Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass
Abstract
1. Introduction
2. Methodology
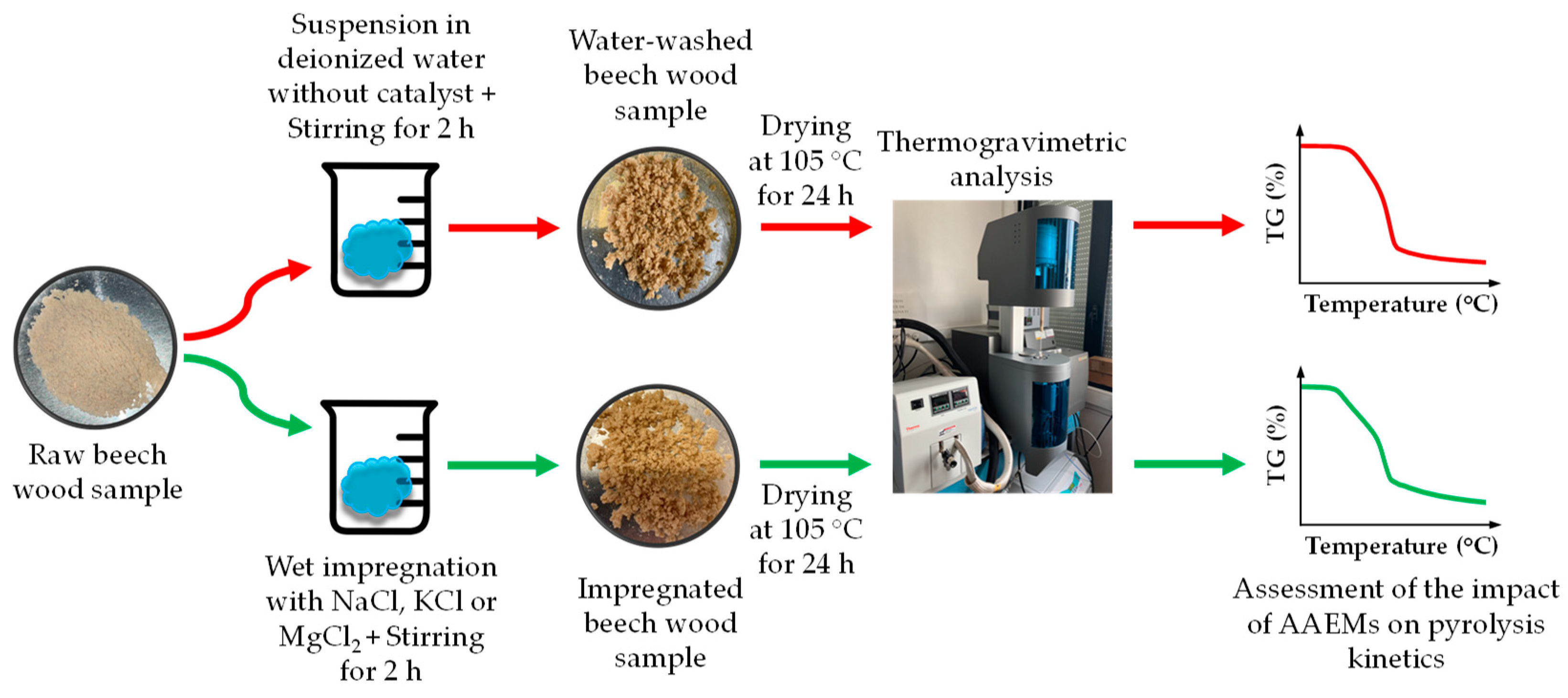
2.1. Feedstocks and Sample Preparation
2.2. Experimental Apparatus and Procedure
2.3. Kinetic Modeling
2.3.1. Isoconversional Models
2.3.2. Methodology Allowing to Identify a Suitable Reaction Model
Model Fitting Approach
Master Plot
3. Results and Discussion
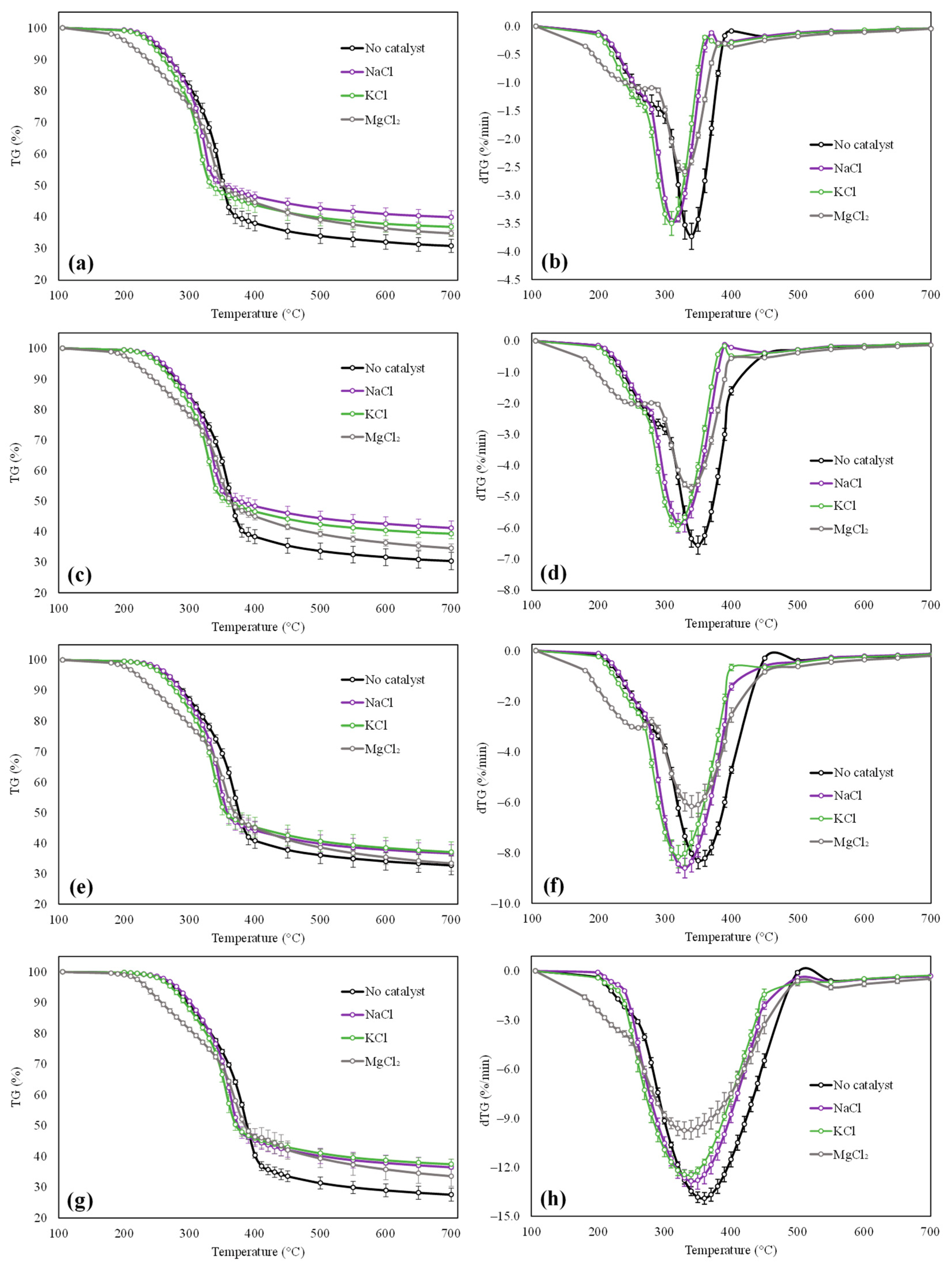
3.1. Experimental Characterization of the Pyrolysis Behavior of Raw and Impregnated Beech Wood Samples
3.2. Kinetic Analysis
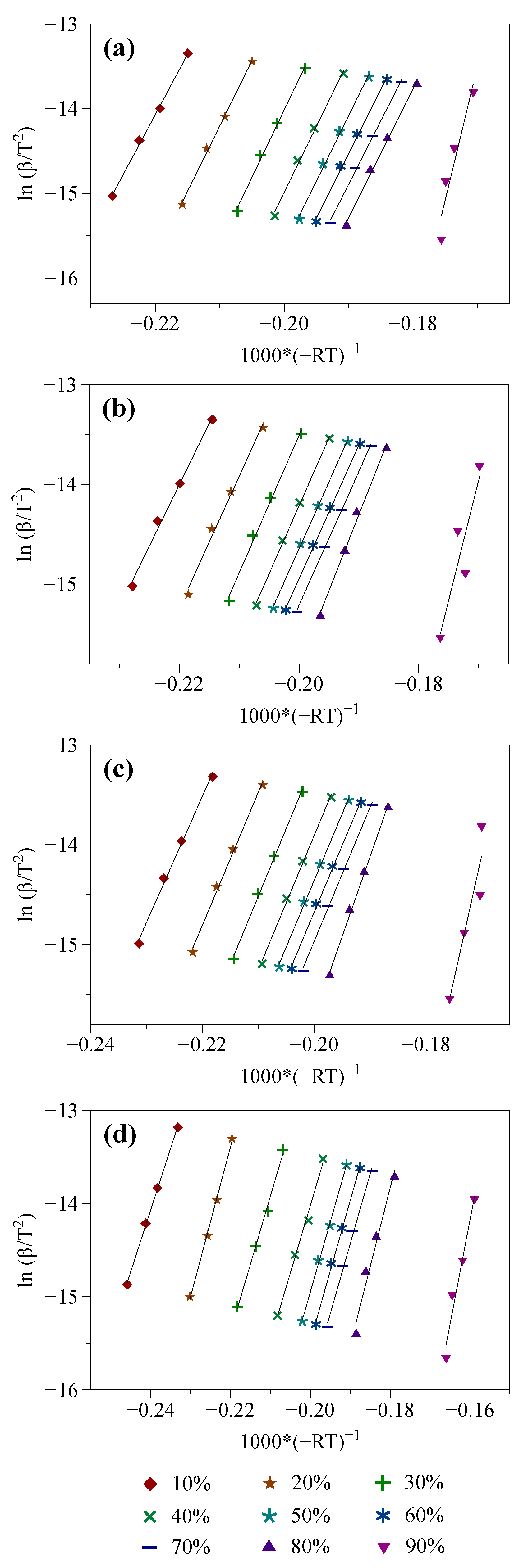
3.2.1. Isoconversional Modeling of TGA Results
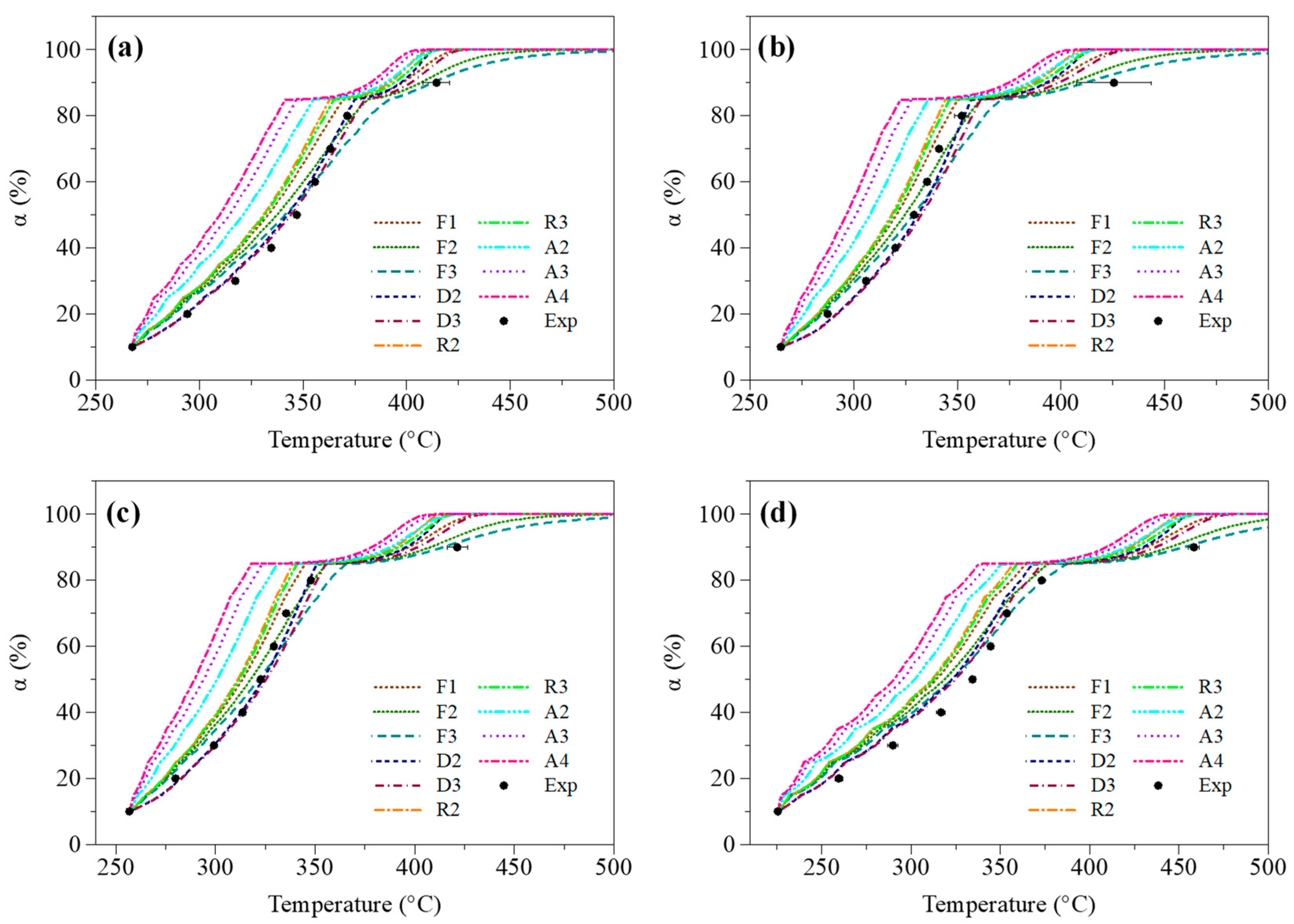
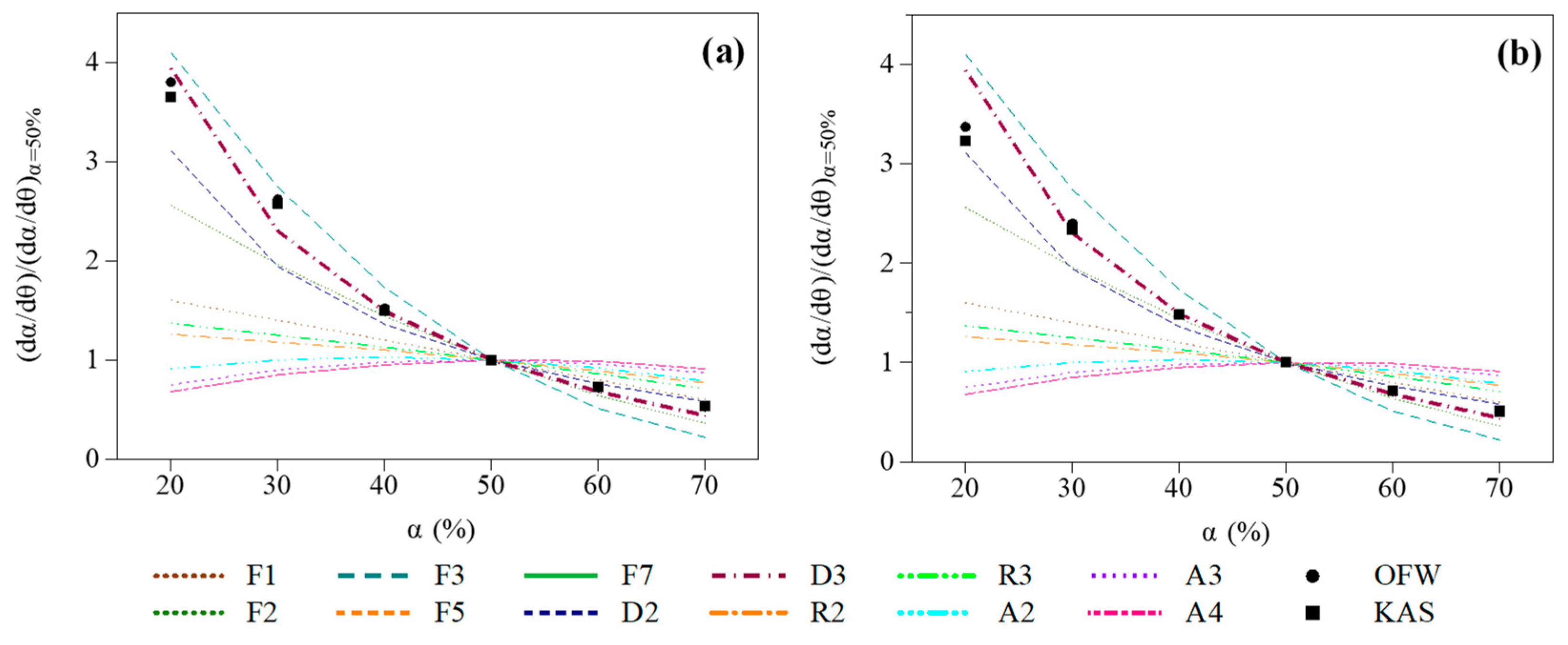
3.2.2. Identification of a Proper Reaction Model
Selection of a Proper Formulation through Model-Fitting Calculations
Validation of the Selected Formulation through Direct Calculations
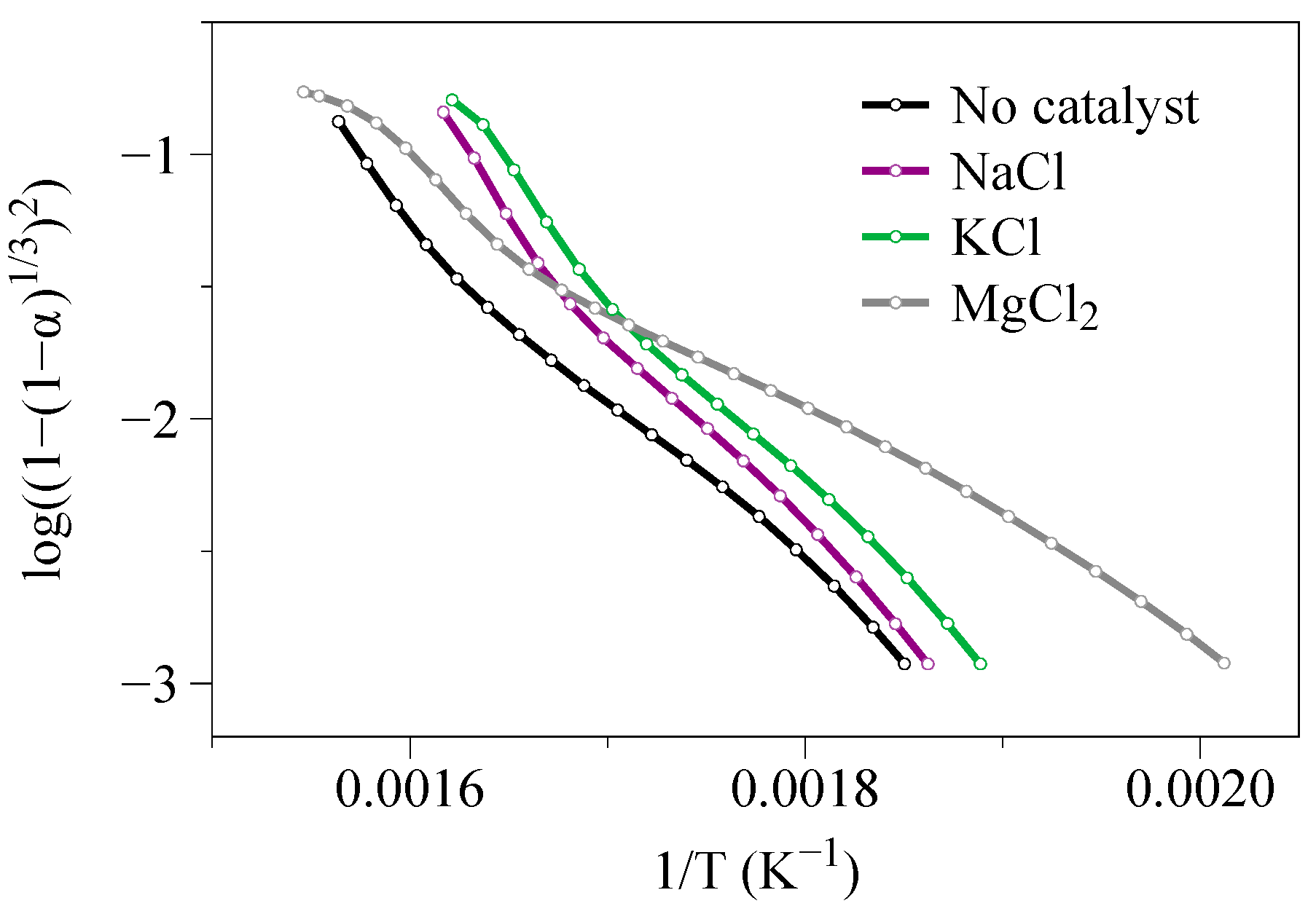
Validation of the Selected Formulation through Master Plot Calculations
3.2.3. Simulation of Conversion Degree Profiles
4. Summary of the Mechanisms Underlying the Catalytic Impact of AAEMs on Biomass Pyrolysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Broumand, M.; Albert-Green, S.; Yun, S.; Hong, Z.; Thomsson, M.J. Spray combustion of fast pyrolysis bio-oils: Applications, challenges, and potential solutions. Prog. Energy Combust. Sci. 2020, 79, 100834. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research progress in the preparation of high-quality liquid fuels and chemicals by catalytic pyrolysis of biomass: A review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Wang, Y.; Li, X.; Li, T.; Guo, C. Pyrolysis kinetics and behavior of potassium-impregnated pine wood in TGA and a fixed-bed reactor. Energy Convers. Manag. 2016, 130, 184–191. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Zhang, S. Catalytic pyrolysis of biomass with potassium compounds for co-production of high-quality biofuels and porous carbons. Energy 2020, 190, 116431. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, S.; Cho, T.-S.; Choi, I.-G.; Choi, J.W. Fast pyrolysis of potassium impregnated poplar wood and characterization of its influence on the formation as well as properties of pyrolytic products. Bioresour. Technol. 2013, 150, 359–366. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Shao, S.; Xiao, R. The effects of potassium on distributions of bio-oils obtained from fast pyrolysis of agricultural and forest biomass in a fluidized bed. Appl. Energy 2017, 208, 867–877. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of lignin for phenols with alkaline additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Martínez, I.; Martínez, J.D.; López, J.M.; Navarro, M.V.; Callén, M.S.; Murillo, R.; García, T. Catalytic pyrolysis of wood biomass in an auger reactor using calcium-based catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef]
- Santana, J.A.; Sousa, N.G.; Cardoso, C.R.; Carvalho, W.S.; Ataíde, C.H. Sodium, zinc and magnesium chlorides as additives for soybean hulls pyrolysis: Influence on the temperature range of reactions and product selectivity. J. Therm. Anal. Calorim. 2016, 125, 471–481. [Google Scholar] [CrossRef]
- Hwang, H.; Oh, S.; Choi, I.-G.; Choi, J.W. Catalytic effects of magnesium on the characteristics of fast pyrolysis products—Bio-oil, bio-char, and non-condensed pyrolytic gas fractions. J. Anal. Appl. Pyrolysis 2015, 113, 27–34. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim. Acta 1999, 340–341, 53–68. [Google Scholar] [CrossRef]
- Lemaire, R.; Menage, D.; Seers, P. Study of the high heating rate devolatilization of bituminous and subbituminous coals-Comparison of experimentally monitored devolatilization profiles with predictions issued from single rate, two-competing rate, distributed activation energy and chemical percolation devolatilization models. J. Anal. Appl. Pyrolysis 2017, 123, 255–268. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Liu, S.; Zhu, D.; Huang, F.; Yang, H.; Guan, H.; Song, A.; Xu, D.; Sun, L.; et al. Catalytic pyrolysis of biomass with Ni/Fe-CaO-based catalysts for hydrogen-rich gas: DFT and experimental study. Energy Convers. Manag. 2022, 254, 115246. [Google Scholar] [CrossRef]
- Sharifzadeh, M.; Sadeqzadeh, M.; Guo, M.; Borhani, T.N.; Murthy Konda, N.V.S.N.; Garcia, M.C.; Wang, L.; Hallett, J.; Shah, N. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: Review of the state of art and future research directions. Prog. Energy Combust. Sci. 2019, 71, 1–80. [Google Scholar] [CrossRef]
- Zhou, L.; Jia, Y.; Nguyen, T.-H.; Adesina, A.A.; Liu, Z. Hydropyrolysis characteristics and kinetics of potassium-impregnated pine wood. Fuel Process. Technol. 2013, 116, 149–157. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Zhang, M.; Chen, M.; Zhu, X.; Min, F.; Tan, Z. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Wang, W.-L.; Ren, X.-Y.; Li, L.-F.; Chang, J.-M.; Cai, L.-P.; Geng, J. Catalytic effect of metal chlorides on analytical pyrolysis of alkali lignin. Fuel Process. Technol. 2015, 134, 345–351. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.-J.; Chen, W.-H.; Langauer, D.; Chang, J.-S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Balasundram, V.; Ibrahim, N.; Md Kasmani, R.; Hamid, M.K.A.; Isha, R.; Hasbullah, H.; Ali, R.R. Thermogravimetric catalytic pyrolysis and kinetic studies of coconut copra and rice husk for possible maximum production of pyrolysis oil. J. Clean. Prod. 2017, 167, 218–228. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Wang, Y.; Yang, H.; Jin, L.; Hu, H. Insight into co-pyrolysis interactions of Pingshuo coal and high-density polyethylene via in-situ Py-TOF-MS and EPR. Fuel 2021, 303, 121199. [Google Scholar] [CrossRef]
- Senum, G.I.; Yang, R.T. Rational approximations of the integral of the Arrhenius function. J. Therm. Anal. 1977, 11, 445–447. [Google Scholar] [CrossRef]
- Flynn, J.H. The isoconversional method for determination of energy of activation at constant heating rates: Corrections for the Doyle approximation. J. Therm. Anal. 1983, 27, 95–102. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Khawam, A.; Flanagan, D.R. Complementary use of model-free and modelistic methods in the analysis of solid-state kinetics. J. Phys. Chem. B 2005, 109, 10073–10080. [Google Scholar] [CrossRef]
- Moriana, R.; Zhang, Y.; Mischnick, P.; Li, J.; Ek, M. Thermal degradation behavior and kinetic analysis of spruce glucomannan and its methylated derivatives. Carbohydr. Polym. 2014, 106, 60–70. [Google Scholar] [CrossRef]
- Wang, S.; Lin, H.; Ru, B.; Dai, G.; Wang, X.; Xiao, G.; Luo, Z. Kinetic modeling of biomass components pyrolysis using a sequential and coupling method. Fuel 2016, 185, 763–771. [Google Scholar] [CrossRef]
- Prasad Nath, H.; Kumar Dutta, B.; Kalita, D.; Saikia, B.K.; Saikia, N. Evaluation of the effect of high sulfur subbituminous coal on the devolatilization of biomass residue by using model free, model fitting and combined kinetic methods. Fuel 2022, 310, 122235. [Google Scholar] [CrossRef]
- Chen, C.; Ma, X.; He, Y. Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresour. Technol. 2012, 117, 264–273. [Google Scholar] [CrossRef]
- He, Y.; Chang, C.; Li, P.; Han, X.; Li, H.; Fang, S.; Chen, J.; Ma, X. Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis. Bioresour. Technol. 2018, 259, 294–303. [Google Scholar] [CrossRef]
- Florentino-Madiedo, L.; Vega, M.F.; Diaz-Faes, E.; Barriocanal, C. Evaluation of synergy during co-pyrolysis of torrefied sawdust, coal and paraffin. A kinetic and thermodynamic dataset. Data Brief 2021, 37, 107170. [Google Scholar] [CrossRef]
- Škvára, F.; Šesták, J. Computer calculation of the mechanism and associated kinetic data using a non-isothermal integral method. J. Therm. Anal. 1975, 8, 477–489. [Google Scholar] [CrossRef]
- Chen, F.; Fu, L.; Feng, L.; Liu, C.; Ren, B. Non-isothermal decomposition kinetics of diosgenin. Russ. J. Phys. Chem. 2013, 87, 1611–1614. [Google Scholar] [CrossRef]
- Zhao, N.; Li, J.; Gong, H.; An, T.; Zhao, F.; Yang, A.; Hu, R.; Ma, H. Effects of α-Fe2O3 nanoparticles on the thermal behavior and non-isothermal decomposition kinetics of nitrocellulose. J. Anal. Appl. Pyrolysis 2016, 120, 165–173. [Google Scholar] [CrossRef]
- Zhao, B.; Xu, X.; Li, H.; Chen, X.; Zeng, F. Kinetics evaluation and thermal decomposition characteristics of co-pyrolysis of municipal sewage sludge and hazelnut shell. Bioresour. Technol. 2018, 247, 21–29. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J. Anal. Appl. Pyrolysis 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Lv, X.; Huang, R.; Wu, Q.; Xu, B.; Zhang, J. Non-isothermal reduction kinetics during vacuum carbothermal reduction of ilmenite concentrate. Vacuum 2019, 160, 139–145. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Zhang, Y. Thermal behavior and kinetic decomposition of sweet potato starch by non-isothermal procedures. Arch. Thermodyn. 2019, 40, 67–82. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Chrissafis, K.; Di Lorenzo, M.L.; Koga, N.; Pijolat, M.; Roduit, B.; Sbirrazzuoli, N.; Sunol, J.J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochim. Acta 2014, 590, 1–23. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Criado, J.M. Generalized master plots as a straightforward approach for determining the kinetic model: The case of cellulose pyrolysis. Thermochim. Acta 2013, 552, 54–59. [Google Scholar] [CrossRef]
- Helsen, L.; Van den Bulck, E. Kinetics of the low-temperature pyrolysis of chromated copper arsenate-treated wood. J. Anal. Appl. Pyrolysis 2000, 53, 51–79. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Chen, M.; Min, F.; Zhang, S.; Ren, Z.; Yan, Y. Catalytic effects of six inorganic compounds on pyrolysis of three kinds of biomass. Thermochim. Acta 2006, 444, 110–114. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Jones, J.M.; Brydson, R.M.D.; Ross, A.B. Potassium catalysis in the pyrolysis behaviour of short rotation willow coppice. Fuel 2007, 86, 2389–2402. [Google Scholar] [CrossRef]
- Shimada, N.; Kawamoto, H.; Saka, S. Different action of alkali/alkaline earth metal chlorides on cellulose pyrolysis. J. Anal. Appl. Pyrolysis 2008, 81, 80–87. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, D.; Wu, H. Formation and characteristics of reaction intermediates from the fast pyrolysis of NaCl- and MgCl2- loaded celluloses. Energy Fuels 2014, 28, 245–253. [Google Scholar] [CrossRef]
- Gao, P.; Xue, L.; Lu, Q.; Dong, C. Effects of alkali and alkaline earth metals on N-containing species release during rice straw pyrolysis. Energies 2015, 8, 13021–13032. [Google Scholar] [CrossRef]
- Leng, E.; Wang, Y.; Gong, X.; Zhang, B.; Zhang, Y.; Xu, M. Effect of KCl and CaCl2 loading on the formation of reaction intermediates during cellulose fast pyrolysis. Proc. Combust. Inst. 2017, 36, 2263–2270. [Google Scholar] [CrossRef]
- Jensen, A.; Dam-Johansen, K.; Wójtowicz, M.A.; Serio, M.A. TG-FTIR study of the influence of potassium chloride on wheat straw pyrolysis. Energy Fuels 1998, 12, 929–938. [Google Scholar] [CrossRef]
- Shah, M.H.; Deng, L.; Bennadji, H.; Fisher, E.M. Pyrolysis of potassium-doped wood at the centimeter and submillimeter scales. Energy Fuels 2015, 29, 7350–7357. [Google Scholar] [CrossRef]
- Zhu, C.; Maduskar, S.; Paulsen, A.D.; Dauenhauer, P.J. Alkaline-earth-metal-catalyzed thin-film pyrolysis of cellulose. ChemCatChem 2016, 8, 818–829. [Google Scholar] [CrossRef]
- Dalluge, D.L.; Kim, K.H.; Brown, R.C. The influence of alkali and alkaline earth metals on char and volatile aromatics from fast pyrolysis of lignin. J. Anal. Appl. Pyrolysis 2017, 127, 385–393. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Tang, W.; Chen, D.; Sheng, Y. Study on non-isothermal decomposition kinetics of ephedrini hydrochloridum. Wuhan Univ. J. Nat. Sci. 2003, 8, 443–446. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Huang, M.-X.; Zhou, C.-R.; Han, X.-W. Investigation of thermal decomposition kinetics of taurine. J. Therm. Anal. Calorim. 2013, 113, 589–593. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Berger, A.; Zobel, N. How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel 2014, 123, 230–240. [Google Scholar] [CrossRef]
- Mishra, G.; Bhaskar, T. Non isothermal model free kinetics for pyrolysis of rice straw. Bioresour. Technol. 2014, 169, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Chen, Z.; Wang, S.; Guo, D.; Ma, C.; Zhou, Y.; Chen, J.; Laghari, M.; Fazal, S.; Xiao, B.; et al. Thermogravimetric kinetics of lignocellulosic biomass slow pyrolysis using distributed activation energy model, Fraser–Suzuki deconvolution, and iso-conversional method. Energy Convers. Manag. 2016, 118, 1–11. [Google Scholar] [CrossRef]
- Rony, A.H.; Kong, L.; Lu, W.; Dejam, M.; Adidharma, H.; Gasem, K.A.M.; Zheng, Y.; Norton, U.; Fan, M. Kinetics, thermodynamics, and physical characterization of corn stover (Zea mays) for solar biomass pyrolysis potential analysis. Bioresour. Technol. 2019, 284, 466–473. [Google Scholar] [CrossRef]
- Masawat, N.; Atong, D.; Sricharoenchaikul, V. Thermo-kinetics and product analysis of the catalytic pyrolysis of Pongamia residual cake. J. Environ. Manag. 2019, 242, 238–245. [Google Scholar] [CrossRef]
- Hu, L.; Wei, X.-Y.; Guo, X.-H.; Lv, H.-P.; Wang, G.-H. Investigation on the kinetic behavior, thermodynamic and volatile products analysis of chili straw waste pyrolysis. J. Environ. Chem. Eng. 2021, 9, 105859. [Google Scholar] [CrossRef]
- Masnadi, M.S.; Habibi, R.; Kopyscinski, J.; Hill, J.M.; Bi, X.; Lim, C.J.; Ellis, N.; Grace, J.R. Fuel characterization and co-pyrolysis kinetics of biomass and fossil fuels. Fuel 2014, 117, 1204–1214. [Google Scholar] [CrossRef]
- Ding, Y.; Ezekoye, O.A.; Lu, S.; Wang, C.; Zhou, R. Comparative pyrolysis behaviors and reaction mechanisms of hardwood and softwood. Energy Convers. Manag. 2017, 132, 102–109. [Google Scholar] [CrossRef]
- Zhao, N.; Li, B.-X. The effect of sodium chloride on the pyrolysis of rice husk. Appl. Energy 2016, 178, 346–352. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, X.; Yu, Z.; Cai, Z. A kinetic study on the effects of alkaline earth and alkali metal compounds for catalytic pyrolysis of microalgae using thermogravimetry. Appl. Therm. Eng. 2014, 73, 357–361. [Google Scholar] [CrossRef]



| Sample | Proximate Analysis | Ultimate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Carbon (wt%, db †) | Volatiles (wt%, db) | Ash (wt%, db) | C (wt%, daf ‡) | H (wt%, daf) | O * (wt%, daf) | N (wt%, daf) | S (wt%, daf) | |
| Beech wood | 14.36 | 84.48 | 1.16 | 51.6 | 5.4 | 43.0 | - | - |
| 5 °C/min | 10 °C/min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Ti (°C) | Tf (°C) | Tp (°C) | dTGmax (%/min) | TG700°C (wt%) | Ti (°C) | Tf (°C) | Tp (°C) | dTGmax (%/min) | TG700°C (wt%) |
| Wood | 257.5 (±0.9) | 411.9 (±37.1) | 340 (±2) | −3.74 (±0.23) | 30.9 (±2.1) | 267.5 (±0.2) | 414.3 (±6.5) | 350 (±1) | −6.55 (±0.29) | 30.4 (±2.8) |
| Wood + NaCl | 254.7 (±0.6) | 408.8 (±11.0) | 315 (±1) | −3.49 (±0.04) | 40.0 (±2.0) | 264.7 (±1.1) | 425.5 (±18.0) | 325 (±2) | −5.88 (±0.31) | 41.2 (±2.3) |
| Wood + KCl | 246.7 (±0.8) | 411.1 (±5.0) | 309 (±1) | −3.51 (±0.21) | 36.9 (±2.8) | 256.8 (±0.5) | 421.4 (±5.1) | 320 (±3) | −5.92 (±0.19) | 39.3 (±1.7) |
| Wood + MgCl2 | 216.0 (±5.1) | 451.6 (±3.4) | 329(±0) | −2.59 (±0.08) | 34.9 (±1.0) | 225.4 (±1.6) | 458.2 (±3.1) | 340 (±1) | −4.72 (±0.11) | 34.5 (±1.4) |
| 15 °C/min | 30 °C/min | |||||||||
| Sample | Ti(°C) | Tf(°C) | Tp(°C) | dTGmax (%/min) | TG700°C (wt%) | Ti(°C) | Tf(°C) | Tp(°C) | dTGmax (%/min) | TG700°C (wt%) |
| Wood | 275.5 (±2.4) | 419.7 (±10.3) | 353 (±1) | −8.32 (±0.32) | 32.7 (±3.1) | 286.4 (±2.6) | 431.6 (±3.3) | 361 (±3) | −13.9 (±0.4) | 27.6 (±2.1) |
| Wood + NaCl | 273.7 (±1.4) | 420.1 (±5.8) | 329 (±2) | −8.61 (±0.39) | 36.6 (±2.9) | 287.6 (±1.5) | 435.0 (±5.1) | 344 (±1) | −12.9 (±0.5) | 36.5 (±2.7) |
| Wood + KCl | 264.4 (±1.0) | 433.1 (±11.6) | 323 (±1) | −8.15 (±0.45) | 37.1 (±3.3) | 278.0 (±3.2) | 434.2 (±4.9) | 337 (±3) | −12.5 (±0.3) | 37.5 (±1.6) |
| Wood + MgCl2 | 231.4 (±1.1) | 470.2 (±21.7) | 342 (±3) | −6.18 (±0.44) | 33.4 (±2.6) | 242.6 (±13.4) | 484.4 (±55.6) | 331 (±5) | −9.7 (±0.5) | 33.6 (±3.3) |
| Wood | Wood + NaCl | Wood + KCl | Wood + MgCl2 | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | |||||
| OFW | ||||||||
| 10% | 143.7 | 0.9978 | 125.4 | 0.9938 | 128.6 | 0.9961 | 134.4 | 0.9991 |
| 20% | 155.8 | 0.9982 | 134.0 | 0.9926 | 135.1 | 0.9967 | 161.0 | 0.9983 |
| 30% | 160.5 | 0.9963 | 140.0 | 0.9953 | 138.2 | 0.9974 | 147.6 | 0.9946 |
| 40% | 157.9 | 0.9954 | 139.0 | 0.9984 | 137.1 | 0.9980 | 146.9 | 0.9937 |
| 50% | 156.5 | 0.9966 | 137.3 | 0.9993 | 136.3 | 0.9983 | 152.3 | 0.9986 |
| 60% | 154.4 | 0.9971 | 136.8 | 0.9994 | 136.3 | 0.9983 | 153.2 | 0.9978 |
| 70% | 153.0 | 0.9971 | 137.2 | 0.9993 | 137.3 | 0.9982 | 152.7 | 0.9955 |
| 80% | 155.0 | 0.9967 | 155.0 | 0.9949 | 162.1 | 0.9972 | 171.2 | 0.9744 |
| 90% | 309.5 | 0.9096 | 239.0 | 0.8348 | 248.5 | 0.8853 | 224.7 | 0.9647 |
| KAS | ||||||||
| 10% | 142.1 | 0.9976 | 122.9 | 0.9927 | 126.3 | 0.9954 | 133.0 | 0.9990 |
| 20% | 154.4 | 0.9979 | 131.6 | 0.9914 | 132.8 | 0.9962 | 160.5 | 0.9981 |
| 30% | 159.0 | 0.9958 | 137.6 | 0.9945 | 135.8 | 0.9969 | 145.9 | 0.9939 |
| 40% | 156.0 | 0.9947 | 136.3 | 0.9981 | 134.3 | 0.9977 | 144.7 | 0.9929 |
| 50% | 154.3 | 0.9961 | 134.3 | 0.9991 | 133.4 | 0.9980 | 150.1 | 0.9984 |
| 60% | 151.8 | 0.9966 | 133.7 | 0.9993 | 133.3 | 0.9979 | 150.8 | 0.9974 |
| 70% | 150.3 | 0.9966 | 134.1 | 0.9992 | 134.3 | 0.9979 | 150.1 | 0.9947 |
| 80% | 152.2 | 0.9962 | 152.6 | 0.9941 | 160.1 | 0.9967 | 169.2 | 0.9709 |
| 90% | 314.2 | 0.9034 | 239.9 | 0.8211 | 249.9 | 0.8757 | 223.9 | 0.9607 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass. Molecules 2022, 27, 7662. https://doi.org/10.3390/molecules27227662
Wang W, Lemaire R, Bensakhria A, Luart D. Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass. Molecules. 2022; 27(22):7662. https://doi.org/10.3390/molecules27227662
Chicago/Turabian StyleWang, Wei, Romain Lemaire, Ammar Bensakhria, and Denis Luart. 2022. "Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass" Molecules 27, no. 22: 7662. https://doi.org/10.3390/molecules27227662
APA StyleWang, W., Lemaire, R., Bensakhria, A., & Luart, D. (2022). Thermogravimetric Analysis and Kinetic Modeling of the AAEM-Catalyzed Pyrolysis of Woody Biomass. Molecules, 27(22), 7662. https://doi.org/10.3390/molecules27227662








