A Novel Fluorescent Probe for Determination of pH and Viscosity Based on a Highly Water-Soluble 1,8-Naphthalimide Rotor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design and Synthesis
2.2. Chemosensing Properties of Probe 3
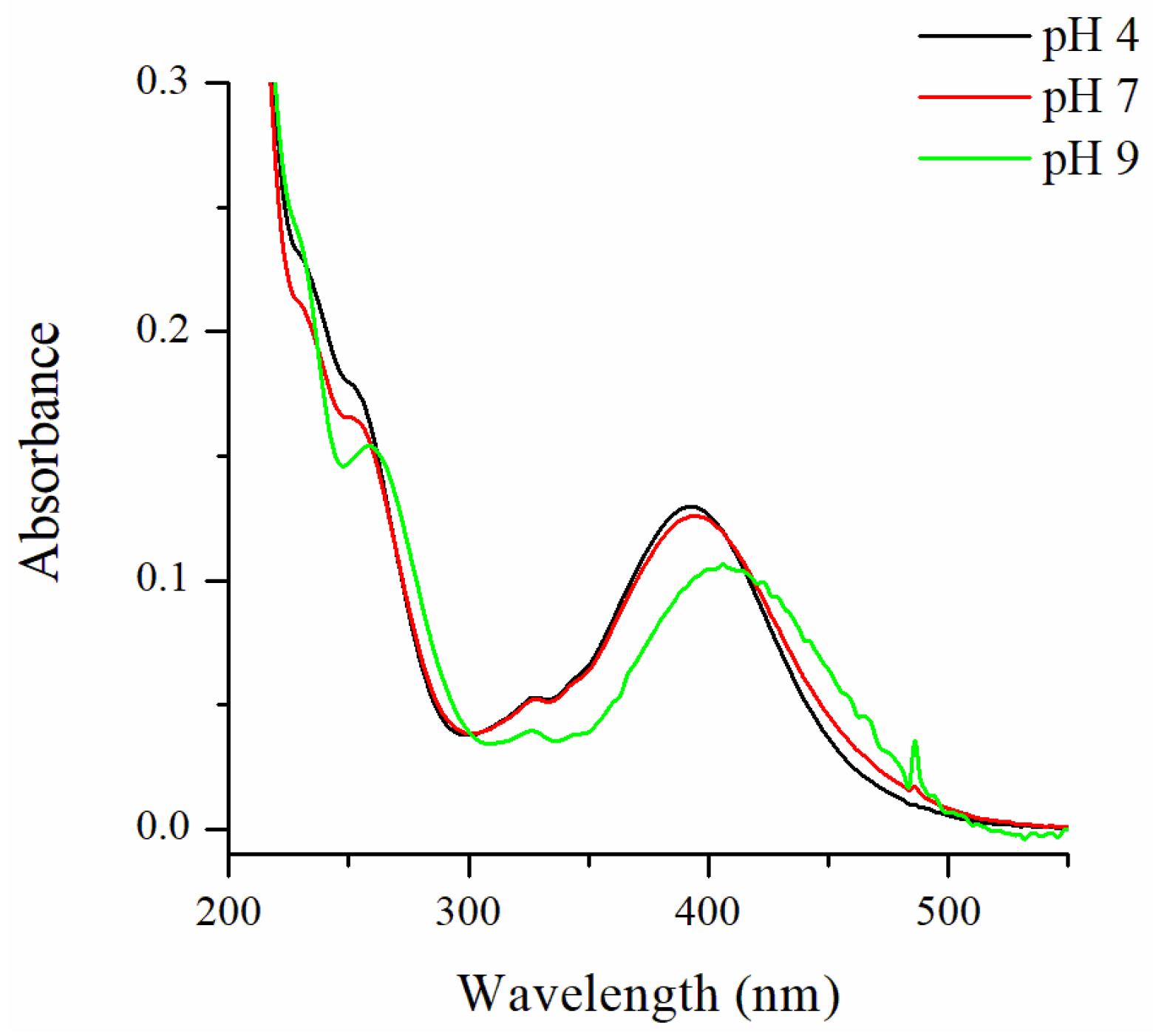
2.2.1. pH-Sensing Properties
2.2.2. Viscosity-Sensing Properties
2.2.3. Molecular Logic
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthetic Procedures
3.3.1. Synthesis N-Amino-4-chloro-1,8-naphthalimide 2
3.3.2. Synthesis of Probe 3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of pH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.P. Crossing the divide: Experiences of taking fluorescent PET (photoinduced electron transfer) sensing/switching systems from solution to solid. Dye. Pigment. 2022, 204, 110453. [Google Scholar] [CrossRef]
- Hamilton, G.; Sahoo, S.; Kamila, S.; Singh, N.; Kaur, N.; Hyland, B.; Callan, J. Optical probes for the detection of protons, and alkali and alkaline earth metal cations. Chem. Soc. Rev. 2015, 44, 4415–4432. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Das, G. Exploring the Aggregation and Light-Harvesting Aptitude of Naphthalimide-Based Amphiphile and Non-amphiphile AIEgen. Langmuir 2022, 38, 6158–6163. [Google Scholar] [CrossRef]
- Zhang, Q.; Rao, S.-J.; Xie, T.; Li, X.; Xu, T.-Y.; Li, D.-W.; Qu, D.-H.; Long, Y.-T.; Tian, H. Muscle-like Artificial Molecular Actuators for Nanoparticles. Chem 2018, 4, 2670–2684. [Google Scholar] [CrossRef] [Green Version]
- Shirai, Y.; Morin, J.-F.; Sasaki, T.; Guerrero, J.; Tour, J. Recent progress on nanovehicles. Chem. Soc. Rev. 2006, 35, 1043–1055. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; He, Q.; Chi, X.; Lynch, V.; Zhang, Z.; Su, J.; Tian, H.; Sessler, J. Molecular Cursor Caliper: A Fluorescent Sensor for Dicarboxylate Dianions. J. Am. Chem. Soc. 2019, 141, 14798–14806. [Google Scholar] [CrossRef]
- Slavcheva, S.; Mendoza, J.; Stanimirov, S.; Petkov, I.; Basílio, N.; Pina, F.; Petrov, V. On the multistate of 2′-hydroxyflavylium-flavanone system. Illustrating the concept of a timer with reset at the molecular level. Dye. Pigment. 2018, 158, 465–473. [Google Scholar] [CrossRef]
- Georgiev, N.; Marinova, N.; Bojinov, V. Design and synthesis of light-harvesting rotor based on 1,8-naphthalimide units. J. Photochem. Photobiol. A Chem. 2020, 401, 112733. [Google Scholar] [CrossRef]
- Nakashima, K.; Georgiev, A.; Yordanov, D.; Matsushima, Y.; Hirashima, S.; Miura, T.; Antonov, L. Solvent-Triggered Long-Range Proton Transport in 7-Hydroxyquinoline Using a Sulfonamide Transporter Group. J. Org. Chem. 2022, 87, 6794–6806. [Google Scholar] [CrossRef]
- Ling, J.; Naren, G.; Kelly, J.; Moody, T.; de Silva, A.P. Building pH sensors intopaper-based small-molecular logic systems for very simple detection of edgesof objects. J. Am. Chem. Soc. 2015, 137, 3763–3766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, S.; Xing, X.; Liu, Y.; Gao, H. A hemicyanine-based fluorescence probe for selective detection of CYP2D6 in living cells and tumor-bearing mice. Dye. Pigment. 2022, 198, 109959. [Google Scholar] [CrossRef]
- Georgiev, N.; Krasteva, P.; Bakov, V.; Bojinov, V. A Highly Water-Soluble and Solid State Emissive 1,8-Naphthalimide as a Fluorescent PET Probe for Determination of pHs, Acid/Base Vapors, and Water Content in Organic Solvents. Molecules 2022, 27, 4229. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Du, J.; Hou, J.; Shi, P.; Wang, K.; Zhang, S.; Han, T.; Li, Z. Rewritable optical data storage based on mechanochromic fluorescence materials with aggregation-induced emission. Dye. Pigment. 2019, 160, 830–838. [Google Scholar] [CrossRef]
- Cafferty, B.; Ten, A.; Fink, M.; Morey, S.; Preston, D.; Mrksich, M.; Whitesides, G. Storage of Information Using Small Organic Molecules. ACS Cent. Sci. 2019, 5, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Ruskowitz, E.; Comerford, M.; Badeau, B.; DeFores, C. Logical stimuli-triggered delivery of small molecules from hydrogel biomaterials. Biomater. Sci. 2019, 7, 542–546. [Google Scholar] [CrossRef]
- Romero, M.; Mateus, P.; Matos, B.; Acuña, Á.; García-Río, L.; Arteaga, J.; Pischel, U.; Basílio, N. Binding of Flavylium Ions to Sulfonatocalix[4]arene and Implication in the Photorelease of Biologically Relevant Guests in Water. J. Org. Chem. 2019, 84, 10852–10859. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Georgieva, S.; Tchekalarova, J.; Vitkova, V.; Antonova, K.; Georgiev, A. Synthesis, characterization and anticonvulsant activity of new azobenzene-containing UV-hemorphin-5 bio photoswitch. Amino Acids 2019, 51, 549–563. [Google Scholar] [CrossRef]
- Turan, I.; Gunaydin, G.; Ayan, S.; Akkaya, E. Molecular demultiplexer as a terminator automaton. Nat. Commun. 2018, 9, 805. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, N.I.; Bryaskova, R.G.; Ismail, S.R.; Philipova, N.D.; Uzunova, V.P.; Bakov, V.V.; Tzoneva, R.D.; Bojinov, V.B. Aggregation induced emission in 1,8-naphthalimide embedded nanomicellar architecture as a platform for fluorescent ratiometric pH-probe with biomedical applications. J. Photochem. Photobiol. A Chem. 2021, 418, 113380. [Google Scholar] [CrossRef]
- Yang, X.; Lovell, J.F.; Murthy, N.; Zhang, Y. Organic Fluorescent Probes for Diagnostics and Bio-Imaging. Top. Med. Chem. 2020, 34, 33–53. [Google Scholar]
- Shen, R.; Qian, Y. A mitochondria-oriented fluorescent probe for ultrafast and ratiometric detection of HSO3− based on naphthalimide-hemicyanine. New J. Chem. 2019, 43, 7606–7612. [Google Scholar] [CrossRef]
- Guo, F.-F.; Wu, W.-N.; Zhao, X.-L.; Wang, Y.; Fan, Y.-C.; Zhang, C.-X.; Xu, Z.-H. A deep-red lysosome-targetable fluorescent probe for detection of hypochlorous acid in pure water and its imaging application in living cells and zebrafish. Spectrochim. Acta Part A 2022, 264, 120270. [Google Scholar] [CrossRef] [PubMed]
- Gond, S.; Yadava, P.; Chauhan, B.; Srikrishna, S.; Singh, V. Development of an ‘OFF-ON-OFF’ colorimetric and fluorometric pH sensor for the study of physiological pH and its bioimaging application. J. Mol. Struct. 2022, 1252, 132147. [Google Scholar] [CrossRef]
- Johnson, A.D.; Zammit, R.; Vella, J.; Valentino, M.; Buhagiar, J.A.; Magri, D.C. Aminonaphthalimide hybrids of mitoxantrone and amonafide as anticancer and fluorescent cellular imaging agents. Bioorg. Chem. 2019, 93, 103287. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Kang, J.H.; Han, J.; Lee, H.; Lim, M.H.; Kim, K.-T.; Kim, C. A novel “off-on” type fluorescent chemosensor for detection of Zn2+ and its zinc complex for “on-off” fluorescent sensing of sulfide in aqueous solution, in vitro and in vivo. Sens. Actuators B Chem. 2018, 267, 58–69. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Song, B.; Huang, C. Spectroscopic behavior and intracellular application of a highly sensitive UV-fluorescence double ratio probe based on water-soluble indole for detection acid pH. Dye. Pigment. 2021, 188, 109205. [Google Scholar] [CrossRef]
- Georgiev, N.; Said, A.; Toshkova, R.; Tzoneva, R.; Bojinov, V. A novel water-soluble perylenetetracarboxylic diimide as a fluorescent pH probe: Chemosensing, biocompatibility and cell imaging. Dye. Pigment. 2019, 160, 28–36. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659. [Google Scholar] [CrossRef]
- Ozdemir, M. Two Colorimetric and Fluorescent Dual-Channel Chemosensors for the Selective Detection of pH in Aqueous Solutions. ChemistrySelect 2020, 5, 14340–14348. [Google Scholar] [CrossRef]
- Thottiparambil, A.; Kumar, P.; Chakkumkumarath, L. Styrylcyanine-based ratiometric and tunable fluorescent pH sensors. RSC Adv. 2014, 4, 56063–56067. [Google Scholar] [CrossRef]
- Wang, X.; Huang, D.; Niu, C.; Guo, L.; Cui, J.; Hu, L.; Zeng, G. An internal reference fluorescent pH sensor with two pH-sensitive fluorophores carrier. Sens. Actuators B 2016, 234, 593–601. [Google Scholar] [CrossRef]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH sensor covering the range from pH 0-14 compatible with mobile-device readout and based on a set of rationally designed indicator dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Koenig, M.; Storti, B.; Bizzarri, R.; Guldi, D.; Brancato, G.; Bottari, G. A fluorescent molecular rotor showing vapochromism, aggregation-induced emission, and environmental sensing in living cells. Mater. Chem. C 2016, 4, 3018–3027. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liu, X.; Spring, D.; Qian, X.; Cui, J.; Xu, Z. Quantitatively Mapping Cellular Viscosity with Detailed Organelle Information via a Designed PET Fluorescent Probe. Sci. Rep. 2014, 4, 5418. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-Y.; Cui, J.-N.; Qian, X.-H.; Liu, T.-Y. A 4-aminonaphthalimide based environmentally sensitive fluorescence probe. Chin. Chem. Lett. 2013, 24, 359–361. [Google Scholar] [CrossRef]
- Han, J.; Burgess, K. Fluorescent Indicators for Intracellular pH. Chem. Rev. 2010, 110, 2709–2728. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Tian, Y. Micro Electrochemical pH Sensor Applicable for Real-Time Ratiometric Monitoring of pH Values in Rat Brains. Anal. Chem. 2016, 88, 2113–2118. [Google Scholar] [CrossRef]
- Nan, M.; Niu, W.; Fan, L.; Lu, W.; Shuang, S.; Li, C.; Dong, C. Indole-based pH probe with ratiometric fluorescence behavior for intracellular imaging. RSC Adv. 2015, 5, 99739–99744. [Google Scholar] [CrossRef]
- Song, H.; Zhang, W.; Zhang, Y.; Yin, C.; Huo, F. Viscosity activated NIR fluorescent probe for visualizing mitochondrial viscosity dynamic and fatty liver mice. Chem. Eng. J. 2022, 445, 136448. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Zhang, X.-Q.; Xu, Y.-J.; Sun, R.; Ge, J.-F. Fluorescent probes based 1,8-naphthalimide-nitrogen heterocyclic for monitoring the fluctuation of mitochondrial viscosity. Dye. Pigment. 2021, 194, 109559. [Google Scholar] [CrossRef]
- Sasaki, S.; Drummen, G.; Konishi, G. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 2016, 4, 2731–2743. [Google Scholar] [CrossRef] [Green Version]
- Raja, S.; Sivaraman, G.; Mukherjee, A.; Duraisamy, C.; Gulyani, A. Facile Synthesis of Highly Sensitive, Red-Emitting, Fluorogenic Dye for Microviscosity and Mitochondrial Imaging in Embryonic Stem Cells. ChemistrySelect 2017, 2, 4609–4616. [Google Scholar] [CrossRef]
- Das, M.; Brahma, M.; Krishnamoorthy, G. Controlling the photoswitching of 2-(4′-diethylamino-2′-hydroxyphenyl)-1H-imidazo-[4,5-b]pyridine by pH. J. Photochem. Photobiol. A Chem. 2021, 421, 113504. [Google Scholar] [CrossRef]
- Rudebeck, E.; Cox, R.; Bell, T.; Acharya, R.; Feng, Z.; Gueven, N.; Ashton, T.; Pfeffer, F. Mixed alkoxy/hydroxy 1,8-naphthalimides: Expanded fluorescence colour palette and in vitro bioactivity. Chem. Commun. 2020, 56, 6866–6869. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Ahmad, I. Theoretical study on photophysical properties of multifunctional star-shaped molecules with 1,8-naphthalimide core for organic light-emitting diode and organic solar cell application. Theor. Chem. Acc. 2015, 134, 89. [Google Scholar] [CrossRef]
- Saini, A.; Bhasin, A.; Singh, N.; Kaur, N. Development of a Cr(III) ion selective fluorescence probe using organic nanoparticles and its real time applicability. New J. Chem. 2016, 40, 278–284. [Google Scholar] [CrossRef]
- Szakacs, Z.; Rousseva, S.; Bojtar, M.; Hessz, D.; Bitter, I.; Kallay, M.; Hilbers, M.; Zhang, H.; Kubinyi, M. Experimental evidence of TICT state in 4-piperidinyl-1,8-naphthalimide—A kinetic and mechanistic study. Phys. Chem. Chem. Phys. 2018, 20, 10155–10164. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Qi, J.; Huang, J.; Zhou, X.; Niu, L.; Yan, Z.; Wang, J. A nontoxic, photostable and high signal-to-noise ratio mitochondrial probe with mitochondrial membrane potential and viscosity detectivity. Spectrochim. Acta Part A 2018, 189, 634–641. [Google Scholar] [CrossRef]
- Lynch, S.; Rice, T.; Moody, T.; Gunaratne, H.; de Silva, A. Structural effects on the pH-dependent fluorescence of naphthalenic derivatives and consequences for sensing/switching. Photochem. Photobiol. Sci. 2012, 11, 1675–1681. [Google Scholar]
- Chi, W.; Chen, J.; Qiao, Q.; Gao, Y.; Xu, Z.; Liu, X. Revealing the switching mechanisms of an OFF-ON-OFF fluorescent logic gate system. Phys. Chem. Chem. Phys. 2019, 21, 16798–16803. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, J.; Johnson, A.; Denisov, S.; Jonusauskas, G.; McClenaghan, N.; Magri, D. A fluorescent AND logic gate based on a ferrocene-naphthalimide-piperazine format responsive to acidity and oxidizability. Dye. Pigment. 2018, 157, 278–283. [Google Scholar] [CrossRef] [Green Version]
- Pais, V.; Remón, P.; Collado, D.; Andréasson, J.; Pérez-Inestrosa, E.; Pischel, U. OFF-ON-OFF Fluorescence Switch with T-Latch Function. Org. Lett. 2011, 13, 5572–5575. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Yang, W.; Xu, D.; Zhang, K. Water soluble 1,8-naphthalimide fluorescent pH probes and their application to bioimagings. J. Photochem. Photobiol. A Chem. 2011, 223, 111–118. [Google Scholar] [CrossRef]
- Georgiev, N.; Dimov, S.; Asiri, A.; Alamry, K.; Obaid, A.; Bojinov, V. Synthesis, selective pH-sensing activity and logic behavior of highly water-soluble 1,8-naphthalimide and dihydroimidazonaphthalimide derivatives. J. Lumin. 2014, 149, 325–332. [Google Scholar] [CrossRef]
- Liu, J.; de Silva, A.P. Path-selective photoinduced electron transfer (PET) in a membrane-associated system studied by pH-dependent fluorescence. Inorg. Chim. Acta 2012, 381, 243–246. [Google Scholar] [CrossRef]
- Marinova, N.; Georgiev, N.; Bojinov, V. Synthesis and photophysical properties of novel 1,8-naphthalimide lightharvesting antennae based on benzyl aryl ether architecture. J. Lumin. 2018, 204, 253–260. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Shang, C.; Kang, R.; Fang, Y. Naphthalimide-Based Fluorophore for Soft Anionic Interface Monitoring. ACS Appl. Mater. Interfaces 2017, 9, 35419–35426. [Google Scholar] [CrossRef]
- Reynolds, G.; Drexhage, K. New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt. Commun. 1975, 13, 222–225. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakov, V.V.; Georgiev, N.I.; Bojinov, V.B. A Novel Fluorescent Probe for Determination of pH and Viscosity Based on a Highly Water-Soluble 1,8-Naphthalimide Rotor. Molecules 2022, 27, 7556. https://doi.org/10.3390/molecules27217556
Bakov VV, Georgiev NI, Bojinov VB. A Novel Fluorescent Probe for Determination of pH and Viscosity Based on a Highly Water-Soluble 1,8-Naphthalimide Rotor. Molecules. 2022; 27(21):7556. https://doi.org/10.3390/molecules27217556
Chicago/Turabian StyleBakov, Ventsislav V., Nikolai I. Georgiev, and Vladimir B. Bojinov. 2022. "A Novel Fluorescent Probe for Determination of pH and Viscosity Based on a Highly Water-Soluble 1,8-Naphthalimide Rotor" Molecules 27, no. 21: 7556. https://doi.org/10.3390/molecules27217556
APA StyleBakov, V. V., Georgiev, N. I., & Bojinov, V. B. (2022). A Novel Fluorescent Probe for Determination of pH and Viscosity Based on a Highly Water-Soluble 1,8-Naphthalimide Rotor. Molecules, 27(21), 7556. https://doi.org/10.3390/molecules27217556







