Phenol Derivatives Obtained from Grape Seed Extract Show Virucidal Activity against Murine Norovirus
Abstract
:1. Introduction
2. Results
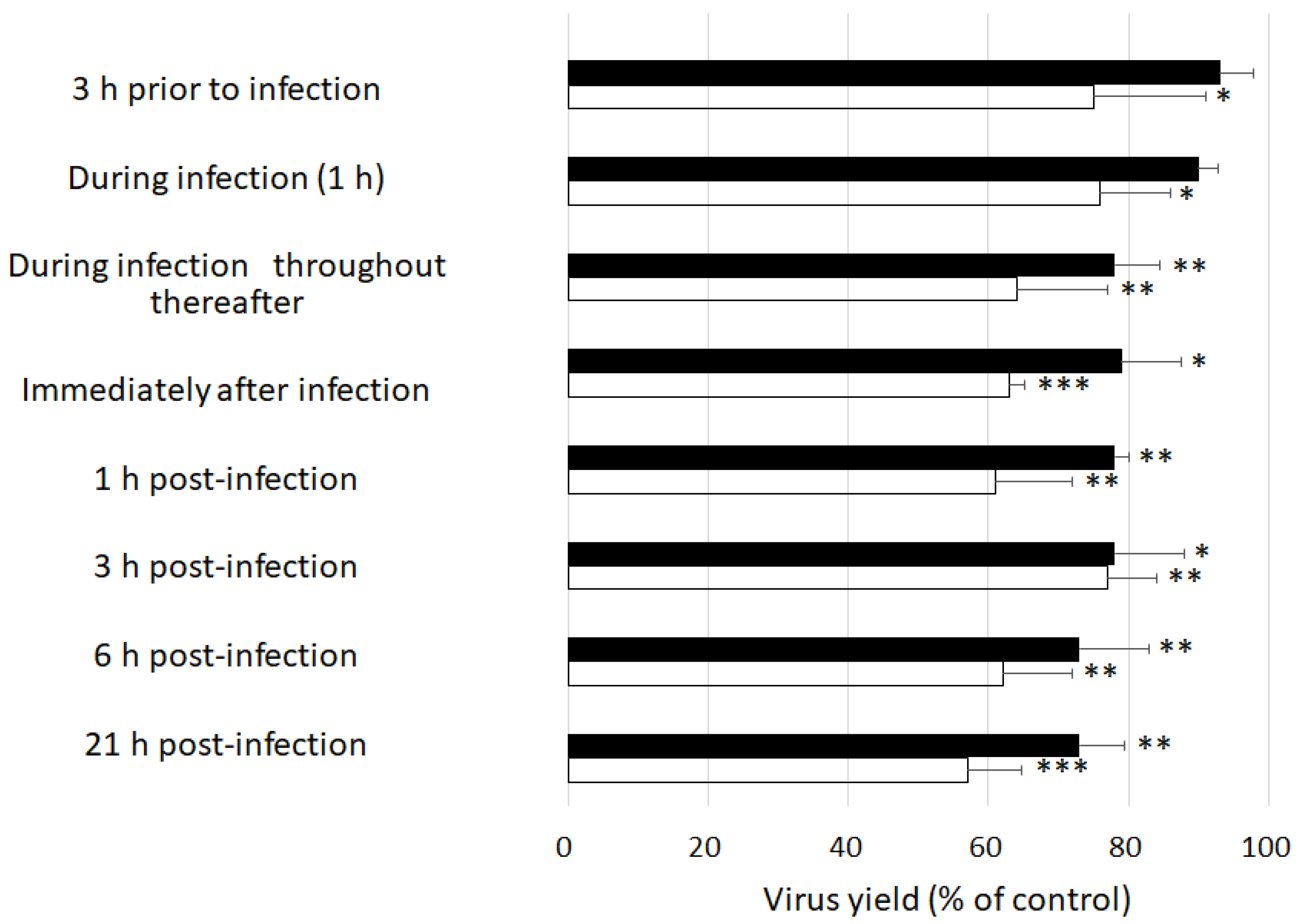
2.1. Effects of GSE Treatment Enveloped and Non-Enveloped Viral Replication
2.2. Enveloped and Non-Enveloped Viral Inactivation Using GSE Treatment
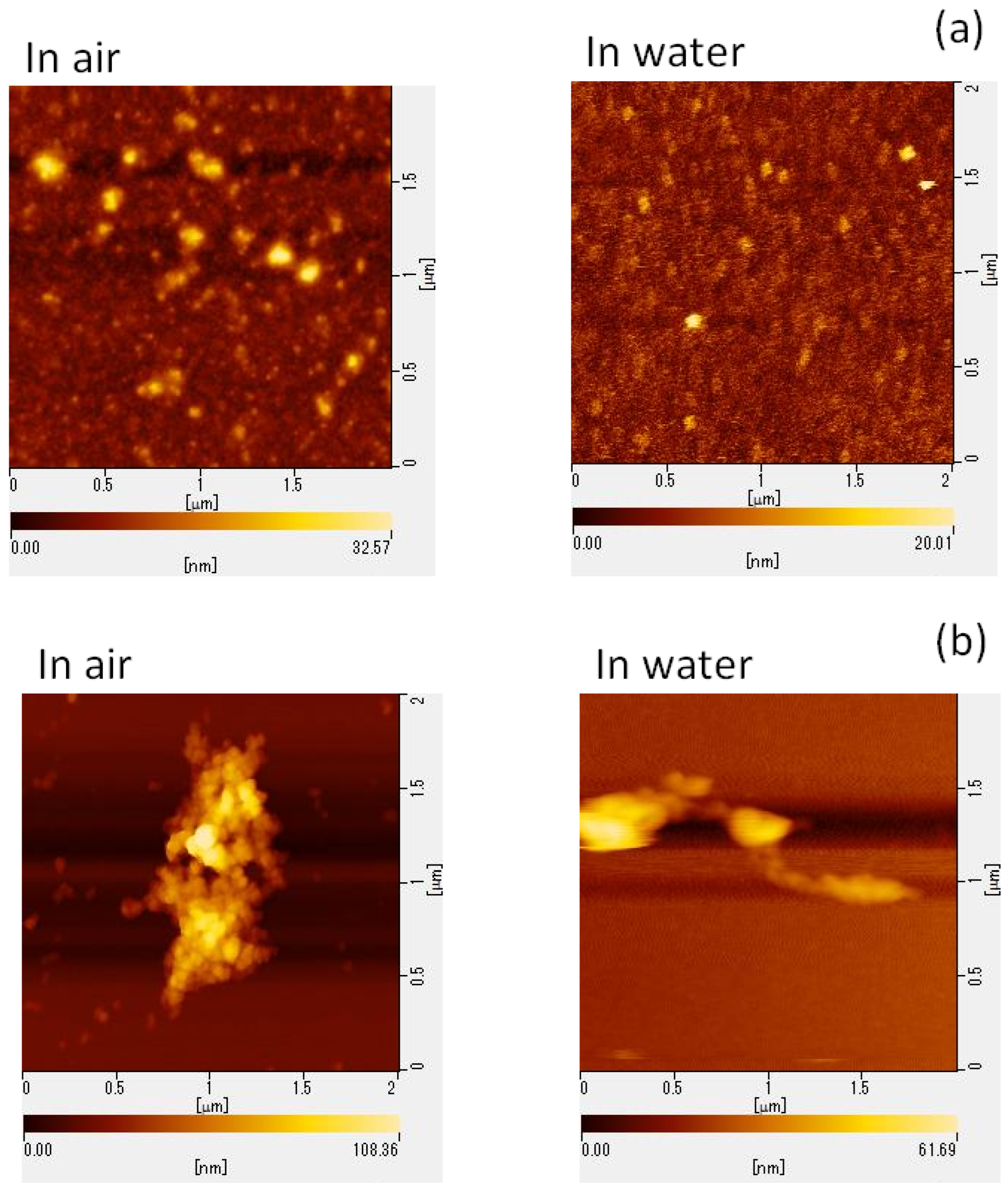
2.3. AFM Images
2.4. Evaluation of the Irreversibility of GSE’s Virucidal Effect in Vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cells and Viruses
4.3. Antiviral Assay
4.4. Virucidal Assay
4.5. Time-of-Addition Experiments
4.6. Morphological Analysis of Viral Particles via AFM
4.7. In Vivo Animal Experiments
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Anastasidi, M.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Haroutounian, S.A. Antilisterial activities of polyphenol-rich extract of grapes and vinification by-products. J. Agric. Food Chem. 2009, 57, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, V.; Singh, T.; Katiyar, S.K. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008, 269, 378–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Mandair, R.; Agarwal, R.; Agarwal, C. Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr. Cancer 2008, 60, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharaf, M.; EI-Deeb, N.; Eladawi, H. The potentiality of grape seed extract as a novel anti-hepatitis C virus agent. J. Med. Sci. 2012, 12, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; D’Souza, D.H. Grape seed extract for control of human enteric viruses. Appl. Environ. Microbiol. 2011, 77, 3982–3987. [Google Scholar] [CrossRef] [Green Version]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Development of antiviral and bacteriostatic chitosan-based food packaging material with grape seed extract for murine norovirus, Escherichia coli and Listeia innocua control. Food Sci. Nutr. 2020, 8, 6174–6181. [Google Scholar] [CrossRef]
- Weiss, H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 2004, 11 (Suppl. 1), 24A–35A. [Google Scholar] [PubMed]
- World Health Organization. Evolution of a Pandemic: A (H1N1) 2009, April 2009—August 2010, 2nd ed.; World Health Organization: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/handle/10665/78414 (accessed on 3 November 2022).
- Filipe, A.M.M.; Rincao, V.P.; Beneti, F.J.; Linhares, R.E.C.; Galina, K.J.; Toledo, C.E.M.; Lopes, G.C.; de Mello, J.C.P.; Nozawa, C. Antiviral Effect of Guazuma ulmifolia and Stryphnodendron adstringens on Poliovirus and Bovine Herpesvirus. Biol. Pharm. Bull. 2006, 29, 1092–1095. [Google Scholar] [CrossRef] [Green Version]
- Casanova, V.; Sousa, F.H.; Stevens, C.; Barlow, P.G. Antiviral therapeutic approaches for human rhinovirus infections. Future Virol. 2018, 13, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Payne, D.C.; Vinjé, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 2013, 368, 1121–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treanor, J.J.; Atmar, R.L.; Frey, S.E.; Gormley, R.; Chen, W.H.; Ferreira, J.; Goodwin, R.; Borkowski, A.; Clemens, R.; Mendelman, P.M. A novel intramuscular bivalent-reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J. Infect. Dis. 2014, 210, 1763–1771. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, Y.; Tohya, Y.; Ike, F.; Kajita, A.; Park, S.J.; Ishii, Y.; Kyuwa, S.; Yoshikawa, Y. Indirect ELISA and indirect immunofluorescent antibody assay for detecting the antibody against murine norovirus S7 in mice. Exp. Anim. 2010, 59, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.A.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P.; et al. Antiviral activity of Vitis vinifera leaf extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1263. [Google Scholar] [CrossRef] [PubMed]
- Lipson, S.M.; Ozen, F.S.; Karthikeyan, L.; Bulut, O.; Hyka, X.; Sullivan, G.L.; Gordon, R.E. Flavonoid-associated direct loss of rotavirus antigen/antigen activity in cell-free suspension. J. Med. Act. Plants 2013, 2, 10–24. [Google Scholar] [CrossRef]
- Derksen, A.; Hensel, A.; Hafezi, W.; Herrmann, F.; Schmidt, T.J.; Ehrhardt, C.; Ludwig, S.; Kuhn, J. 3-O-Galloylated procyanidins from Rumex acetosa L. inhibit the attachment of influenza A virus. PLoS ONE 2014, 9, e110089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-W.; Kim, Y.I.; Im, C.-N.; Kim, S.W.; Kim, S.J.; Min, S.; Joo, Y.H.; Yim, S.-V.; Chung, N. Grape seed proanthocyanidin inhibits mucin synthesis and viral replication by suppression of AP-1 and NF-κB via p38 MAPKs/JNK signaling pathways in respiratory syncytial virus-infected A549 cells. J. Agric. Food Chem. 2017, 65, 4472–4483. [Google Scholar] [CrossRef]
- Trivedi, T.K.; DeSalvo, T.; Lee, L.; Palumbo, A.; Moll, M.; Curns, A.; Hall, A.J.; Patel, M.; Parashar, U.D.; Lopman, B.A. Hospitalization and mortality associated with norovirus outbreaks in nursing homes. J. Am. Med. Assoc. 2012, 308, 1668–1675. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.; Vergoulidou, M.; SchreirSchreier, E.; Loddenkemper, C.; Reinwald, M.; Schmidt-Hieber, M.; Flegel, W.A.; Thiel, E.; Schneider, T. Norovirus gastroenteritis causes severe and lethal complicatons after chemotherapy and hematropoietic stem cell transplantation. Blood 2011, 117, 5850–5856. [Google Scholar] [CrossRef] [Green Version]
- Bagci, S.; Eis-Hübinger, A.M.; Yassin, A.F.; Simon, A.; Bartmann, P.; Franz, A.R.; Mueller, A. Clinical characteristics of viral intestinal infection in preterm and term neonates. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1079–1084. [Google Scholar] [CrossRef]
- Bok, K.; Green, K.Y. Norovirus gastroenteritis in immunocompromised patients. N. Engl. J. Med. 2012, 367, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henke-Gendo, C.; Harste, G.; Juergens-Saathoff, B.; MttnerMattner, F.; Deppe, H.; Heim, A. New real-time PCR detects prolonged norovirus excretion in highly immunosuppressed patients and children. J. Clin. Microbiol. 2009, 47, 2855–2862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, A.; Adams, O.; Laws, H.J.; Schroten, H.; Tenenbaum, T. Quantitative detection of norovirus excretion in pediatric patients with cancer and prolonged gastroenteritis and shedding of norovirus. J. Med. Virol. 2008, 80, 1461–1467. [Google Scholar] [CrossRef]
- SchromSchorn, R.; Höhne, M.; Meerbach, A.; Bossart, W.; Wüthrich, R.P.; Schreier, E.; Müller, N.J.; Fehr, T. Chronic norovirus infection after kidney transplantation: Molecular evidence for immune-driven viral evolution. Clin. Infect. Dis. 2010, 51, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar] [CrossRef]
- Hayashi, K.; Komatsu, S.; Kuno, H.; Asai, S.; Matsuura, I.; Kudkyal, V.R.; Kawahara, T. Virucidal and immunostimulating activities of monogalactosyl diacylglyceride from Coccomyxa sp. KJ, a green microalga, against murine norovirus and feline calicivirus. Mar. Drugs 2022, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Asai, S.; Umezawa, K.; Kakizoe, H.; Miyachi, H.; Morita, M.; Akaike, T.; Kuno, H.; Komatsu, S.; Watanabe, T.; et al. Virucidal effect of monogalactosyl diacylglyceride from a green microalga, Coccomyxa sp. KJ, against clinical isolates of SARS-CoV-2 as assessed by a plaque assay. J. Clin. Lab. Anal. 2022, 36, e24146. [Google Scholar] [CrossRef]
- Kawahara, T.; Hiramatsu, H.; Suzuki, Y.; Nakakita, S.; Ohno, Y.; Maehashi, K.; Matsumoto, K.; Okamoto, K.; Matsuba, T.; Utsunomiya, R. Development of nano-carbon biosensors using glycan for host range detection of influenza virus. Condens. Matter 2016, 1, 7. [Google Scholar] [CrossRef]


| Antiviral Activity | ||||
|---|---|---|---|---|
| Envelope | CC50 (μg/mL) | EC50 (μg/mL) | CC50/EC50 | |
| Herpes simplex virus type 2 | + | 140 | 40 | 3.5 |
| Influenza A virus | + | 80 | 3.8 | 21 |
| Poliovirus type 1 | - | 150 | 33 | 4.5 |
| Human rhinovirus | - | 120 | 28 | 4.3 |
| Murine norovirus | - | 21 | 160 | 0.13 |
| GSE | Relative Infectivity (%) | ||||
|---|---|---|---|---|---|
| (μg/mL) | 1 min | 5 min | 10 min | 30 min | |
| Herpes simplex virus type 2 | 0 | 96 ± 4.2 | 93 ± 3.5 | 90 ± 2.1 | 81 ± 6.4 |
| 1 | 68 ± 6.4 * | 49 ± 5.7 * | 34 ± 4.2 ** | 23 ± 4.9 ** | |
| 10 | 0.8 ± 0.57 *** | 0 *** | 0 *** | 0 ** | |
| 50 | 0 *** | 0 *** | 0 *** | 0 ** | |
| Influenza A virus | 0 | 93 ± 12 | 90 ± 5.7 | 85 ± 11 | 82 ± 7.8 |
| 1 | 47 ± 7.1 * | 44 ± 8.5 * | 13 ± 4.2 * | 12 ± 4.2 ** | |
| 10 | 14 ± 3.5 * | 10 ± 4.2 ** | 9.3 ± 0.99 * | 2.3 ± 0.35 ** | |
| 50 | 0 ** | 0 ** | 0 ** | 0 ** | |
| Poliovirus type 1 | 0 | 97 ± 3.5 | 97 ± 7.8 | 93 ± 3.5 | 88 ± 4.9 |
| 1 | 43 ± 7.8 * | 34 ± 5.7 * | 16 ± 4.2 ** | 10 ± 3.5 ** | |
| 10 | 4.0 ± 1.5 *** | 0 ** | 0 *** | 0 *** | |
| 50 | 0 *** | 0 ** | 0 *** | 0 *** | |
| Human rhinovirus | 0 | 98 ± 2.1 | 94 ± 4.9 | 90 ± 9.2 | 87 ± 7.1 |
| 1 | 54 ± 9.2 * | 36 ± 6.4 ** | 19 ± 4.2 ** | 11 ± 2.8 ** | |
| 10 | 2.8 ± 1.3 *** | 0.94 ± 0.23 *** | 0 ** | 0 ** | |
| 50 | 0 *** | 0 *** | 0 ** | 0 ** | |
| Murine norovirus | 0 | 95 ± 2.8 | 94 ± 5.7 | 91 ± 6.4 | 80 ± 6.4 |
| 1 | 33 ± 6.4 ** | 12 ± 4.9 ** | 1.9 ± 0.57 ** | 0 ** | |
| 10 | 1.6 ± 0.28 *** | 0 ** | 0 ** | 0 ** | |
| 50 | 0 *** | 0 ** | 0 ** | 0 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudkyal, V.R.; Matsuura, I.; Hiramatsu, H.; Hayashi, K.; Kawahara, T. Phenol Derivatives Obtained from Grape Seed Extract Show Virucidal Activity against Murine Norovirus. Molecules 2022, 27, 7739. https://doi.org/10.3390/molecules27227739
Kudkyal VR, Matsuura I, Hiramatsu H, Hayashi K, Kawahara T. Phenol Derivatives Obtained from Grape Seed Extract Show Virucidal Activity against Murine Norovirus. Molecules. 2022; 27(22):7739. https://doi.org/10.3390/molecules27227739
Chicago/Turabian StyleKudkyal, Vyankatesh Raml, Iori Matsuura, Hiroaki Hiramatsu, Kyoko Hayashi, and Toshio Kawahara. 2022. "Phenol Derivatives Obtained from Grape Seed Extract Show Virucidal Activity against Murine Norovirus" Molecules 27, no. 22: 7739. https://doi.org/10.3390/molecules27227739
APA StyleKudkyal, V. R., Matsuura, I., Hiramatsu, H., Hayashi, K., & Kawahara, T. (2022). Phenol Derivatives Obtained from Grape Seed Extract Show Virucidal Activity against Murine Norovirus. Molecules, 27(22), 7739. https://doi.org/10.3390/molecules27227739






