Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor
Abstract
1. Introduction
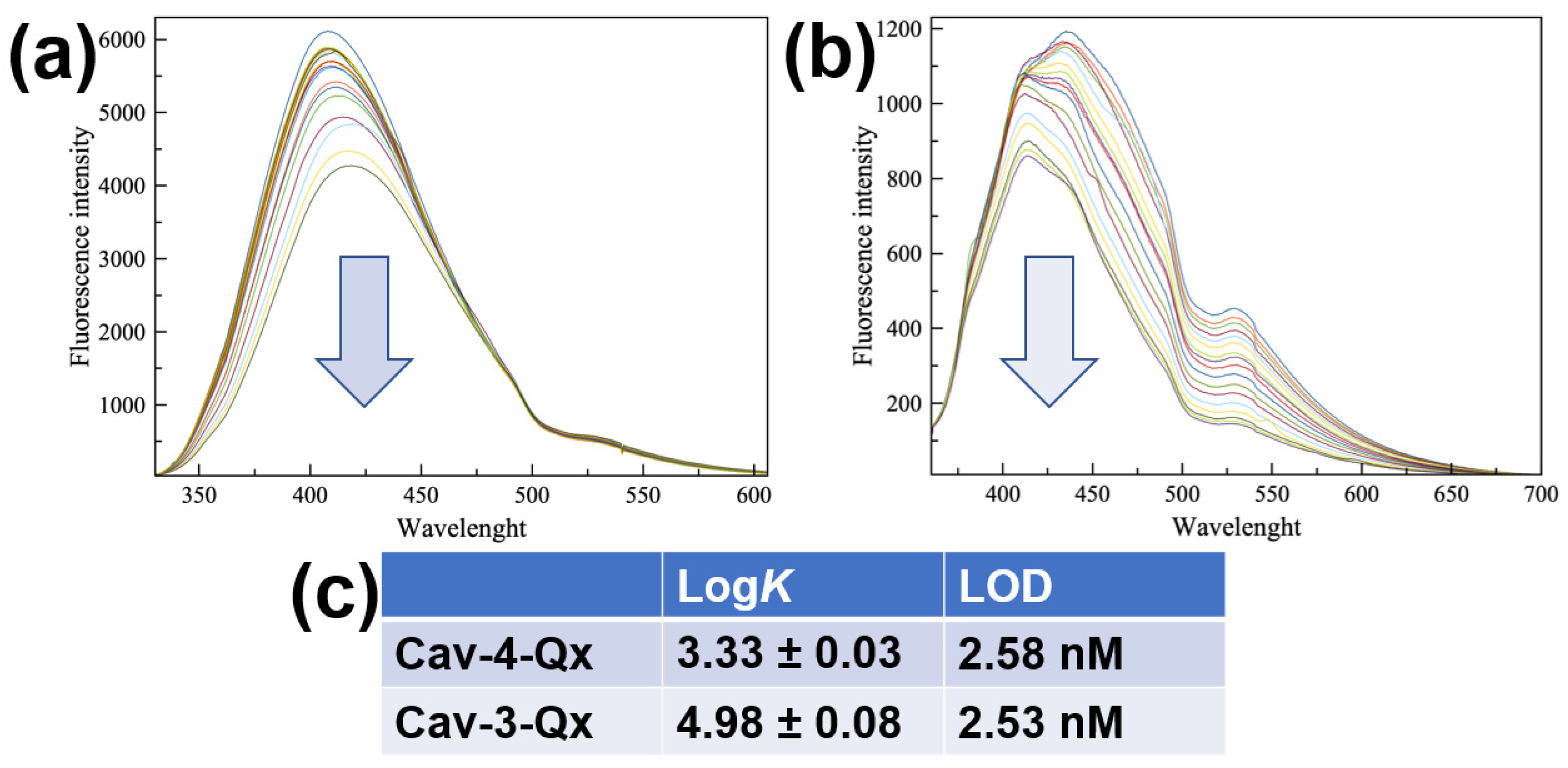
2. Results
3. Materials and Methods
3.1. General Experimental Methods
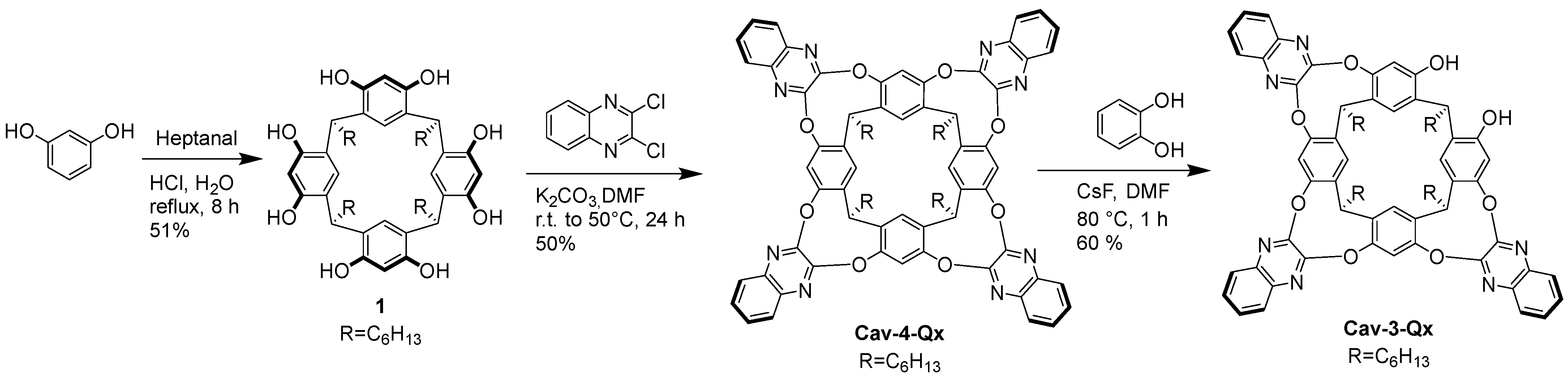
3.2. Synthesis of 1
3.3. Synthesis of Cav-4-Qx
3.4. Synthesis of Cav-3-Qx
3.5. Procedure for Fluorescence Titrations
3.6. Determination of Stoichiometry
3.7. Preparation of Solid Support
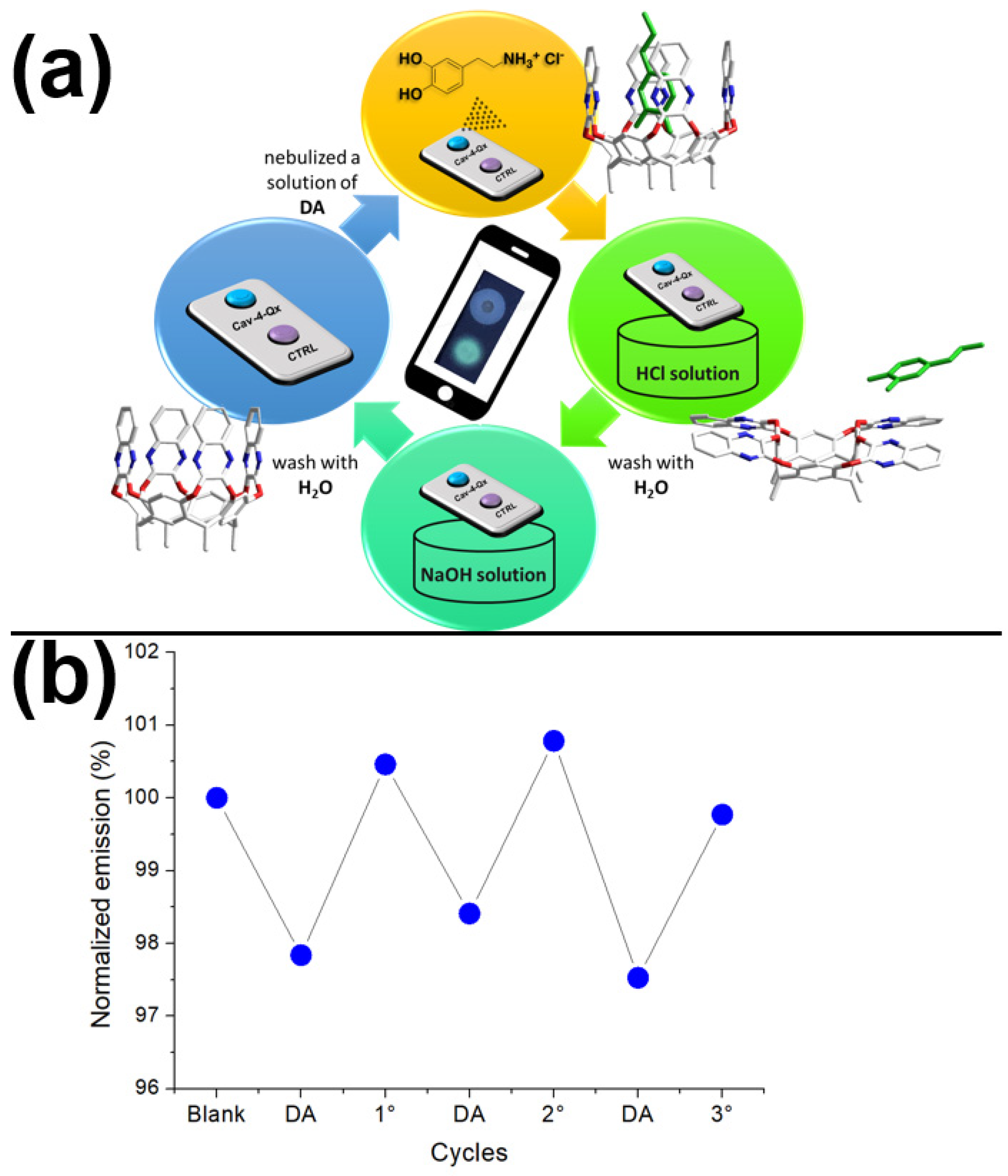
3.8. Procedure for Sensing by Strip Test
3.9. Procedure for Sensing by Strip Test-Experimental Setup
3.10. Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, Y.; Kang, K.; Wang, S.; Kang, W.; Cheng, C.; Niu, L.M.; Guo, Z. A novel label-free fluorescence aptasensor for dopamine detection based on an Exonuclease III- and SYBR Green I- aided amplification strategy. Sens. Actuators B 2020, 305, 127348. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Miletich, R.S.; Kohn, P.D.; Esposito, G.; Carson, R.E.; Quarantelli, M.; Weinberger, D.R.; Berman, K.F. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat. Neurosci. 2002, 5, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Secomandi, E.; Castiglioni, A.; Melone, M.A.B.; Isidoro, C. Resveratrol protects neuronal-like cells expressing mutant Huntingtin from dopamine toxicity by rescuing ATG4-mediated autophagosome formation. Neurochem. Int. 2018, 117, 174–187. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.; Yu, P.; Xiong, E.; Zhang, X.; Chen, J. A simple label-free electrochemical aptasensor for dopamine detection. RSC Adv. 2014, 4, 52250–52255. [Google Scholar] [CrossRef]
- Li, B.-R.; Hsieh, Y.-J.; Chen, Y.-X.; Chung, Y.-T.; Pan, C.-Y.; Chen, Y.-T. An ultrasensitive nanowire-transistor biosensor for detecting dopamine release from living PC12 cells under hypoxic stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef]
- Turkmen, D.; Bakhshpour, M.; Gokturk, I.; Asir, S.; Yilmaz, F.; Denizli, A. Selective dopamine detection by SPR sensor signal amplification using gold nanoparticles. New J. Chem. 2021, 45, 18296–18306. [Google Scholar] [CrossRef]
- Okumura, T.; Nakajima, Y.; Matsuok, M.; Takamatsu, T. Study of salivary catecholamines using fully automated column-switching high-performance liquid chromatography. J. Chromatogr. B 1997, 694, 305–316. [Google Scholar] [CrossRef]
- Ji, D.; Liu, Z.; Liu, L.; Low, S.S.; Lu, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef]
- Syslova, K.; Rambousek, L.; Kuzma, M.; Najmanovà, V.; Bubenikova-Valesova, V.; Slamberova, R.; Kacer, P. Monitoring of dopamine and its metabolites in brain microdialysates: Method combining freeze-drying with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3382–3391. [Google Scholar] [CrossRef]
- Claude, B.; Nehme, R.; Morin, P. Analysis of urinary neurotransmitters by capillary electrophoresis: Sensitivity enhancement using field-amplified sample injection and molecular imprinted polymer solid phase extraction. Anal. Chim. Acta 2011, 699, 242–248. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Lee, J.-J. Electrochemical Dopamine Sensors Based on Graphene. J. Electrochem. Sci. Technol. 2019, 10, 185–195. [Google Scholar]
- Sun, X.; Zhang, L.; Zhang, X.; Liu, X.; Jian, J.; Kong, D.; Zeng, D.; Yuan, H.; Feng, S. Electrochemical dopamine sensor based on superionic conducting potassium ferrite. Biosens. Bioelectron. 2020, 153, 112045. [Google Scholar] [CrossRef] [PubMed]
- Eddin, F.B.K.; Fen, Y.W.; Sadrolhosseini, A.R.; Liew, J.Y.C.; Daniyal, W.M.E.M.M. Optical Property Analysis of Chitosan-Graphene Quantum Dots Thin Film and Dopamine Using Surface Plasmon Resonance Spectroscopy. Plasmonic 2022, 17, 1985–1997. [Google Scholar] [CrossRef]
- Rithesh Raj, D.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic dopamine sensor using green synthesized silver nanoparticles. Sens. Actuators B 2016, 224, 600–606. [Google Scholar] [CrossRef]
- Rostami, S.; Mehdinia, A.; Jabbari, A.; Kowsari, E.; Niroum, R.; Booth, T.J. Colorimetric sensing of dopamine using hexagonal silver nanoparticles decorated by task-specific pyridinum based ionic liquid. Sens. Actuators, B 2018, 271, 64–72. [Google Scholar] [CrossRef]
- Yildirim, A.; Mehmet, M. Turn-on Fluorescent Dopamine Sensing Based on in Situ Formation of Visible Light Emitting Polydopamine Nanoparticles. Anal. Chem. 2014, 86, 5508–5512. [Google Scholar] [CrossRef]
- Baluta, S.; Malecha, K.; Zajac, D.; Soloducho, J.; Cabaj, J. Dopamine sensing with fluorescence strategy based on low temperature co-fired ceramic technology modified with conducting polymers. Sens. Actuators B 2017, 252, 803–812. [Google Scholar] [CrossRef]
- Li, N.; Nan, C.; Mei, X.; Sun, Y.; Feng, H.; Li, Y. Electrochemical sensor based on dual-template molecularly imprinted polymer and nanoporous gold leaf modified electrode for simultaneous determination of dopamine and uric acid. Microchim. Acta 2018, 187, 496. [Google Scholar] [CrossRef]
- Ghosh, S.; Nagarjun, N.; Nandi, S.; Dhakshinamoorthy, A.; Biswas, S. Two birds with one arrow: A functionalized Al (iii) MOF acts as a fluorometric sensor of dopamine in bio-fluids and a recyclable catalyst for the Biginelli reaction. J. Mater. Chem. C 2022, 10, 6717–6727. [Google Scholar] [CrossRef]
- Gajendar, S.; Amisha, K.; Manu, S. Mildly acidic pH and room temperature triggered peroxidase-mimics of rGO–Cu3(OH)2(MoO4)2 cuboidal nanostructures: An effective colorimetric detection of neurotransmitter dopamine in blood serum and urine samples. CrystEngComm 2021, 23, 599–616. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, A.; Mukherjee, P.; Saikia, T.; Pal, K.; Sahu, S.K. A design of fluorescence-based sensor for the detection of dopamine via FRET as well as live cell imaging. Microchem. J. 2020, 159, 105590. [Google Scholar] [CrossRef]
- Moghzi, F.; Soleimannejad, J.; Sañudo, E.C.; Janczak, J. Dopamine Sensing Based on Ultrathin Fluorescent Metal–Organic Nanosheets. ACS Appl. Mater. Interf. 2020, 12, 44499. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, S.; Guan, L.; Li, H.; Fang, J.; Zhang, P.; Du, J.; Zhu, C. Facile fabrication of fluorescent Fe-doped carbon quantum dots for dopamine sensing and bioimaging application. Analyst 2019, 144, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Jiang, K.; Sun, S.; Qian, S.; Wang, Y.; Lin, H. A conjugated carbon-dot–tyrosinase bioprobe for highly selective and sensitive detection of dopamine. Analyst 2019, 144, 468–473. [Google Scholar] [CrossRef]
- Wang, K.; Song, J.; Duan, X.; Mu, J.; Wang, Y. Perovskite LaCoO3 nanoparticles as enzyme mimetics: Their catalytic properties, mechanism and application in dopamine biosensing. New J. Chem. 2017, 41, 8554–8564. [Google Scholar] [CrossRef]
- He, X.-P.; Zeng, Y.-L.; Tang, X.-Y.; Li, N.; Zhou, D.-M.; Chen, G.-R.; Tian, T. Rapid Identification of the Receptor-Binding Specificity of Influenza A Viruses by Fluorogenic Glycofoldamers. Angew. Chem. Int. Ed. 2016, 55, 13995–13999. [Google Scholar] [CrossRef]
- Dou, W.-T.; Han, H.-H.; Sedgwick, A.-C.; Zhu, G.-B.; Zang, Y.; Yang, X.-R.; Yoon, J.; James, T.D.; Li, J.; He, X.P. Fluorescent probes for the detection of disease-associated biomarkers. Sci. Bull. 2022, 67, 853–878. [Google Scholar] [CrossRef]
- Kwon, H.J.; Rivera, E.C.; Neto, M.R.C.; Marsh, D.; Swerdlow, J.J.; Summerscales, R.L.; Tadi, P.P. Development of smartphone-based ECL sensor for dopamine detection: Practical approaches. Results Chem. 2020, 2, 100029. [Google Scholar] [CrossRef]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-Based Electrochemical Potentiostat Detection System Using PEDOT: PSS/Chitosan/Graphene Modified Screen-Printed Electrodes for Dopamine Detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef]
- Muralidharan, R.; Chandrashekhar, V.; Butler, D.; Ebrahimi, A. A Smartphone-Interfaced, Flexible Electrochemical Biosensor Based on Graphene Ink for Selective Detection of Dopamine. IEEE Sens. J. 2020, 20, 13204–13211. [Google Scholar] [CrossRef]
- Dadkhah, S.; Mehdinia, A.; Jabbari, A.; Manbohi, A. Rapid and sensitive fluorescence and smartphone dual-mode detection of dopamine based on nitrogen-boron co-doped carbon quantum dots. Microchim. Acta 2020, 187, 569. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Bao, X.; Han, J.; Xia, J.; Tian, X.; Ni, L. A smartphone-based colorimetric reader coupled with a remote server for rapid on-site catechols analysis. Talanta 2016, 160, 194–204. [Google Scholar] [CrossRef]
- Chellasamy, G.; Ankireddy, S.R.; Lee, K.-N.; Govindaraju, S.; Yun, K. Smartphone-integrated colorimetric sensor array-based reader system and fluorometric detection of dopamine in male and female geriatric plasma by bluish-green fluorescent carbon quantum dots. Mater Today Bio 2021, 12, 100168. [Google Scholar] [CrossRef]
- Bhaiyya, M.; Kulkarni, M.B.; Pattnaik, P.K.; Goel, S. Internet of things-enabled photomultiplier tube- and smartphone-based electrochemiluminescence platform to detect choline and dopamine using 3D-printed closed bipolar electrodes. Luminescence 2022, 37, 357–365. [Google Scholar] [CrossRef]
- Zheng, G.; Shen, C.; Huan, L.; Zhao, R.; Chen, M.; Diao, G. Electrochemical detection dopamine by Ester-calix[n]arenes/graphene nanosheets modified electrodes. J. Electroanal. Chem. 2017, 804, 16–22. [Google Scholar] [CrossRef]
- Halawa, M.; Wu, I.; Fereja, T.H.; Lou, B.; Xu, G. One-pot green synthesis of supramolecular β-cyclodextrin functionalized gold nanoclusters and their application for highly selective and sensitive fluorescent detection of dopamine. Sens. Actuators B 2018, 254, 1017–1024. [Google Scholar] [CrossRef]
- Fragoso, A.; Almirall, E.; Cao, R.; Echegoyen, L.; Gonzalez-Jonte, R. A supramolecular approach to the selective detection of dopamine in the presence of ascorbate. Chem. Commun. 2004, 19, 2230–2231. [Google Scholar] [CrossRef]
- Palomar-Pardavè, M.; Corona-Avendano, S.; Romero-Romo, M.; Alarcon-Angeles, G.; Merkoci, A.; Ramirez-Silva, M.T. Supramolecular interaction of dopamine with β-cyclodextrin: An experimental and theoretical electrochemical study. J. Electroanal. Chem. 2014, 717, 103–109. [Google Scholar] [CrossRef]
- Harley, C.C.; Annibaldi, V.; Yu, T.; Breslin, C.B.; Carmel, B. The selective electrochemical sensing of dopamine at a polypyrrole film doped with an anionic β−cyclodextrin. J. Electroanal. Chem. 2019, 855, 113614. [Google Scholar]
- Kurzatkowska, K.; Sayin, S.; Yilmaz, M.; Radecka, H.; Radecki, J. Calix[4]arene derivatives as dopamine hosts in electrochemical sensors. Sens. Actuators B Chem. 2015, 218, 111–121. [Google Scholar] [CrossRef]
- Mei, Y.; Zhang, Q.-W.; Gu, Q.; Liu, Z.; He, X.; Tian, Y. Pillar[5]arene-Based Fluorescent Sensor Array for Biosensing of Intracellular Multi-neurotransmitters through Host–Guest Recognitions. J. Am. Chem. Soc. 2022, 144, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-D.; Shi, L.; Guo, L.-H.; Wang, J.-W.; Zhang, X. Determination of dopamine hydrochloride by host-guest interaction based on water-soluble pillar[5]arene. Spectrochim. Acta A 2017, 173, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, V.; Cejas, M.A.; Raymo, F.M.; Chen, W.; Parker, S.E.; Kaifer, A.E. Supramolecular Assembly of 2,7-Dimethyldiazapyrenium and Cucurbit[8]uril: A New Fluorescent Host for Detection of Catechol and Dopamine. Chem. Eur. J. 2005, 11, 7054–7059. [Google Scholar] [CrossRef]
- Kasera, S.; Walsh, Z.; Del Barrio, J.; Scherman, O.A. A selective supramolecular photochemical sensor for dopamine. Supramol. Chem. 2014, 26, 280–285. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by a supramolecular complex composed of tin(IV) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Danylyuk, O.; Worzakowsk, M.; Osypiuk-Tomasik, J.; Sashuk, V.; Kedra-Krolik, K. Solution-mediated and single-crystal to single-crystal transformations of cucurbit[6]uril host–guest complexes with dopamine. CrystEngComm 2020, 22, 634–638. [Google Scholar] [CrossRef]
- Mecozzi, S.; Rebek, J., Jr. The 55 % Solution: A Formula for Molecular Recognition in the Liquid State. Chem. Eur. J. 1998, 4, 1016. [Google Scholar] [CrossRef]
- Mosca, S.; Yang, Y.; Rebek, J., Jr. Preparative scale and convenient synthesis of a water-soluble, deep cavitand. Nat. Prot. 2016, 11, 1371. [Google Scholar] [CrossRef]
- Trusso Sfrazzetto, G.; Satriano, C.; Tomaselli, G.A.; Rizzarelli, E. Synthetic fluorescent probes to map metallostasis and intracellular fate of zinc and copper. Coord. Chem. Rev. 2016, 311, 125–167. [Google Scholar] [CrossRef]
- Giuffrida, M.L.; Rizzarelli, E.; Tomaselli, G.A.; Satriano, C.; Trusso Sfrazzetto, G. A novel fully water-soluble Cu(I) probe for fluorescence live cell imaging. Chem. Commun. 2014, 50, 9835–9838. [Google Scholar] [CrossRef] [PubMed]
- Ballistreri, F.P.; Brancatelli, G.; Demitri, N.; Geremia, S.; Guldi, D.M.; Melchionna, M.; Pappalardo, A.; Prato, M.; Tomaselli, G.A.; Trusso Sfrazzetto, G. Recognition of C60 by tetra- and tri-quinoxaline cavitands. Supramol. Chem. 2016, 28, 601–607. [Google Scholar] [CrossRef]
- Legnani, L.; Puglisi, R.; Pappalardo, A.; Chiacchio, M.A.; Trusso Sfrazzetto, G. Supramolecular recognition of phosphocholine by an enzyme-like cavitand receptor. Chem. Commun. 2020, 56, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, R.; Pappalardo, A.; Gulino, A.; Trusso Sfrazzetto, G. Multitopic Supramolecular Detection of Chemical Warfare Agents by Fluorescent Sensors. ACS Omega 2019, 4, 7550–7555. [Google Scholar] [CrossRef]
- Tuccitto, N.; Riela, L.; Zammataro, A.; Spitaleri, L.; Li Destri, G.; Sfuncia, G.; Nicotra, G.; Pappalardo, A.; Capizzi, G.; Trusso Sfrazzetto, G. Functionalized Carbon Nanoparticle-Based Sensors for Chemical Warfare Agents. ACS Appl. Nano Mater. 2020, 3, 8182–8191. [Google Scholar] [CrossRef]
- Puglisi, R.; Pappalardo, A.; Gulino, A.; Trusso Sfrazzetto, G. Supramolecular recognition of a CWA simulant by metal–salen complexes: The first multi-topic approach. Chem. Commun. 2018, 54, 11156–11159. [Google Scholar] [CrossRef]
- Trusso Sfrazzetto, G.; Millesi, S.; Pappalardo, A.; Tomaselli, G.A.; Ballistreri, F.P.; Toscano, R.M.; Fragalà, I.; Gulino, A. Nerve Gas Simulant Sensing by a Uranyl–Salen Monolayer Covalently Anchored on Quartz Substrates. Chem. Eur. J. 2017, 23, 1576–1583. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preblisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Chaisiwamongkhol, K.; Batchelor-McAuley, C.; Compton, R.G. Chemical analysis in saliva and the search for salivary biomarkers—A tutorial review. Analyst 2018, 143, 81–99. [Google Scholar] [CrossRef]
- Tuccitto, N.; Catania, G.; Pappalardo, A.; Trusso Sfrazzetto, G. Agile Detection of Chemical Warfare Agents by Machine Vision: A Supramolecular Approach. Chem. Eur. J. 2021, 27, 13715–13718. [Google Scholar] [CrossRef]
- Pagliusi, P.; Lagugne-Labarthet, F.; Shenoy, D.K.; Dalcanale, E.; Shen, Y.R. Sensing Vase-to-Kite Switching of Cavitands by Sum-Frequency Vibrational Spectroscopy. J. Am. Chem. Soc. 2006, 128, 12610–12611. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonocito, R.; Tuccitto, N.; Pappalardo, A.; Trusso Sfrazzetto, G. Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor. Molecules 2022, 27, 7503. https://doi.org/10.3390/molecules27217503
Santonocito R, Tuccitto N, Pappalardo A, Trusso Sfrazzetto G. Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor. Molecules. 2022; 27(21):7503. https://doi.org/10.3390/molecules27217503
Chicago/Turabian StyleSantonocito, Rossella, Nunzio Tuccitto, Andrea Pappalardo, and Giuseppe Trusso Sfrazzetto. 2022. "Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor" Molecules 27, no. 21: 7503. https://doi.org/10.3390/molecules27217503
APA StyleSantonocito, R., Tuccitto, N., Pappalardo, A., & Trusso Sfrazzetto, G. (2022). Smartphone-Based Dopamine Detection by Fluorescent Supramolecular Sensor. Molecules, 27(21), 7503. https://doi.org/10.3390/molecules27217503









