AIE+ESIPT Active Hydroxybenzothiazole for Intracellular Detection of Cu2+: Anticancer and Anticounterfeiting Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Material and Methods
2.2. Characterization and Measurements
2.3. UV-Visible and Fluorescence Studies
2.4. Sample Preparation for Cu2+ Analysis in Real Samples
2.5. Anticancer Studies (MTT Assay)
2.6. The Cell Imaging
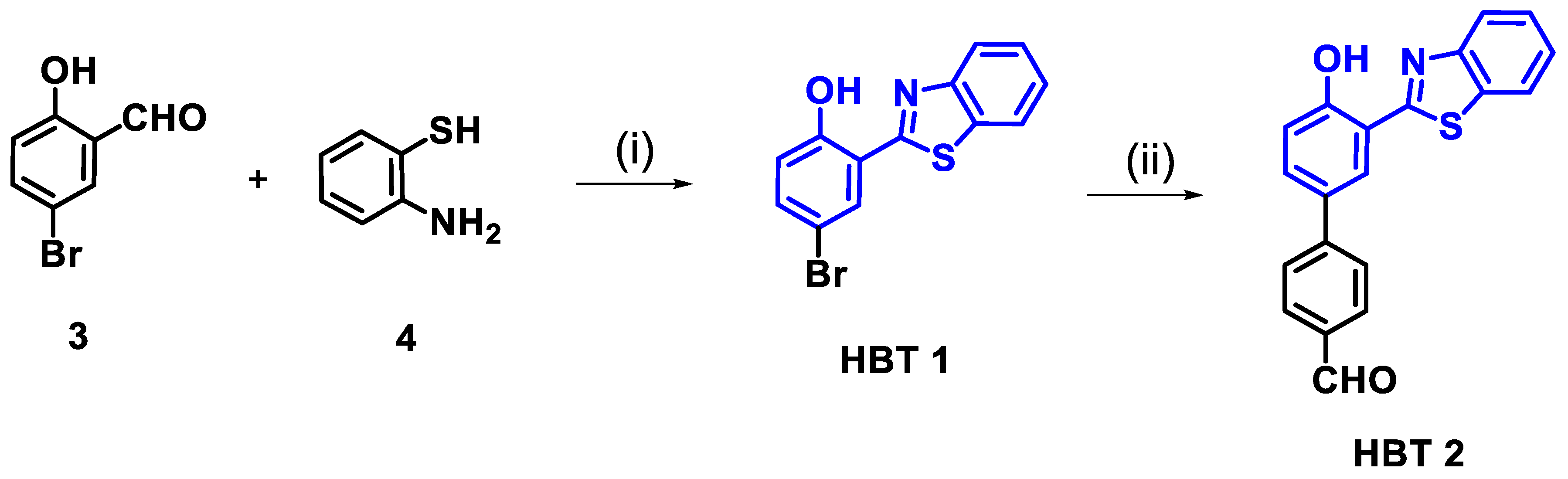
2.7. Synthesis of Derivative HBT 1
2.8. Synthesis of Derivative HBT 2
3. Results and Discussion
3.1. Synthetic Design
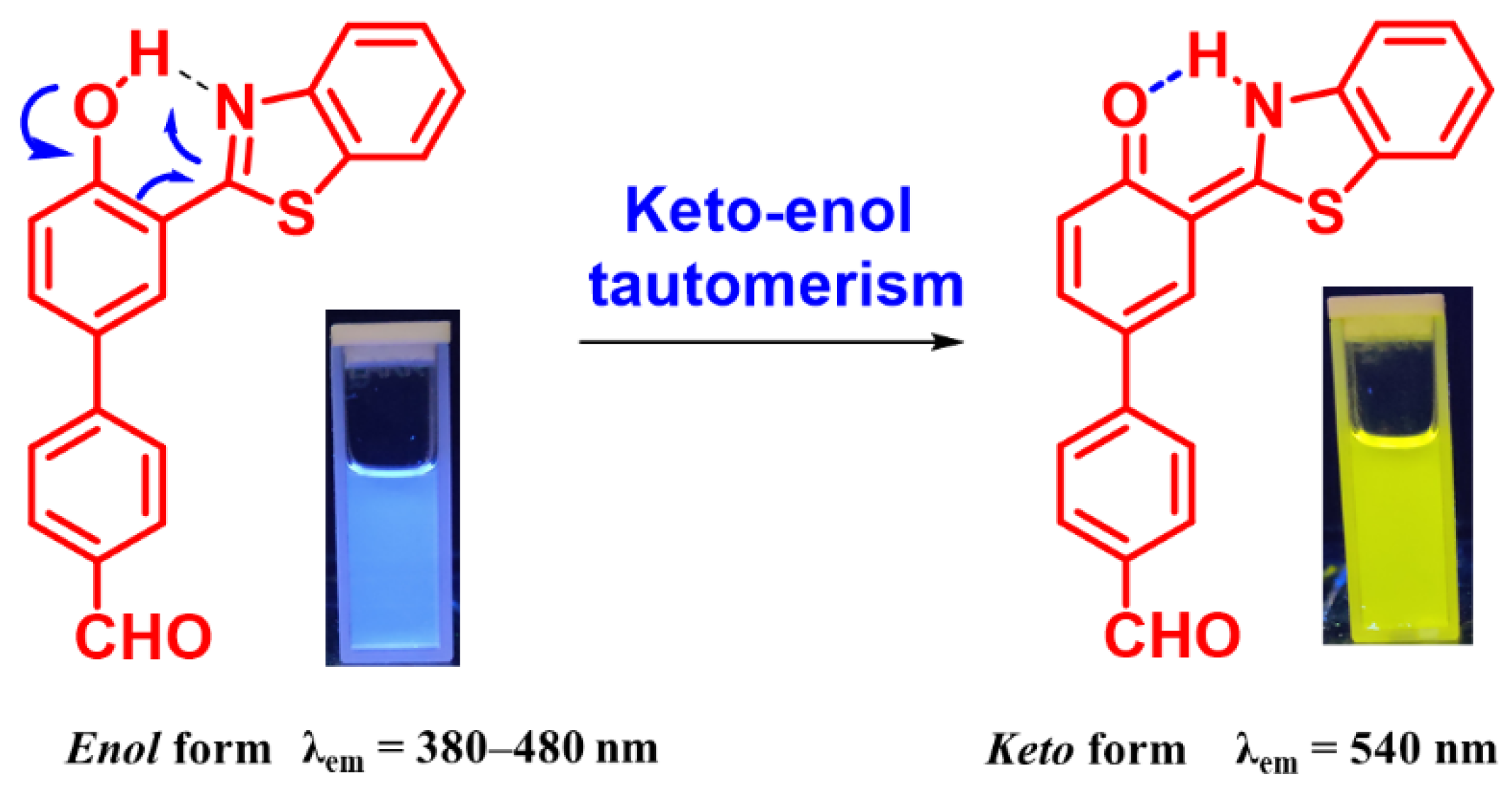
3.2. Photophysical Studies to Support AIE+ESIPT Features in HBT 2
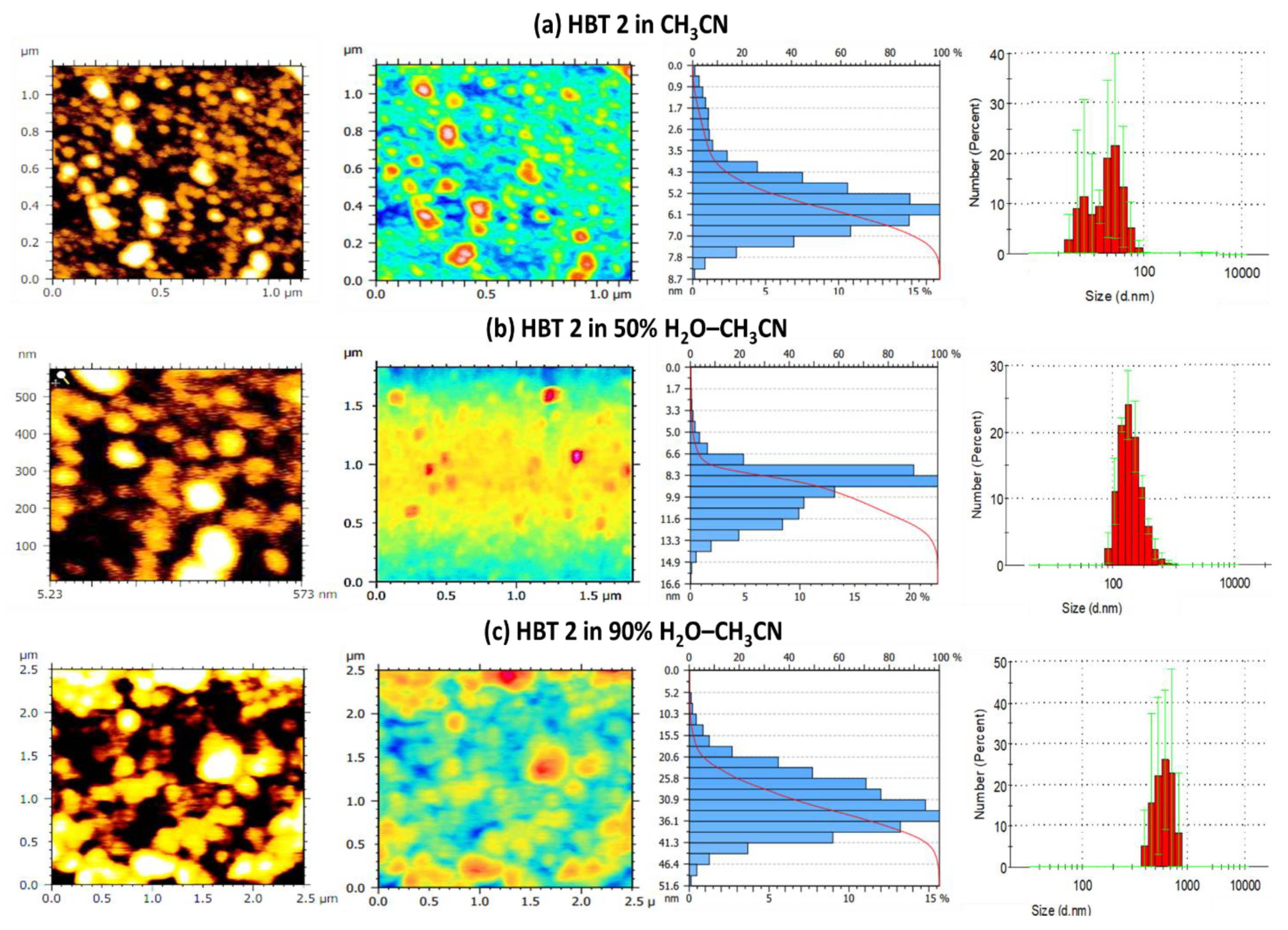
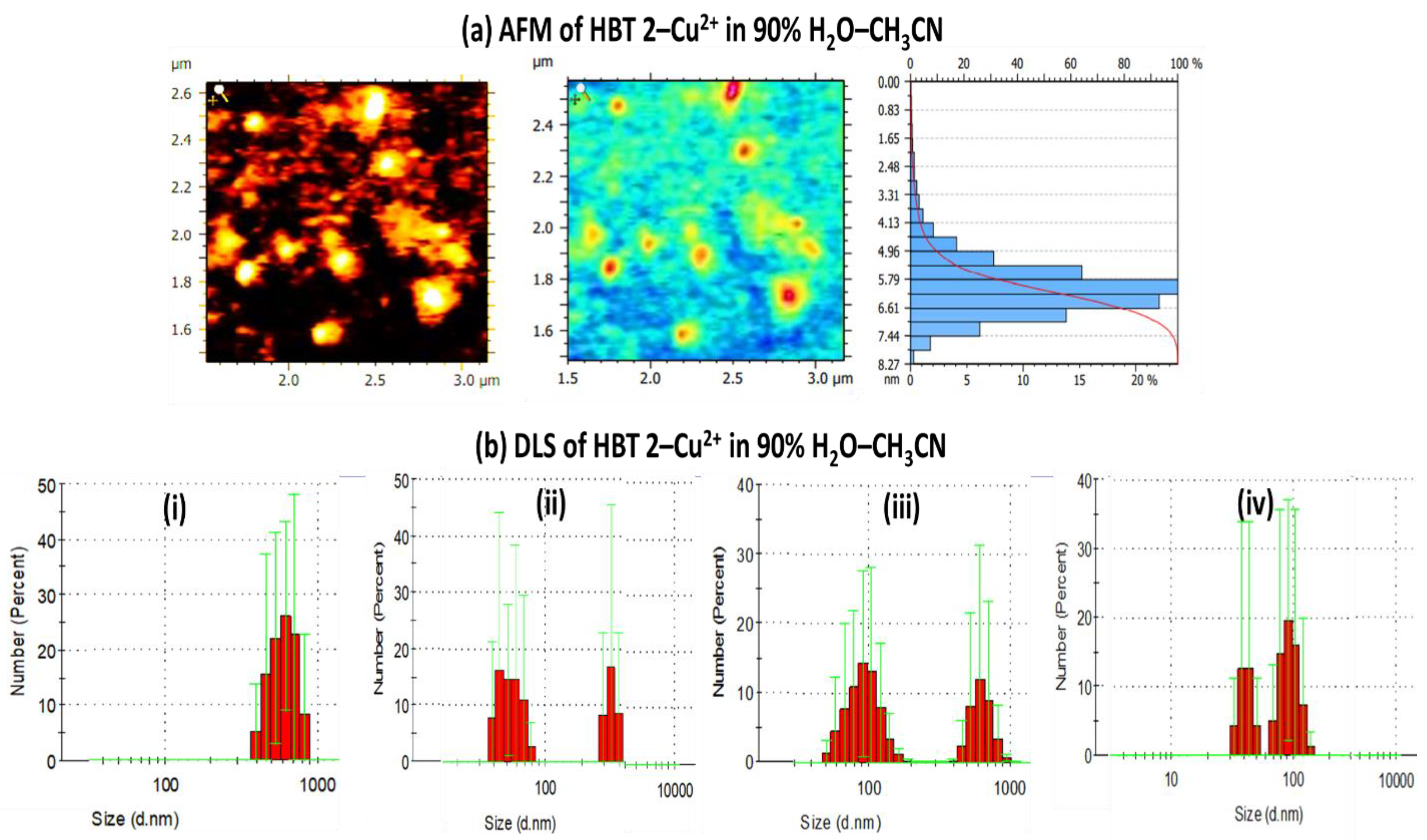
3.3. Validation of Aggregation in HBT 2 Using AFM and DLS Techniques
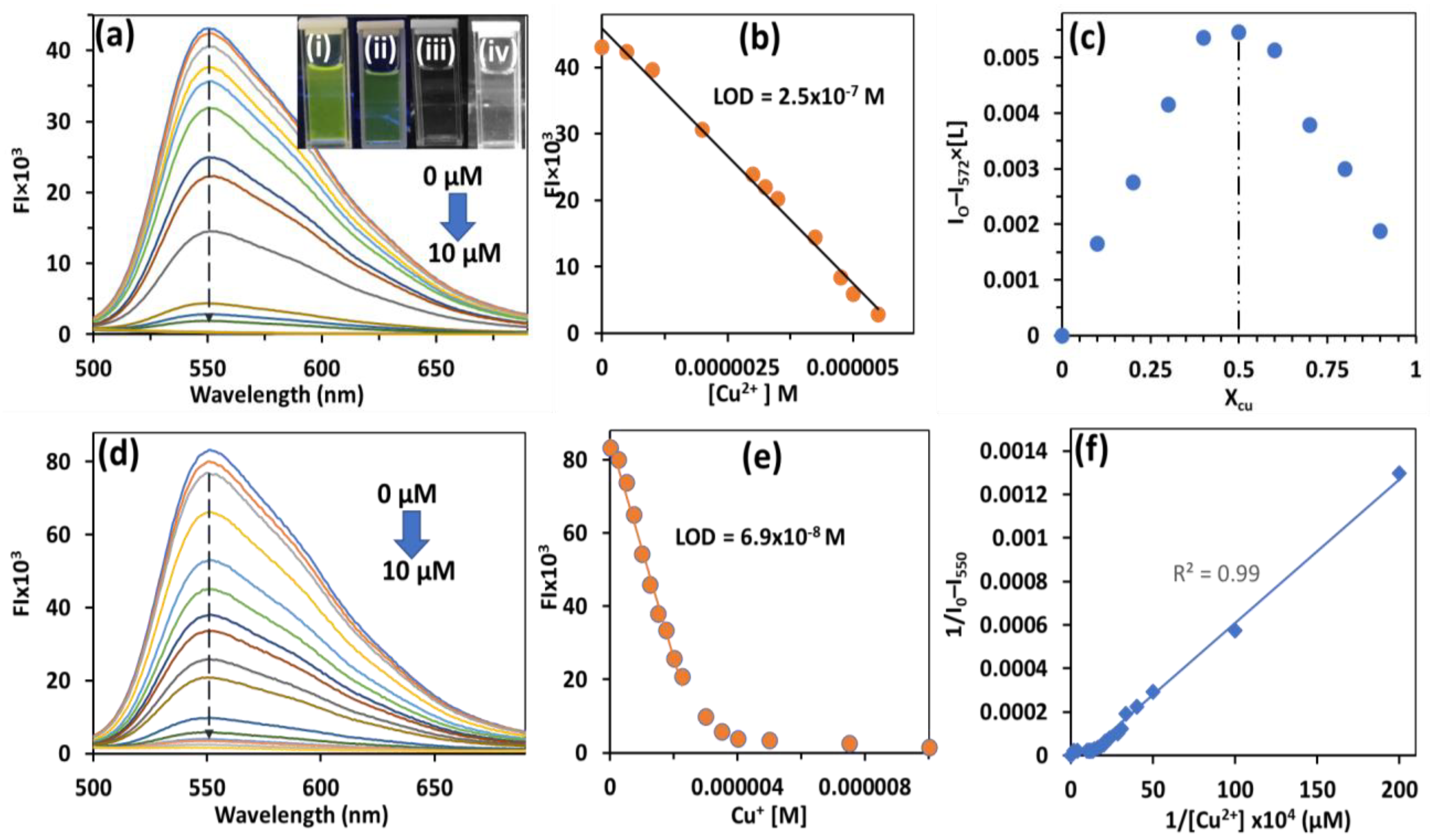
3.4. Application of HBT 2 for Detection of Cu2+/Cu+ Ions
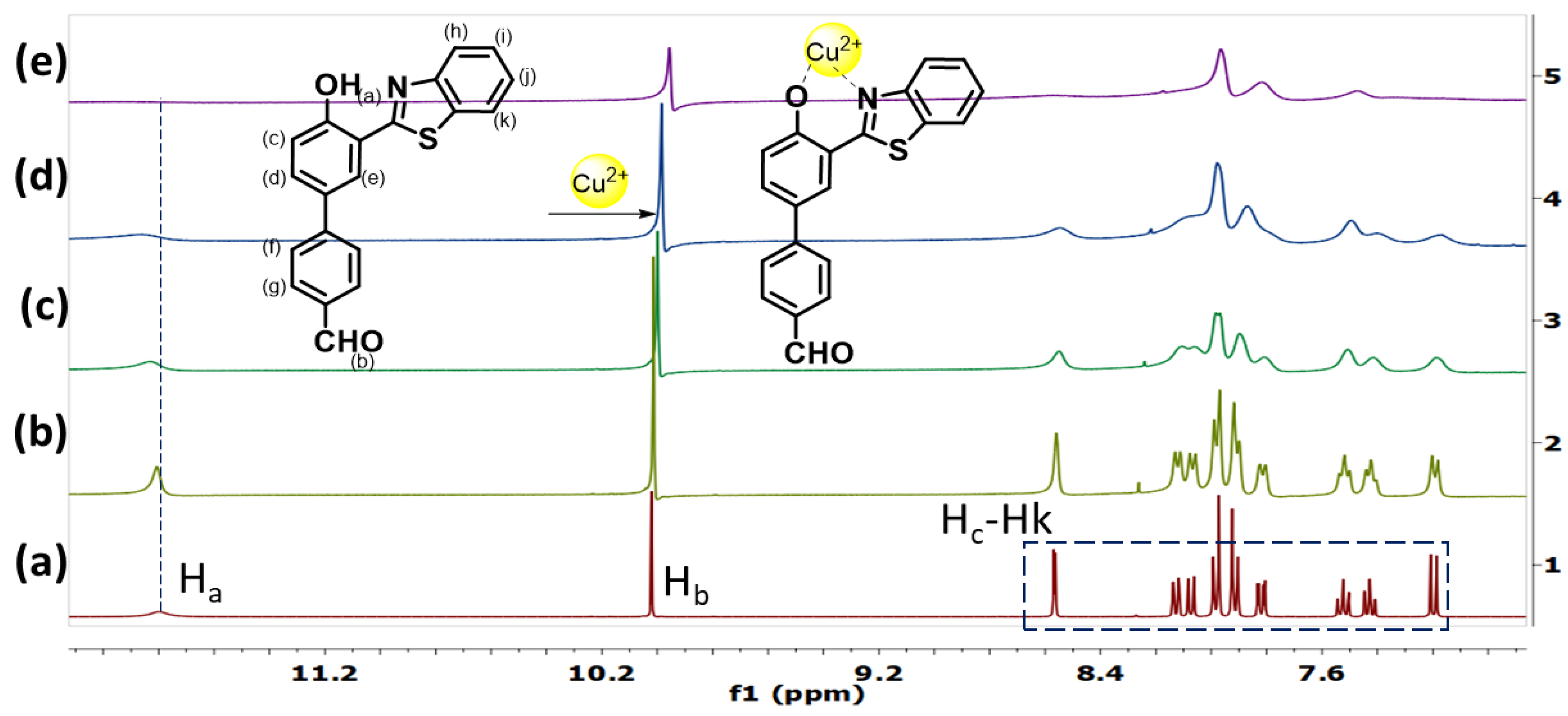
3.5. NMR, DLS and AFM Studies of HBT 2 with Cu2+ Ions
3.6. Application in Determination of Cu2+ in Real Water Samples
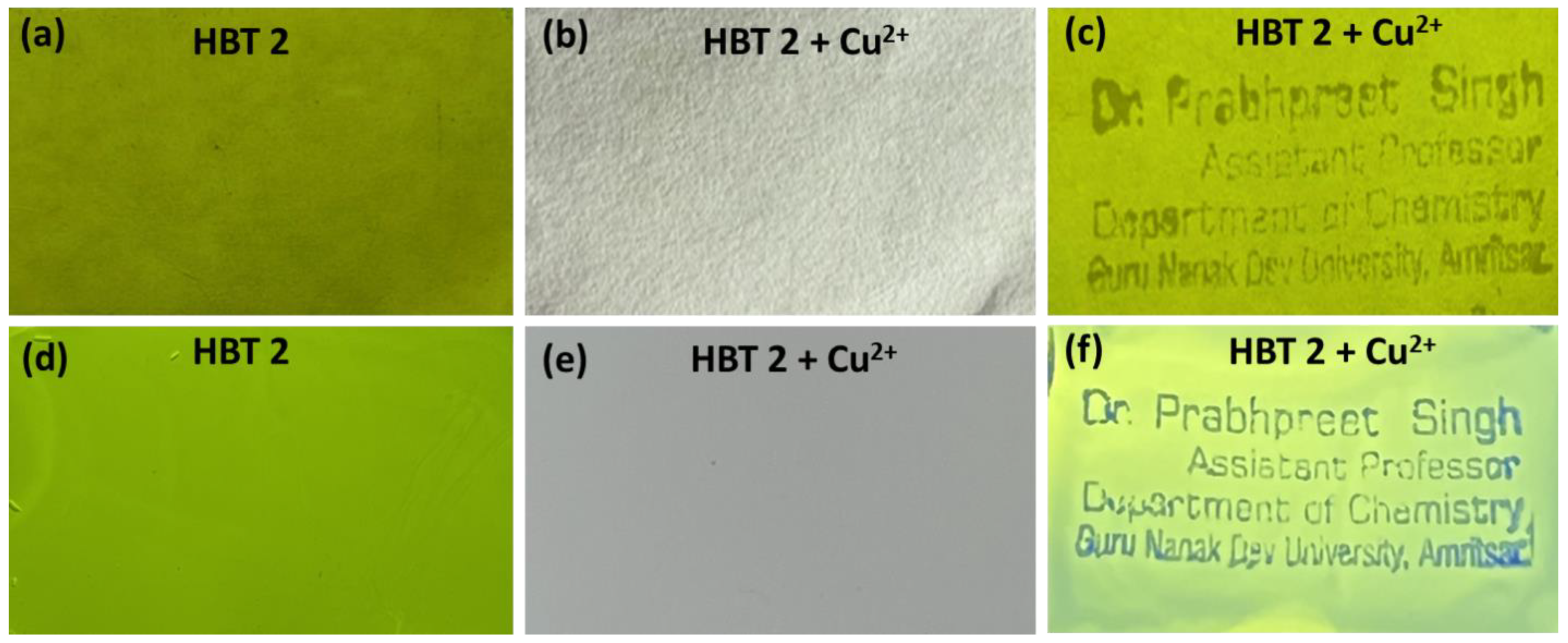
3.7. Application of HBT 2 as Anti-Counterfeiting Labels
3.8. Intracellular Detection of Cu2+ in A549 Cells
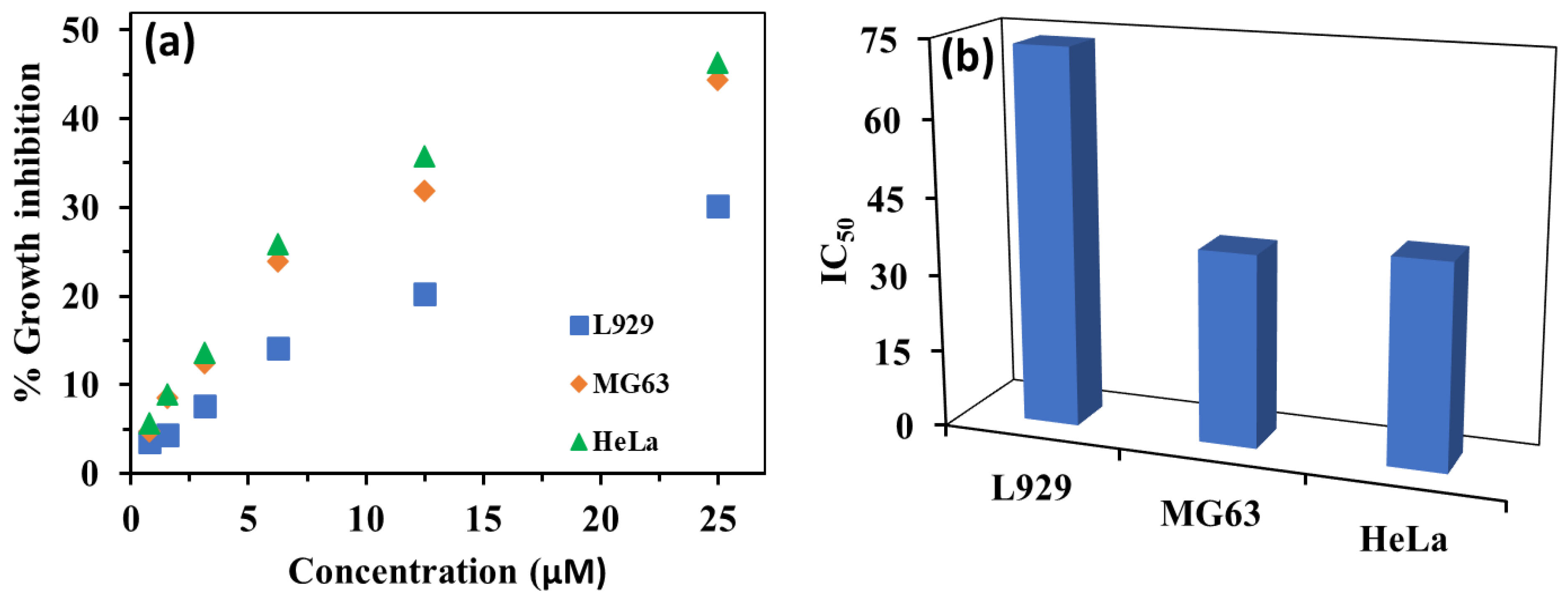
3.9. Anticancer Activity of HBT 2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Das, S.; Indurthi, H.K.; Asati, P.; Saha, P.; Sharma, D.K. Benzothiazole based fluorescent probes for the detection of biomolecules, physiological conditions, and ions responsible for diseases. Dye. Pigm. 2022, 199, 110074. [Google Scholar] [CrossRef]
- Yin, J.; Huang, L.; Wu, L.; Li, J.; James, T.D.; Lin, W. Small molecule based fluorescent chemosensors for imaging the microenvironment within specific cellular regions. Chem. Soc. Rev. 2021, 50, 12098–12150. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udhaykumari, D.; Inbaraj, V. A Review on Schiff Base Fluorescent Chemosensors for Cell Imaging Applications. J. Fluoresc. 2020, 30, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Novel Quercetin Aggregation-Induced Emission Luminogen (AIEgen) with Excited-State Intramolecular Proton Transfer for In Vivo Bioimaging. Adv. Funct. Mater. 2018, 28, 11. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Pathak, N.; Rathi, E.; Kumar, N.; Kini, S.G.; Rao, C.M. A Review on Anticancer Potentials of Benzothiazole Derivatives. Mini Rev. Med. Chem. 2020, 20, 12–23. [Google Scholar] [CrossRef]
- Jiang, J.; Bian, Y.; Lin, C.; Liu, X.; Yu, B.; Han, C.; Gong, L.; Wang, C.; Gao, Y. Rational Modification of Two-Dimensional Donor-Acceptor Covalent Organic Frameworks for Enhanced Visible Light Photocatalytic Activity. ACS Appl. Mater. Inter. 2021, 23, 27041–27048. [Google Scholar]
- Chen, W.; Wang, L.; Mo, D.; He, F.; Wen, Z.; Wu, X.; Xu, H.; Chen, L. Modulating Benzothiadiazole-Based Covalent Organic Frameworks via Halogenation for Enhanced Photocatalytic Water Splitting. Angew. Chem. Int. Ed. Engl. 2020, 59, 16902–16909. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Sharma, R.; Bhargava, G.; Kumar, S. Diphenylpyrimidinone–salicylideneamine–new ESIPT based AIEgens with applications in latent fingerprinting. J. Mater. Chem. C 2016, 4, 11180–11189. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, R.; Bhargava, G.; Kumar, S.; Singh, P. AIE+ESIPT based red fluorescent aggregates for visualization of latent fingerprints. N. J. Chem. 2018, 42, 12900–12907. [Google Scholar] [CrossRef]
- Ahmad, M.; Kumar, G.; Luxami, V.; Kaur, S.; Singh, P.; Kumar, S. Fluorescence Imaging of Surface-Versatile Latent Fingerprints at the Second and Third level using double ESIPT-based AIE fluorophore. N. J. Chem. 2021, 45, 7705–7713. [Google Scholar] [CrossRef]
- Makau, J.N.; Kitagawa, A.; Kitamura, K.; Yamaguchi, T.; Mizuta, S. Design and Development of an HBT-Based Ratiometric Fluorescent Probe to Monitor Stress-Induced Premature Senescence. ACS Omega 2020, 5, 11299–11307. [Google Scholar] [CrossRef]
- Zhou, P.; Han, K. ESIPT-based AIE luminogens: Design strategies, applications, and mechanisms. Aggregate 2022, 3, e160. [Google Scholar] [CrossRef]
- Azarifar, D.; Maleki, B.; Hojati, S.F.; Veisi, H.; Gholizadeh, M.; Salehabadi, H.; Moghadam, M.K. Efficient 2,4,6-Trichloro-1,3,5-triazine-Catalyzed Synthesis of 2-Arylbenzothiazoles and Bisbenzothiazoles by Condensation of 2-Aminithiophenol with Aldehydes under Mild Conditions. J. Hetero. Chem. 2011, 48, 449–453. [Google Scholar]
- Azarifar, D.; Malekim, B.; Setayeshnazar, M. A Simple, Microwave-Assisted, and Solvent-Free Synthesis of 2-Arylbenzothiazoles by Acetic Acid–Promoted Condensation of Aldehydes with 2- Aminothiophenol in Air. Phosphorus Sulfur Silicon 2009, 184, 2097–2102. [Google Scholar] [CrossRef]
- Kwon, J.E.; Park, S.Y. Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process. Adv. Mater. 2011, 23, 3615–3642. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Tang, K.-C.; Chou, P.-T. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [CrossRef]
- Zhou, P.; Han, K. Unraveling the Detailed Mechanism of Excited-State Proton Transfer. Acc. Chem. Res. 2018, 51, 1681–1690. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Liang, J.; Tang, B.Z.; Liu, B. Specific light-up bioprobes based on AIEgen conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goswami, S.; Bag, R.; Sikdar, Y.; Sahu, S.; Mukhopadhyay, C.D.; Drew, M. Benzimidazole based ESIPT active chemosensors enable nano-molar detection of Cu2+ in 90% aqueous solution, MCF-7 cells and plants. J. Photochem. Photobio. A 2022, 431, 114006. [Google Scholar]
- Liang, N.; Zhao, L.; Pan, W.; Yang, X.; Wu, L. AIE-ESIPT based colorimetric and “OFF-ON-OFF” fluorescence Schiff base sensor for visual and fluorescent determination of Cu2+ in aqueous media. J. Photochem. Photobio. A 2021, 420, 113506. [Google Scholar]
- Iyer, S.K.; Enbanathan, S.; Munusamy, S.; Jothi, D.; Kumar, S.M.; Gopal, A.P. A new fast-responding fluorometric “turn-on” sensor based on benzothiazole-phenanthridine for the sensitive, selective, and reversible detection of Cu2+ in real water samples and its use in bio-imaging. Dye. Pigm. 2022, 205, 111514. [Google Scholar]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharma. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, X.; Wang, D.; Zhou, X.; Ma, C.; Liu, M.; Huang, H.; Zhang, X. An amphiphilic fluorogen with aggregation-induced emission characteristic for highly sensitive and selective detection of Cu2+ in aqueous solution and biological system. Arab. J. Chem. 2021, 14, 103351. [Google Scholar]
- Yilmaz, I.; Savran, T.; Elmas, S.N.K.; Geyik, G.A.; Bostanci, A.; Aydin, D.; Arslan, F.N. “Turn-on” Fluorescence Chemosensor Based Probing of Cu2+ with Excellent Sensitivity: Experimental Study, DFT Calculations and Application in Living Cells and Natural Waters. Chem. Select. 2021, 6, 6286–6294. [Google Scholar]
- Bu, H.; Han, J.; Wang, Z.; Jia, N.; Zhou, X. Cu(I)-catalysed click reaction-triggered 3D DNA walker for constructing an “OFF-ON” Fluorescent Biosensor for Cu2+ detection. ACS Appl. Bio Mater. 2021, 4, 3571–3578. [Google Scholar]
- Liu, J.; Li, G.; Zhang, T.; Guan, P.; Liu, W.; Zhao, G.; Fang, Y.; Fug, H.; Guif, J.F. Copper stress induces zebrafish central neural system yelin defects via WNT/NOTCH-hoxb5b signalling and pou3f1/fam168a/168b DNA methylation. BBA Gene Regu. Mech. 2020, 1863, 194612. [Google Scholar]
- Stern, B.R. Essentiality and Toxicity in Copper health Risk Assessment: Overview, update and regulatory considerations. J. Toxico. Environ. Health Part A 2010, 73, 114–127. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Fanni, D.; Gerosa, C.; Nemolato, S.; Faa, G. Copper-related diseases: From chemistry to molecular pathology. Coord. Chem. Rev. 2010, 254, 876–889. [Google Scholar] [CrossRef]
- Kozlowski, H.; Luczkowski, M.; Remelli, M.; Valensin, D. Copper, zinc and iron in neurodegenerative diseases (Alzheimer’s, Parkinson’s and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. [Google Scholar] [CrossRef]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.-C.; Hsiao, C.-D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef]
- Tsade, H.K. Atomic absorption spectroscopic determination of heavy metal concentrations in Kulufo River, Arbaminch, Gamo Gofa, Ethiopia. J. Environ. Anal. Chem. 2016, 3, 1–3. [Google Scholar]
- Alkas, F.B.; Shaban, J.A.; Sukuroglu, A.A.; Kurt, M.A.; Battal, D.; Saygi, S. Monitoring and assessment of heavy metal/metalloid concentration by inductively coupled plasma mass spectroscopy (ICP-MS) method in Gonyeli Lake, Cyprus. Environ. Monit. Assess. 2017, 189, 1–8. [Google Scholar] [CrossRef]
- Sangi, M.R.; Jayatissa, D.; Kim, J.P.; Hunter, K.A. Determination of labile Cu2+ in fresh waters by chemiluminescence: Interference by iron and other cations. Talanta 2004, 62, 924–930. [Google Scholar] [CrossRef]
- Mei, C.J.; Yusof, N.A.; Ahmad, S.A.A. Electrochemical Determination of Lead & Copper Ions Using Thiolated Calix [4]arene-Modified Screen-Printed Carbon Electrode. Chemosensors 2021, 9, 157. [Google Scholar]
- Cotruvo, J.J.A.; Aron, A.T.; Ramos-Torres, K.M.; Chang, C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015, 44, 4400–4414. [Google Scholar] [CrossRef] [Green Version]
- Udhayakumari, D.; Naha, S.; Velmathi, S. Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013–2015. Anal. Methods 2017, 9, 552–578. [Google Scholar] [CrossRef]
- Li, L.; Feng, Q.; Niu, G.; Zhang, W.; Li, Y.; Kang, M.; Xu, K.; He, Z.; Hou, H.; Tang, B.Z. Benzothiazole-based AIEgen with tunable excited-state intramolecular proton transfer (ESIPT) and restricted intramolecular rotation (RIR) processes for highly sensitive physiological pH sensing. ACS Sens. 2018, 3, 920–928. [Google Scholar] [CrossRef]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent Chemosensors Based on Spiroring-Opening of Xanthenes and Related Derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Kang, R.; Grimm, L.M.; Cola, L.D.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P. A perylene diimide-based near-IR ratiometric sensor for detection of Cu2+ ions: Ensemble for discrimination of CN− and S22− ions. Anal. Methods 2020, 12, 758–767. [Google Scholar]
- Singh, P.; Kumar, K.; Kaur, S.; Bhargava, G.; Kumar, S. Perylene diimide-Cu2+ based fluorescent nanoparticles for the detection of spermine in clinical and food samples: A step toward the development of a diagnostic kit as a POCT tool for spermine. J. Mater. Chem. B 2019, 7, 7218–7227. [Google Scholar]
- Singh, P.; Singh, H.; Bhargava, G.; Kumar, S. Triple-signaling mechanism-based three-in-one multi-channel chemosensor for discriminating Cu2+, acetate and ion pair mimicking AND, NOR, INH and IMP logic functions. J. Mater. Chem. C 2015, 3, 5524–5532. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P.; Sharma, N.; Kaur, S. Ratiometric ‘lightening up’ intracellular probe for Cu2+ and ClO− and applications for real time detection. J. Photochem. Photobio. A 2022, 423, 113574. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, P.; Kaur, S.; Kaur, S. Near-IR oxime-based solvatochromic perylene diimide probe as a chemosensor for Pd species and Cu2+ ions in water and live cells. Photochem. Photobiol. Sci. 2020, 19, 504. [Google Scholar]
- Singh, P.; Singh, H.; Vanita, V.; Sharma, R.; Bhargava, G.; Kumar, S. Nanomolar Cu2+ Detection in water on Disassembly of AIEgen: Applications in blood Serum, Cell imaging and complex Logic circuits. Chem. Sel. 2016, 1, 6880–6887. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, R.; Sharma, P.; Kumar, S.; Kumar, S. Hydroxyphenyl Benzothiazole Derivative, its Method of Preparation and Use Thereof. Indian Patent Applications 202211017786.
- Guchhait, N.; Bhattacharyya, A.; Makhal, S.C.; Mandal, S. Exploring the hidden potential of a methoxy substituted HBT derivative as an efficient example of coupling of AIE and ESIPT process and as an energy harvesting platform. New J. Chem. 2019, 43, 15087–15096. [Google Scholar]
- Kim, C.; Heo, J.S.; Suh, B. Selective detection of Cu2+ by benzothiazole-based colorimetric chemosensor: A DFT study. J. Chem. Sci. 2022, 43, 134. [Google Scholar]
- Pu, S.; Wang, R.; Dong, X.; Leo, G.; Leo, P. A novel sensitive sensor for Cu2+ and multi-switch based on a diarylethene with a 2-(2′-hydroxyphenyl)benzothiazole unit. Tetrahedron 2016, 72, 2935–2942. [Google Scholar]
- Han, Y.; Yang, C.; Chen, Y.; Wu, K.; Wei, T.; Wang; Zhang, J.S. A reactive probe for Cu2+ based on the ESIPT mechanism and its application in live-cell imaging. Anal. Methods 2015, 7, 3327. [Google Scholar]
- Xing, Z.; Li, J.; Wang, J.; Tian, Z.; Wu, D.; Xiang, Y. A dual-functional probe for sensing pH changes and ratiometric detection of Cu2+. Spectrochim. Acta Part A Mol. Biomol. Spec. 2020, 235, 11831. [Google Scholar]
- Chansaenpak, K.; Nootem, J.; Daengngern, R.; Sattayanon, C.; Wattanathana, W.; Wannapaiboon, S.; Rashatasakhon, P. The synergy of CHEF and ICT toward fluorescence ‘turn-on’ probes based on push-pull benzothiazoles for selective detection of Cu2+ in acetonitrile/ water mixture. J. Photochem. Photobio. 2021, 415, 113318. [Google Scholar]



| Technique Used | Samples Taken from | Cu2+ Added (µM) | Cu2+ Found (µM) | % Age Recovery |
|---|---|---|---|---|
| Fluorescence | Amritsar | 2.6 | 2.5 | 98.03 |
| 3.4 | 3.5 | 102.94 | ||
| 5.6 | 5.5 | 99.09 | ||
| 7.7 | 7.5 | 99.29 | ||
| Bathinda (Rural) | 3.5 | 3.0 | 95.38 | |
| 4.5 | 4.5 | 95.23 | ||
| 6.2 | 6.0 | 100.00 | ||
| 7.9 | 7.5 | 95.54 | ||
| Bathinda (Town) | 1.6 | 1.5 | 95.23 | |
| 3.2 | 3.0 | 93.75 | ||
| 4.7 | 4.5 | 96.77 | ||
| 7.6 | 7.5 | 99.33 | ||
| Muktsar | 1.6 | 1.5 | 96.15 | |
| 2.7 | 2.5 | 94.33 | ||
| 4.6 | 4.5 | 97.82 | ||
| 7.5 | 7.5 | 100.00 | ||
| Faridkot | 1.7 | 1.5 | 90.90 | |
| 2.5 | 2.5 | 99.00 | ||
| 4.5 | 4.5 | 100.00 | ||
| 6.2 | 6.5 | 105.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, R.; Kour, R.; Marok, S.S.; Kaur, S.; Singh, P. AIE+ESIPT Active Hydroxybenzothiazole for Intracellular Detection of Cu2+: Anticancer and Anticounterfeiting Applications. Molecules 2022, 27, 7678. https://doi.org/10.3390/molecules27227678
Kaur R, Kour R, Marok SS, Kaur S, Singh P. AIE+ESIPT Active Hydroxybenzothiazole for Intracellular Detection of Cu2+: Anticancer and Anticounterfeiting Applications. Molecules. 2022; 27(22):7678. https://doi.org/10.3390/molecules27227678
Chicago/Turabian StyleKaur, Rajdeep, Rasdeep Kour, Satwinder Singh Marok, Satwinderjeet Kaur, and Prabhpreet Singh. 2022. "AIE+ESIPT Active Hydroxybenzothiazole for Intracellular Detection of Cu2+: Anticancer and Anticounterfeiting Applications" Molecules 27, no. 22: 7678. https://doi.org/10.3390/molecules27227678
APA StyleKaur, R., Kour, R., Marok, S. S., Kaur, S., & Singh, P. (2022). AIE+ESIPT Active Hydroxybenzothiazole for Intracellular Detection of Cu2+: Anticancer and Anticounterfeiting Applications. Molecules, 27(22), 7678. https://doi.org/10.3390/molecules27227678






