
| 1 | 2,3-dimethyl-1,4-benzoquinone | C8H8O2 | 5.43 |
| 2 | 1,3-dihydroxy-2-methylbenzene | C7H8O2 | 8.91 |
| 3 | 2,5-dimethyl hydroquinone | C8H10O2 | 10.58 |
| 4 | tetracosane | C24H50 | 25.74 |
| 5 | oleic acid | C18H34O2 | 26.05 |
| 6 | hexacosane | C26H54 | 29.81 |
| 7 | pentacosane | C25H52 | 31.05 |
| 8 | 7-hexadecenal | C16H30O | 32.31 |
| 9 | tert-hexadecanethiol | C16H34S | 35.18 |
| Treatment | Concentration (µL) | Mitotic Index (% ± SD) | Chromosomal Aberration (% ± SD) |
|---|---|---|---|
| Control (distilled water) | 100 | 11.29 ± 0.32 | NIL |
| 200 | 12.65 ± 0.25 | NIL | |
| 300 | 14.44 ± 0.37 | NIL | |
| 400 | 14.55 ± 0.32 | NIL | |
| 500 | 15.58 ± 0.30 | NIL | |
| Experiment | 100 | 9.50 ± 0.50 | 11.17 ± 0.29 |
| (defensive gland extract) | 200 | 6.57 ± 0.27 | 13.61 ± 0.27 |
| 300 | 5.64 ± 0.24 | 14.46 ± 0.42 | |
| 400 | 3.57 ± 0.36 | 15.35 ± 0.36 | |
| 500 | 3.33 ± 0.33 | 16.33 ± 0.33 |
| Sl. No | Assay | IC50 Value (µg/mL) |
|---|---|---|
| 1 | DPPH radical scavenging | 108.3 ± 2.1 |
| 2 | ABTS radical scavenging | 95.6 ± 1.5 |
| 3 | H2O2 scavenging | 69.4 ± 3.5 |
| 4 | Ferric reducing antioxidant power | 34.1 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabira, O.; Vignesh, A.R.; Ajaykumar, A.P.; Varma, S.R.; Jayaraj, K.N.; Sebastin, M.; Nikhila, K.; Babu, A.; Rasheed, V.A.; Binitha, V.S.; et al. The Chemical Composition and Antimitotic, Antioxidant, Antibacterial and Cytotoxic Properties of the Defensive Gland Extract of the Beetle, Luprops tristis Fabricius. Molecules 2022, 27, 7476. https://doi.org/10.3390/molecules27217476
Sabira O, Vignesh AR, Ajaykumar AP, Varma SR, Jayaraj KN, Sebastin M, Nikhila K, Babu A, Rasheed VA, Binitha VS, et al. The Chemical Composition and Antimitotic, Antioxidant, Antibacterial and Cytotoxic Properties of the Defensive Gland Extract of the Beetle, Luprops tristis Fabricius. Molecules. 2022; 27(21):7476. https://doi.org/10.3390/molecules27217476
Chicago/Turabian StyleSabira, Ovungal, Attuvalappil Ramdas Vignesh, Anthyalam Parambil Ajaykumar, Sudhir Rama Varma, Kodangattil Narayanan Jayaraj, Merin Sebastin, Kalleringal Nikhila, Annet Babu, Vazhanthodi Abdul Rasheed, Valiyaparambil Sivadasan Binitha, and et al. 2022. "The Chemical Composition and Antimitotic, Antioxidant, Antibacterial and Cytotoxic Properties of the Defensive Gland Extract of the Beetle, Luprops tristis Fabricius" Molecules 27, no. 21: 7476. https://doi.org/10.3390/molecules27217476
APA StyleSabira, O., Vignesh, A. R., Ajaykumar, A. P., Varma, S. R., Jayaraj, K. N., Sebastin, M., Nikhila, K., Babu, A., Rasheed, V. A., Binitha, V. S., Vasu, Z. k., & Sujith, M. S. (2022). The Chemical Composition and Antimitotic, Antioxidant, Antibacterial and Cytotoxic Properties of the Defensive Gland Extract of the Beetle, Luprops tristis Fabricius. Molecules, 27(21), 7476. https://doi.org/10.3390/molecules27217476









