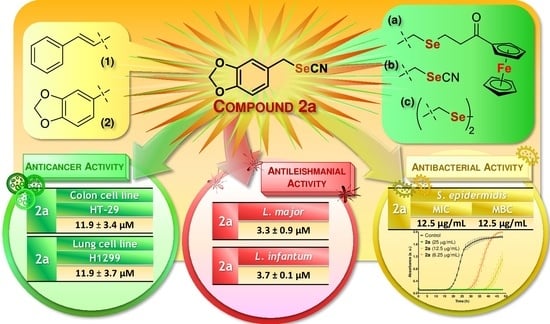
Unveiling a New Selenocyanate as a Multitarget Candidate with Anticancer, Antileishmanial and Antibacterial Potential
Abstract
1. Introduction
2. Results and Discussion
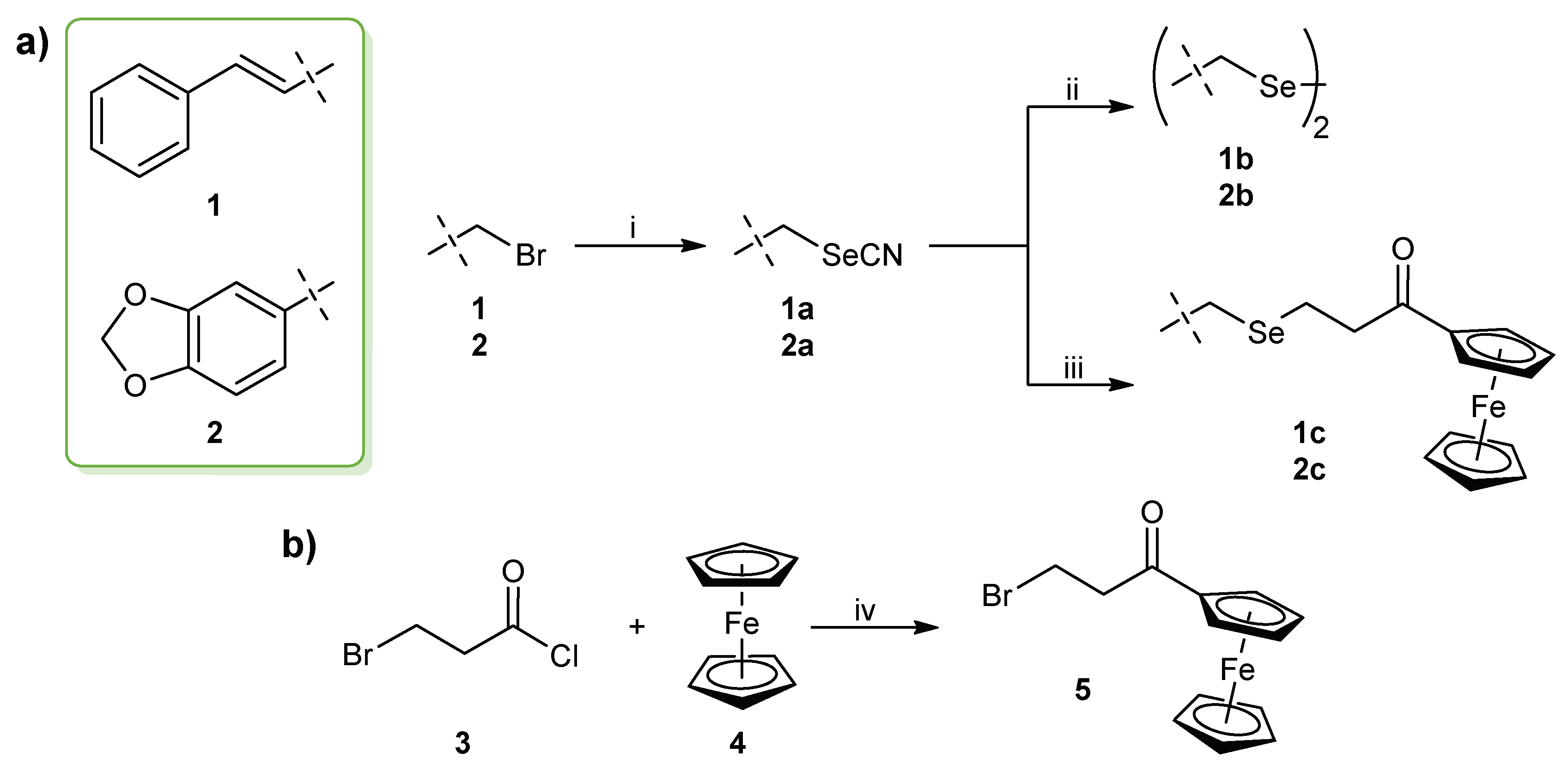
2.1. Chemistry and Characterization
2.2. Biological Evaluation
2.2.1. Antiproliferative Activity on Tumor and Nonmalignant Cells
2.2.2. Antileishmanial Activity on Promastigote Forms
2.2.3. Antibacterial Activity
2.2.4. Antioxidant Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Synthesis of Selenocyanate Derivatives 1–2a
3.1.2. General Synthesis of Diselenide Derivatives 1–2b
3.1.3. Synthesis of (3-bromo-1-oxopropyl)ferrocene (5)
3.1.4. General Synthesis of Ferrocene-Containing Selenide Derivatives 1–2c
3.2. Biology
3.2.1. Cell Culture Conditions
3.2.2. Cell Viability Assay
3.2.3. Culture Conditions of Leishmania Promastigotes
3.2.4. Parasite Viability Assay
3.2.5. Antibacterial Activity: MIC and MBC Assays
3.2.6. Growth Curve Studies
3.2.7. DPPH Radical Scavenging Assay
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Nii-Trebi, N.I. Emerging and neglected infectious diseases: Insights, advances, and challenges. Biomed. Res. Int. 2017, 2017, 5245021. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C., Jr. Discovering new antimicrobial agents. Int. J. Antimicrob. Agents 2011, 37, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Yang, T.; Chen, Y.; Miao, Y.; Xu, Y.; Jiang, H.; Yang, M.; Mao, C. Emulating interactions between microorganisms and tumor microenvironment to develop cancer theranostics. Theranostics 2022, 12, 2833–2859. [Google Scholar] [CrossRef]
- Vandeven, N.; Nghiem, P. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol. Res. 2014, 2, 9–14. [Google Scholar] [CrossRef]
- Rashidi, S.; Fernández-Rubio, C.; Manzano-Román, R.; Mansouri, R.; Shafiei, R.; Ali-Hassanzadeh, M.; Barazesh, A.; Karimazar, M.; Hatam, G.; Nguewa, P. Potential therapeutic targets shared between leishmaniasis and cancer. Parasitology 2021, 148, 655–671. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet. 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Fuertes, M.A.; Nguewa, P.A.; Castilla, J.; Alonso, C.; Pérez, J.M. Anticancer compounds as leishmanicidal drugs: Challenges in chemotherapy and future perspectives. Curr. Med. Chem. 2008, 15, 433–439. [Google Scholar]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updat. 2020, 50, 100682. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Gupta, O.; Pradhan, T.; Bhatia, R.; Monga, V. Recent advancements in anti-leishmanial research: Synthetic strategies and structural activity relationships. Eur. J. Med. Chem. 2021, 223, 113606. [Google Scholar] [CrossRef]
- Radomska, D.; Czarnomysy, R.; Radomski, D.; Bielawski, K. Selenium compounds as novel potential anticancer agents. Int. J. Mol. Sci. 2021, 22, 1009. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S.; Fernández-Rubio, C.; Mansouri, R.; Ali-Hassanzadeh, M.; Ghani, E.; Karimazar, M.; Manzano-Román, R.; Nguewa, P. Selenium and protozoan parasitic infections: Selenocompounds and selenoproteins potential. Parasitol. Res. 2022, 121, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lobanov, A.V.; Hatfield, D.L.; Gladyshev, V.N. Eukaryotic selenoproteins and selenoproteomes. Biochim. Biophys. Acta 2009, 1790, 1424–1428. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological activity of selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Wells, M.; Basu, P.; Stolz, J.F. The physiology and evolution of microbial selenium metabolism. Metallomics 2021, 13, mfab024. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Gromer, S.; Salinas, G.; Gladyshev, V.N. Selenium metabolism in Trypanosoma: Characterization of selenoproteomes and identification of a Kinetoplastida-specific selenoprotein. Nucleic Acids Res. 2006, 34, 4012–4024. [Google Scholar] [CrossRef]
- Kim, S.J.; Choi, M.C.; Park, J.M.; Chung, A.S. Antitumor effects of selenium. Int. J. Mol. Sci. 2021, 22, 11844. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.T.; Silva-Jardim, I.; Thiemann, O.H. Biological implications of selenium and its role in trypanosomiasis treatment. Curr. Med. Chem. 2014, 21, 1772–1780. [Google Scholar] [CrossRef]
- Martins, I.L.; Miranda, J.P.; Oliveira, N.G.; Fernandes, A.S.; Gonçalves, S.; Antunes, A.M. Synthesis and biological activity of 6-selenocaffeine: Potential modulator of chemotherapeutic drugs in breast cancer cells. Molecules 2013, 18, 5251–5264. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Fu, X.; Xiong, Z.; Zhang, H.; Hill, S.M.; Rowan, B.G.; Dong, Y. Methylseleninic acid enhances paclitaxel efficacy for the treatment of triple-negative breast cancer. PLoS ONE 2012, 7, e31539. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, M.; Farajzadeh, S.; Sharifi, I.; Khazaeli, P.; Sharifi, H. Leishmanicidal effects of amphotericin B in combination with selenium loaded on niosome against Leishmania tropica. J. Parasit. Dis. 2019, 43, 176–185. [Google Scholar] [CrossRef]
- Mostafavi, M.; Khazaeli, P.; Sharifi, I.; Farajzadeh, S.; Sharifi, H.; Keyhani, A.; Parizi, M.H.; Kakooei, S. A novel niosomal combination of selenium coupled with glucantime against Leishmania tropica. Korean J. Parasitol. 2019, 57, 1–8. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and therapeutic potential of selenazo compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef]
- Karelia, D.; Kim, S.; Pandey, M.K.; Plano, D.; Amin, S.; Lu, J.; Sharma, A. Novel seleno-aspirinyl compound AS-10 induces apoptosis, G1 arrest of pancreatic ductal adenocarcinoma cells, inhibits their NF-κB signaling, and synergizes with gemcitabine cytotoxicity. Int. J. Mol. Sci. 2021, 22, 4966. [Google Scholar] [CrossRef]
- Sabir, S.; Yu, T.T.; Kuppusamy, R.; Almohaywi, B.; Iskander, G.; Das, T.; Willcox, M.D.P.; Black, D.S.; Kumar, N. Novel seleno- and thio-urea containing dihydropyrrol-2-one analogues as antibacterial agents. Antibiotics (Basel) 2021, 10, 321. [Google Scholar]
- Mosolygó, T.; Kincses, A.; Csonka, A.; Tönki, Á.S.; Witek, K.; Sanmartín, C.; Marć, M.A.; Handzlik, J.; Kieć-Kononowicz, K.; Domínguez-Álvarez, E.; et al. Selenocompounds as novel antibacterial agents and bacterial efflux pump inhibitors. Molecules 2019, 24, 1487. [Google Scholar] [CrossRef]
- Fernández-Rubio, C.; Larrea, E.; Peña Guerrero, J.; Sesma Herrero, E.; Gamboa, I.; Berrio, C.; Plano, D.; Amin, S.; Sharma, A.K.; Nguewa, P.A. Leishmanicidal activity of isoselenocyanate derivatives. Antimicrob. Agents Chemother. 2019, 63, e00904-18. [Google Scholar] [CrossRef] [PubMed]
- Al-Tamimi, A.S.; Etxebeste-Mitxeltorena, M.; Sanmartín, C.; Jiménez-Ruiz, A.; Syrjänen, L.; Parkkila, S.; Selleri, S.; Carta, F.; Angeli, A.; Supuran, C.T. Discovery of new organoselenium compounds as antileishmanial agents. Bioorg. Chem. 2019, 86, 339–345. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Ramos-Inza, S.; Aydillo, C.; Talavera, I.; Encío, I.; Plano, D.; Sanmartín, C. Novel N,N’-disubstituted acylselenoureas as potential antioxidant and cytotoxic agents. Antioxidants (Basel) 2020, 9, 55. [Google Scholar] [CrossRef]
- Reddy, N.D.; Shoja, M.H.; Biswas, S.; Nayak, P.G.; Kumar, N.; Rao, C.M. An appraisal of cinnamyl sulfonamide hydroxamate derivatives (HDAC inhibitors) for anti-cancer, anti-angiogenic and anti-metastatic activities in human cancer cells. Chem. Biol. Interact. 2016, 253, 112–124. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Q.H.; Li, X.J.; Wang, S.Q.; Chen, X.B.; Yu, B.; Liu, H.M. Discovery of a cinnamyl piperidine derivative as new neddylation inhibitor for gastric cancer treatment. Eur. J. Med. Chem. 2021, 226, 113896. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Hameedi, S.; Mousa, A. Design, synthesis and biological evaluation of novel benzodioxole derivatives as COX inhibitors and cytotoxic agents. BMC Chem. 2020, 14, 54. [Google Scholar] [CrossRef]
- Parise-Filho, R.; Pasqualoto, K.F.; Magri, F.M.; Ferreira, A.K.; da Silva, B.A.; Damião, M.C.; Tavares, M.T.; Azevedo, R.A.; Auada, A.V.; Polli, M.C.; et al. Dillapiole as antileishmanial agent: Discovery, cytotoxic activity and preliminary SAR studies of dillapiole analogues. Arch. Pharm. 2012, 345, 934–944. [Google Scholar] [CrossRef]
- Spasova, M.; Kortenska-Kancheva, V.; Totseva, I.; Ivanova, G.; Georgiev, L.; Milkova, T. Synthesis of cinnamoyl and hydroxycinnamoyl amino acid conjugates and evaluation of their antioxidant activity. J. Pept. Sci. 2006, 12, 369–375. [Google Scholar] [CrossRef]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef]
- Álvarez-Pérez, M.; Ali, W.; Marć, M.A.; Handzlik, J.; Domínguez-Álvarez, E. Selenides and diselenides: A review of their anticancer and chemopreventive activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef]
- Nie, Y.; Zhong, M.; Li, S.; Li, X.; Zhang, Y.; Zhang, Y.; He, X. Synthesis and potential anticancer activity of some novel selenocyanates and diselenides. Chem. Biodivers. 2020, 17, e1900603. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, V.; Moreno, E.; Etxebeste-Mitxeltorena, M.; Navarro-Blasco, I.; González-Peñas, E.; Jiménez-Ruiz, A.; Irache, J.M.; Sanmartín, C.; Espuelas, S. 3,5-Dimethyl-4-isoxazoyl selenocyanate as promising agent for the treatment of Leishmania infantum-infected mice. Acta Trop. 2021, 215, 105801. [Google Scholar] [CrossRef] [PubMed]
- Garnica, P.; Etxebeste-Mitxeltorena, M.; Plano, D.; Moreno, E.; Espuelas, S.; Antonio Palop, J.; Jiménez-Ruiz, A.; Sanmartín, C. Pre-clinical evidences of the antileishmanial effects of diselenides and selenocyanates. Bioorg. Med. Chem. Lett. 2020, 30, 127371. [Google Scholar] [CrossRef] [PubMed]
- Pesarico, A.P.; Sartori, G.; dos Santos, C.F.; Neto, J.S.; Bortolotto, V.; Santos, R.C.; Nogueira, C.W.; Prigol, M. 2,2’-Dithienyl diselenide pro-oxidant activity accounts for antibacterial and antifungal activities. Microbiol. Res. 2013, 168, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Abbas, G.; Del Prete, S.; Capasso, C.; Supuran, C.T. Selenides bearing benzenesulfonamide show potent inhibition activity against carbonic anhydrases from pathogenic bacteria Vibrio cholerae and Burkholderia pseudomallei. Bioorg. Chem. 2018, 79, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Kumar, V. Has ferrocene really delivered its role in accentuating the bioactivity of organic scaffolds? J. Med. Chem. 2021, 64, 16865–16921. [Google Scholar] [CrossRef]
- Peter, S.; Aderibigbe, B.A. Ferrocene-based compounds with antimalaria/anticancer activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Yan, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-containing hybrids as potential anticancer agents: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020, 190, 112109. [Google Scholar] [CrossRef]
- Vale-Costa, S.; Vale, N.; Matos, J.; Tomás, A.; Moreira, R.; Gomes, P.; Gomes, M.S. Peptidomimetic and organometallic derivatives of primaquine active against Leishmania infantum. Antimicrob. Agents Chemother. 2012, 56, 5774–5781. [Google Scholar] [CrossRef]
- Mendoza-Martínez, C.; Galindo-Sevilla, N.; Correa-Basurto, J.; Ugalde-Saldivar, V.M.; Rodríguez-Delgado, R.G.; Hernández-Pineda, J.; Padierna-Mota, C.; Flores-Alamo, M.; Hernández-Luis, F. Antileishmanial activity of quinazoline derivatives: Synthesis, docking screens, molecular dynamic simulations and electrochemical studies. Eur. J. Med. Chem. 2015, 92, 314–331. [Google Scholar] [CrossRef]
- Lewandowski, E.M.; Szczupak, Ł.; Wong, S.; Skiba, J.; Guśpiel, A.; Solecka, J.; Vrček, V.; Kowalski, K.; Chen, Y. Antibacterial properties of metallocenyl-7-ADCA derivatives and structure in complex with CTX-M β-lactamase. Organometallics 2017, 36, 1673–1676. [Google Scholar] [CrossRef]
- Bugarinović, J.P.; Pešić, M.S.; Minić, A.; Katanić, J.; Ilić-Komatina, D.; Pejović, A.; Mihailović, V.; Stevanović, D.; Nastasijević, B.; Damljanović, I. Ferrocene-containing tetrahydropyrazolopyrazolones: Antioxidant and antimicrobial activity. J. Inorg. Biochem. 2018, 189, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Inza, S.; Plano, D.; Sanmartín, C. Metal-based compounds containing selenium: An appealing approach towards novel therapeutic drugs with anticancer and antimicrobial effects. Eur. J. Med. Chem. 2022, 244, 114834. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Karelia, D.N.; Pandey, M.K.; Spallholz, J.E.; Amin, S.; Sharma, A.K. Design, synthesis, and biological evaluation of novel selenium (Se-NSAID) molecules as anticancer agents. J. Med. Chem. 2016, 59, 1946–1959. [Google Scholar] [CrossRef]
- Baquedano, Y.; Alcolea, V.; Toro, M.; Gutiérrez, K.J.; Nguewa, P.; Font, M.; Moreno, E.; Espuelas, S.; Jiménez-Ruiz, A.; Palop, J.A.; et al. Novel heteroaryl selenocyanates and diselenides as potent antileishmanial agents. Antimicrob. Agents Chemother. 2016, 60, 3802–3812. [Google Scholar] [CrossRef]
- Kowalski, K.; Koceva-Chyła, A.; Pieniazek, A.; Bernasińska, J.; Skiba, J.; Rybarczyk-Pirek, A.J.; Jóźwiak, Z. The synthesis, structure, electrochemistry and in vitro anticancer activity studies of ferrocenyl-thymine conjugates. J. Organomet. Chem. 2012, 700, 58–68. [Google Scholar] [CrossRef]
- Nasim, M.J.; Witek, K.; Kincses, A.; Abdin, A.Y.; Zesławska, E.; Marć, M.A.; Gajdács, M.; Spengler, G.; Nitek, W.; Latacz, G.; et al. Pronounced activity of aromatic selenocyanates against multidrug resistant ESKAPE bacteria. New J. Chem. 2019, 43, 6021–6031. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, W.; Zadlo, A.; Kozinska, A.; Sarna, T.; Hamblin, M.R. Antimicrobial photodynamic inactivation is potentiated by the addition of selenocyanate: Possible involvement of selenocyanogen? J. Biophotonics 2018, 11, e201800029. [Google Scholar] [CrossRef]
- Day, B.J.; Bratcher, P.E.; Chandler, J.D.; Kilgore, M.B.; Min, E.; LiPuma, J.J.; Hondal, R.J.; Nichols, D.P. The thiocyanate analog selenocyanate is a more potent antimicrobial pro-drug that also is selectively detoxified by the host. Free Radic. Biol. Med. 2020, 146, 324–332. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.Y.; Ahmed, S.; Wang, F.N.; Gu, Y.F.; Zhang, C.N.; Chai, X.M.; Wu, Y.L.; Cai, J.X.; Cheng, G.Y. Antimicrobial activity and resistance: Influencing factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]


| Compounds | Colon Cell Lines | Lung Cell Lines | Breast Cell Lines | |||
|---|---|---|---|---|---|---|
| HT-29 | HCT-116 | H1299 | HTB-54 | MDA-MB-231 | MCF-7 | |
| 1a | 61.2 ± 9.7 | 68.8 ± 6.3 | 82.5 ± 5.9 | 58.7 ± 9.0 | 71.0 ± 10.3 | 55.0 ± 2.3 |
| 1b | 36.0 ± 5.4 | 36.6 ± 6.7 | 28.7 ± 2.6 | 50.1 ± 5.9 | 56.3 ± 5.3 | >100 |
| 1c | >100 | >100 | 70.6 ± 6.6 | 32.3 ± 3.2 | 32.1 ± 3.0 | 18.5 ± 5.4 |
| 2a | 11.9 ± 3.4 | 69.7 ± 5.3 | 11.9 ± 3.7 | 45.2 ± 8.2 | 43.8 ± 5.7 | 59.3 ± 6.5 |
| 2b | 10.1 ± 1.2 | 11.3 ± 1.0 | 3.6 ± 0.8 | 41.6 ± 9.4 | 46.6 ± 12.1 | >100 |
| 2c | 21.4 ± 4.3 | >100 | 45.2 ± 7.2 | 29.7 ± 2.9 | 30.9 ± 1.3 | 14.8 ± 4.3 |
| Compounds | Breast Cell Lines | ||||
|---|---|---|---|---|---|
| 184B5 | MDA-MB-231 | SI a | MCF-7 | SI b | |
| 1a | 55.0 ± 6.7 | 71.0 ± 10.3 | 0.8 | 55.0 ± 2.3 | 1.0 |
| 1b | >100 | 56.3 ± 5.3 | 1.8 | >100 | nc c |
| 1c | 24.6 ± 0.4 | 32.1 ± 3.0 | 0.8 | 18.5 ± 5.4 | 1.3 |
| 2a | 48.6 ± 2.1 | 43.8 ± 5.7 | 1.1 | 59.3 ± 6.5 | 0.8 |
| 2b | >100 | 46.6 ± 12.1 | 2.2 | >100 | nc |
| 2c | 50.1 ± 4.5 | 30.9 ± 1.3 | 1.6 | 14.8 ± 4.3 | 3.4 |
| Compounds | Leishmania major | Leishmania infantum |
|---|---|---|
| 1a | 4.6 ± 1.7 | 3.3 ± 1.8 |
| 1b | 47.6 ± 16.0 | 39.4 ± 5.1 |
| 1c | 25.8 ± 2.7 | 23.2 ± 10.7 |
| 2a | 3.3 ± 0.9 | 3.7 ± 0.1 |
| 2b | 49.4 ± 16.7 | 56.1 ± 8.1 |
| 2c | 11.2 ± 3.0 | 7.2 ± 3.6 |
| Miltefosine | 66.6 ± 11.6 | 31.9 ± 2.3 |
| Paromomycin | 71.0 ± 3.3 | 18.0 ± 1.0 |
| Compounds | Gram-Negative Bacteria | Gram-Positive Bacteria | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. coli | K. pneumoniae | C. freundii | S. aureus | S. faecalis | S. epidermidis | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| 1a | 100 | >200 | 100 | 100 | 100 | 100 | 50 | 50 | 50 | >200 | 12.5 | 12.5 |
| 1b | >200 | nd a | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd |
| 1c | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd |
| 2a | 50 | 50 | 50 | 50 | 100 | 100 | 25 | >200 | 25 | >200 | 12.5 | 12.5 |
| 2b | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd |
| 2c | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd | >200 | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Inza, S.; Henriquez-Figuereo, A.; Moreno, E.; Berzosa, M.; Encío, I.; Plano, D.; Sanmartín, C. Unveiling a New Selenocyanate as a Multitarget Candidate with Anticancer, Antileishmanial and Antibacterial Potential. Molecules 2022, 27, 7477. https://doi.org/10.3390/molecules27217477
Ramos-Inza S, Henriquez-Figuereo A, Moreno E, Berzosa M, Encío I, Plano D, Sanmartín C. Unveiling a New Selenocyanate as a Multitarget Candidate with Anticancer, Antileishmanial and Antibacterial Potential. Molecules. 2022; 27(21):7477. https://doi.org/10.3390/molecules27217477
Chicago/Turabian StyleRamos-Inza, Sandra, Andreina Henriquez-Figuereo, Esther Moreno, Melibea Berzosa, Ignacio Encío, Daniel Plano, and Carmen Sanmartín. 2022. "Unveiling a New Selenocyanate as a Multitarget Candidate with Anticancer, Antileishmanial and Antibacterial Potential" Molecules 27, no. 21: 7477. https://doi.org/10.3390/molecules27217477
APA StyleRamos-Inza, S., Henriquez-Figuereo, A., Moreno, E., Berzosa, M., Encío, I., Plano, D., & Sanmartín, C. (2022). Unveiling a New Selenocyanate as a Multitarget Candidate with Anticancer, Antileishmanial and Antibacterial Potential. Molecules, 27(21), 7477. https://doi.org/10.3390/molecules27217477