Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities
Abstract
:1. Introduction
2. Material and Methods
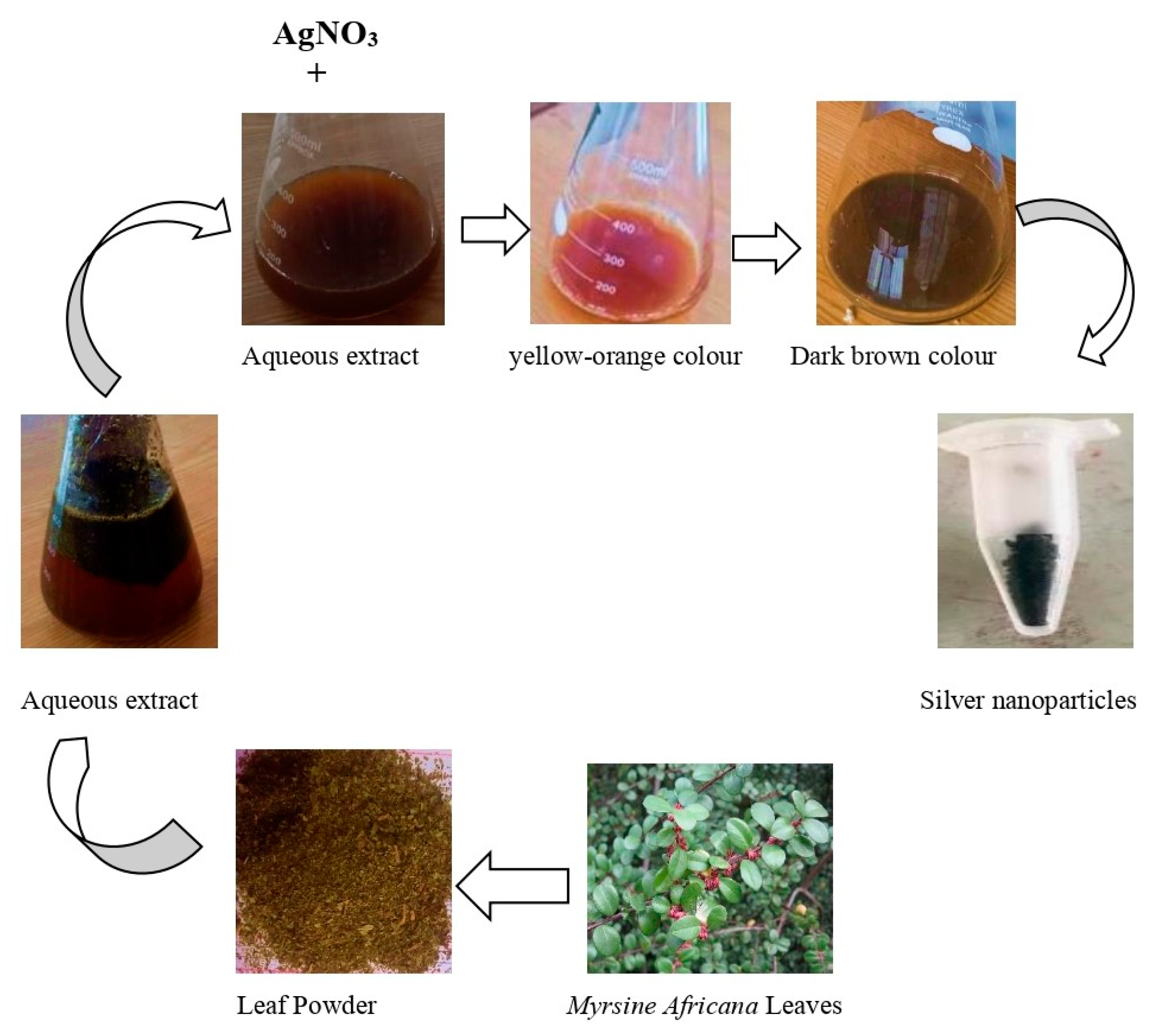
2.1. Collection of Sample and Preparation of Plant Extract
2.2. Green Synthesis of AgNPs
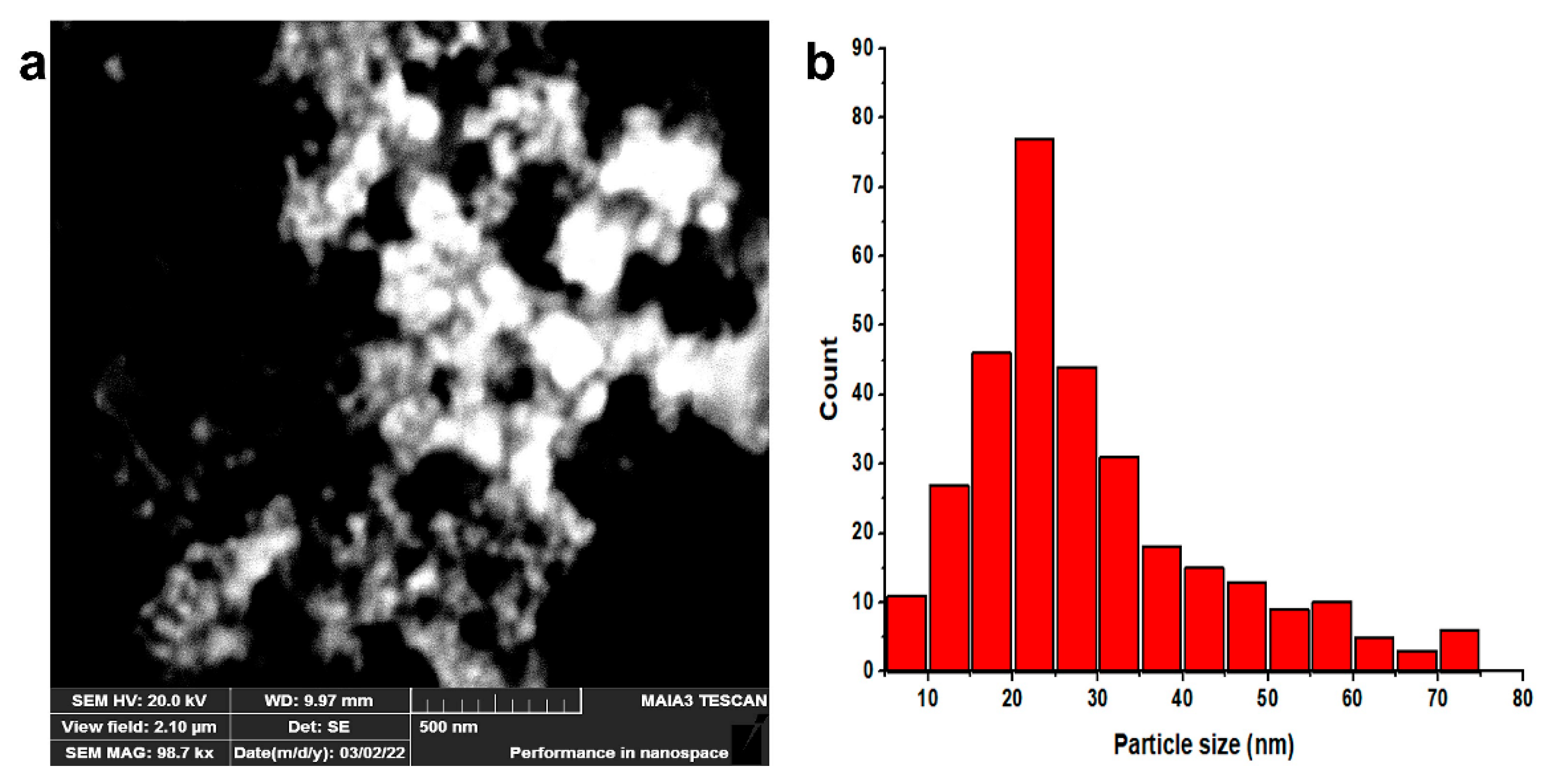
2.3. Characterization of Silver Nanoparticles
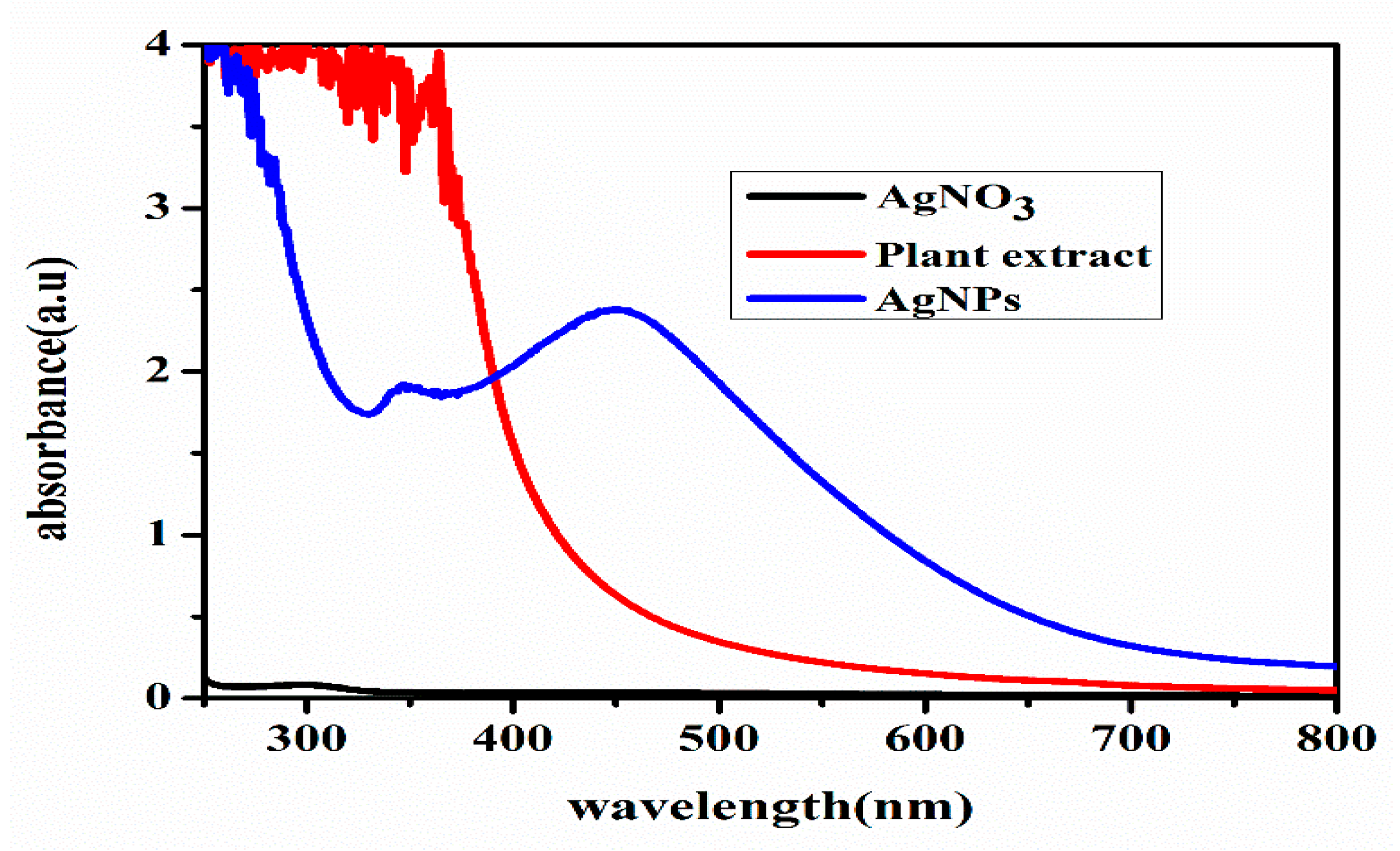
2.3.1. UV-Visible Spectroscopy
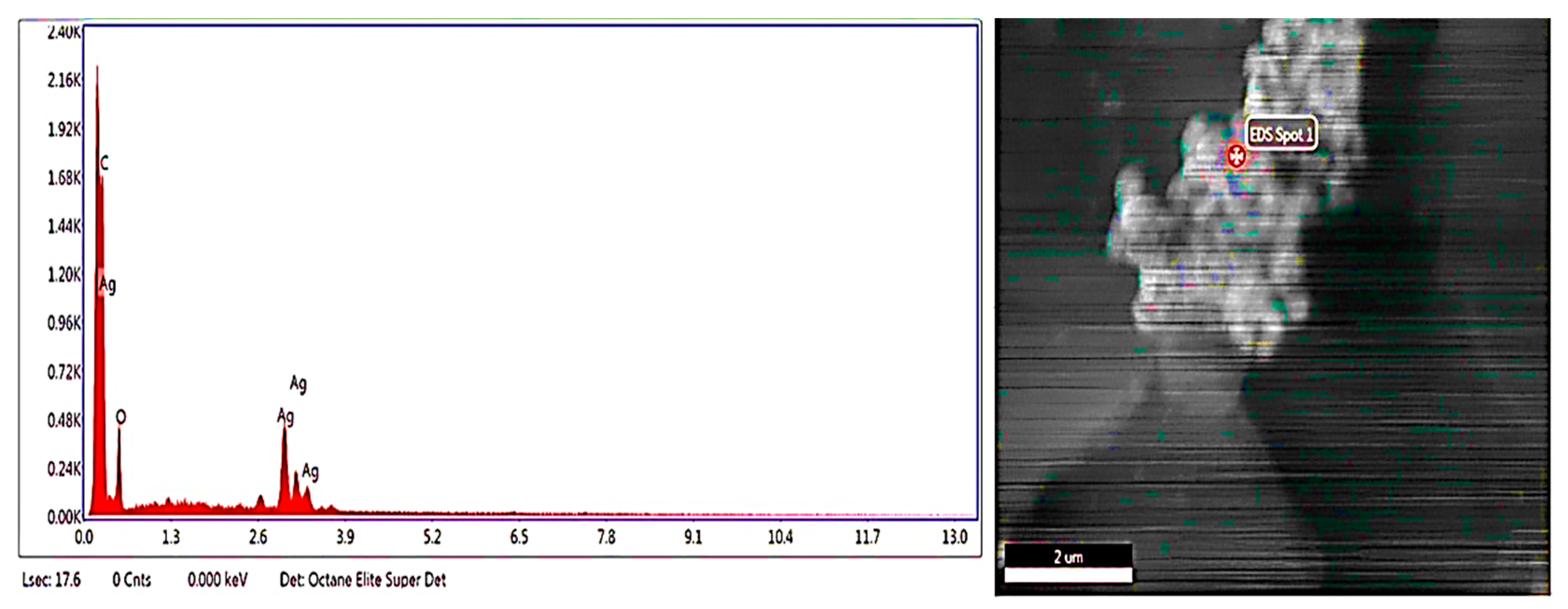
2.3.2. SEM-EDX
2.3.3. XRD
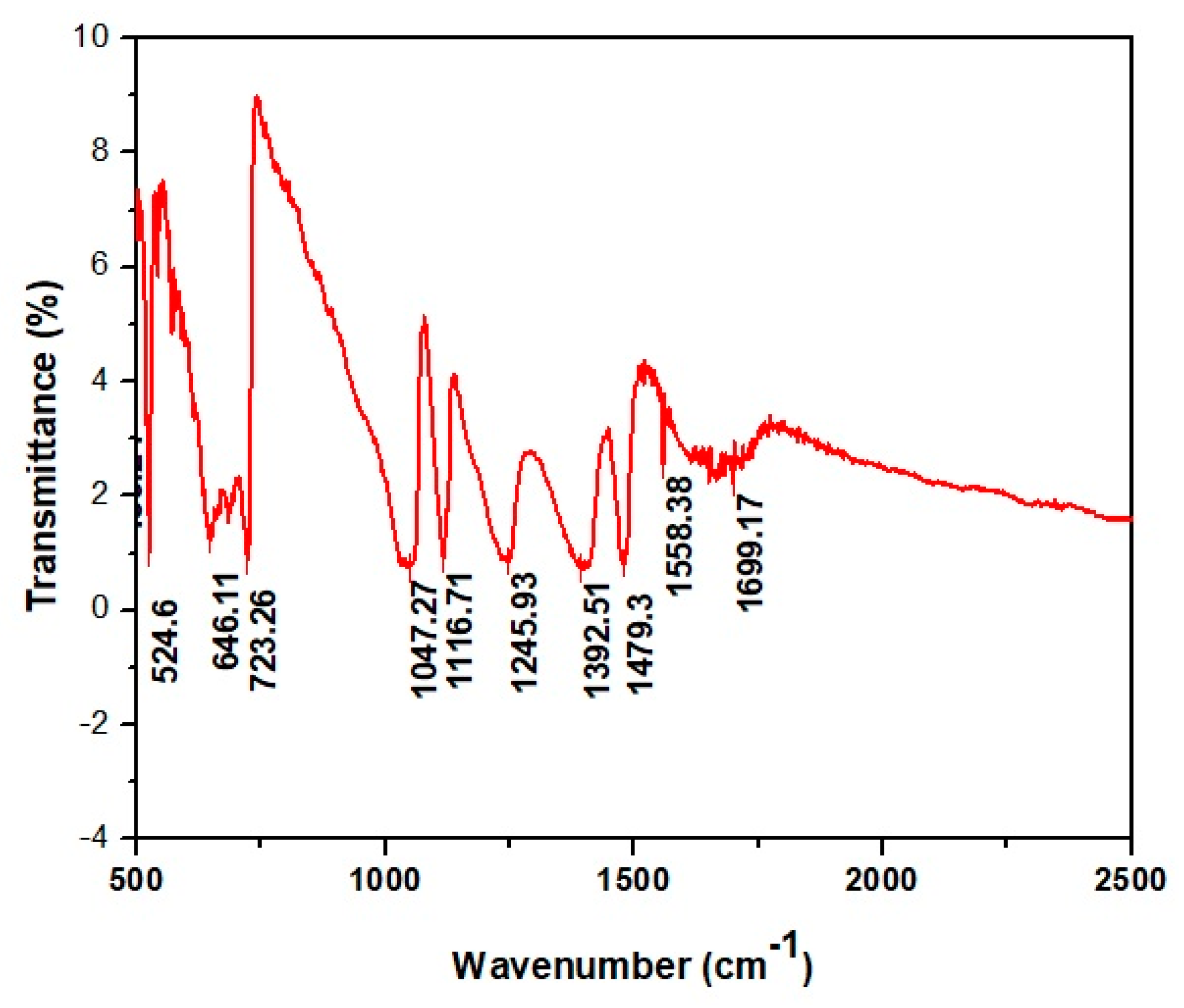
2.3.4. FT-IR
2.4. Biological Activities
2.4.1. Antibacterial Activity
2.4.2. Antioxidant Assay
2.5. Phytotoxicity Assesement of AgNPs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Green Synthesis and Characterization of AgNPs
3.2. Biological Activities
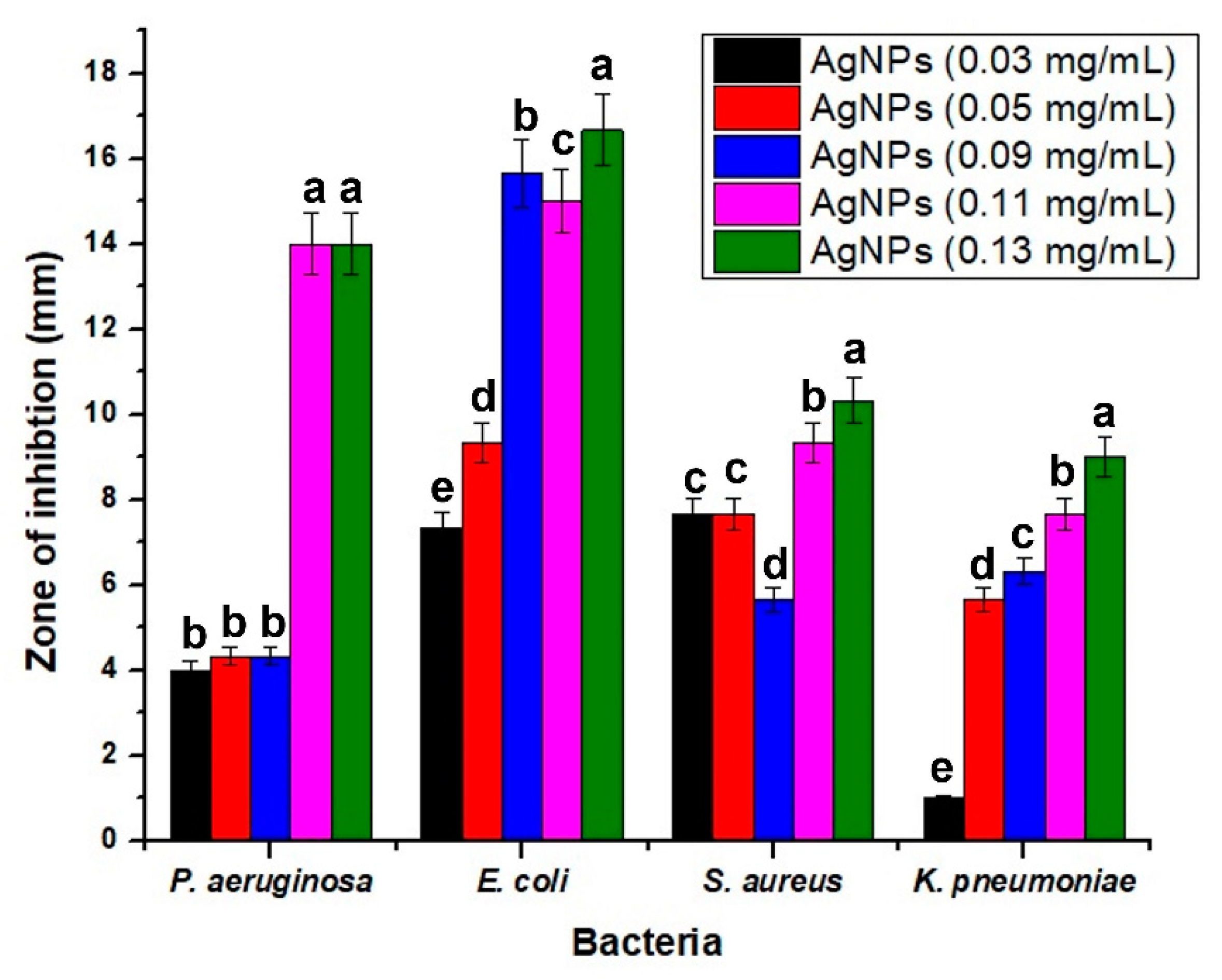
3.2.1. Anti-Bacterial Activity
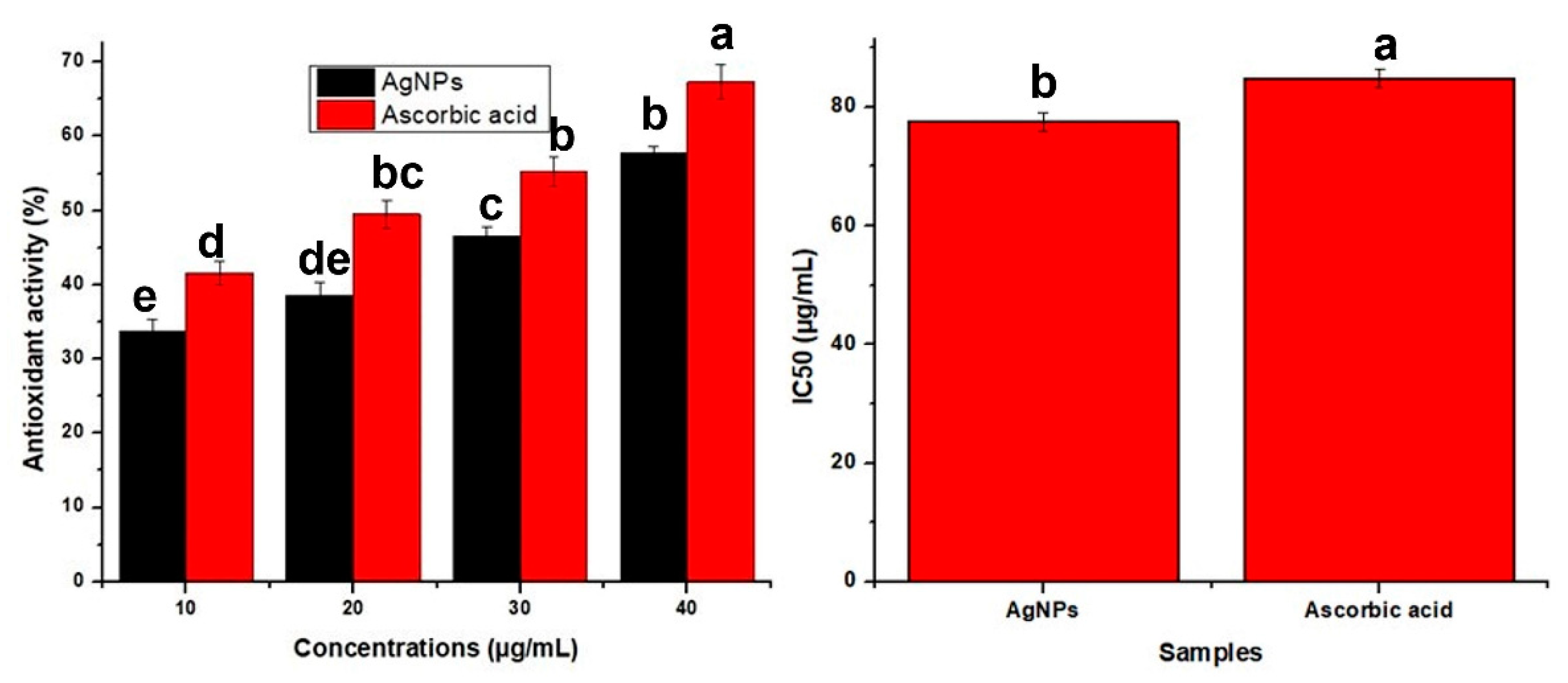
3.2.2. Antioxidant Activity
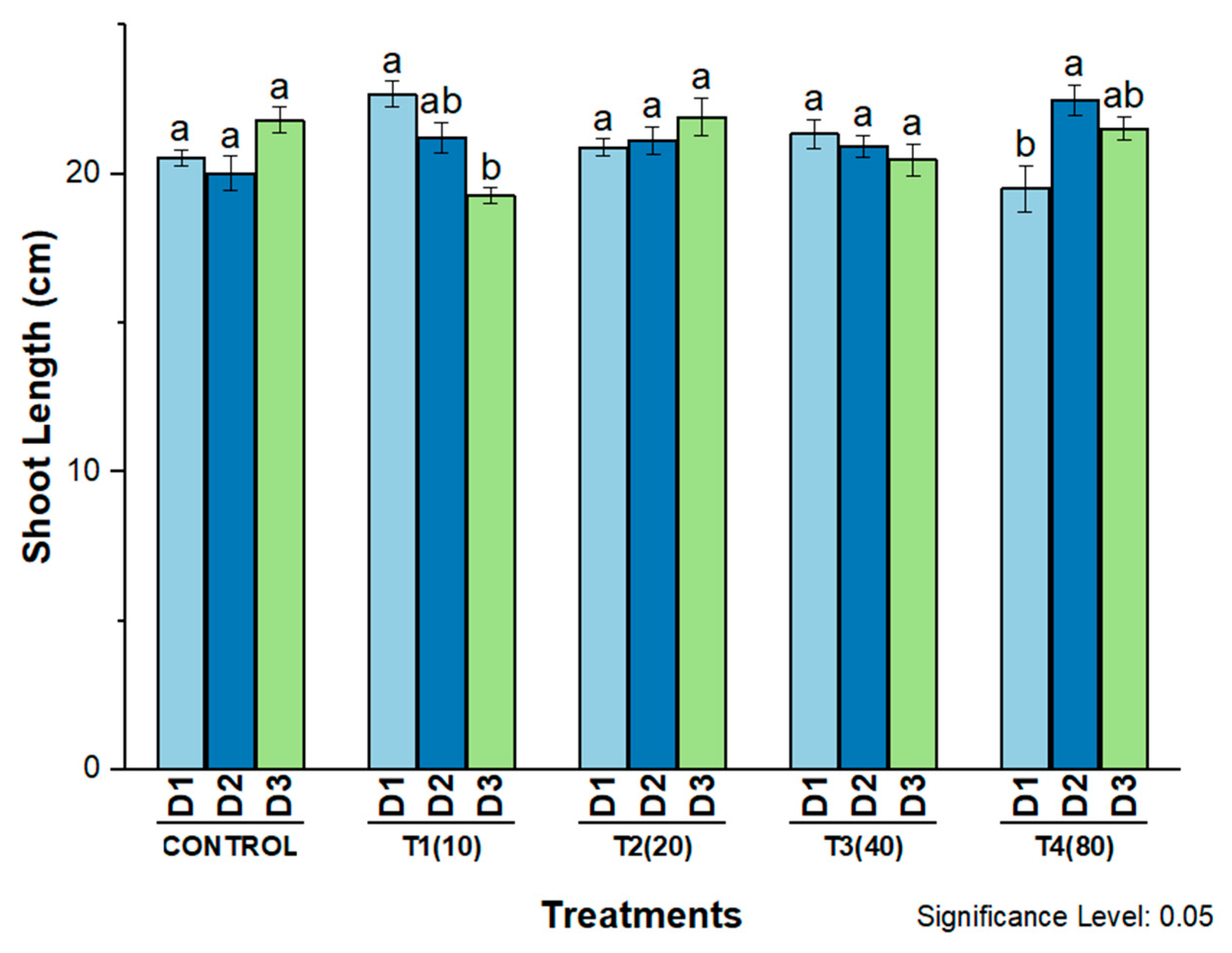
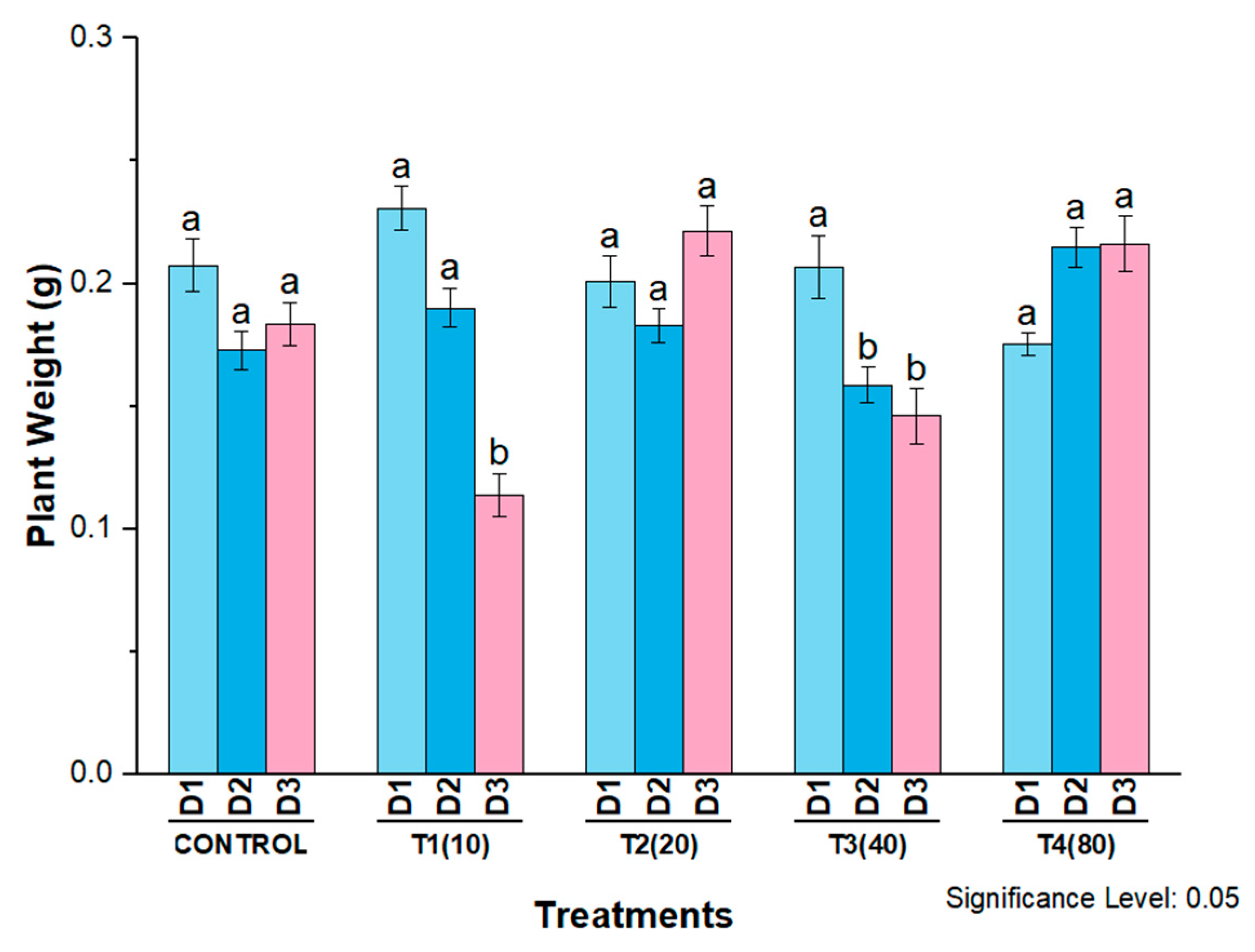
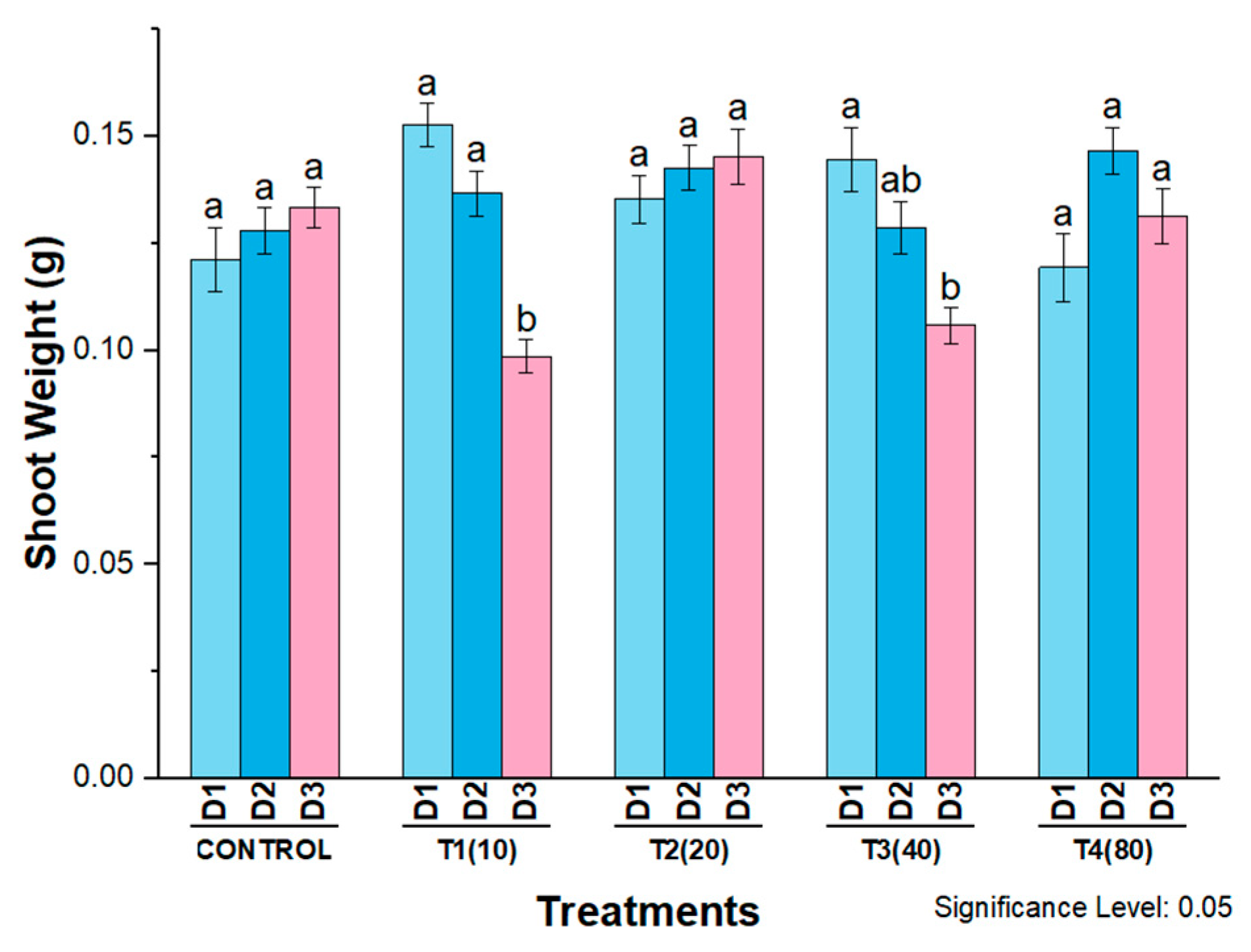
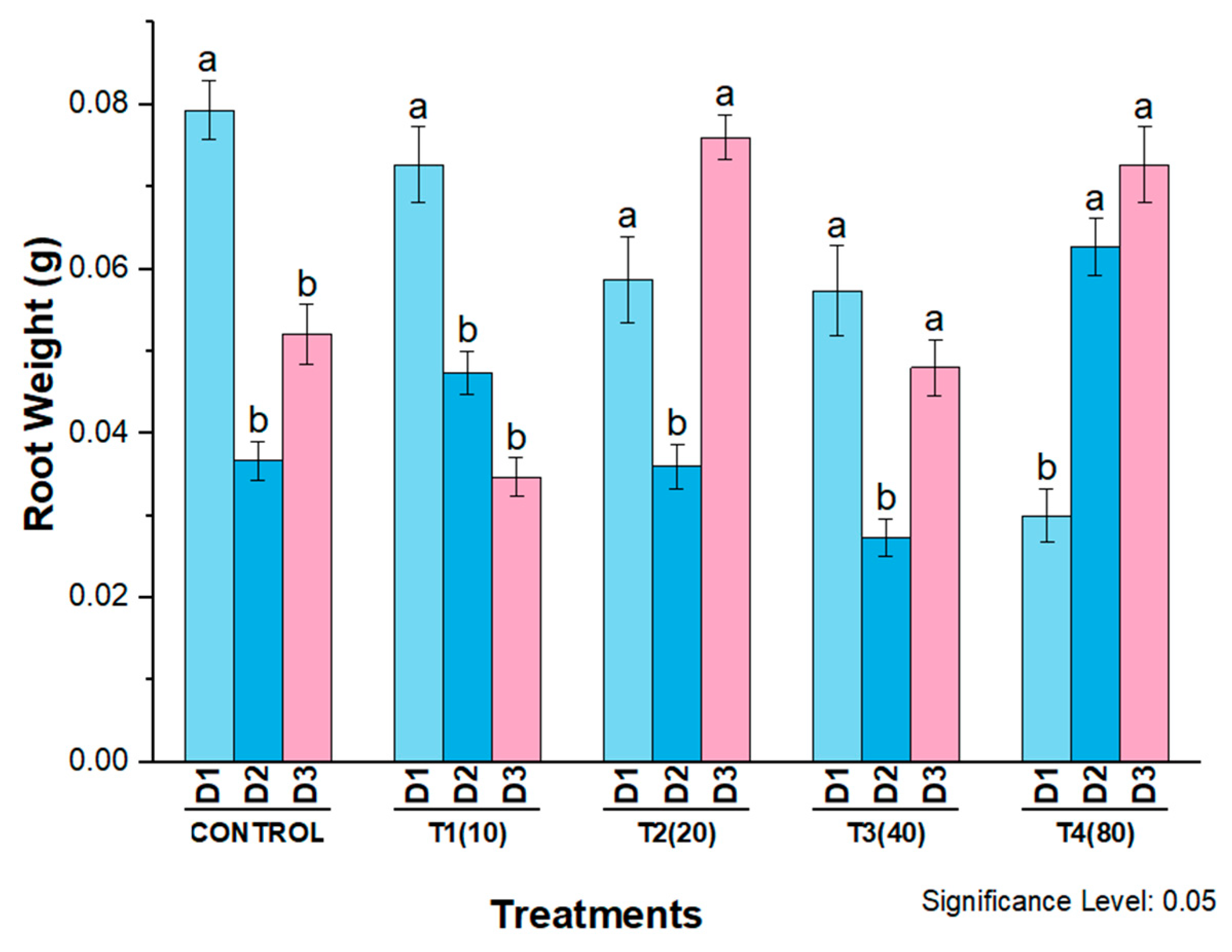
4. Phytotoxicity of AgNPs
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Emran, T.B.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ. Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.-Y. Synthesis, bioactive properties, and biomedical applications of intrinsically therapeutic nanoparticles for disease treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Ramamurthy, C.; Sampath, K.; Arunkumar, P.; Kumar, M.S.; Sujatha, V.; Premkumar, K.; Thirunavukkarasu, C. Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioprocess Biosyst. Eng. 2013, 36, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Alhumaydhi, F.A. Green Synthesis of Gold Nanoparticles Using Extract of Pistacia chinensis and Their In Vitro and In Vivo Biological Activities. J. Nanomater. 2022, 2022, 5544475. [Google Scholar] [CrossRef]
- Arya, A.; Chundawat, T.S. Metal nanoparticles from algae: A green approach for the synthesis, characterization and their biological activity. Nanosci. Nanotechnol. Asia 2020, 10, 185–202. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Ji, Y.; Thomas, C.; Tulin, N.; Lodhi, N.; Boamah, E.; Kolenko, V.; Tulin, A.V. Charon mediates immune deficiency–driven PARP-1–dependent immune responses in Drosophila. J. Immunol. 2016, 197, 2382–2389. [Google Scholar] [CrossRef]
- Nii-Trebi, N.I. Emerging and neglected infectious diseases: Insights, advances, and challenges. BioMed Res. Int. 2017, 2017, 5245021. [Google Scholar] [CrossRef]
- Tong, C.; Zou, W.; Ning, W.; Fan, J.; Li, L.; Liu, B.; Liu, X. Synthesis of DNA-guided silver nanoparticles on a graphene oxide surface: Enhancing the antibacterial effect and the wound healing activity. RSC Adv. 2018, 8, 28238–28248. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Das, C.A.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Govindaraju, K.; Joselin, J.M.; Baalamurugan, J. Antibacterial activity of silver nanoparticles (biosynthesis): A short review on recent advances. Biocatal. Agric. Biotechnol. 2020, 27, 101593. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Contreras-Ruiz, J.; Coutts, P.; Fierheller, M.; Rothman, A.; Woo, K. Bacteriology, inflammation, and healing: A study of nanocrystalline silver dressings in chronic venous leg ulcers. Adv. Ski. Wound Care 2007, 20, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lee, P.y.; Ho, C.m.; Lui, V.C.; Chen, Y.; Che, C.m.; Tam, P.K.; Wong, K.K. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. ChemMedChem 2010, 5, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of oxidative stress on the heart and vasculature: Part 2 of a 3-part series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Amjad, M.S.; Mehmood, A.; Mustafa, G.; Binish, Z.; Khan, S.; Arshad, H.; Proćków, J.; Pérez de la Lastra, J.M. Antibacterial, Antioxidant, and Phytotoxic Potential of Phytosynthesized Silver Nanoparticles Using Elaeagnus umbellata Fruit Extract. Molecules 2022, 27, 5847. [Google Scholar] [CrossRef]
- Nagaich, U.; Gulati, N.; Chauhan, S. Antioxidant and antibacterial potential of silver nanoparticles: Biogenic synthesis utilizing apple extract. J. Pharm. 2016, 2016, 7141523. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J.; Murtaza, G.; Rashid, F.; Iqbal, J. Eco-friendly green synthesis of silver nanoparticles and their potential applications as antioxidant and anticancer agents. Drug Dev. Ind. Pharm. 2019, 45, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Abourehab, M.A.; Khan, S.; Thu, H.E. Silver nanoparticles: A promising nanoplatform for targeted delivery of therapeutics and optimized therapeutic efficacy. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–173. [Google Scholar]
- Tehri, N.; Vashishth, A.; Gahlaut, A.; Hooda, V. Biosynthesis, antimicrobial spectra and applications of silver nanoparticles: Current progress and future prospects. Inorg. Nano-Met. Chem. 2022, 52, 1–19. [Google Scholar] [CrossRef]
- Fibrich, B.; Gao, X.; Puri, A.; Banga, A.K.; Lall, N. In vitro antioxidant, anti-inflammatory and skin permeation of myrsine africana and its isolated compound myrsinoside B. Front. Pharmacol. 2020, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, Y.; Wang, H.; Wu, F.; Zhao, Y.; Liu, X.; Wu, H.; Wang, L.; Su, H. Green synthesis of silver nanoparticles using mushroom Flammulina velutipes Extract and their antibacterial activity against aquatic pathogens. Food Bioprocess Technol. 2020, 13, 1908–1917. [Google Scholar] [CrossRef]
- Juibari, M.M.; Abbasalizadeh, S.; Jouzani, G.S.; Noruzi, M. Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater. Lett. 2011, 65, 1014–1017. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Park, H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014, 140, 185–192. [Google Scholar] [CrossRef]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; El-Belely, E.F.; Awad, M.A.; Hassan, S.E.-D.; Al-Faifi, Z.E.; Hamza, M.F. Enhanced antimicrobial, cytotoxicity, larvicidal, and repellence activities of brown algae, cystoseira crinita-mediated green synthesis of magnesium oxide nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Khan, F.A.; Zahoor, M.; Jalal, A.; Rahman, A.U. Green synthesis of silver nanoparticles by using Ziziphus nummularia leaves aqueous extract and their biological activities. J. Nanomater. 2016, 2016, 8026843. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef] [PubMed]
- Valgas, C.; Souza, S.M.D.; Smânia, E.F.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Vanaja, M.; Gnanajobitha, G.; Paulkumar, K.; Rajeshkumar, S.; Malarkodi, C.; Annadurai, G. Phytosynthesis of silver nanoparticles by Cissus quadrangularis: Influence of physicochemical factors. J. Nanostruct. Chem. 2013, 3, 17. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Chae, J.B.; Kim, C. A multi-functional chemosensor for highly selective ratiometric fluorescent detection of silver (I) ion and dual turn-on fluorescent and colorimetric detection of sulfide. R. Soc. Open Sci. 2018, 5, 180293. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, K.; Velmurugan, S. Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Alqadi, M.; Abo Noqtah, O.; Alzoubi, F.; Alzouby, J.; Aljarrah, K. PH effect on the aggregation of silver nanoparticles synthesized by chemical reduction. Mater. Sci. Pol. 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Ehiri, R.C.; Nnaji, N.J. Phytosynthesis of silver nanoparticles using aqueous leaf extracts of Lippia citriodora: Antimicrobial, larvicidal and photocatalytic evaluations. Mater. Sci. Eng. C 2017, 75, 980–989. [Google Scholar] [CrossRef]
- Loo, Y.Y.; Rukayadi, Y.; Nor-Khaizura, M.-A.-R.; Kuan, C.H.; Chieng, B.W.; Nishibuchi, M.; Radu, S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018, 9, 1555. [Google Scholar] [CrossRef]
- Elmusa, F.; Aygun, A.; Gulbagca, F.; Seyrankaya, A.; Göl, F.; Yenikaya, C.; Sen, F. Investigation of the antibacterial properties of silver nanoparticles synthesized using Abelmoschus esculentus extract and their ceramic applications. Int. J. Environ. Sci. Technol. 2021, 18, 849–860. [Google Scholar] [CrossRef]
- Saravanan, M.; Barik, S.K.; MubarakAli, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef]
- Mo, F.; Li, H.; Li, Y.; Chen, X.; Wang, M.; Li, Z.; Deng, N.; Yang, Y.; Huang, X.; Zhang, R.; et al. Physiological, biochemical, and transcriptional regulation in a leguminous forage Trifolium pratense L. responding to silver ions. Plant Physiol. Biochem. 2021, 162, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.S.; Shaheen, M.S.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J. Saudi Chem. Soc. 2014, 18, 356–363. [Google Scholar] [CrossRef]
- Nayak, S.P.; Ramamurthy, S.S.; Kumar, J.K. Green synthesis of silver nanoparticles decorated reduced graphene oxide nanocomposite as an electrocatalytic platform for the simultaneous detection of dopamine and uric acid. Mater. Chem. Phys. 2020, 252, 123302. [Google Scholar] [CrossRef]
- Khan, G.A.; Bouraine, S.; Wege, S.; Li, Y.; de Carbonnel, M.; Berthomieu, P.; Poirier, Y.; Rouached, H. Coordination between zinc and phosphate homeostasis involves the transcription factor PHR1, the phosphate exporter PHO1, and its homologue PHO1; H3 in Arabidopsis. J. Exp. Bot. 2014, 65, 871–884. [Google Scholar] [CrossRef]
- Goncharova, N.; Isamukhamedov, A.S.; Glushenkova, A. Glycolipids and phospholipids of the fruit of Elaeagnus angustifolia. Chem. Nat. Compd. 1993, 29, 569–573. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the ions: The effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Gardea-Torresdey, J.L.; Rico, C.M.; White, J.C. Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ. Sci. Technol. 2014, 48, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Mustafa, G.; Hasan, M.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. A comparative proteomic analysis of engineered and bio synthesized silver nanoparticles on soybean seedlings. J. Proteom. 2020, 224, 103833. [Google Scholar] [CrossRef]
- Hasan, M.; Mehmood, K.; Mustafa, G.; Zafar, A.; Tariq, T.; Hassan, S.G.; Loomba, S.; Zia, M.; Mazher, A.; Mahmood, N.; et al. Phytotoxic evaluation of phytosynthesized silver nanoparticles on lettuce. Coatings 2021, 11, 225. [Google Scholar] [CrossRef]
- Mustafa, G.; Sakata, K.; Komatsu, S. Proteomic analysis of soybean root exposed to varying sizes of silver nanoparticles under flooding stress. J. Proteom. 2016, 148, 113–125. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwer, Q.; Amjad, M.S.; Mehmood, A.; Binish, Z.; Mustafa, G.; Farooq, A.; Qaseem, M.F.; Abasi, F.; Pérez de la Lastra, J.M. Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities. Molecules 2022, 27, 7612. https://doi.org/10.3390/molecules27217612
Sarwer Q, Amjad MS, Mehmood A, Binish Z, Mustafa G, Farooq A, Qaseem MF, Abasi F, Pérez de la Lastra JM. Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities. Molecules. 2022; 27(21):7612. https://doi.org/10.3390/molecules27217612
Chicago/Turabian StyleSarwer, Qudsia, Muhammad Shoaib Amjad, Ansar Mehmood, Zakia Binish, Ghazala Mustafa, Atikah Farooq, Mirza Faisal Qaseem, Fozia Abasi, and José Manuel Pérez de la Lastra. 2022. "Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities" Molecules 27, no. 21: 7612. https://doi.org/10.3390/molecules27217612
APA StyleSarwer, Q., Amjad, M. S., Mehmood, A., Binish, Z., Mustafa, G., Farooq, A., Qaseem, M. F., Abasi, F., & Pérez de la Lastra, J. M. (2022). Green Synthesis and Characterization of Silver Nanoparticles Using Myrsine africana Leaf Extract for Their Antibacterial, Antioxidant and Phytotoxic Activities. Molecules, 27(21), 7612. https://doi.org/10.3390/molecules27217612






