MOF-Based Mycotoxin Nanosensors for Food Quality and Safety Assessment through Electrochemical and Optical Methods
Abstract
:1. Introduction
2. MOFs: Types, Various Synthesis Methods, and Applications
3. Electrochemical Platforms for Sensing Different Mycotoxins in Various Foods
| MOF Type | Technique | Target Mycotoxins | Modifier | Detection Limit (LOD) | Linear Range | Recovery (%) | Reference |
|---|---|---|---|---|---|---|---|
| AFB1-PBP-cDNA-Apt-MPA-AuNP-NiMOF-GCE | DPV | Aflatoxin B1 (AFB1) | AuNP/Ni-MOF | 1.0 × 10−6 mg·L−1 | 5.0 × 10−6–0.15 mg·L−1 | 8.4–101.3 | [61] |
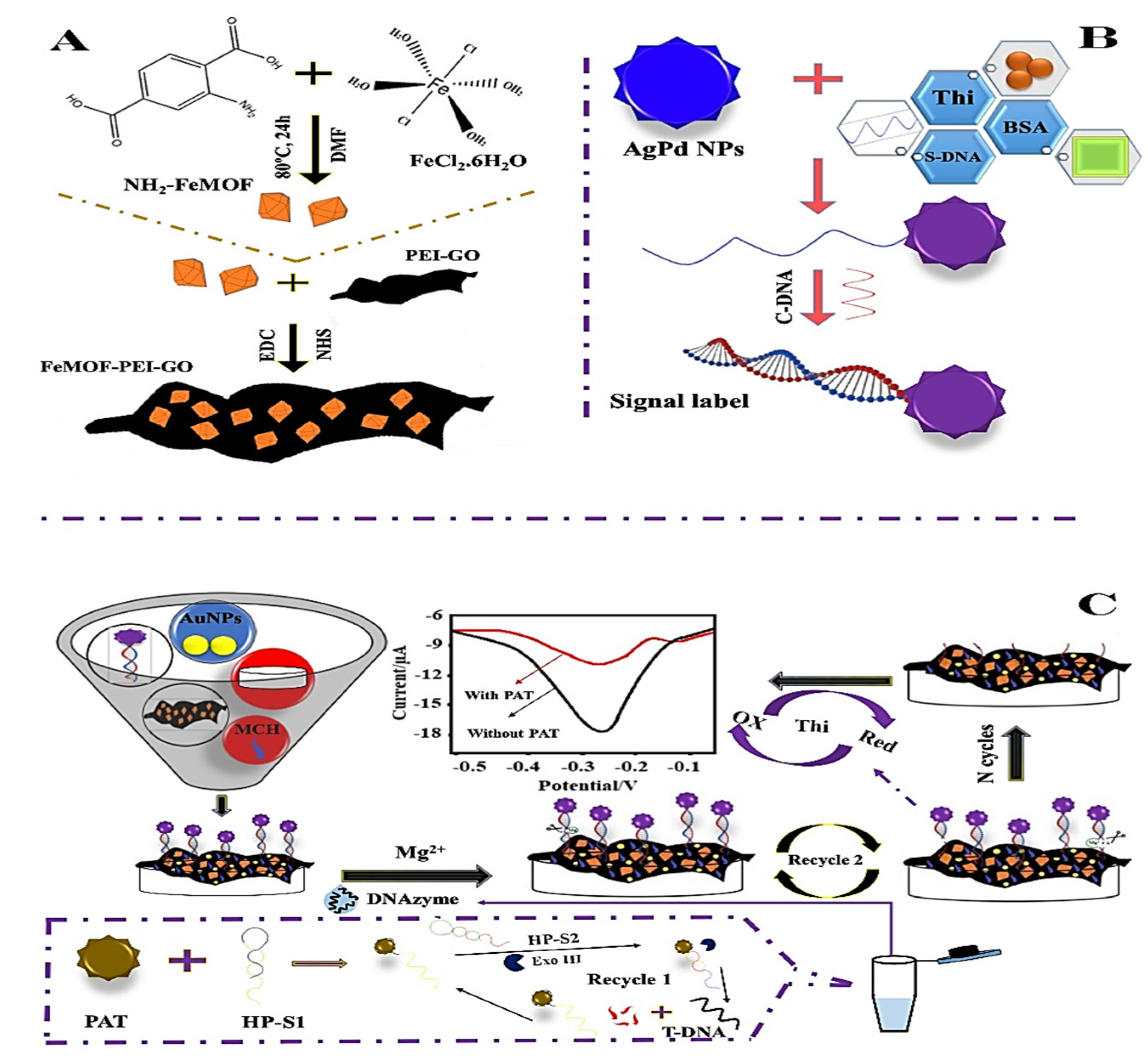
| AuNPs/FeMOF-PEIGO; AgPdNPs | DPV | Patulin (PAT) | AuNPs/FeMOF-PEI-GO | 2.17 × 10−10 mg·L−1 | 5 × 10−10–0.005 mg·L−1 | 91.0–103 | [58] |
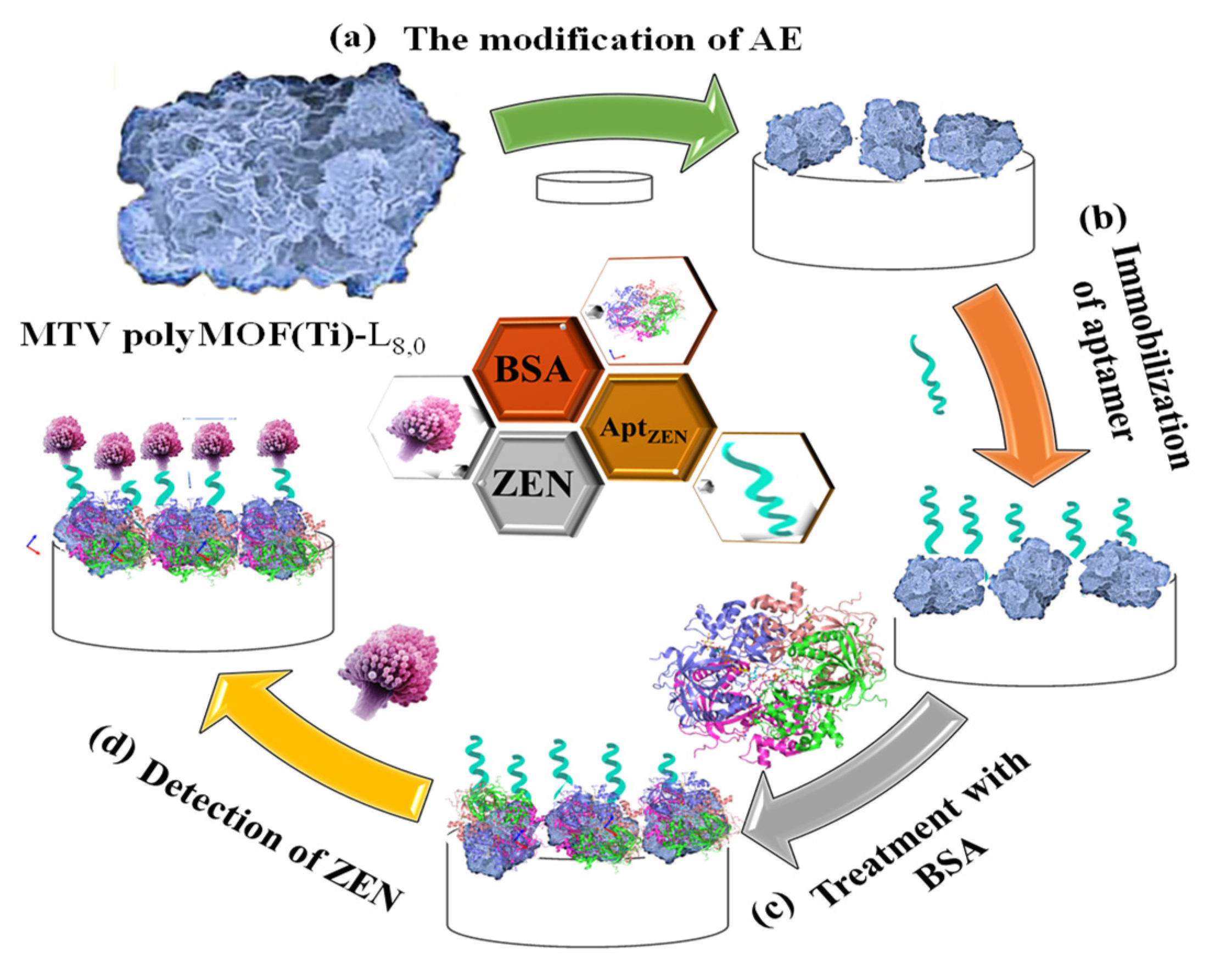
| MTV polyMOF-L8,0 | DPV and EIS | Zearalenone | ligand of L8 or L0 | 7 × 10−9 mg·L−1 and 3.5 × 10−9 mg·L−1 | 10 × 10−9 mg L−1 to 0.01 mg·L−1 | 95.72–106.32 | [64] |
| MoS2 QDs@UiO-66-NH2 composite | CV and EIS | Aflatoxin M1 (AFM1) | UiO-66-NH2 | 6.0 × 10−5 mg·L−1 | 0.0002−0.01 mg·L−1 | - | [60] |
| N–Cu–MOF | DPV | Deoxynivalenol [16] | N-Doped | 8.0 × 10−6 mg·L−1 | 2.0 × 10−5–0.02 mg·L−1 | 95.6–105.9 | [72] |
| Zr-MOF | EIS | Ochratoxin A (OTA) | - | 2.4 × 10−8 mg·L−1 | 1.0 × 10−7–0.14 × 10−3 mg·L−1 | - | [67] |
| CuMOF-GCE | EIS | Aflatoxin B1 (AFB1) | CuMOF | 8.3 × 10−7 mg·L−1 | 1.0 × 10−7–0.2 mg·L−1 | 97.8–105.5 | [62] |
| NH2/MIL-101@CoPc6:1 | EIS and CV | Ochratoxin A (OTA) | CoPc6:1 | 0.063 × 10−9 mg·L−1 | 1.0 × 10−10–1.0 × 10−4 mg·L−1 | 98.2–110.0 | [68] |
| Cu-MOF/Fe3O4-GO | DPASV | Zearalenone (ZEA) | Cu-MOF | 0.023 14 mg·L−1 | 0.1592–2.8652 mg·L−1 | 96.4–97.3 | [66] |
| MOFLISA (MOFs@Ab2) | chromogenic system | Aflatoxin B1 (AFB1) | ELISA | 9.0 × 10−6 mg·L−1 | 1.0 × 10−5 to 0.02 mg·L−1 | 86.41–99.77 | [63] |
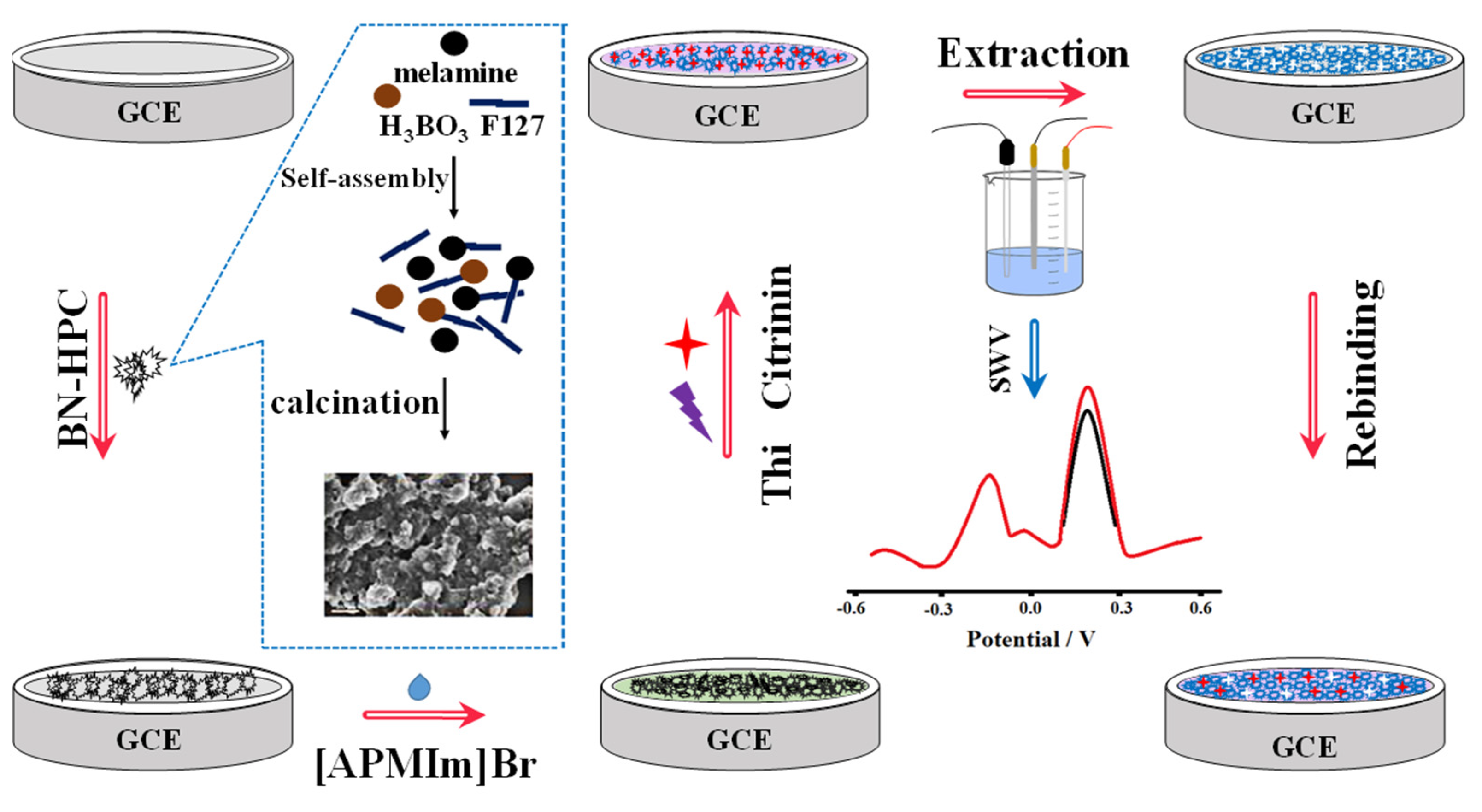
| MIP/BN-HPC/[APMIm]Br/GCE | SWV | Citrinin | [APMIm]Br/BN | 1.0 × 10−7 mg·L−1 | 1.0 × 10−6−0.01 mg·L−1 | 97–110 | [71] |
| SA/AgPt/PCN-223-Fe | DPV | Ochratoxin A (OTA) | AgPt | 14 × 10−9 mg·L−1 | 20 × 10−9–0.002 mg·L−1 | 95.5–104.0 | [69] |
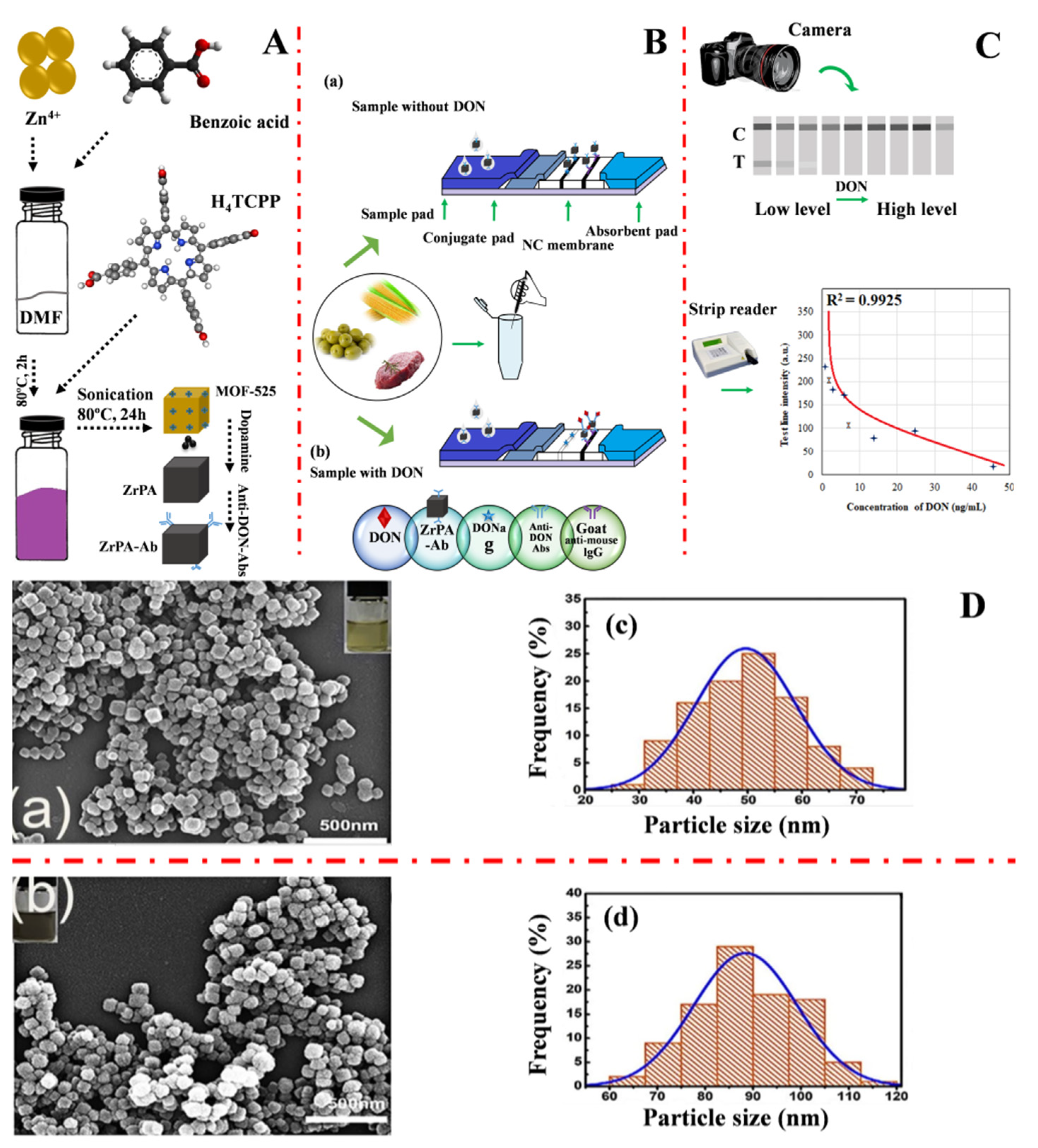
| ZrPA-ICA | Immunochromatographic | Deoxynivalenol [16] | ZrPA | 0.18 × 10−3 mg·L−1 | 0.18 × 10−3–0.05 mg·L−1 | 97.8–109.5 | [59] |
| Cu-MOF/AuNPs/S4 | DPV | Aflatoxin B1 (AFB1) | DNA (S4) | 6.7 × 10−10 mg·L−1 | 1.0 × 10−9–0.001 mg·L−1 | 96–103 | [73] |
| SA/Au NPs@Cd/MOF-74 | DPV | Ochratoxin A (OTA) | Cd-MOF-74 | 1.0 × 10−5 mg·L−1 | 5.0 × 10−5–0.1 mg·L−1 | 91.1–105.2 | [74] |
| BSA/Apt-PtNP/MIL–101/GCE | EIS | Aflatoxin M1 (AFM1) | MIL–101 | 2.0 × 10–6 mg·L−1 | 1.0 × 10–5–0.08 mg·L−1 | 93.0–108.0 | [75] |
| AuNP/MIP-MOF | LSV | Aflatoxin B1 (AFB1) | AuNP | 0.3 × 10−9 mg·L−1 | 0.0000032 nM–3200 nM | - | [76] |
| AuE/DLS/OBA-TSS/UiO-66/MCH | SWV | Ochratoxin A (OTA) | UiO-66 | 7.9 × 10−8 nM | 10−7–2000 nM | 98.5–103.7 | [77] |
| Zr-MOFs-PEI-rGO/Fe-MOFs/Pt@AuNRs | DPV | Patulin (PAT) | MB@Zr-MOFs-cDNA | 4.14 × 10−8 mg·L−1 | 5.0 × 10−8–0.0005 mg·L−1 | 87–101 | [78] |
| DNA-PtNi@Co-MOF/AuNRs/CoSe2 | DPV | Zearalenone [44] | PtNi@Co-MOF | 1.37 × 10−9 mg·L−1 | 10×10−9–0.01 mg·L−1 | 93.6–103.4 | [79] |
| MIP-Au@Cu-MOF/N-GQDs/GCE | DPV | Patulin (PAT) | Au@Cu-MOF | 7.0 × 10−7 mg·L−1 | 1.0 × 10−6–0.07 mg·L−1 | 97.6–99.4 | [80] |
| MIP-Au@PANI-SeS2@Co MO | DPV | Patulin (PAT) | SeS2@Co MO | 0.001–100 nM | 0.001–100 nM | 94.5–106.4 | [39] |
| CoNi-MOF | EIS | Deoxynivalenol [16] | CoNi | 5.0 × 10−8 mg·L−1 | 1.0 × 10−6–0.0005 mg·L−1 | 95.7–102.6 | [81] |
| Ag NPs/2D MOF sheets | DPV | Ochratoxin A (OTA) | Ag NPs | 0.08 × 10−9 mg·L−1 | 0.10 × 10−9–1 mg·L−1 | 99.27–101.20 | [82] |
| MIP/COFs-AuNPs/AuE | ELISA | Aflatoxin B1 (AFB1) | COFs-AuNPs | 2.8 × 10−6 mg·L−1 | 5.0 × 10−5–0.075 mg·L−1 | 87.0−101.7 | [83] |
4. Optical Sensing Platforms for Sensing Different Mycotoxins in Various Food
5. Conclusions
6. Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraemer, K.; Cordaro, J.; Fanzo, J.; Gibney, M.; Kennedy, E.; Labrique, A.; Steffen, J.; Eggersdorfer, M. The Critical Role of Food Safety in Ensuring Food Security. In Good Nutrition: Perspectives for the 21st Century; Karger Publishers: Basel, Switzerland, 2016; pp. 312–325. [Google Scholar]
- Mogopodi, D.; Mbisana, M.; Raditloko, S.; Chibua, I.; Paphane, B. Toward Safe Food Systems: Analyses of Mycotoxin Contaminants in Food and Preventive Strategies Thereof for Their Formation and Toxicity; IntechOpen: London, UK, 2022. [Google Scholar]
- Kussaga, J.B.; Jacxsens, L.; Tiisekwa, B.P.; Luning, P.A. Food safety management systems performance in African food processing companies: A review of deficiencies and possible improvement strategies. J. Sci. Food Agric. 2014, 94, 2154–2169. [Google Scholar] [CrossRef]
- Vågsholm, I.; Arzoomand, N.S.; Boqvist, S. Food security, safety, and sustainability—Getting the trade-offs right. Front. Sustain. Food Syst. 2020, 4, 16. [Google Scholar] [CrossRef]
- Krivohlavek, A. Food Safety, standards and norms against bioterrorism: Food safety and hazards. In Defence Against Bioterrorism; Springer: Dordrecht, The Netherlands, 2018; pp. 239–248. [Google Scholar]
- Bennett, J.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- TT, W.; IJ, A.A.; Braide, W. Mycotoxins and mycotoxicoses, detection and analysis: A review in retrospect. Asian J. Appl. Sci. Technol. 2021, 5, 43–67. [Google Scholar]
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [Green Version]
- Picardo, M.; Filatova, D.; Nunez, O.; Farré, M. Recent advances in the detection of natural toxins in freshwater environments. TrAC Trends Anal. Chem. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Selvaraj, J.N.; Lu, Z.; Yan, W.; Zhao, Y.-j.; Xing, F.-g.; Dai, X.-f.; Yang, L. Mycotoxin detection—Recent trends at global level. J. Integr. Agric. 2015, 14, 2265–2281. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Wang, F.; He, P. Recent advances in immunoassays and biosensors for mycotoxins detection in feedstuffs and foods. J. Anim. Sci. Biotechnol. 2021, 12, 1–19. [Google Scholar] [CrossRef]
- Chen, F.; Luan, C.; Wang, L.; Wang, S.; Shao, L. Simultaneous determination of six mycotoxins in peanut by high-performance liquid chromatography with a fluorescence detector. J. Sci. Food Agric. 2017, 97, 1805–1810. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Jedziniak, P. Dilute-and-shoot HPLC-UV method for determination of urinary creatinine as a normalization tool in mycotoxin biomonitoring in pigs. Molecules 2020, 25, 2445. [Google Scholar] [CrossRef]
- Zhou, D.; Li, Y.; Huang, L.; Qian, M.; Li, D.; Sun, G.; Yang, B. A reliable and cost-efficient TLC-HPLC method for determining total florfenicol residues in porcine edible tissues. Food Chem. 2020, 303, 125399. [Google Scholar] [CrossRef]
- He, H.; Sun, D.-W.; Wu, Z.; Pu, H.; Wei, Q. On-off-on fluorescent nanosensing: Materials, detection strategies and recent food applications. Trends Food Sci. Technol. 2022, 119, 243–256. [Google Scholar] [CrossRef]
- Shen, J.; Xia, X.; Jiang, H.; Li, C.; Li, J.; Li, X.; Ding, S. Determination of chloramphenicol, thiamphenicol, florfenicol, and florfenicol amine in poultry and porcine muscle and liver by gas chromatography-negative chemical ionization mass spectrometry. J. Chromatogr. B 2009, 877, 1523–1529. [Google Scholar] [CrossRef]
- Tao, X.; Peng, Y.; Liu, J. Nanomaterial-based fluorescent biosensors for veterinary drug detection in foods. J. Food Drug Anal. 2020, 28, 575. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Wu, D.; Li, X.; Yu, Y.; Luo, P.; Chen, J.; Dai, C.; Wu, Y. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. TrAC Trends Anal. Chem. 2019, 121, 115669. [Google Scholar] [CrossRef]
- Righetti, L.; Dreolin, N.; Celma, A.; McCullagh, M.; Barknowitz, G.; Sancho, J.V.; Dall’Asta, C. Travelling wave ion mobility-derived collision cross section for mycotoxins: Investigating interlaboratory and interplatform reproducibility. J. Agric. Food Chem. 2020, 68, 10937–10943. [Google Scholar] [CrossRef]
- Javanmardi, F.; Khodaei, D.; Sheidaei, Z.; Bashiry, M.; Nayebzadeh, K.; Vasseghian, Y.; Mousavi Khaneghah, A. Decontamination of aflatoxins in edible oils: A comprehensive review. Food Rev. Int. 2022, 38, 1410–1426. [Google Scholar] [CrossRef]
- Sohrabi, H.; Khataee, A.; Ghasemzadeh, S.; Majidi, M.R.; Orooji, Y. Layer double hydroxides (LDHs)-based electrochemical and optical sensing assessments for quantification and identification of heavy metals in water and environment samples: A review of status and prospects. Trends Environ. Anal. Chem. 2021, 31, e00139. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2021, 107, 20–30. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical immuno-and aptasensors for mycotoxin determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Muniandy, S.; Teh, S.J.; Thong, K.L.; Thiha, A.; Dinshaw, I.J.; Lai, C.W.; Ibrahim, F.; Leo, B.F. Carbon nanomaterial-based electrochemical biosensors for foodborne bacterial detection. Crit. Rev. Anal. Chem. 2019, 49, 510–533. [Google Scholar] [CrossRef]
- McDonagh, C.; Burke, C.S.; MacCraith, B.D. Optical chemical sensors. Chem. Rev. 2008, 108, 400–422. [Google Scholar] [CrossRef]
- Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.-Z.; Wang, S. Recent progress on luminescent metal-organic framework-involved hybrid materials for rapid determination of contaminants in environment and food. Polymers 2020, 12, 691. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.-J.; Wu, C.; Fang, M.; Ding, B.; Liu, P.-P.; Zhou, M.-X.; Gong, Z.-Y.; Ma, D.-L.; Leung, C.-H. Application of metal–organic framework for the adsorption and detection of food contamination. TrAC Trends Anal. Chem. 2021, 143, 116384. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef]
- Sohrabi, H.; Sani, P.S.; Orooji, Y.; Majidi, M.R.; Yoon, Y.; Khataee, A. MOF-based sensor platforms for rapid detection of pesticides to maintain food quality and safety. Food Chem. Toxicol. 2022, 165, 113176. [Google Scholar] [CrossRef]
- Nong, W.; Liu, X.; Wang, Q.; Wu, J.; Guan, Y. Metal-organic framework-based materials: Synthesis, stability and applications in food safety and preservation. ES Food Agrofor. 2020, 1, 11–40. [Google Scholar] [CrossRef]
- Stavila, V.; Talin, A.A.; Allendorf, M.D. MOF-based electronic and opto-electronic devices. Chem. Soc. Rev. 2014, 43, 5994–6010. [Google Scholar] [CrossRef] [Green Version]
- Marimuthu, M.; Arumugam, S.S.; Jiao, T.; Sabarinathan, D.; Li, H.; Chen, Q. Metal organic framework based sensors for the detection of food contaminants. TrAC Trends Anal. Chem. 2022, 154, 116642. [Google Scholar] [CrossRef]
- Cheng, W.; Tang, X.; Zhang, Y.; Wu, D.; Yang, W. Applications of metal-organic framework (MOF)-based sensors for food safety: Enhancing mechanisms and recent advances. Trends Food Sci. Technol. 2021, 112, 268–282. [Google Scholar] [CrossRef]
- Ling, P.; Lei, J.; Zhang, L.; Ju, H. Porphyrin-encapsulated metal–organic frameworks as mimetic catalysts for electrochemical DNA sensing via allosteric switch of hairpin DNA. Anal. Chem. 2015, 87, 3957–3963. [Google Scholar] [CrossRef]
- An, H.; Li, M.; Gao, J.; Zhang, Z.; Ma, S.; Chen, Y. Incorporation of biomolecules in metal-organic frameworks for advanced applications. Coord. Chem. Rev. 2019, 384, 90–106. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, N.; Lou, Y.; Ren, S.; Pang, S.; He, Y.; Chen, X.-B.; Shi, Z.; Feng, S. A stable nanoscaled Zr-MOF for the detection of toxic mycotoxin through a pH-modulated ratiometric luminescent switch. Chem. Commun. 2020, 56, 5389–5392. [Google Scholar] [CrossRef]
- Selvam, S.P.; Kadam, A.N.; Maiyelvaganan, K.R.; Prakash, M.; Cho, S. Novel SeS2-loaded Co MOF with Au@ PANI comprised electroanalytical molecularly imprinted polymer-based disposable sensor for patulin mycotoxin. Biosens. Bioelectron. 2021, 187, 113302. [Google Scholar] [CrossRef]
- Majdinasab, M.; Ben Aissa, S.; Marty, J.L. Advances in colorimetric strategies for mycotoxins detection: Toward rapid industrial monitoring. Toxins 2020, 13, 13. [Google Scholar] [CrossRef]
- Lai, H.; Dai, H.; Li, G.; Zhang, Z. Rapid determination of pesticide residues in fruit and vegetable using Au@ AgNPs decorated 2D Ni-MOF nanosheets as efficient surface-enhanced Raman scattering substrate. Sens. Actuators B Chem. 2022, 369, 132360. [Google Scholar] [CrossRef]
- Hitabatuma, A.; Wang, P.; Su, X.; Ma, M. Metal-Organic Frameworks-Based Sensors for Food Safety. Foods 2022, 11, 382. [Google Scholar] [CrossRef]
- Villalonga, A.; Sánchez, A.; Mayol, B.; Reviejo, J.; Villalonga, R. Electrochemical biosensors for food bioprocess monitoring. Curr. Opin. Food Sci. 2022, 43, 18–26. [Google Scholar] [CrossRef]
- Campagnol, N.; Souza, E.R.; De Vos, D.E.; Binnemans, K.; Fransaer, J. Luminescent terbium-containing metal–organic framework films: New approaches for the electrochemical synthesis and application as detectors for explosives. Chem. Commun. 2014, 50, 12545–12547. [Google Scholar] [CrossRef]
- Bo, Q.-B.; Pang, J.-J.; Wang, H.-Y.; Fan, C.-H.; Zhang, Z.-W. Hydrothermal synthesis, characterization and photoluminescent properties of the microporous metal organic frameworks with 1, 3-propanediaminetetraacetate ligand and its auxiliary ligand. Inorg. Chim. Acta 2015, 428, 170–175. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Yang, Q.; Wei, Q.; Xie, G.; Chen, S. Mixed-metal–organic frameworks (M′ MOFs) from 1D to 3D based on the “organic” connectivity and the inorganic connectivity: Syntheses, structures and magnetic properties. CrystEngComm 2015, 17, 3312–3324. [Google Scholar] [CrossRef]
- Dong, W.; Liu, X.; Shi, W.; Huang, Y. Metal–organic framework MIL-53 (Fe): Facile microwave-assisted synthesis and use as a highly active peroxidase mimetic for glucose biosensing. RSC Adv. 2015, 5, 17451–17457. [Google Scholar] [CrossRef]
- Singh, N.K.; Hardi, M.; Balema, V.P. Mechanochemical synthesis of an yttrium based metal–organic framework. Chem. Commun. 2013, 49, 972–974. [Google Scholar] [CrossRef]
- Xue, Y.; Peng, Y.; Geng, Z.; Wang, Y.; Ung, C.O.L.; Hu, H. Metal–organic frameworks (MOFs) based analytical techniques for food safety evaluation. eFood 2021, 2, 1–12. [Google Scholar] [CrossRef]
- Sharanyakanth, P.; Radhakrishnan, M. Synthesis of metal-organic frameworks (MOFs) and its application in food packaging: A critical review. Trends Food Sci. Technol. 2020, 104, 102–116. [Google Scholar] [CrossRef]
- Ensafi, A.A. An Introduction to Sensors and Biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–10. [Google Scholar]
- Das, J.; Mishra, H.N. Recent advances in sensors for detecting food pathogens, contaminants, and toxins: A review. Eur. Food Res. Technol. 2022, 248, 1125–1148. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Tang, X.; Zhang, Z.; Li, P. Recent advances in electrochemical sensors for mycotoxin detection in food. Electroanalysis 2021. [Google Scholar] [CrossRef]
- Fortunato, J.; Jordan, J.W.; Newton, G.N.; Walsh, D.A.; Augustyn, V. Electrochemical reactivity of atomic and molecular species under solid-state confinement. Curr. Opin. Electrochem. 2022, 34, 101014. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Mukunzi, D.; Habimana, J.; Li, Z.; Zou, X. Mycotoxins detection: View in the lens of molecularly imprinted polymer and nanoparticles. Crit. Rev. Food Sci. Nutr. 2022, 1–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Lou, Y.; Guo, C.; Jia, Q.; Song, Y.; Tian, J.-Y.; Zhang, S.; Wang, M.; He, L.; Du, M. Metal–organic frameworks (MOFs) based chemosensors/biosensors for analysis of food contaminants. Trends Food Sci. Technol. 2021, 118, 569–588. [Google Scholar] [CrossRef]
- Lu, X.; He, B.; Liang, Y.; Wang, J.; Jiao, Q.; Liu, Y.; Guo, R.; Wei, M.; Jin, H.; Ren, W. An electrochemical aptasensor based on dual-enzymes-driven target recycling strategy for patulin detection in apple juice. Food Control 2022, 137, 108907. [Google Scholar] [CrossRef]
- Li, R.; Bu, T.; Zhao, Y.; Sun, X.; Wang, Q.; Tian, Y.; Bai, F.; Wang, L. Polydopamine coated zirconium metal-organic frameworks-based immunochromatographic assay for highly sensitive detection of deoxynivalenol. Anal. Chim. Acta 2020, 1131, 109–117. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, S.; Singh, S.; Bhardwaj, N.; Deep, A. Selective and Sensitive Electrochemical Sensor for Aflatoxin M1 with a Molybdenum Disulfide Quantum Dot/Metal–Organic Framework Nanocomposite. ACS Omega 2022, 7, 17600–17608. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z.; Benvidi, A. Development of an electrochemical aptasensor based on Au nanoparticles decorated on metal–organic framework nanosheets and p-biphenol electroactive label for the measurement of aflatoxin B1 in a rice flour sample. Anal. Bioanal. Chem. 2022, 414, 1973–1985. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. A non-label electrochemical aptasensor based on Cu metal–organic framework to measure aflatoxin B1 in wheat flour. Food Anal. Methods 2022, 15, 192–202. [Google Scholar] [CrossRef]
- Xu, Z.; Long, L.-L.; Chen, Y.-Q.; Chen, M.-L.; Cheng, Y.-H. A nanozyme-linked immunosorbent assay based on metal–organic frameworks (MOFs) for sensitive detection of aflatoxin B1. Food Chem. 2021, 338, 128039. [Google Scholar] [CrossRef]
- Duan, F.; Rong, F.; Guo, C.; Chen, K.; Wang, M.; Zhang, Z.; Pettinari, R.; Zhou, L.; Du, M. Electrochemical aptasensing strategy based on a multivariate polymertitanium-metal-organic framework for zearalenone analysis. Food Chem. 2022, 385, 132654. [Google Scholar] [CrossRef]
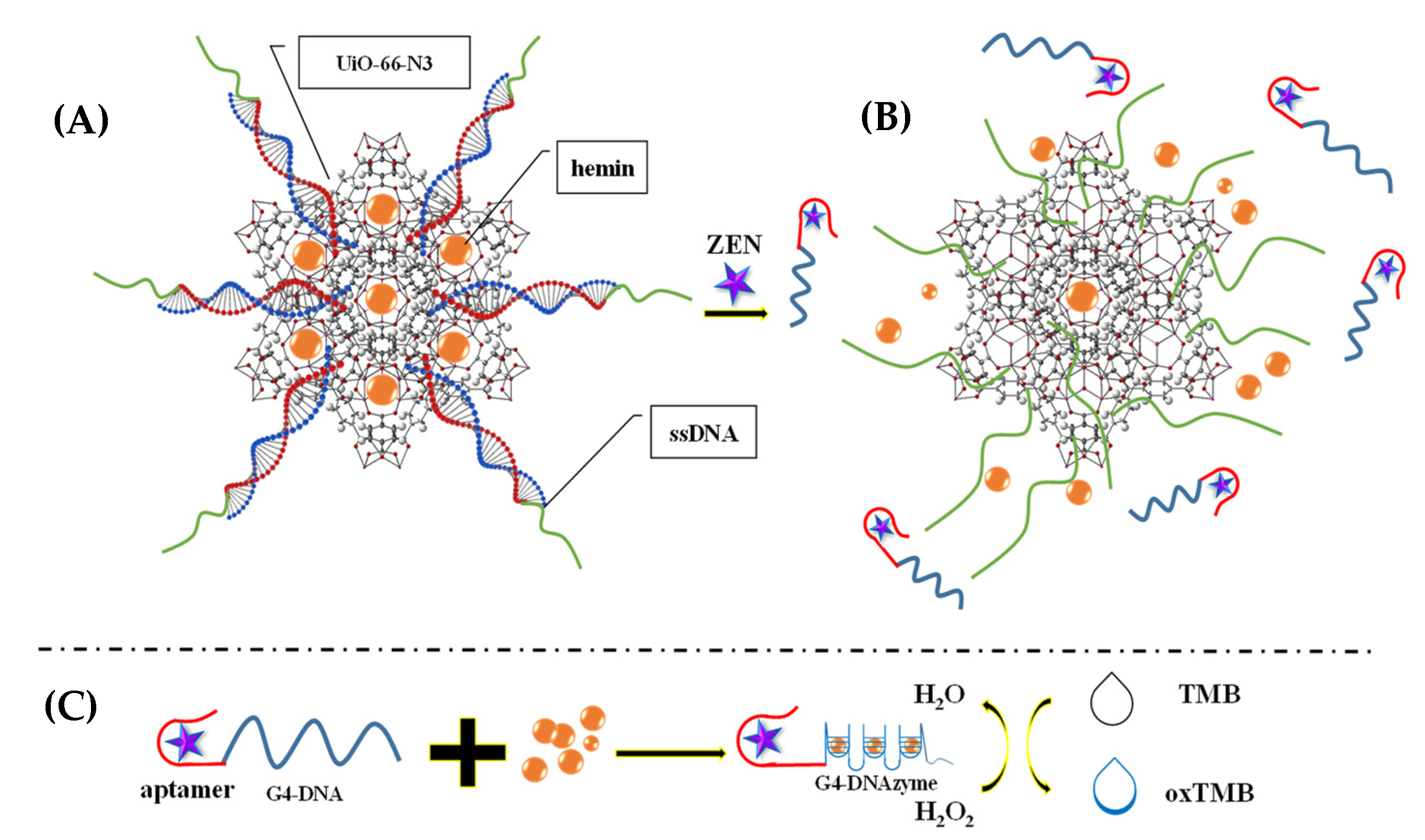
- Sun, Y.; Lv, Y.; Qi, S.; Zhang, Y.; Wang, Z. Sensitive colorimetric aptasensor based on stimuli-responsive metal-organic framework nano-container and trivalent DNAzyme for zearalenone determination in food samples. Food Chem. 2022, 371, 131145. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Camarada, M.B.; Lu, X.; Tang, K.; Li, W.; Qiu, D.; Wen, Y.; Wu, G.; Luo, Q.; Bai, L. Detection and electrocatalytic mechanism of zearalenone using nanohybrid sensor based on copper-based metal-organic framework/magnetic Fe3O4-graphene oxide modified electrode. Food Chem. 2022, 370, 131024. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Hu, X.; Shi, Y.; Liang, N.; Huang, X.; Wang, X.; Shen, T.; Zou, X.; Shi, J. Simple Design Concept for Dual-Channel Detection of Ochratoxin A Based on Bifunctional Metal–Organic Framework. ACS Appl. Mater. Interfaces 2022, 14, 5615–5623. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, L.; Zhang, S.; Liu, X.; Chen, K.; Jia, Q.; Zhang, Z.; Du, M. Novel impedimetric sensing strategy for detecting ochratoxin A based on NH2-MIL-101 (Fe) metal-organic framework doped with cobalt phthalocyanine nanoparticles. Food Chem. 2021, 351, 129248. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Qiang, Y. Ultrasensitive electrochemical aptasensor for ochratoxin A detection using AgPt bimetallic nanoparticles decorated iron-porphyrinic metal-organic framework for signal amplification. Sens. Actuators B Chem. 2020, 312, 127964. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Z.; Guan, Y.; Chen, Y.; Liu, W.; Liu, Y. Zeolitic imidazolate frameworks-derived hollow Co/N-doped CNTs as oxidase-mimic for colorimetric-fluorescence immunoassay of ochratoxin A. Sens. Actuators B Chem. 2022, 359, 131609. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Xia, Y.; Zhao, F.; Zeng, B. A novel ratiometric electrochemical sensor for the selective detection of citrinin based on molecularly imprinted poly (thionine) on ionic liquid decorated boron and nitrogen co-doped hierarchical porous carbon. Food Chem. 2021, 363, 130385. [Google Scholar] [CrossRef]
- Wen, X.; Huang, Q.; Nie, D.; Zhao, X.; Cao, H.; Wu, W.; Han, Z. A multifunctional n-doped cu–mofs (N–cu–mof) nanomaterial-driven electrochemical aptasensor for sensitive detection of deoxynivalenol. Molecules 2021, 26, 2243. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Jiang, X.; Zhou, B. Electrochemical determination of aflatoxin B1 (AFB1) using a copper-based metal-organic framework (Cu-MOF) and gold nanoparticles (AuNPs) with exonuclease III (Exo III) assisted recycling by differential pulse voltammetry (DPV). Anal. Lett. 2019, 52, 2439–2453. [Google Scholar] [CrossRef]
- Li, D.-l.; Zhang, X.; Ma, Y.; Deng, Y.; Hu, R.; Yang, Y. Preparation of an OTA aptasensor based on a metal–organic framework. Anal. Methods 2018, 10, 3273–3279. [Google Scholar] [CrossRef]
- Jahangiri–Dehaghani, F.; Zare, H.R.; Shekari, Z. Measurement of aflatoxin M1 in powder and pasteurized milk samples by using a label–free electrochemical aptasensor based on platinum nanoparticles loaded on Fe–based metal–organic frameworks. Food Chem. 2020, 310, 125820. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Braiek, M.; Florea, A.; Chrouda, A.; Farre, C.; Bonhomme, A.; Bessueille, F.; Vocanson, F.; Zhang, A.; Jaffrezic-Renault, N. Aflatoxin B1 detection using a highly-sensitive molecularly-imprinted electrochemical sensor based on an electropolymerized metal organic framework. Toxins 2015, 7, 3540–3553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, W.; Gao, F.; Yano, N.; Kataoka, Y.; Handa, M.; Yang, W.; Tanaka, H.; Wang, Q. Specific coordination between Zr-MOF and phosphate-terminated DNA coupled with strand displacement for the construction of reusable and ultrasensitive aptasensor. Anal. Chem. 2020, 92, 11332–11340. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Dong, X.N. BbvCI powered DNA walking machine-based Zr-MOFs-labeled electrochemical aptasensor using Pt@ AuNRs/Fe-MOFs/PEI-rGO as electrode modification material for patulin detection. Chem. Eng. J. 2021, 405, 126642. [Google Scholar] [CrossRef]
- He, B.; Yan, X. Ultrasensitive electrochemical aptasensor based on CoSe2/AuNRs and 3D structured DNA-PtNi@ Co-MOF networks for the detection of zearalenone. Sens. Actuators B Chem. 2020, 306, 127558. [Google Scholar] [CrossRef]
- Hatamluyi, B.; Rezayi, M.; Beheshti, H.R.; Boroushaki, M.T. Ultra-sensitive molecularly imprinted electrochemical sensor for patulin detection based on a novel assembling strategy using Au@ Cu-MOF/N-GQDs. Sens. Actuators B Chem. 2020, 318, 128219. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Li, Z.; He, L.; Hu, M.; He, L.; Zhang, Z.; Du, M. A bimetallic CoNi-based metal− organic framework as efficient platform for label-free impedimetric sensing toward hazardous substances. Sens. Actuators B Chem. 2020, 311, 127927. [Google Scholar] [CrossRef]
- Qiao, X.; Ma, X.; Ma, X.; Yue, T.; Sheng, Q. A label-free aptasensor for ochratoxin a detection with signal amplification strategies on ultrathin micron-sized 2D MOF sheets. Sens. Actuators B Chem. 2021, 334, 129682. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly (o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B Chem. 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Tong, P.; Liang, J.; Jiang, X.; Li, J. Research progress on metal-organic framework composites in chemical sensors. Crit. Rev. Anal. Chem. 2020, 50, 376–392. [Google Scholar] [CrossRef]
- Khataee, A.; Sohrabi, H.; Ehsani, M.; Agaei, M.; Sisi, A.J.; Abdi, J.; Yoon, Y. State-of-the-art progress of metal-organic framework-based electrochemical and optical sensing platforms for determination of bisphenol A as an endocrine disruptor. Environ. Res. 2022, 113536. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Sun, K.; Chen, H.; Jiang, Y.; Wu, L.; Mei, J.; Ding, Z.; Xie, J. Nanoscale metal-organic frameworks as fluorescence sensors for food safety. Antibiotics 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü.; Timur, S.; Dursun, Z. Metal organic frameworks in electrochemical and optical sensing platforms: A review. Microchim. Acta 2019, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, W.; Pan, Q. Integration of fluorescent probes into metal–organic frameworks for improved performances. RSC Adv. 2020, 10, 33879–33893. [Google Scholar] [CrossRef]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Jiang, W.-J.; Cai, Z.-H.; Li, D.-L.; Liu, Y.-L.; Chen, Z.-Z. Recent Progress in Metal-Organic Framework Based Fluorescent Sensors for Hazardous Materials Detection. Molecules 2022, 27, 2226. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Yu, W.; Tong, X.; Li, C.; Duan, N.; Wang, Z.; Wu, S. Application of Nanomaterials for Coping with Mycotoxin Contamination in Food Safety: From Detection to Control. Crit. Rev. Anal. Chem. 2022, 1–34. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Ye, Y.; Qiu, Y.; Liu, J.; Huang, L.; Liang, B.; Chen, B. A Fluorescent Metal–Organic Framework for Food Real-Time Visual Monitoring. Adv. Mater. 2021, 33, 2008020. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal–organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Z.; Jia, H.; Lu, R.; Zhang, S.; Pan, C.; Zhang, Z. An ultralow concentration of Al-MOFs for turn-on fluorescence detection of aflatoxin B1 in tea samples. Food Chem. 2022, 383, 132389. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Xian, J.; Gao, J.; Zhang, L.; Wang, W.; Fu, Z. Highly Sensitive Chemiluminescent Immunoassay of Mycotoxins Using ZIF-8-Derived Yolk-Shell Co Single-Atom Site Catalysts as Superior Fenton-like Probes. Anal. Chem. 2022, 94, 3400–3407. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, G.; Wang, X.; Zhang, Y.; Li, Z.; Liu, P.; Yu, B.; Zhang, J. A metal-organic framework/aptamer system as a fluorescent biosensor for determination of aflatoxin B1 in food samples. Talanta 2020, 219, 121342. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Quan, H.; Zhang, J.; Zhang, Q.; Fu, Y.; Ying, Y.; Li, Y. Adsorptive and responsive hybrid sponge of melamine foam and metal organic frameworks for rapid collection/removal and detection of mycotoxins. Chem. Eng. J. 2021, 410, 128268. [Google Scholar] [CrossRef]
- Yu, Y.; Li, G. Design of terbium (III)-functionalized covalent organic framework as a selective and sensitive turn-on fluorescent switch for ochratoxin A monitoring. J. Hazard. Mater. 2022, 422, 126927. [Google Scholar] [CrossRef]
- Tan, X.; Yu, W.; Wang, Y.; Song, P.; Xu, Q.; Ming, D.; Yang, Y. A switchable and signal-amplified aptasensor based on metal organic frameworks as the quencher for turn-on detection of T-2 mycotoxin. Anal. Bioanal. Chem. 2021, 413, 6595–6603. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Huang, H.; Bai, J.; Peng, Y.; Li, S.; Han, D.; Ren, S. A fluorescence aptasensor for the sensitive detection of T-2 toxin based on FRET by adjusting the surface electric potentials of UCNPs and MIL-101. Anal. Chim. Acta 2021, 1160, 338450. [Google Scholar] [CrossRef]
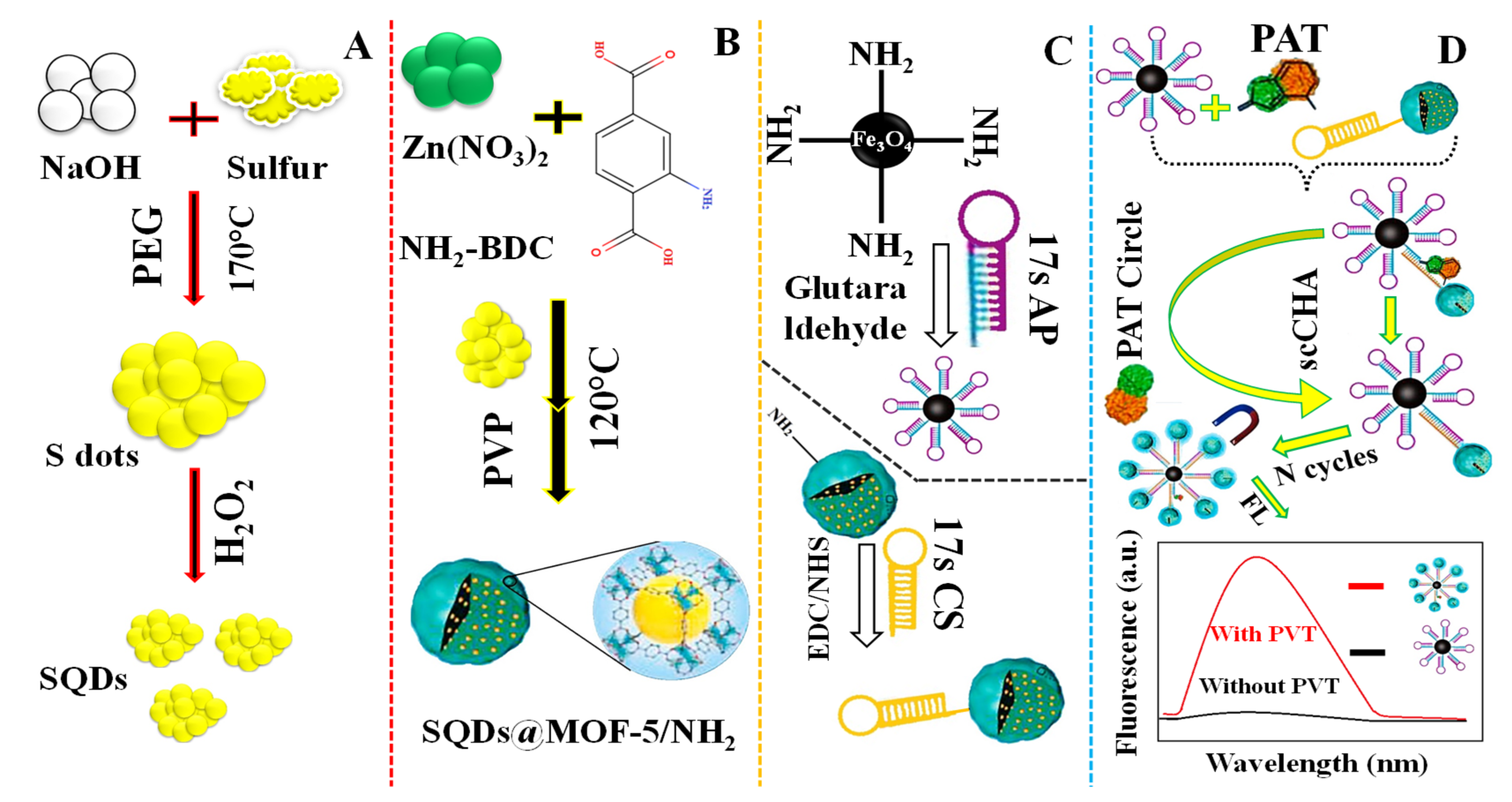
- Yan, X.; Zhao, Y.; Du, G.; Guo, Q.; Chen, H.; He, Q.; Zhao, Q.; Ye, H.; Wang, J.; Yuan, Y. Magnetic capture of sulfur quantum dots encapsulated in MOF-5-NH2 via a target-driven self-cycling catalyzed hairpin assembly for the sensitive detection of patulin. Chem. Eng. J. 2022, 433, 133624. [Google Scholar] [CrossRef]
- Yang, S.-L.; Liu, W.-S.; Li, G.; Bu, R.; Li, P.; Gao, E.-Q. A pH-Sensing Fluorescent Metal–Organic Framework: pH-Triggered Fluorescence Transition and Detection of Mycotoxin. Inorg. Chem. 2020, 59, 15421–15429. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Fu, Y.; Guo, Y.; Zhang, Q.; Zhang, Q.; Yang, H.; Li, Y. A water-stable luminescent metal–organic framework for effective detection of aflatoxin B1 in walnut and almond beverages. RSC Adv. 2019, 9, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Effective detection of mycotoxins by a highly luminescent metal–organic framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Wang, Y.; Li, J.; Huo, B.; Qin, Y.; Zhang, J.; Chen, M.; Peng, Y.; Bai, J.; Li, S. A fluorescence aptasensor based on controlled zirconium–based MOFs for the highly sensitive detection of T–2 toxin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119893. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Wang, Z. A “turn-on” FRET aptasensor based on the metal-organic framework-derived porous carbon and silver nanoclusters for zearalenone determination. Sens. Actuators B Chem. 2021, 347, 130661. [Google Scholar] [CrossRef]
- Tian, D.; Liu, X.-J.; Feng, R.; Xu, J.-L.; Xu, J.; Chen, R.-Y.; Huang, L.; Bu, X.-H. Microporous luminescent metal–organic framework for a sensitive and selective fluorescence sensing of toxic mycotoxin in moldy sugarcane. ACS Appl. Mater. Interfaces 2018, 10, 5618–5625. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Li, J.; Zhang, Z. Exonuclease I-assisted fluorescent method for ochratoxin A detection using iron-doped porous carbon, nitrogen-doped graphene quantum dots, and double magnetic separation. Anal. Bioanal. Chem. 2019, 411, 2405–2414. [Google Scholar] [CrossRef]
- Durmus, Z.; Zengin Kurt, B.; Gazioğlu, I.; Sevgi, E.; Kizilarslan Hancer, C. Spectrofluorimetric determination of Aflatoxin B1 in winter herbal teas via magnetic solid phase extraction method by using metal–organic framework (MOF) hybrid structures anchored with magnetic nanoparticles. Appl. Organomet. Chem. 2020, 34, e5375. [Google Scholar] [CrossRef]




| MOF Type | Target Mycotoxins | Fluorescence Response | Detection Limit (LOD) | Linear Range | Recovery (%) | Real Sample | Reference |
|---|---|---|---|---|---|---|---|
| Zr-MOF | Ochratoxin A (OTA) | Turn-on | 2.4 × 10−8 mg·L−1 | 1.0 × 107−0.14 × 10−3 mg·L−1 | - | Corn | [67] |
| NH2-MIL-53 | Aflatoxin B1 (AFB1) | Turn-on | 0.01167 mg·L−1 | 0.05–9.61 μM | 80.99 ± 0.67 to 115.29 ± 3.85 | Biluochun tea Junshan silver needle tea Pu’er tea | [96] |
| Co/NCNT/ZIF-8@ZIF-67 | Ochratoxin A (OTA) | Turn-on | 1.7 × 10−7 mg·L−1 | 1.0 × 10−6–0.01 mg·L−1 | 95.0−103.8 | Corn and Millet | [70] |
| Co SASCs derived from ZIF-8@SiO2 | Aflatoxin B1 (AFB1) | Turn-off | 4.4 × 10−7 mg·L−1 | 1.0 × 10−5−0.001 mg·L−1 | G·uralensis 88.4−95.4 P·notoginseng 94.4−111.9 | Glycyrrhiza uralensis and Panax notoginseng | [97] |
| MOFs (H2dtoaCu) MOF (H2 dtoa = dithiooxamide anion) | T-2 mycotoxin | Turn-on | 0.00039 mg·L−1 | 0.1–0.5 mg·L−1 | Corn flour 98.02 ± 4.76 to 100.11 ± 3.32 Wheat flour 99.05 ± 1.33 to 00.40 ± 2.82 | Corn flour and Wheat flour | [101] |
| SQDs@MOF-5-NH2 | Patulin (PAT) | Turn-on | 7.53 × 10−7 mg·L−1 | 1.0 × 10−6–0.1 mg·L−1 | 89.03–107.67 | Apple juice sample | [103] |
| Dpy-NhBtCOF@Tb3+ | Ochratoxin A (OTA) | Turn-on | 0.0135 μM | 0–10 μM | 97.3–98.6 | Wheat | [100] |
| TAMRA aptamer/UiO-66-NH2 | Aflatoxin B1 (AFB1) | Turn-on | 0.35 × 10−3 mg·L−1 | 0–0.18 mg·L−1 | Milk 103.10–10414 Corn 96.38–97.36 Rice 90.42–94.21 | Milk Corn Rice | [98] |
| Zr-LMOF | Aflatoxin B1 (AFB1) | Turn-off | 0.01997 mg·L−1 | 0.0312–15.6 mg·L−1 | - | Water | [99] |
| Zr-LMOF/M.F. | Aflatoxin B1 (AFB1) | Turn-off | 0.0016 mg·L−1 | 0.0234–7.8 mg·L−1 | - | Water | [99] |
| Zr-CAU-24 | Aflatoxin B1 (AFB1) | Turn-off | 0.01997 mg·L−1 | 75–25,000 μM | 91–108 | Walnut beverage Almond beverage | [105] |
| [Zn2(bpdc)2(tppe)]LMOF-241 | Aflatoxin B1 (AFB1) Aflatoxin B2 (AFB2) OchratoxinA | Turn-off | 0.046 mg·L−1 | - | - | - | [106] |
| NH2–UiO–66/Cy3–aptamer | T-2 mycotoxin | Turn-on | 0·239 × 10−3 mg·L−1 | 0.5 × 10−3–0.1 mg·L−1 | Milk 89.86–108.99 Beer 99.17–111.51 | Milk and Beer | [107] |
| DNA-templated AgNCs/MOFderived/Fe3O4/carbonoctahedra | Zearalenone [44] | Turn-on | 2 × 10−6 mg·L−1 | 1.0 × 10−5–0.25 mg·L−1 | Maize 97.3–102.9 Wheat 96.0–101 | Maize and Wheat | [108] |
| Dye-functionalized MOF (FITC@1)0(Cd(NO3)2·6H2O/DMF/C2H5OH) = 1 | 3-nitropropionic acid (3-NPA) | Turn-off | 135,000 μM | - | - | Moldy sugarcane | [109] |
| MPC–NGQDs-Ap | Ochratoxin A (OTA) | Turn-on | 0.00405 mg·L−1 | 0.01–5 mg·L−1 | - | Wheat and Corn | [110] |
| MPC–NGQDs-Ap/exonuclease I (ExoI) | Ochratoxin A (OTA) | Turn-on | 0.00228 mg·L−1 | 0.01–5 mg·L−1 | wheat 86.66–99.14 Corn 96.06–109.9 | Wheat and Corn | [110] |
| MIL53-SiO2@Fe3O4 | Aflatoxin B1 (AFB1) | Turn-on | 0.0005 mg·L−1 | 0.0005–0.15 mg·L−1 | 70.7–96.5 | Linden Chamomile Purple a Purple Easternholly Cinnamon Clove Sage leave Lemon Ginger | [111] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohrabi, H.; Salahshour Sani, P.; Zolfaghari, R.; Majidi, M.R.; Yoon, Y.; Khataee, A. MOF-Based Mycotoxin Nanosensors for Food Quality and Safety Assessment through Electrochemical and Optical Methods. Molecules 2022, 27, 7511. https://doi.org/10.3390/molecules27217511
Sohrabi H, Salahshour Sani P, Zolfaghari R, Majidi MR, Yoon Y, Khataee A. MOF-Based Mycotoxin Nanosensors for Food Quality and Safety Assessment through Electrochemical and Optical Methods. Molecules. 2022; 27(21):7511. https://doi.org/10.3390/molecules27217511
Chicago/Turabian StyleSohrabi, Hessamaddin, Parya Salahshour Sani, Ramin Zolfaghari, Mir Reza Majidi, Yeojoon Yoon, and Alireza Khataee. 2022. "MOF-Based Mycotoxin Nanosensors for Food Quality and Safety Assessment through Electrochemical and Optical Methods" Molecules 27, no. 21: 7511. https://doi.org/10.3390/molecules27217511






