Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Polar Glycosides from SCP
2.2. Qualitative and Quantitative Analysis of Polar Compounds in the Aqueous Extracts by LC/MS Analysis
2.3. Determination of Total Phenolics, Flavonoid Content, and Antioxidant Capacity (DPPH and FRAP) in Aqueous Extracts
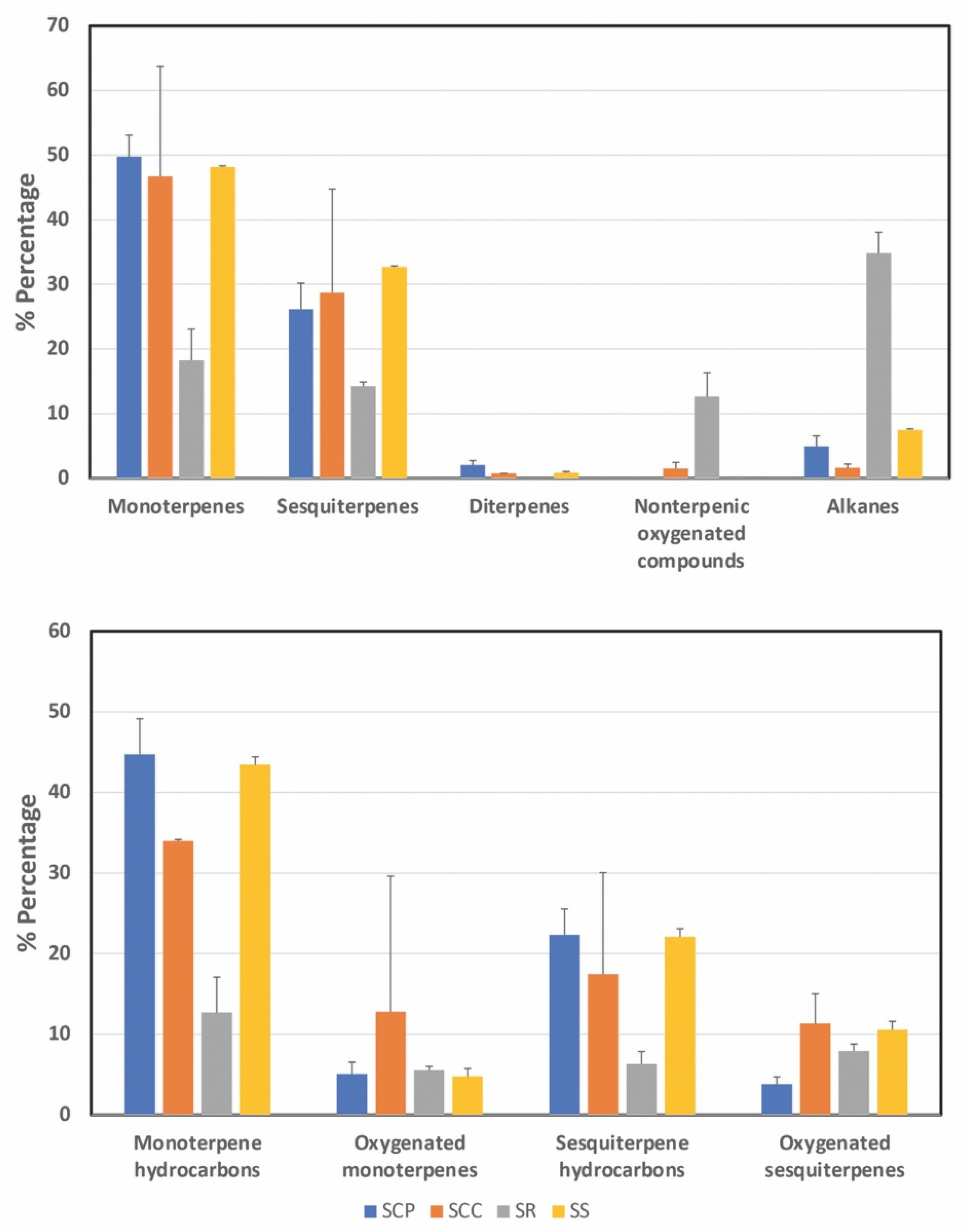
2.4. GC/MS Determination of Volatile Compounds in Petroleum Ether Extracts
3. Materials and Methods
3.1. Plant Material
3.2. Isolation of Polar Compounds from SCP Extracts
3.3. Determination of Polar and Volatile Metabolites in Different Sideritis Samples
3.3.1. Extraction
3.3.2. Gas Chromatography–Mass Spectrometry (GC/MS)
3.3.3. Liquid Chromatography–Mass Spectrometry (LC/MS)
3.4. Determination of Total Phenolics, Flavonoids Content, and Antioxidant Capacity (DPPH and FRAP) in Aqueous Buffer Extracts
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraga, B.M. Phytochemistry and Chemotaxonomy of Sideritis Species from the Mediterranean Region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, Chemical Composition and Pharmacological Activities—A Review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, Section Empedoclia in Southeastern Europe and Turkey—Studies in Ethnopharmacology and Recent Progress of Biological Activities. DARU J. Pharm. Sci. 2019, 27, 407–421. [Google Scholar] [CrossRef]
- Ibraliu, A.; Trendafilova, A.B.; Anđelković, B.; Qazimi, B.; Gođevac, D.; Bebeci, E.; Stefkov, G.; Zdunic, G.; Aneva, I.; Pasho, I.; et al. Comparative Study of Balkan Sideritis Species from Albania, Bulgaria and Macedonia. Eur. J. Med. Plants 2015, 5, 328–340. [Google Scholar] [CrossRef]
- Trikka, F.; Michailidou, S.; Makris, A.M.; Argiriou, A. Biochemical Fingerprint of Greek Sideritis spp.: Implications for Potential Drug Discovery and Advanced Breeding Strategies. Med. Aromat. Plants 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S.; Alipieva, K.; Bankova, V.; Aneva, I.; Evstatieva, L.N. Chemotaxonomic Contribution to the Sideritis Species Dilemma on the Balkans. Biochem. Syst. Ecol. 2015, 61, 477–487. [Google Scholar] [CrossRef]
- Pappas, C.S.; Xagoraris, M.; Kimbaris, A.; Korakis, G.; Tarantilis, P.A. Chemometric-Infrared Spectroscopic Model for the Taxonomy of Medicinal Herbs—The Case of Perennial Sideritis Species. Biomed. J. Sci. Tech. Res. 2020, 32, 24707–24712. [Google Scholar] [CrossRef]
- Petreska, J.; Stefova, M.; Ferreres, F.; Moreno, D.A.; Tomás-Barberán, F.A.; Stefkov, G.; Kulevanova, S.; Gil-Izquierdo, A. Potential Bioactive Phenolics of Macedonian Sideritis Species Used for Medicinal “Mountain Tea”. Food Chem. 2011, 125, 13–20. [Google Scholar] [CrossRef]
- Strid, A.; Tan, K. Mountain Flora of Greece; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1986; ISBN 978-0-521-25737-4. [Google Scholar]
- Koedam, A. Volatile Oil Composition of Greek Mountain Tea (Sideritis spp.). J. Sci. Food Agric. 1986, 37, 681–684. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Chinou, I.B.; Mitakou, S.; Gikas, E.; Tsarbopoulos, A. Composition and Antimicrobial Activity of the Essential Oils of Five Taxa of Sideritis from Greece. J. Agric. Food Chem. 2001, 49, 811–815. [Google Scholar] [CrossRef]
- Koutsaviti, A.; Bazos, I.; Milenkovi, M.; Pavlovi, M. Antimicrobial Activity and Essential Oil Composition of Five Sideritis Taxa of Empedoclia and Hesiodia Sect. from Greece. Rec. Nat. Prod. 2013, 7, 6–14. [Google Scholar]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Phytochemistry and Nematicidal Activity of the Essential Oils from 8 Greek Lamiaceae Aromatic Plants and 13 Terpene Components. J. Agric. Food Chem. 2010, 58, 7856–7863. [Google Scholar] [CrossRef] [PubMed]
- Linardaki, Z.I.; Vasilopoulou, C.G.; Constantinou, C.; Iatrou, G.; Lamari, F.N.; Margarity, M. Differential Antioxidant Effects of Consuming Tea from Sideritis Clandestina subsp. Peloponnesiaca on Cerebral Regions of Adult Mice. J. Med. Food 2011, 14, 1060–1064. [Google Scholar] [CrossRef]
- Vasilopoulou, C.G.; Kontogianni, V.G.; Linardaki, Z.I.; Iatrou, G.; Lamari, F.N.; Nerantzaki, A.A.; Gerothanassis, I.P.; Tzakos, A.G.; Margarity, M. Phytochemical Composition of “Mountain Tea” from Sideritis Clandestina Subsp. Clandestina and Evaluation of Its Behavioral and Oxidant/Antioxidant Effects on Adult Mice. Eur. J. Nutr. 2013, 52, 107–116. [Google Scholar] [CrossRef]
- Dimaki, V.D.; Iatrou, G.; Lamari, F.N. Effect of Acidic and Enzymatic Pretreatment on the Analysis of Mountain Tea (Sideritis spp.) Volatiles via Distillation and Ultrasound-Assisted Extraction. J. Chromatogr. A 2017, 1524, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Serrilli, A.M.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M.; Bianco, A. Monoterpenoids from Stachys Glutinosa L. Nat. Prod. Res. 2006, 20, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Kloukina, C.; Vassiliou, R.; Tomou, E.-M.; Skaltsa, H.; Tzortzakis, N. Cultivation Strategy to Improve Chemical Profile and Anti-Oxidant Activity of Sideritis Perfoliata L. Subsp. Perfoliata. Ind. Crops Prod. 2019, 140, 111694. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Lytra, K.; Chrysargyris, A.; Christofi, M.-D.; Miltiadous, P.; Corongiu, G.L.; Tziouvelis, M.; Tzortzakis, N.; Skaltsa, H. Polar Constituents, Biological Effects and Nutritional Value of Sideritis Sipylea Boiss. Nat. Prod. Res. 2022, 36, 4200–4204. [Google Scholar] [CrossRef]
- Koleva, I.I.; Linssen, J.P.; van Beek, T.A.; Evstatieva, L.N.; Kortenska, V.; Handjieva, N. Antioxidant Activity Screening of Extracts from Sideritis Species (Labiatae) Grown in Bulgaria. J. Sci. Food Agric. 2003, 83, 809–819. [Google Scholar] [CrossRef]
- Lytra, K.; Tomou, E.; Chrysargyris, A.; Christofi, M.; Miltiadous, P.; Tzortzakis, N.; Skaltsa, H. Bio-Guided Investigation of Sideritis Cypria Methanol Extract Driven by In Vitro Antioxidant and Cytotoxic Assays. Chem. Biodivers. 2021, 18, e2000966. [Google Scholar] [CrossRef]
- Axiotis, E.; Petrakis, E.A.; Halabalaki, M.; Mitakou, S. Phytochemical Profile and Biological Activity of Endemic Sideritis Sipylea Boiss. in North Aegean Greek Islands. Molecules 2020, 25, 2022. [Google Scholar] [CrossRef]
- Yu, H.; Yang, G.; Sato, M.; Yamaguchi, T.; Nakano, T.; Xi, Y. Antioxidant Activities of Aqueous Extract from Stevia Rebaudiana Stem Waste to Inhibit Fish Oil Oxidation and Identification of Its Phenolic Compounds. Food Chem. 2017, 232, 379–386. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ozer, M.S.; Istifli, E.S.; Sahinler, S.S.; Tepe, B. Chromatographic Profile and Antioxidant and Enzyme Inhibitory Activity of Sideritis Leptoclada: An Endemic Plant from Turkey. S. Afr. J. Bot. 2021, 143, 393–405. [Google Scholar] [CrossRef]
- Özkan, G. Comparison of Antioxidant Phenolics of Ethanolic Extracts and Aqueous Infusions from Sideritis Species. Asian J. Chem. 2009, 21, 1024–1028. [Google Scholar]
- Venditti, A.; Frezza, C.; Lorenzetti, L.; Maggi, F.; Serafini, M.; Bianco, A. Reassessment of the Polar Fraction of Stachys Alopecuros (L.) Benth. Subsp. Divulsa (Ten.) Grande (Lamiaceae) from the Monti Sibillini National Park and Its Potential Pharmacologic Uses. J. Intercult. Ethnopharmacol. 2017, 6, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Charami, M.-T.; Lazari, D.; Karioti, A.; Skaltsa, H.; Hadjipavlou-Litina, D.; Souleles, C. Antioxidant and Antiinflammatory Activities of Sideritis Perfoliata subsp. Perfoliata (Lamiaceae). Phytother. Res. 2008, 22, 450–454. [Google Scholar] [CrossRef]
- Mendoza, D.; Arias, J.P.; Cuaspud, O.; Esturau-Escofet, N.; Hernández-Espino, C.C.; de San Miguel, E.R.; Arias, M. 1H-NMR-Based Metabolomic of Plant Cell Suspension Cultures of Thevetia Peruviana Treated with Salicylic Acid and Methyl Jasmonate. Ind. Crops Prod. 2019, 135, 217–229. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Fayvush, G.; Tamanyan, K.; Khutsishvili, M.; Atha, D.; Borris, R.P. Phytochemical Investigations of Aethionema Armenum Boiss. (Brassicaceae). Biochem. Syst. Ecol. 2018, 81, 37–41. [Google Scholar] [CrossRef]
- Hanoğlu, D.Y.; Hanoğlu, A.; Yusufoğlu, H.; Demirci, B.; Başer, K.H.C.; Çalış, İ.; Yavuz, D.Ö. Phytochemical Investigation of Endemic Sideritis Cypria Post. Rec. Nat. Prod. 2019, 14, 105–115. [Google Scholar] [CrossRef]
- Garayev, E.; Di Giorgio, C.; Herbette, G.; Mabrouki, F.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. Bioassay-Guided Isolation and UHPLC-DAD-ESI-MS/MS Quantification of Potential Anti-Inflammatory Phenolic Compounds from Flowers of Inula montana L. J. Ethnopharmacol. 2018, 226, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tsao, R.; Liu, Z.; Liu, S.; Yang, R.; Young, J.C.; Zhu, H.; Deng, Z.; Xie, M.; Fu, Z. Isolation and Purification of Acteoside and Isoacteoside from Plantago Psyllium L. by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1063, 161–169. [Google Scholar] [CrossRef]
- Owen, R.W.; Haubner, R.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Isolation, Structure Elucidation and Antioxidant Potential of the Major Phenolic and Flavonoid Compounds in Brined Olive Drupes. Food Chem. Toxicol. 2003, 41, 703–717. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Šavikin, K.; Janković, T.; Zdunić, G.; Ristić, M.; Godjevac, D.; Konić-Ristić, A. Chemical Properties of the Cultivated Sideritis Raeseri Boiss. & Heldr. Subsp. Raeseri. Food Chem. 2011, 124, 226–233. [Google Scholar] [CrossRef]
- Stanoeva, J.P.; Bagashovska, D.; Stefova, M. Characterization of Urinary Bioactive Phenolic Metabolites Excreted after Consumption of a Cup of Mountain Tea (Sideritis scardica) Using Liquid Chromatography Tandem Mass Spectrometry. Maced. J. Chem. Chem. Eng. 2012, 31, 229–243. [Google Scholar] [CrossRef]
- Menkovi, N.; Gođevac, D.; Šavikin, K.; Zdunić, G.; Milosavljević, S.; Bojadži, A.; Avramoski, O. Bioactive Compounds of Endemic Species Sideritis Raeseri Subsp. Raeseri Grown in National Park Galičica. Rec. Nat. Prod. 2013, 7, 161–168. [Google Scholar]
- Petreska Stanoeva, J.; Stefova, M. Assay of Urinary Excretion of Polyphenols after Ingestion of a Cup of Mountain Tea (Sideritis scardica) Measured by HPLC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2013, 61, 10488–10497. [Google Scholar] [CrossRef] [PubMed]
- Petreska, J.; Stefova, M.; Ferreres, F.; Moreno, D.A.; Tomás-Barberán, F.A.; Stefkov, G.; Kulevanova, S.; Gil-Izquierdo, A. Dietary Burden of Phenolics per Serving of “Mountain Tea” (Sideritis) from Macedonia and Correlation to Antioxidant Activity. Nat. Prod. Commun. 2011, 6, 1305–1314. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Serafini, M.; Giacomello, G.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Lucarini, D.; et al. Secondary Metabolites, Glandular Trichomes and Biological Activity of Sideritis montana L. subsp. Montana from Central Italy. Chem. Biodivers. 2016, 13, 1380–1390. [Google Scholar] [CrossRef]
- Alipieva, K.; Petreska, J.; Gil-Izquierdo, Á.; Stefova, M.; Evstatieva, L.; Bankova, V. Influence of the Extraction Method on the Yield of Flavonoids and Phenolics from Sideritis spp. (Pirin Mountain Tea). Nat. Prod. Commun. 2010, 5, 51–54. [Google Scholar] [CrossRef]
- Karapandzova, M.; Qazimi, B.; Stefkov, G.; Bačeva, K.; Stafilov, T.; Panovska, T.K.; Kulevanova, S. Chemical Characterization, Mineral Content and Radical Scavenging Activity of Sideritis scardica and S. raeseri from R. Macedonia and R. Albania. Nat. Prod. Commun. 2013, 8, 639–644. [Google Scholar] [CrossRef]
- Qazimi, B.; Karapandzova, M.; Stefkov, G.; Kulevanova, S. Chemical Composition of Ultrasonic-Assisted n-Hexane Extracts of Sideritis scardica Grieseb. and Sideritis raeseri Boiss. & Heldr. (Lamiaceae) from Macedonia and Albania. Maced. Pharm. Bull. 2011, 56, 45–56. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectorscopy, 4th ed.; Allured Pub. Corp: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- König, W.A.; Joulain, D.; Hochmuth, D.H. Available online: https://massfinder.com/wiki/Terpenoids_Library (accessed on 25 May 2021).
- Qazimi, B.; Stefkov, G.; Karapandzova, M.; Cvetkovikj, I.; Kulevanova, S. Aroma Compounds of Mountain Tea (Sideritis scardica and S. raeseri) from Western Balkan. Nat. Prod. Commun. 2014, 9, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Hodaj-Çeliku, E.; Tsiftsoglou, O.; Shuka, L.; Abazi, S.; Hadjipavlou-Litina, D.; Lazari, D. Antioxidant Activity and Chemical Composition of Essential Oils of Some Aromatic and Medicinal Plants from Albania. Nat. Prod. Commun. 2017, 12, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Tzakou, O. The Essential Oil of Sideritis raeseri Boiss. et Heldr. Ssp. Attica (Heldr.) Pap. et Kok. J. Essent. Oil Res. 2002, 14, 376–377. [Google Scholar] [CrossRef]
- Romanucci, V.; Di Fabio, G.; D’Alonzo, D.; Guaragna, A.; Scapagnini, G.; Zarrelli, A. Traditional Uses, Chemical Composition and Biological Activities of Sideritis raeseri Boiss. & Heldr.: Uses, Composition and Activities of S. raeseri. J. Sci. Food Agric. 2017, 97, 373–383. [Google Scholar] [CrossRef]
- Kostadinova, E.; Nikolova, D.; Alipieva, K.; Stefova, M.; Stefkov, G.; Evstatieva, L.; Matevski, V.; Bankova, V. Chemical Constituents of the Essential Oils of Sideritis Scardica Griseb. and Sideritis raeseri Boiss and Heldr. from Bulgaria and Macedonia. Nat. Prod. Res. 2007, 21, 819–823. [Google Scholar] [CrossRef]
- Kloukina, C.; Tomou, E.-M.; Skaltsa, H. Essential oil composition of two Greek cultivated Sideritis spp. Nat. Volatiles Essent. Oils 2019, 6, 16–23. [Google Scholar]
- Trendafilova, A.B.; Todorova, M.N.; Evstatieva, L.N.; Antonova, D.V. Variability in the Essential-Oil Composition of Sideritis scardica Griseb. from Native Bulgarian Populations. Chem. Biodivers. 2013, 10, 484–492. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Kirimer, N.; Tümen, G. Essential Oil of Sideritis Scardica Griseb. Subsp. Scardica. J. Essent. Oil Res. 1997, 9, 205–207. [Google Scholar] [CrossRef]
- Komaitis, M.E.; Melissari-Panagiotou, E.; Infanti-Papatragianni, N. Constituents of the Essential Oil of Sideritis scardica. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1992; Volume 28, pp. 411–415. ISBN 978-0-444-88558-6. [Google Scholar]
- Todorova, M.; Trendafilova, A.; Evstatieva, L.; Antonova, D. Volatile Components in Sideritis scardica Griseb. Cultivar. Proc. Bulg. Acad. Sci. 2013, 66, 507–512. [Google Scholar] [CrossRef]
- Todorova, M.N.; Christov, R.C.; Evstatieva, L.N. Essential Oil Composition of Three Sideritis Species from Bulgaria. J. Essent. Oil Res. 2000, 12, 418–420. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST) WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 20 April 2021).
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Zeliou, K.; Papasotiropoulos, V.; Manoussopoulos, Y.; Lamari, F.N. Physical and Chemical Quality Characteristics and Antioxidant Properties of Strawberry Cultivars (Fragaria × Ananassa Duch.) in Greece: Assessment of Their Sensory Impact: Quality Factors Determining Sensory Characteristics of Strawberry Cultivars in Greece. J. Sci. Food Agric. 2018, 98, 4065–4073. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef]
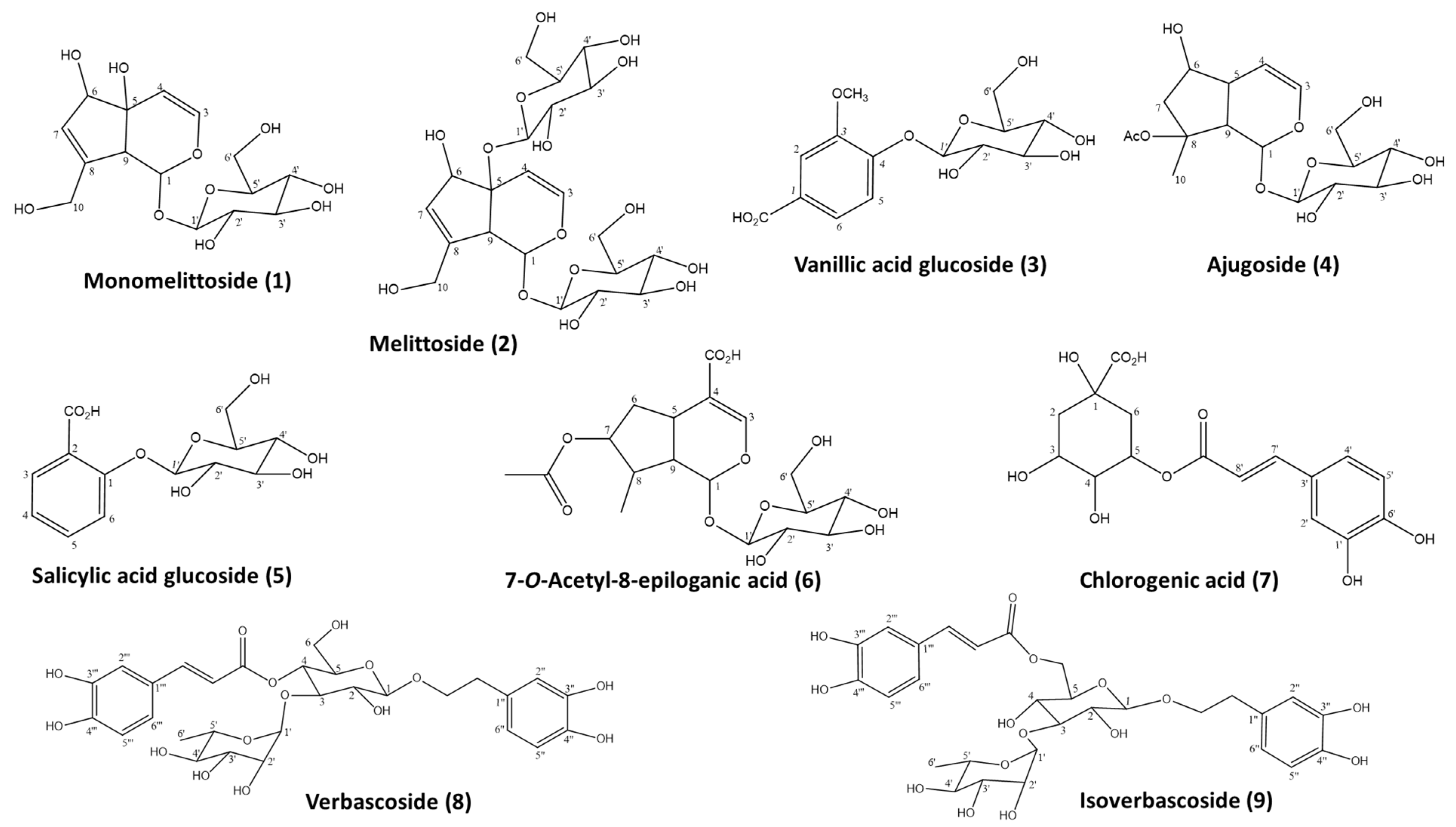
| a/a | tR (min) | Components | M.W. | [M-H]− | Other Negative Ions | SCP | SCC | SR | SS |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 6.96 | Melittoside * | 524 | 523 | 583 [M+Hac-H]− 1070 [2M+Na-H]− | 19.18 ± 2.95 | 15.52 ± 0.49 | 15.49 ± 3.01 | n.d. |
| C2 | 12.00 | Unknown | 374 | 373 | 747 [2M-H]− 769 [2M-2H+Na]− | n.d. | 21.17 ± 4.89 | n.d. | n.d. |
| C3 | 13.30 | Unknown | 374 | 373 | 747 [2M-H]− 769 [2M-2H+Na]− | n.d. | 28.57 ± 6.87 | n.d. | n.d. |
| C4 | 17.65 | Unknown | 488 | 487 | 975 [2M-H]− | 9.20 ± 5.28 | 29.19 ± 3.55 | n.d. | 29.66 ± 0.99 |
| C5 | 18.20 | Unknown | 376 | 375 | 751 [2M-H]− 773 [2M-2H+Na]− | n.d. | 7.57 ± 5.71 | n.d. | n.d. |
| C6 | 18.80 | Chlorogenic acid * | 354 | 353 | 191 (quinic acid) 375 [M+Na-2H]− 707 [2M-H]− 729 [2M-2H+Na]− | 33.23 ± 7.45 | 56.81 ± 11.81 | 61.21 ± 2.57 | 65.65 ± 8.96 |
| C7 | 22.85 | β-Hydroxyverbascoside isomer [34] | 640 | 639 | 661 [M+Na-2H]− | 8.55 ± 3.05 | 11.45 ± 3.56 | n.q. | 16.80 ± 6.95 |
| C8 | 23.65 | β-Hydroxyverbascoside isomer [34] | 640 | 639 | 661 [M+Na-2H]− | 9.20 ± 4.14 | 15.48 ± 3.81 | 9.78 ± 0.32 | 19.34 ± 6.79 |
| C9 | 24.81 | 7-O-Acetyl-8-epi- loganic acid * | 418 | 417 | 835 [2M-H]− 857 [2M+Na-2H]− | 7.29 ± 4.68 | n.d. | 11.76 ± 0.17 | n.d. |
| C10 | 25.57 | Ajugoside * | 390 | 449 [M+Hac-H] 779 [2M-H]− | n.q. | n.d. | 27.62 ± 3.84 | n.d. | |
| C11 | 28.60 | Forsythoside B or Lavandulofolioside [34,35] | 756 | 755 | 377 [M-2H]−2 1512 [2M-H]− | n.d. | n.q. | 29.42 ± 3.56 | n.d. |
| C12 | 30.00 | All-Glc-ISC [8] | 610 | 609 | 1220 [2M-H]− | n.d. | n.d. | n.d. | 25.46 ± 1.34 |
| C13 | 30.30 | Verbascoside * | 624 | 623 | 311 [M-2H]−2 1248 [2M-H]− | n.q. | 23.31 ± 10.52 | 52.53 ± 5.22 | 39.05 ± 2.31 |
| C14 | 31.80 | All-Glc-HYP [8] | 626 | 625 | 1251 [2M-H]− | 8.59 ± 0.94 | n.d. | 18.13 ± 4.01 | 28.79 ± 1.44 |
| C15 # | 34.78 | Allysonoside/Forsythoside B or lavandulofolioside [6,8,22,37] | 770/756 | 769/755 | n.d. | n.d. | 11.58 ± 0.63 | n.d. | |
| C16 # | 35.60 | Leucoseptoside isomer/ Isoverbascoside * [8,37] | 638/624 | 637/623 | n.d. | n.d. | n.q. | 7.57 ± 1.39 | |
| C17 | 36.40 | AcO-All-Glc-ISC or AcO-All-Glc-LUT [8,37] | 652 | 652 | 325 [M-2H]−2 | n.d. | n.d. | n.d. | 14.82 ± 0.82 |
| C18 | 36.90 | All-Glc-LUT [8] | 610 | 609 | 1219 [2M-H]− | 15.86 ± 4.56 | 18.29 ± 0.44 | 16.36 ± 5.71 | 20.72 ± 3.95 |
| C19 | 37.00 | AcO-All-Glc-HYP [6,8] | 668 | 667 | n.d. | n.d. | 9.44 ± 2.08 | 7.51 ± 0.04 | |
| C20 | 38.50 | All-Glc-HYP-Me [6,8] | 640 | 639 | 1279 [2M-H]− | 11.82 ± 1.60 | 10.33 ± 1.43 | 15.29 ± 3.24 | 11.28 ± 1.69 |
| C21 | 39.30 | AcO-All-Glc-HYP [6] | 668 | 667 | 1335 [2M-H]− | n.q. | n.d. | 28.71 ± 0.59 | 38.55 ± 1.21 |
| C22 | 44.50 | AcO-All-Glc-ISC or AcO-All-Glc-LUT [8,35,37] | 652 | 651 | 1303 [2M-H]− | 9.07 ± 3.21 | 11.42 ± 1.78 | 16.25 ± 0.29 | 13.09 ± 0.58 |
| C23 | 44.90 | AcO-All-Glc-HYP-Me [35,37] | 682 | 681 | 1364 [2M-H]− | 7.25 ± 0.66 | 7.16 ± 0.88 | 12.85 ± 0.55 | n.q. |
| C24 | 45.80 | AcO-All-Glc-HYP [8,35,37] | 668 | 667 | 1336 [2M-H]− | n.q. | n.d. | 11.71 ± 0.65 | n.q. |
| C25 | 48.45 | (AcO)2-All-Glc-HYP [8,35,36] | 710 | 709 | 1419 [2M-H]− | n.q. | n.d. | 11.90 ± 2.00 | 10.60 ± 0.29 |
| C26 | 49.09 | AcO-All-Glc-ISC-Me [8,35,36] | 666 | 665 | 1332 [2M-H]− 1354 [2M-2H+Na]− | n.d. | 6.29 ± 0.44 | n.d. | n.d. |
| C27 # | 49.95 | (AcO)2-All-Glc-ISC/ (AcO)2-All-Glc-HYP-Me [8,35,37] | 694/724 | 693/723 | 733.7 [M+Hac-H]− 1388 [2M-H]− 1448 [2M-H]− | 6.30 ± 1.12 | n.q. | 13.11 ± 1.68 | n.q. |
| Total | 145.55 | 262.56 | 373.12 | 348.90 |
| TPC (mg GAE/g) 1 | TFC (mg QE/g) 2 | FRAP Assay (mmol FeII/g) 3 | DPPH Assay IC50 (mg/mL) 4 | |
|---|---|---|---|---|
| SCP | 3.19 ± 0.44 d | 2.40 ± 0.41 c | 19.48 ± 0.88 d | 5.44 ± 0.63 a |
| SCC | 4.62 ± 0.67 c | 2.94 ± 0.43 c | 39.94 ± 5.82 c | 3.11 ± 0.59 b |
| SR | 12.38 ± 1.23 a | 10.67 ± 1.20 a | 94.89 ± 7.41 a | 1.79 ± 0.19 c |
| SS | 9.53 ± 1.69 b | 9.18 ± 1.43 b | 78.41 ± 11.09 b | 1.73 ± 0.31 c |
| TPC | TFC | FRAP | DPPH IC50 | |
|---|---|---|---|---|
| TPC | 1 | |||
| TFC | 0.982 ** | 1 | ||
| FRAP | 0.987 ** | 0.981 ** | 1 | |
| DPPH IC50 | −0.853 ** | −0.849 ** | −0.919 ** | 1 |
| SCP | SCC | SR | SS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | RI cal 1 | RI lit 2 | Components | % | mg/100 g | % | mg/100 g | % | mg/100 g | % | mg/100 g |
| 1 | 901 | 900 | Nonane | 4.26 | 1.11 ± 0.35 | 1.17 | 0.52 ± 0.03 * | 11.25 | 2.13 ± 0.03 | 7.48 | 1.31 ± 0.17 |
| 2 | 923 | 924 | α-Thujene | 0.74 | 0.32 ± 0.02 | 0.29 | 0.17 ± 0.03 | n.d. | n.d. | n.q. | n.q. |
| 3 | 929 | 932 | α-Pinene | 20.01 | 4.70 ± 0.29 | 20.40 | 8.31 ± 0.57 | 2.64 | 0.54 ± 0.15 | 18.26 | 3.18 ± 0.42 * |
| 4 | 969 | 969 | Sabinene | 1.61 | 0.51 ± 0.06 | 0.73 | 0.35 ± 0.07 | n.q. | n.q. | 6.44 | 1.13 ± 0.06 |
| 5 | 971 | 974 | β-Pinene | 7.73 | 1.91 ± 0.21 | 6.47 | 2.68 ± 0.02 | 3.89 | 0.78 ± 0.26 | 6.04 | 1.06 ± 0.01 |
| 6 | 979 | 974 | 1-Octen-3-ol | n.d. | n.d. | 0.43 | 0.23 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 7 | 991 | 988 | β-Myrcene | 5.86 | 1.48 ± 0.25 | n.d. | n.d. | n.d. | n.d. | 2.28 | 0.41 ± 0.08 |
| 8 | 1000 | 1000 | n-Decane | 0.69 | 0.30 ± 0.07 | n.d. | n.d. | 23.59 | 4.42 ± 0.32 | n.d. | n.d. |
| 9 | 1002 | 1002 | α-Phellandrene | 0.74 | 0.31 ± 0.02 | 1.02 | 0.47 ± 0.17 | n.d. | n.d. | n.d. | n.d. |
| 10 | 1007 | 1008 | 3-Carene | 0.87 | 0.34 ± 0.05 | 1.44 | 0.64 ± 0.18 | 0.56 | 0.15 ± 0.01 * | n.d. | n.d. |
| 11 | 1014 | 1014 | α-Terpinene | 0.57 | 0.28 ± 0.05 | n.d. | n.d. | n.d. | n.d. | 1.69 | 0.30 ± 0.07 |
| 12 | 1022 | 1022 | o-Cymene | 0.94 | 0.36 ± 0.09 | 0.37 | 0.20 ± 0.05 | 2.83 | 0.58 ± 0.38 | 1.75 | 0.31 ± 0.16 |
| 13 | 1025 | 1025 | Sylvestrene | 5.52 | 1.41 ± 0.24 | 3.10 | 1.31 ± 0.41 | 1.39 | 0.31 ± 0.06 | 4.76 | 0.85 ± 0.29 |
| 14 | 1027 | 1026 | 1,8-Cineole | 1.30 | 0.46 ± 0.33 | 0.58 | 0.28 ± 0.17 | n.q. | n.q. | n.q. | n.q. |
| 15 | 1046 | 1053 | trans-Decahydro-naphthalene | 0.31 | 0.22 ± 0.03 | n.d. | n.d. | 1.65 | 0.35 ± 0.03 | 1.50 | 0.27 ± 0.01 |
| 16 | 1056 | 1054 | γ-Terpinene | n.d. | n.d. | n.d. | n.d. | n.q. | n.q. | 0.72 | 0.14 ± 0.01 |
| 17 | 1085 | 1086 | Terpinolene | n.d. | n.d. | 0.17 | 0.12 ± 0.01 * | n.d. | n.d. | n.d. | n.d. |
| 18 | 1101 | 1095 | Linalool | 3.73 | 1.02 ± 0.24 | 13.90 | 5.57 ± 0.11 * | n.q. | n.q. | 3.59 | 0.63 ± 0.14 |
| 19 | 1138 | 1141 | Camphor | n.d. | n.d. | 2.68 | 1.12 ± 0.05 * | n.d. | n.d. | n.d. | n.d. |
| 20 | 1151 | 1155 | Isoborneol | n.d. | n.d. | 0.41 | 0.22 ± 0.01 * | n.d. | n.d. | n.d. | n.d. |
| 21 | 1160 | 1165 | Borneol | n.d. | n.d. | 1.48 | 0.64 ± 0.02 * | n.d. | n.d. | n.d. | n.d. |
| 22 | 1174 | 1174 | Terpinen-4-ol | n.d. | n.d. | 0.72 | 0.34 ± 0.01 * | n.d. | n.d. | n.d. | n.d. |
| 23 | 1187 | 1186 | α-Terpineol | n.d. | n.d. | 0.72 | 0.34 ± 0.06 | n.d. | n.d. | 0.69 | 0.13 ± 0.01 |
| 24 | 1200 | 1200 | n-Dodecane | n.d. | n.d. | 0.45 | 0.24 ± 0.09 | n.d. | n.d. | n.d. | n.d. |
| 25 | 1260 | 1254 | Linalool acetate | n.d. | n.d. | 3.76 | 1.55 ± 0.15 * | n.d. | n.d. | n.d. | n.d. |
| 26 | 1332 # | 1324 | Bicycloelemene | 3.49 | 0.95 ± 0.22 | 0.71 | 0.34 ± 0.16 | n.d. | n.d. | n.d. | n.d. |
| 27 | 1355 | 1356 | Eugenol | n.d. | n.d. | n.d. | n.d. | 3.43 | 0.69 ± 0.15 | 0.47 | 0.10 ± 0.03 |
| 28 | 1360 | 1369 | Cyclosativene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.75 | 0.15 ± 0.02 |
| 29 | 1370 | 1370 | α-Copaene | n.q. | n.q. | 0.93 | 0.44 ± 0.08 * | n.q. | n.q. | 4.05 | 0.72 ± 0.14 |
| 30 | 1378 | 1387 | β-Bourbonene | 0.62 | 0.29 ± 0.06 | 0.33 | 0.19 ± 0.01 * | 0.91 | 0.22 ± 0.01 * | 1.60 | 0.30 ± 0.12 |
| 31 | 1388 | 1389 | β-Elemene | n.q. | n.q. | 0.22 | 0.14 ± 0.01 * | n.q. | n.q. | 0.32 | 0.07 ± 0.01 * |
| 32 | 1413 | 1417 | β-Caryophyllene | 3.82 | 1.03 ± 0.22 | 4.86 | 2.04 ± 1.12 | 4.28 | 0.84 ± 0.08 | 4.33 | 0.77 ± 0.24 |
| 33 | 1423 | 1430 | β-Copaene | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.39 | 0.08 ± 0.02 |
| 34 | 1444 | 1451 | trans-Muurola,3,5-diene | n.d. | n.d. | 0.25 | 0.15 ± 0.01 * | n.d. | n.d. | n.d. | n.d. |
| 35 | 1446 | 1452 | α-Humulene | n.d. | n.d. | 0.28 | 0.17 ± 0.02 * | n.d. | n.d. | n.d. | n.d. |
| 36 | 1456 | 1456 | β-Farnesene | n.d. | n.d. | 0.92 | 0.43 ± 0.03 §, * | n.d. | n.d. | 1.10 | 0.21 ± 0.15 |
| 37 | 1457 | 1464 | α-Acoradiene | 0.54 | 0.27 ± 0.04 | n.d. | §, * | n.d. | n.d. | n.d. | n.d. |
| 38 | 1461 # | 1464 | 9-epi-(E)-β-Caryophyllene | n.d. | n.d. | 3.12 | 1.33 ± 0.57 | n.d. | n.d. | n.d. | n.d. |
| 39 | 1474 | 1478 | γ-Muurolene | n.d. | n.d. | 1.89 | 0.83 ± 0.97 | n.d. | n.d. | n.d. | n.d. |
| 40 | 1474 | 1480 | Germacrene D | 5.28 | 1.37 ± 0.28 | n.d. | n.d. | 1.35 | 0.30 ± 0.06 | 2.60 | 0.47 ± 0.15 |
| 41 | 1477 | 1481 | γ-Curcumene | 0.71 | 0.30 ± 0.02 | 0.38 | 0.21 ± 0.01 * | n.d. | n.d. | n.d. | n.d. |
| 42 | 1480 | 1480 | a-Curcumene | 0.87 | 0.35 ± 0.06 | 0.44 | 0.23 ± 0.03 * | n.d. | n.d. | 0.64 | 0.12 ± 0.07 |
| 43 | 1490 | 1493 | epi-Cubebol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.90 | 0.17 ± 0.01 |
| 44 | 1490 | 1500 | Bicyclogermacrene | 6.01 | 1.54 ± 0.54 | 1.35 | 0.61 ± 0.33 | n.d. | n.d. | n.q. | n.q. |
| 45 | 1493 | 1493 | α-Zingiberene | 1.03 | 0.38 ± 0.06 | 0.70 | 0.34 ± 0.02 * | n.d. | n.d. | n.d. | n.d. |
| 46 | 1506 | 1505 | β-Bisabolene | n.d. | n.d. | 1.55 | 0.68 ± 0.16 | 0.48 | 0.14 ± 0.01 * | n.d. | n.d. |
| 47 | 1509 | 1514 | β-Curcumene | n.d. | n.d. | 0.60 | 0.30 ± 0.03 * | n.d. | n.d. | n.d. | n.d. |
| 48 | 1510 | 1514 | Cubebol | n.d. | n.d. | 0.62 | 0.31 ± 0.04 * | n.d. | n.d. | 1.23 | 0.23 ± 0.03 |
| 49 | 1518 | 1528 | cis-Calamenene | n.d. | n.d. | 0.93 | 0.43 ± 0.03 * | n.d. | n.d. | 1.39 | 0.26 ± 0.10 |
| 50 | 1518 | 1522 | δ-Cadinene | n.d. | n.d. | 0.38 | 0.21 ± 0.01 * | n.d. | n.d. | 4.75 | 0.84 ± 0.08 |
| 51 | 1519 # | 1522 | Dihydroactinidiolide | n.d. | n.d. | n.d. | n.d. | 2.13 | 0.44 ± 0.03 | n.d. | n.d. |
| 52 | 1526 | 1534 | trans-Cadina-1(2)4-diene | n.d. | n.d. | 1.58 | 0.70 ± 0.01 * | n.d. | n.d. | 0.34 | 0.08 ± 0.01 * |
| 53 | 1543 | 1549 | Elemol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.95 | 0.18 ± 0.11 |
| 54 | 1568 | 1578 | Spathulenol | 2.06 | 0.62 ± 0.12 | 0.31 | 0.18 ± 0.08 | n.d. | n.d. | n.d. | n.d. |
| 55 | 1572 | 1583 | Caryophyllene oxide | n.q. | n.q. | 1.29 | 0.58 ± 0.07 | 4.17 | 0.82 ± 0.13 | 2.63 | 0.48 ± 0.12 |
| 56 | 1583 | 1590 | Globulol | n.d. | n.d. | 0.17 | 0.12 ± 0.02 * | n.d. | n.d. | 1.74 | 0.31 ± 0.10 |
| 57 | 1621 | 1628 | 1-epi-Cubenol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.72 | 0.14 ± 0.03 |
| 58 | 1621 | 1631 | Muurola-4,10(14)-dien-1β-οl | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.57 | 0.11 ± 0.02 |
| 59 | 1634 | 1642 | α-epi-Muurolol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.37 | 0.08 ± 0.01 * |
| 60 | 1654 | 1666/1668 | 14-Hydroxy-(Z)-caryophyllene/14-Hydroxy-9-epi-(E)-caryophyllene | n.d. | n.d. | 0.17 | 0.12 ± 0.01 * | n.d. | n.d. | n.d. | n.d. |
| 61 | 1660 | 1668 | trans-Calamenen-10-ol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.81 | 0.16 ± 0.04 |
| 62 | 1660 | 1675 | Valeranone | n.d. | n.d. | 0.78 | 0.36 ± 0.02 * | n.d. | n.d. | n.d. | n.d. |
| 63 | 1677 | 1683 | α-epi-Bisabolol | n.d. | n.d. | 3.42 | 1.45 ± 0.19 | n.d. | n.d. | n.d. | n.d. |
| 64 | 1677 | 1685 | Germacra-4(15),5,10(14)-trien-1-a-ol | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.67 | 0.13 ± 0.04 |
| 65 | 1678 | 1685 | α-Bisabolol | 1.75 | 0.56 ± 0.19 | 5.42 | 2.26 ± 0.66 | 3.75 | 0.74 ± 0.02 | n.d. | n.d. |
| 66 | 1754 | 1759 | Benzyl benzoate | n.d. | n.d. | n.d. | n.d. | 1.56 | 0.34 ± 0.01 | n.d. | n.d. |
| 67 | 1975 | 1997 | Kaur-15-ene | 1.26 | 0.42 ± 0.08 | 0.74 | 0.35 ± 0.01 | n.d. | n.d. | n.d. | n.d. |
| 68 | 1979 | 1960 | Hexadecanoic acid | n.d. | n.d. | n.d. | n.d. | 11.09 | 2.09 ± 0.58 | n.d. | n.d. |
| 69 | 1995 | 1987/2009 | Manool oxide/ 13-epi-Manool oxide | 1.10 | 0.40 ± 0.07 | n.d. | n.d. | n.d. | n.d. | 0.86 | 0.16 ± 0.01 |
| 70 | 2066 # | 2060 | Oleyl alcohol | n.d. | n.d. | 1.10 | 0.51 ± 0.40 | n.d. | n.d. | n.d. | n.d. |
| Total | 82.88 | 23.05 ± 2.78 | 78.75 | 41.31 ± 0.79 | 79.97 | 15.64 ± 0.41 | 89.20 | 16.01 ± 0.67 | |||
| Number of compounds | 31 | 50 | 24 | 38 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimaki, V.D.; Zeliou, K.; Nakka, F.; Stavreli, M.; Bakratsas, I.; Papaioannou, L.; Iatrou, G.; Lamari, F.N. Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations. Molecules 2022, 27, 7613. https://doi.org/10.3390/molecules27217613
Dimaki VD, Zeliou K, Nakka F, Stavreli M, Bakratsas I, Papaioannou L, Iatrou G, Lamari FN. Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations. Molecules. 2022; 27(21):7613. https://doi.org/10.3390/molecules27217613
Chicago/Turabian StyleDimaki, Virginia D., Konstantina Zeliou, Fotini Nakka, Michaela Stavreli, Ioannis Bakratsas, Ligeri Papaioannou, Gregoris Iatrou, and Fotini N. Lamari. 2022. "Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations" Molecules 27, no. 21: 7613. https://doi.org/10.3390/molecules27217613
APA StyleDimaki, V. D., Zeliou, K., Nakka, F., Stavreli, M., Bakratsas, I., Papaioannou, L., Iatrou, G., & Lamari, F. N. (2022). Characterization of Sideritis clandestina subsp. peloponnesiaca Polar Glycosides and Phytochemical Comparison to Other Mountain Tea Populations. Molecules, 27(21), 7613. https://doi.org/10.3390/molecules27217613







